Abstract
Rationale: Neonatal chronic lung disease, known as bronchopulmonary dysplasia (BPD), remains a serious complication of prematurity despite advances in the treatment of extremely low birth weight infants.
Objectives: Given the reported protective actions of bone marrow stromal cells (BMSCs; mesenchymal stem cells) in models of lung and cardiovascular injury, we tested their therapeutic potential in a murine model of BPD.
Methods: Neonatal mice exposed to hyperoxia (75% O2) were injected intravenously on Day 4 with either BMSCs or BMSC-conditioned media (CM) and assessed on Day 14 for lung morphometry, vascular changes associated with pulmonary hypertension, and lung cytokine profile.
Measurements and Main Results: Injection of BMSCs but not pulmonary artery smooth muscle cells (PASMCs) reduced alveolar loss and lung inflammation, and prevented pulmonary hypertension. Although more donor BMSCs engrafted in hyperoxic lungs compared with normoxic controls, the overall low numbers suggest protective mechanisms other than direct tissue repair. Injection of BMSC-CM had a more pronounced effect than BMSCs, preventing both vessel remodeling and alveolar injury. Treated animals had normal alveolar numbers at Day 14 of hyperoxia and a drastically reduced lung neutrophil and macrophage accumulation compared with PASMC–CM-treated controls. Macrophage stimulating factor 1 and osteopontin, both present at high levels in BMSC-CM, may be involved in this immunomodulation.
Conclusions: BMSCs act in a paracrine manner via the release of immunomodulatory factors to ameliorate the parenchymal and vascular injury of BPD in vivo. Our study suggests that BMSCs and factor(s) they secrete offer new therapeutic approaches for lung diseases currently lacking effective treatment.
Keywords: bronchopulmonary dysplasia, pulmonary hypertension, mesenchymal stem cells, inflammation, bone marrow stromal cells
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Bone marrow stromal cells (BMSCs) have cytoprotective effects in adult animal models of lung injury through poorly-defined mechanisms, which may include modulation of lung inflammation. Whether BMSC therapy can attenuate lung injury in a neonatal mouse model of bronchopulmonary dysplasia and the potential mechanisms of action are unknown.
What This Study Adds to the Field
Systemic delivery of BMSC-conditioned media inhibits lung inflammation and prevents neonatal lung injury due to hyperoxia, suggesting that these effects are mediated through paracrine mechanisms.
Bronchopulmonary dysplasia (BPD) is a serious and common complication of prematurity (1, 2). The degree of initial respiratory distress, need for mechanical ventilation, and associated infections are some of the factors that have been linked with BPD, as well as a prominent inflammatory response that produces lung injury often complicated by pulmonary hypertension (3). Pathological mechanisms include fibrosis, apoptosis, and abnormal cellular proliferation that can impair normal repair processes, leading to arrested alveolar development and associated abnormal vascular growth. Preventive approaches, including alternative ventilator strategies and use of antiinflammatory medications such as corticosteroids, have limited success and unacceptable side effects (4).
Recent advances in stem cell research hold promise for the prevention and treatment of chronic debilitating diseases. Bone marrow stromal cells (BMSCs, also known as mesenchymal stem cells, MSCs) have been shown to differentiate into a variety of tissue cell types (5–10). A number of studies in pulmonary medicine have demonstrated that BMSC treatment can ameliorate bleomycin, monocrotaline, endotoxin, or lipopolysaccharide (LPS)-induced lung injury (9, 11–15). Mechanisms for this protection are not limited to tissue repair, such as engraftment and differentiation of BMSCs into specific lung cell types, but also include immunomodulation, the latter being associated with an increase in circulating levels of specific growth factors or lower levels of inflammatory cytokines (9, 11, 13–15). Collectively, these studies demonstrate inherent qualities of stem cells, such as the capacity to respond, migrate, and repair damaged tissue and to deliver protection by secretion of specific growth and immunoprotective factors. These properties make BMSC treatment a promising approach for protection from and repair of neonatal lung injury.
In the present study, we hypothesized that BMSCs could confer protection in a neonatal mouse model of BPD. The saccular and alveolar stages of lung development in the mouse occur around the time of birth with alveolarization starting on Postnatal Day 5 and continuing up to 3 to 4 weeks of age. The developmental stage of the mouse lung at birth overlaps with that in the human preterm neonate between 24 and 28 weeks' gestation, making the newborn mouse a good model to study human developmental lung injury. Indeed, hyperoxia-induced lung injury in neonatal mice is similar to BPD with rarification and simplification of alveoli, thickened alveolar septa, and right ventricular hypertrophy. We report here that intravenous delivery of BMSCs in newborn mice conferred significant vascular and immunological protection from hyperoxia-induced injury but had minimal effect in preserving alveolar architecture. Interestingly, concentrated BMSC-conditioned media (BMSC-CM) completely prevented both vascular and alveolar injury resulting in normal alveolar number and thin septa, comparable to normoxic controls. Our findings suggest that BMSCs have important cytoprotective effects in this mouse model of developmental lung injury mimicking BPD, via paracrine mechanisms involving the release of immunomodulatory and vasoprotective mediators.
METHODS
Cell Isolation, Culture, and Differentiation
BMSCs were isolated from femurs and tibiae of 5- to 7-week-old FVB mice as described (16–18). Plastic adherent cells were grown in tissue culture, and at passage two to three immunodepletion was performed as per published protocols and the International Society for Cellular Therapy guidelines (19–21), as detailed in the online supplement. Cells were used for injection or for derivation of conditioned media between passages 7 through 10. The differentiation potential of BMSC cultures was assessed following published protocols (17, 18).
Animals and Hyperoxia Exposure
Timed pregnant FVB mice were either obtained from Charles River Laboratories (Wilmington, MA) or were raised in the Animal Facility at Children's Hospital Boston. Newborn pups from four different litters were subdivided into two groups and exposed to 75% O2 in a Plexiglas chamber (OxyCycler; BioSpherix, Redfield, NY) or 21% O2 within 24 hours of birth. Ventilation was adjusted to remove CO2 so that it did not exceed 5,000 ppm (0.5%) (average range 1,000–3,000 ppm). Ammonia was removed by ventilation and activated charcoal filtration through an air purifier. Dams were rotated from hyperoxia to room air every 24 to 48 hours to prevent excessive oxygen toxicity to the adult animals. Each litter consisted of fewer than 12 pups to control for the effect of litter size on nutrition and growth.
BMSC Transplantation
A suspension of 5 × 104 BMSCs in 50 μL phosphate-buffered saline (PBS) was injected via the superficial temporal vein on Postnatal Day 4. Processing of lungs for bronchoalveolar lavage fluid (BALF) retrieval, lung fixation, and assessment of hearts for Fulton's Index is described in the online supplement.
Assessment of BMSC Engraftment
The number of male donor BMSCs retained in female recipient lungs was determined as detailed in the online supplement.
BMSC-conditioned Media Treatment
BMSC and pulmonary artery smooth muscle cell (PASMC) confluent cultures were incubated in serum-free α-MEM media for 24 hours and conditioned media representing equal number of cells were concentrated 10-fold using Amicon Ultra Centrifugal Filter Device (Millipore Corporation, Billerica, MA) with a molecular weight cutoff of 10 kD. A volume of 50 μL concentrated BMSC-CM was injected via the superficial temporal vein on Postnatal Day 4. PASMC-CM in the same concentration and volume served as control. After an additional 10 days in hyperoxia, animals were killed, lungs perfused and embedded for histopathology, and hearts weighed for assessment of Fulton's Index, as detailed in the online supplement.
Tissue and Vessel Morphometry
Vessel morphometry and assessment of volume density of alveolar wall tissue (VDawt) are described in the online supplement.
Cytokine Levels in BALF
Cytokine profiling in BALF using the Luminex 200 System (Luminex Corp., Austin, TX) is described in the online supplement.
Statistical Analysis
All values were expressed as mean ± SEM. Comparison of results between different groups was performed by one-way analysis of variance or Mann Whitney U test, as appropriate, using GraphPad InStat (GraphPad Software, San Diego, CA). P value less than 0.05 was considered significant.
RESULTS
Assessment of BMSC Engraftment in Neonatal Mouse Lungs
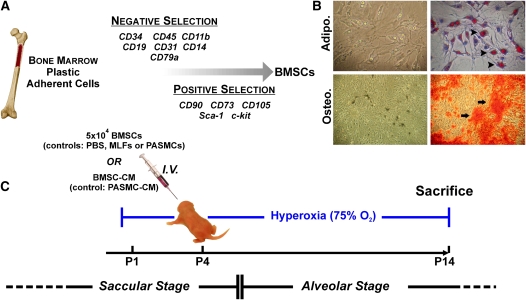
BMSC cultures were prepared from bone marrow of 5- to 7-week-old male mice (Figure 1A) and cells were assessed for differentiation potential into adipocytes and osteocytes (Figure 1B) before transplantation, as described. For the hyperoxia treatment group, newborn pups were placed in 75% O2 on Postnatal Day 1. BMSCs from male donors were injected intravenously into female hyperoxic or normoxic pups on Postnatal Day 4 and animals were continued in hyperoxia for an additional 10 days or maintained under normoxic conditions (Figure 1C). In certain experiments, lungs were harvested on Postnatal Day 5 or Postnatal Day 14 (1 or 10 d post injection, respectively) for assessment of donor cell engraftment. Quantitative polymerase chain reaction assays on DNA from lungs collected on Postnatal Day 5 showed similar levels of Y chromosome in normoxia and hyperoxia groups, and these overall levels decreased significantly over time. However, on Postnatal Day 14, donor BMSCs represented 0.025% of total lung cells in the hyperoxic group and 0.006% in the normoxic group (P < 0.05), indicating a higher retention of transplanted BMSCs in the injured lung (see Figure E1A in the online supplement). To further confirm our findings we visualized the male donor cells in the female recipient mice using fluorescence in situ hybridization for the Y chromosome. Figure E1B, in the online supplement, shows fluorescein isothiocyanate–labeled Y chromosome signal colocalized with the 4′,6-diamidino-2-phenylindole staining of the nucleus in control sections from male mouse lung (upper panels) and hyperoxia-exposed female recipient lung (lower panels). These events were rare in the female recipient animals and paralleled the results of the quantitative assessment of engraftment using real-time polymerase chain reaction for Y chromosome sequences.
Figure 1.
Isolation and differentiation of bone marrow stromal cell (BMSC) cultures and experimental design for in vivo study. (A) Cells were isolated from bone marrow and subjected to negative and positive selection using the indicated cell surface markers. (B) BMSCs were grown in the presence of adipogenic or osteogenic media, as described in Materials and Methods. After 3 weeks of adipogenic induction, lipid droplets could be observed within the cells (upper left panel), which were then stained using Oil Red O, as indicated by arrowheads (upper right panel). After 3 weeks of osteogenic induction (lower left panel), calcium deposition was visualized using Alizarin Red S staining, as indicated by arrows (lower right panel). (C) Neonatal mouse pups were exposed to hyperoxia on Postnatal Day 1, injected with cells or conditioned media on Postnatal Day 4, and killed on Day 14.
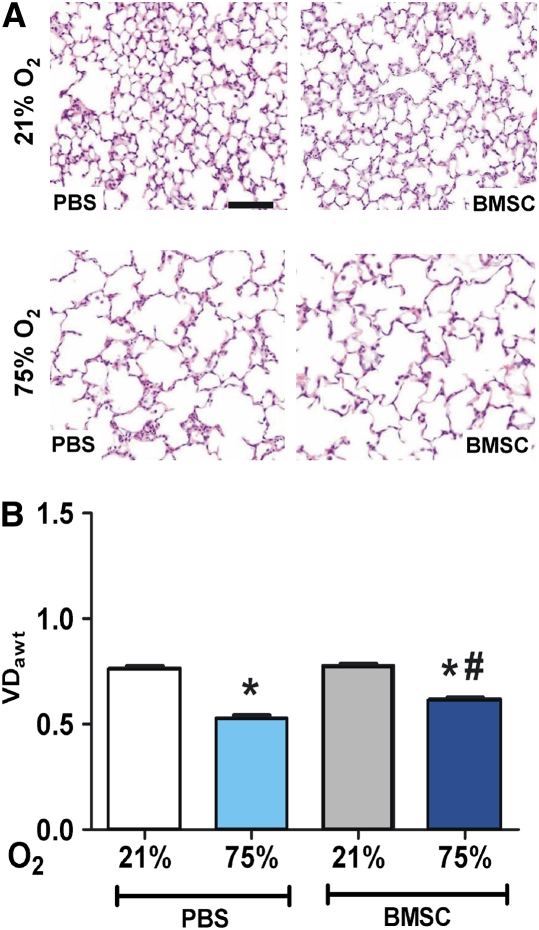
Effect of BMSC Treatment on Hyperoxia-induced Alveolar Injury
Representative histology images of lungs from treated animals are shown in Figure 2A. Lungs from animals in the normoxia group who had received PBS or BMSCs appeared normal with numerous small alveoli and thin alveolar septa (upper panels). Compared with lungs from normoxic animals, lungs from hyperoxia-exposed animals demonstrated enlarged simplified alveoli and thicker septa (Figure 2A, lower panels). Morphometric analysis (Figure 2B) revealed a significant decrease in the VDawt as assessed by number of tissue intercepts in lungs from the hyperoxia group compared with normoxia (0.77 ± 0.01 vs. 0.53 ± 0.01, P < 0.001, normoxia vs. hyperoxia). BMSC treatment resulted in a significant but modest increase in VDawt in the hyperoxia group compared with the respective PBS controls (0.53 ± 0.01 vs. 0.62 ± 0.01, P < 0.05, PBS vs. BMSC treatment). In addition, the mean alveolar septal thickness significantly increased in lungs of animals exposed to 75% O2 compared with normoxia (2.83 ± 0.1 vs. 2.3 ± 0.5, P < 0.001, respectively) and was almost completely reversed by BMSC transplantation but not by PBS (2.4 ± 0.08 vs. 2.83 ± 0.1, P < 0.05, BMSCs vs. PBS treatment). Thus, BMSC treatment had a more pronounced effect on inhibiting hyperoxia-induced septal thickness, a consequence of inflammation and fibrosis, than in preserving alveolar number.
Figure 2.
Effect of bone marrow stromal cell (BMSC) treatment on hyperoxic alveolar injury. (A) Representative hematoxylin and eosin–stained lung sections from normoxic, phosphate-buffered saline (PBS)- or BMSC-treated animals (upper panels) and from animals exposed to hyperoxia for 14 days and treated with PBS or BMSCs, as indicated on lower panels (original magnification ×100). Solid bar scale represents 400 μm and all the panels are under the same magnification. (B) Hyperoxia reduced the volume density of alveolar wall tissue (VDawt) compared with the normoxic group, indicative of lower alveolar count, which was modestly improved with BMSC treatment. Data are expressed as mean ± SEM (n = 10–12 animals per group). *P < 0.001 versus 21% O2 groups; #P < 0.01 versus PBS-treated hyperoxic group.
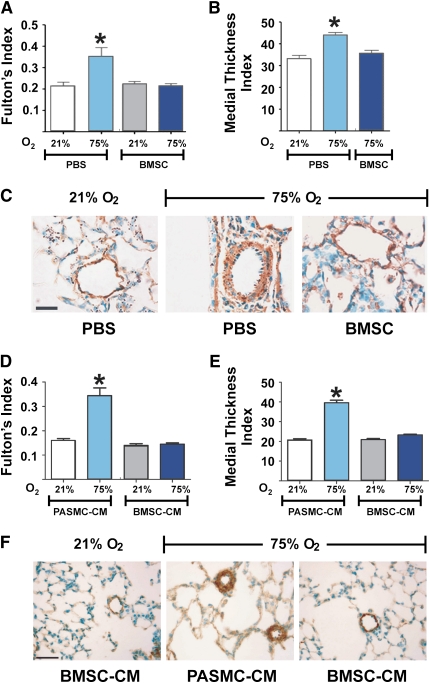
BMSC Treatment Ameliorates Vascular Changes Associated with Hyperoxia-induced Lung Injury
To determine whether exogenous BMSCs protect against the development of pulmonary hypertension in oxygen-induced lung injury, Fulton's index (right ventricle/[left ventricle + septum]), an indicator of right ventricular (RV) hypertrophy, was measured in hearts from both control and hyperoxia-exposed animals (Figure 3A). Under normoxia, there was no difference in Fulton's index between the PBS-injected controls and BMSC-recipient mice. Hyperoxia-exposed animals showed higher Fulton's index compared with the normoxia group indicative of chronically elevated pulmonary artery pressures (0.21 ± 0.01 vs. 0.35 ± 0.04, P < 0.001, normoxia vs. hyperoxia). BMSC recipient mice in the hyperoxia group demonstrated a significantly lower Fulton's index with values comparable to normoxic levels suggesting amelioration of RV hypertrophy in these animals (0.35 ± 0.04 vs. 0.21 ± 0.01, P < 0.001, PBS vs. MSC treatment). In parallel sets of experiments, injection of mouse lung fibroblasts (MLFs) or PASMCs had no protective effect on the degree of RV hypertrophy, similar to PBS treatment (results not shown). We further assessed muscularization by immunostaining for α-smooth muscle actin in the medial vessel wall to test whether chronic changes in the pulmonary hemodynamics were accompanied by alterations in the remodeling of small pulmonary arterioles. Hyperoxia-exposed animals presented a significant increase in the muscularization of intrapulmonary arterioles compared with normoxic animals as assessed by measuring the medial thickness index of α-smooth muscle actin immunostained vessels (33 ± 1.7% vs. 44 ± 1.1%, P < 0.001, normoxia vs. hyperoxia). The degree of muscularization was unaffected by BMSC treatment in the normoxia group (results not shown); however, the hyperoxia-induced muscularization decreased significantly by BMSC treatment compared with the respective PBS-injected controls (44 ± 1.1% vs. 35 ± 1.2%, P < 0.001, PBS vs. BMSC treatment), returning to the levels found in normoxic animals (Figures 3B and 3C).
Figure 3.
Either bone marrow stromal cell (BMSC) or BMSC–conditioned media (CM) treatment prevents vascular changes associated with pulmonary hypertension in hyperoxia-induced lung injury. (A) Hyperoxia-exposed, phosphate-buffered saline (PBS)-treated newborn mice develop significant right ventricular hypertrophy that is significantly reduced by BMSC treatment. Data are expressed as mean ± SEM (n = 10–12 animals per group). *P < 0.001, compared with the two normoxia groups and the hyperoxia group that received BMSC. (B) BMSC treatment significantly reduced the medial wall thickness as compared with the hyperoxia group that received PBS treatment. Data are expressed as mean ± SEM. *P < 0.001, compared with normoxia and the hyperoxia group that received BMSC. (C) Representative pulmonary arterioles immunostained for α–smooth muscle actin, displaying a thickened smooth muscle layer in hyperoxia-exposed mouse lungs as compared with normoxic controls, and absence of muscularization on BMSC treatment. (D) Similar to BMSC treatment, BMSC-CM treatment significantly reduced right ventricular hypertrophy in hyperoxia-exposed animals, and (E) significantly reduced medial wall thickness as compared with the hyperoxia group that received pulmonary artery smooth muscle cell–CM. Data are expressed as mean ± SEM (n = 16–18 animals per group). *P < 0.0001 versus normoxic groups or BMSC-CM treated groups. (F) Representative small pulmonary arterioles, as in (C). Solid bar scale represents 100 μm and all the panels are under the same magnification.
Effect of BMSC-conditioned Media on Hyperoxia-induced Alveolar and Vascular Injury
The above findings indicate that BMSC transplantation confers significant protection against hyperoxia-induced pulmonary vascular remodeling in the developing lung but provides modest preservation of lung architecture. Importantly, these effects are observed in the context of very low BMSC engraftment within the lung epithelium and no BMSC engraftment detected in the wall of the lung vessels where the cytoprotective effect was more pronounced. This suggests mechanisms of BMSC action other than direct tissue repair. To explore potential paracrine action of the BMSCs in this model, we treated 4-day-old mice with BMSC-conditioned media and assessed RV hypertrophy, vascular remodeling, and VDawt. Results are shown in Figures 3D–3F. Recapitulating the results obtained with BMSC transplantation, treatment with BMSC-CM significantly lowered Fulton's index to normoxic levels (Figure 3D) and significantly decreased hyperoxia-induced muscularization of intrapulmonary arterioles (Figures 3E and 3F), whereas PASMC-CM failed to either prevent RV hypertrophy or reduce vascular remodeling. Interestingly, although stem cell injections had minimal protective effect on the lung parenchyma as demonstrated above (Figure 2) treatment with BMSC-CM completely prevented hyperoxic lung injury, resulting in almost normal lung alveolar architecture (Figure 4A). Morphometric analysis revealed a significant increase in VDawt in the hyperoxia group treated with BMSC-CM compared with the hyperoxia group treated with PASMC-CM (Figure 4B), and, in parallel to the observations with BMSC injection, the alveolar septal thickness in the hyperoxia group that received BMSC-CM was significantly less compared with the corresponding PASMC-CM group and similar to normoxic animal values (results not shown).
Figure 4.
Effect of bone marrow stromal cell–conditioned media (BMSC-CM) on hyperoxic alveolar injury. (A) Representative hematoxylin and eosin–stained lung sections from normoxic animals compared with hyperoxic animals treated with either pulmonary artery smooth muscle cell (PASMC)-CM or BMSC-CM, as indicated. Quantitation of volume density of alveolar wall tissue (VDawt) is shown in (B). Treatment with BMSC-CM but not PASMC-CM prevented alveolar loss. Data are expressed as mean ± SEM (n = 16–18 animals per group). *P < 0.0001 versus normoxic controls and BMSC-CM group. Solid bar scale represents 400 μm and all the panels are under the same magnification.
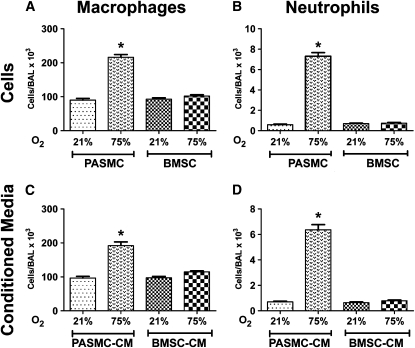
BMSCs and BMSC-CM Suppress Lung Inflammation in Hyperoxia-induced Lung Injury
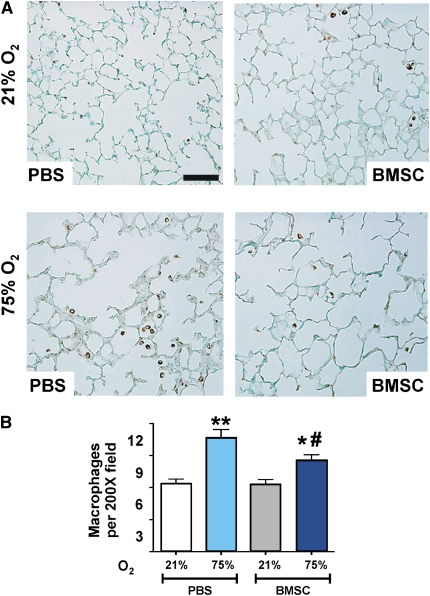
Several reports in the recent years suggest that BMSCs are potent modulators of immune responses resulting in a decrease of proinflammatory cytokines from bleomycin, endotoxin, or LPS-induced injury (9, 11, 13–15). To investigate whether BMSCs or factors secreted in their conditioned media modulate the inflammatory response to hyperoxia in the developing neonatal lung, we quantified macrophage and neutrophil numbers in the BALF of mice in response to BMSC and BMSC-CM treatment. The number of polymorphonuclear cells and macrophages in BALF was significantly higher after 14 days of hyperoxia exposure in control PBS-, MLF-, or PASMC-treated animals (Figure 5 and data not shown). Significantly, hyperoxic animals treated with either BMSC or BMSC-CM demonstrated a dramatic suppression of lung inflammation compared with animals treated with PASMC or PASMC-CM. This was evidenced by a dramatically decreased number of both polymorphonuclear cells and macrophages in BALF that was equal to levels observed in uninjured, normoxic animals (Figures 5A–5D). This reduction in inflammatory cell infiltrate was also observed in lung sections shown by immunohistochemical analysis for resident macrophages stained with Mac-3 (Figure 6A). Hyperoxia-exposed but BMSC-treated animals presented a lower number of lung macrophages per high power field (hpf) compared with the corresponding PBS control group (10.7 ± 0.7/hpf vs. 8.6 ± 0.48/hpf, P < 0.01, PBS vs. BMSC treatment), suggesting a suppressive effect of BMSCs on the recipient animal's immunological response (Figure 6B).
Figure 5.
Effect of stem cells or cell-free conditioned media (CM) treatments on bronchoalveolar lavage fluid (BALF) macrophage and neutrophil counts. (A and B) Differential cell counts for neutrophils and macrophages were performed in the BALF of normoxic or hyperoxic animals revealing marked inhibition of both cell types by treatment with bone marrow stromal cells (BMSCs) but not with pulmonary artery smooth muscle cells. Similarly, BMSC-CM suppressed both (C) macrophage, and (D) neutrophil numbers in bronchoalveolar lavage fluid of hyperoxic animals to levels of normoxic controls. Data are expressed as mean ± SEM (n = 16–18 animals per group). *P < 0.0001 versus normoxia or BMSC- or BMSC-CM–treated hyperoxic groups.
Figure 6.
Effect of bone marrow stromal cell (BMSC) transplantation on lung inflammation. (A) Immunostaining with anti–Mac-3 antibody for macrophages (brown staining) in representative paraffin-embedded lung sections from BMSC recipient or phosphate-buffered saline (PBS) control mice under normoxic conditions (upper panels) or after 14 days of exposure to hyperoxia (lower panels). Original magnification: ×200. (B) Quantitation of Mac-3–positive cells. Hyperoxia-exposed lungs demonstrated a higher macrophage count compared with normoxia that was significantly reduced in the BMSC-treated hyperoxic group compared with the respective PBS control. Data are expressed as mean ± SEM (n = 6–8 animals per group) *P < 0.01, **P < 0.001 compared with normoxia groups; #P < 0.01, compared with the control hyperoxia group that received PBS. Solid bar scale represents 200 μm and all the panels are under the same magnification.
The levels of proinflammatory and antiinflammatory activities in the BALF of BMSC-CM–treated and -untreated animals were measured using a Luminex panel of 21 relevant molecules, as detailed in the online supplement. The entire panel results are presented in Figure E2, with the exception of the results for INFγ, which was below the level of detection in all samples analyzed. Although suggestive trends were detected in a number of molecules, for example IL-1α, IL-1rn (IL-1ra), IL-12, CCL2 (MCP-1), and CXCL9 (MIG), only the levels of tumor necrosis factor (TNF)-α, IL-5, and IL-17, were different between the BMSC-CM–treated and the PASMC-CM–treated hyperoxic groups at a statistically significant level (P < 0.05). A statistically significant but probably physiologically insignificant difference was also observed in the levels of CXCL10 (IP-10) between the two aforementioned groups. These results suggest that one of the effects of BMSC-CM treatment is the suppression of the hyperoxia-induced increases in BALF levels of TNF-α and IL-17, and the down-regulation of the steady-state levels of IL-5, but more robust studies are required to dissect the exact mechanism of BMSC-CM antiinflammatory action.
Major Functional Classes Represented in the BMSC Secretome
The cytoprotective effect of BMSC-CM on both inflammation and lung injury was abrogated with heat treatment, pointing to a heat-labile protein as the active component. To pursue the identification and characterization of the critical mediators secreted by BMSCs that have a physiological effect in the neonatal lung in vivo, we performed a preliminary proteomic analysis of conditioned media active fractions. Mass spectroscopic analysis identified a high abundance of extracellular space proteins, including matrix components, with fibronectin (Fn1), heparan sulfate proteoglycan (Hspg2; perlecan), and collagen (Col6a3) accounting for more than 20% of the peptides detected. The rest of the conditioned media proteome is complex, and a functional categorization of the species reproducibly detected in independent mass spectroscopic analyses was generated through the use of Ingenuity Pathways Analysis (22). Using the standard classification of the Ingenuity System, the majority of the protein species could be classified into four overlapping functional groups, namely molecules associated with (1) proliferation and apoptosis, (2) cell–cell interactions and cell motility, (3) immune responses and inflammation, and (4) respiratory disease (Figure E3A). Among the relatively abundant proteins identified in the BMSC-CM (represented by more than 15 peptides in independent mass spectroscopic analyses), two were of particular interest given their documented role in immune modulation: osteopontin (Spp1; Opn) and macrophage colony stimulating factor 1 (M-csf; Csf1). To verify the reproducibility of these results, we performed Western blot analyses on the conditioned media produced by three independently isolated BMSC cultures. Both Spp1 and Csf1 were detected in similar abundance in all three samples analyzed (Figure E3B). Given their reproducible presence and relative abundance in BMSC-CM, both of these molecules must be included among the candidates for more detailed functional studies to determine if, in isolation or in combination with other secreted proteins, they are responsible for the observed efficacy of BMSC-CM in modulating lung inflammation and neonatal lung injury in the mouse model of BPD.
DISCUSSION
BPD is a multifactorial disease of premature infants resulting from mechanical injury, oxygen toxicity, and infection, subsequently leading to pulmonary inflammation and damage. The disease mainly affects preterm infants as their lungs are more vulnerable to injury due to poorly developed airway supporting structures, surfactant deficiency, decreased compliance, and underdeveloped antioxidant mechanisms. In the current era, with the increased survival of extremely low gestational age infants and the advent of surfactant treatment and gentler modes of ventilation, the pathology of BPD has evolved into a new pattern of injury characterized by impaired alveolarization and dysmorphic vasculogenesis. The prominent pathologic findings include disruption of lung development with decreased septation and alveolar hypoplasia leading to fewer and larger alveoli, thickened alveolar septa, inflammation, bronchial smooth muscle hypertrophy, and interstitial edema (23). Vascular changes characteristic of pulmonary hypertension may also be evident with pulmonary arteriolar muscularization and right ventricular hypertrophy (24). Data are lacking on the exact timing and relative roles of vasculogenesis, angiogenesis, and remodeling during lung development. Interplay between diverse signaling pathways, including transcription factors, growth factors, extracellular matrix, and mechanical forces leading to the precise development of the lung and its circulation is largely unknown. In recent years, a vascular hypothesis for the pathogenesis of BPD has been proposed with accumulating data to suggest that early disruption of vascular growth from endothelial damage leads to impaired growth of the distal airspaces, resulting in reduced alveolarization (25). Disruption of lung vascular growth also sets the stage for late pulmonary hypertension. Lung injury results in increased elastic tissue formation and thickening of the interstitium, which in turn compromises septation and capillary development. At present, all the available interventions for the prevention and/or treatment of BPD lack efficacy and have undesirable side effects. Therefore, the search for an effective preventive and/or treatment modality is of paramount importance.
Research on stem cell and progenitor cell–based therapies on animal models of disease has produced promising results on the ability of BMSCs to repair tissue injury (7–9, 11–13). BMSCs belong to the select group of adult stem cells that have traditionally shown differentiation potential toward mesenchymal tissues such as bone (5), cartilage (26), and fat cells (27), but recent work has demonstrated a greater plasticity beyond mesenchymal cell fate and includes the differentiation into endothelial and neuronal lineages (28–30). In addition to their multilineage differentiating capability, BMSCs produce immunosuppressive cytokines and growth factors that may help in the reparative process (31). The potent immunomodulatory and antiinflammatory properties of BMSCs in a clinical study involved treatment of graft-versus-host disease (32) and cell-based treatments are currently the focus of intensive studies in graft enhancement, tissue protection, and regenerative medicine. We hypothesized that exogenous administration of BMSCs may protect the lung architecture in a neonatal mouse model of bronchopulmonary dysplasia. The saccular and alveolar stages of lung development in the mouse occur after birth, making it a suitable model to study lung injury at similar stages of lung development to those observed in the human preterm neonate between 24 and 28 weeks' gestation, a period most vulnerable to BPD from ventilator- and oxygen-induced injury. Pulmonary hypertension contributes significantly to the morbidity and mortality of patients with BPD (3, 33). Changes such as vascular remodeling and right ventricular hypertrophy due to chronically elevated pulmonary artery pressures have been successfully reproduced in rodent models of the pathology.
In our studies, neonatal mice exposed to 75% O2 showed the histological findings of BPD, including simplification of alveoli, thickened alveolar septa, inflammatory cell infiltration, and higher Fulton's index (right ventricular hypertrophy) and vascular remodeling. Using this well-characterized mouse model of BPD, we demonstrated that systemically administered BMSCs partially protect the lung architecture against hyperoxia-induced injury, whereas a single bolus injection of BMSC-CM is able to confer full protection, preventing alveolar simplification, preserving normal alveolar number, and ameliorating the lung vascular remodeling associated with the disease.
One of the limitations of using BMSCs is the lack of unique cell surface markers to isolate and characterize them with several early studies reporting significant variability in isolation and culture methods. The BMSC isolation and characterization used in this study included both positive and negative selection methods using specific cell surface markers according to and exceeding the criteria set forth by the International Society for Cellular Therapy (21) to achieve a homogeneous cell population. In addition, differentiation assays confirmed that these cells have the ability to differentiate into osteoblasts and adipocytes in vitro. Sex-mismatch experiments with donor male cells and female recipient neonatal mice allowed us to quantify transplanted cells and show persistence of a small number of donor cells 2 weeks after injection. We were unable to further characterize whether the donor cells differentiated into lung cell types or to address cell fusion possibilities. We did observe that a higher number of donor BMSCs can be detected in the injured lung compared with the normal lung at 10 days post injection. Nevertheless, the overall donor cell retention is minimal, in the order of one BMSC per 10,000 lung cells, and we cannot formally exclude the possibility that the observed donor cells represent rare, nonmesenchymal contaminants in our cultures, despite the strict selection regimen we used. Based on these data, we cannot propose that donor BMSCs extensively replace injured lung cells to effectively improve lung architecture. The minimal BMSC engraftment after transplantation combined with the therapeutic efficacy of cell-free conditioned media point to paracrine effects of BMSC action rather than direct tissue repair.
Similar results of low engraftment, associated with a significant beneficial response, have been reported in other studies. Togel and colleagues observed a rapid clearance of BMSCs within 24 hours of intravenous delivery but demonstrated significant protection from ischemic acute renal failure in a rat model of disease (34). A similar “early benefit” effect was observed in a cardiac injury model within 72 hours of BMSC injection that was attributed to the paracrine effects of growth factors released by the transplanted cells (35). However, none of these studies tested the potential in vivo cytoprotective actions of secreted factors derived from cultured BMSCs. More recently it has become apparent that many (but not all) of the beneficial effects of stem and progenitor cells in animal disease models are the result of immunomodulatory and trophic support properties delivered by the transplanted cells acting in a paracrine manner. Using a bleomycin-induced lung inflammation and fibrosis model, Ortiz and colleagues (36) suggested that BMSC-secreted interleukin 1 receptor antagonist (IL1ra) represents a key candidate for the observed beneficial effects of BMSC treatment, and the antiapoptotic effect of BMSCs on neutrophils was shown to depend on IL-6 secretion but not on cell-to-cell contact (37). Chen and colleagues have demonstrated a beneficial effect of BMSC-CM on wound healing compared with fibroblast conditioned media (38). Parekkadan and colleagues have demonstrated a significant survival benefit in rats with fulminant hepatic failure via intravenous administration of sonicated BMSCs (39). Similar paracrine effects of stem or progenitor cells have been observed in other studies (40–42), and the assertion has been advanced that, in certain systems, it may not be just a singular factor but rather a specific milieu of secreted factors that confer the reparative and trophic action of stem or progenitor cells (43). Although paracrine effects may explain certain of the observed cytoprotective properties of BMSCs, cell-to-cell interactions, resulting in reprogramming of immune cells by BMSCs, have been shown to be paramount for protection in an animal model of sepsis (44).
None of the above studies identified the factor(s) responsible for BMSC-CM efficacy in vivo. In our system, the beneficial response appears to be stem cell–specific, because we did not detect any effect with mouse lung fibroblast or PASMC injection. BMSCs were administered after the animals had been already exposed to high oxygen for 3 days and the observed protective effect suggests that BMSCs, or the milieu of proteins they secrete, could be used clinically as a prophylaxis tool to prevent further injury. BMSC-mediated release of growth factors and potential immunosuppressive effects may explain some of the observed physiological benefits. Our mass spectroscopic analysis on BMSC-CM identified protein classes associated with cell proliferation and apoptosis, cell–cell interactions and cell motility, immune modulation, and respiratory disease. A previous gene expression profiling study by Ohnishi and colleagues (45) identified similar classes of molecules expressed in high abundance (> 100-fold) specifically by BMSCs compared with bone marrow–derived mononuclear cells. In our analysis, out of several proteins identified in BMSC-CM, two were of particular interest: Spp1 and Csf1. Spp1, also known as osteopontin (Opn), has been identified as a protein with a pivotal role in immune and vascular models of injury. Opn regulates cytokine production by macrophages, inhibits macrophage accumulation in vascular systems, mediates cell adhesion and migration, and can act as a survival factor (46, 47). Osteopontin can have an antiinflammatory or proinflammatory effect depending on the injury model and stage of injury. Opn is important for Th1-mediated immune and autoimmune disease modulation. Xanthou and colleagues have demonstrated effects of Opn on Th2-mediated allergic disease, observing a proinflammatory effect of Opn on primary systemic sensitization and an antiinflammatory effect during secondary pulmonary antigenic challenge (48). Csf1 is a key differentiation, growth, and survival factor for monocytes/macrophages and its action on these cells results in enhanced cytotoxicity, superoxide production, phagocytosis, chemotaxis, and secondary cytokine production (49). Exogenous administration of recombinant Csf1 was shown to have protective effects in human fungal infections (50). The role of both Spp1 and Csf1 in this neonatal mouse model of disease is unknown and these molecules represent attractive candidates for further functional studies to determine whether in isolation, or in combination with other secreted proteins, they are responsible for the observed BMSC-CM efficacy. It is likely that a combination of these proteins rather than one single factor confers cytoprotective epithelial, vascular, and antiinflammatory effects on the developing neonatal lung.
Our studies indicate that mechanisms of immunological protection may be the major effectors of BMSC treatment. In agreement, several reports have demonstrated immunological protection with BMSC administration, including down-regulation of T cell proliferation and dendritic cell maturation (51–53), and antiproliferative effects on B lymphocytes (54) and natural killer cells (55). We speculate that the low retention of BMSCs into the lung may limit their ability to secrete factor(s) in sufficient amounts to achieve complete tissue recovery in response to hyperoxic or potentially other lung injury. The concentrated administration of active immunomodulators produced by BMSCs in culture may achieve significant in vivo levels to trigger signaling pathways of repair and immunological protection that can have long-lasting effects. Inflammation is considered the key mediator of alveolar and vascular injury in BPD. It likely results from an imbalance between pro- and antiinflammatory mediators within the lung. In their review of BPD, Thompson and colleagues have found higher levels of proinflammatory cytokines (IL1β, IL-6, and IL-16) and lower levels of antiinflammatory cytokines (IL-10 and IL-13) in premature infants with BPD (56). In a multicenter study of extremely low birth weight infants with BPD, Ambalavanan and colleagues demonstrated a correlation of higher IL1β and lower IL-17 serum concentrations with BPD (57). In our study, hyperoxia exposure resulted in significant inflammation within the lungs of animals treated with MLFs or PASMCs, as shown by significant neutrophil and macrophage levels in the BALF. Treatment of hyperoxic animals with BMSCs or BMSC-CM prevented the development of inflammation resulting in a significantly reduced number of neutrophils and macrophages within the BALF. To characterize the inflammation further we measured the cytokine and chemokine profile within the BALF via the Luminex 200 system. Our analysis identified higher levels of proinflammatory cytokines in the BALF of hyperoxic animals treated with PASMC-CM (IL-17, IL-5, and TNF-α) compared with the BMSC-CM. The rest of the proinflammatory cytokine levels, although higher in the hyperoxic animals treated with PASMC-CM compared with BMSC-CM, did not reach statistical significance. One likely explanation is the presence of low cytokine levels in the BALF compared with the levels in serum or whole lung. Also the peak timing of cytokine elevation is unknown and it is possible that some of these cytokines are secreted in higher amounts in the early or late phase of inflammation. We have measured these cytokine levels at 14 days post hyperoxia. Further studies using early and late time frames, as well as measurement of cytokine profile within the lungs in addition to BALF, will determine the exact cytokine and chemokine alterations in BPD.
In conclusion, this report demonstrates a potential beneficial effect of BMSC treatment on the pathophysiology of oxygen-induced lung injury. Further in vivo and in vitro studies are required to optimize dose, timing, and duration of both stem cell and cell-free media treatment and to delineate the mechanisms underlying BMSC protection in our model of BPD. The findings of this study point to the beneficial use of stem cells and/or a pool of factors they secrete in culture as a therapeutic approach to protect the newborn injured lung.
Supplementary Material
Acknowledgments
The authors thank Xianlan Liu and Anne Silkowski for technical expertise and Stephanie Giannetto and Sarah Gately for invaluable assistance in preparing the manuscript.
Supported by National Heart Lung and Blood Institute grants RO1 HL055454 (S.K.), R01 HL085446 (S.K.), and P50 HL067669 (S.K. and S.A.M.). Dr. Baveja was supported by 5T32HD04034 (Gary Fleisher, P.I.). Dr. Aslam was supported by 5T32 HD007466 (S.K., P.I.) and IKARIA Advancing Newborn Medicine Grant (M.A., P.I.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200902-0242OC on August 27, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet 2006;367:1421–1431. [DOI] [PubMed] [Google Scholar]
- 2.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 2005;67:623–661. [DOI] [PubMed] [Google Scholar]
- 3.Abman SH. Pulmonary hypertension in chronic lung disease of infancy. Pathogenesis, pathophysiology and treatment. In: Bland RD, Coalson JJ, editors. Chronic lung disease of infancy. New York: Marcel Dekker; 2000. pp. 619–668.
- 4.Baveja R, Christou H. Pharmacological strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol 2006;30:209–218. [DOI] [PubMed] [Google Scholar]
- 5.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA 1998;95:1142–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 7.Phinney DG, Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des 2005;11:1255–1265. [DOI] [PubMed] [Google Scholar]
- 8.Shah RV, Mitchell RN. The role of stem cells in the response to myocardial and vascular wall injury. Cardiovasc Pathol 2005;14:225–231. [DOI] [PubMed] [Google Scholar]
- 9.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells 2007;25:2896–2902. [DOI] [PubMed] [Google Scholar]
- 11.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, Prockop DJ. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J 2008;22:1226–1236. [DOI] [PubMed] [Google Scholar]
- 13.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 2007;4:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol 2007;293:L131–L141. [DOI] [PubMed] [Google Scholar]
- 15.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 2007;179:1855–1863. [DOI] [PubMed] [Google Scholar]
- 16.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;9:1195–1201. [DOI] [PubMed] [Google Scholar]
- 17.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 2004;103:1662–1668. [DOI] [PubMed] [Google Scholar]
- 18.Dobson KR, Reading L, Haberey M, Marine X, Scutt A. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int 1999;65:411–413. [DOI] [PubMed] [Google Scholar]
- 19.Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol 2009;482:281–294. [DOI] [PubMed] [Google Scholar]
- 20.Wong SH, Lowes KN, Bertoncello I, Quigley AF, Simmons PJ, Cook MJ, Kornberg AJ, Kapsa RM. Evaluation of Sca-1 and c-Kit as selective markers for muscle remodelling by nonhemopoietic bone marrow cells. Stem Cells 2007;25:1364–1374. [DOI] [PubMed] [Google Scholar]
- 21.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 22.Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 1991;67:901–908. [DOI] [PubMed] [Google Scholar]
- 23.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998;29:710–717. [DOI] [PubMed] [Google Scholar]
- 24.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729. [DOI] [PubMed] [Google Scholar]
- 25.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis.” Am J Respir Crit Care Med 2001;164:1755–1756. [DOI] [PubMed] [Google Scholar]
- 26.Magne D, Vinatier C, Julien M, Weiss P, Guicheux J. Mesenchymal stem cell therapy to rebuild cartilage. Trends Mol Med 2005;11:519–526. [DOI] [PubMed] [Google Scholar]
- 27.Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res 2004;295:395–406. [DOI] [PubMed] [Google Scholar]
- 28.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 1999;96:10711–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prockop DJ, Azizi SA, Colter D, Digirolamo C, Kopen G, Phinney DG. Potential use of stem cells from bone marrow to repair the extracellular matrix and the central nervous system. Biochem Soc Trans 2000;28:341–345. [PubMed] [Google Scholar]
- 30.Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA 2005;102:18171–18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol 2006;36:2566–2573. [DOI] [PubMed] [Google Scholar]
- 32.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004;363:1439–1441. [DOI] [PubMed] [Google Scholar]
- 33.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med 2004;170:1006–1013. [DOI] [PubMed] [Google Scholar]
- 34.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 2005;289:F31–F42. [DOI] [PubMed] [Google Scholar]
- 35.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 2006;20:661–669. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 2007;104:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 2008;26:151–162. [DOI] [PubMed] [Google Scholar]
- 38.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One 2007;2:e941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004;109:1292–1298. [DOI] [PubMed] [Google Scholar]
- 41.Cai L, Johnstone BH, Cook TG, Liang Z, Traktuev D, Cornetta K, Ingram DA, Rosen ED, March KL. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells 2007;25:3234–3243. [DOI] [PubMed] [Google Scholar]
- 42.Capone C, Frigerio S, Fumagalli S, Gelati M, Principato MC, Storini C, Montinaro M, Kraftsik R, De Curtis M, Parati E, et al. Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS One 2007;2:e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei X, Du Z, Zhao L, Feng D, Wei G, He Y, Tan J, Lee WH, Hampel H, Dodel R, et al. IFATS collection: the conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells 2009;27:478–488. [DOI] [PubMed] [Google Scholar]
- 44.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells 2007;25:1166–1177. [DOI] [PubMed] [Google Scholar]
- 46.Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer 2004;90:1877–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denhardt DT, Giachelli CM, Rittling SR. Role of osteopontin in cellular signaling and toxicant injury. Annu Rev Pharmacol Toxicol 2001;41:723–749. [DOI] [PubMed] [Google Scholar]
- 48.Xanthou G, Alissafi T, Semitekolou M, Simoes DC, Economidou E, Gaga M, Lambrecht BN, Lloyd CM, Panoutsakopoulou V. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat Med 2007;13:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hubel K, Dale DC, Liles WC. Therapeutic use of cytokines to modulate phagocyte function for the treatment of infectious diseases: current status of granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, macrophage colony-stimulating factor, and interferon-gamma. J Infect Dis 2002;185:1490–1501. [DOI] [PubMed] [Google Scholar]
- 50.Roilides E, Sein T, Holmes A, Chanock S, Blake C, Pizzo PA, Walsh TJ. Effects of macrophage colony-stimulating factor on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Infect Dis 1995;172:1028–1034. [DOI] [PubMed] [Google Scholar]
- 51.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 2007;83:71–76. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev 2004;13:263–271. [DOI] [PubMed] [Google Scholar]
- 53.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005;105:2821–2827. [DOI] [PubMed] [Google Scholar]
- 54.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006;107:367–372. [DOI] [PubMed] [Google Scholar]
- 55.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006;107:1484–1490. [DOI] [PubMed] [Google Scholar]
- 56.Thompson A, Bhandari V. Pulmonary biomarkers of bronchopulmonary dysplasia. Biomark Insights 2008;3:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ambalavanan N, Carlo WA, D'Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics 2009;123:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.