Synopsis
The presence of nutritional deficiencies in overweight and obesity may seem paradoxical in light of excess caloric intake, but several micronutrient deficiencies appear to higher in prevalence in overweight and obese adults and children. Causes are multi-factorial and include decreased consumption of fruits and vegetables, increased intake of high calorie, but nutritionally poor quality foods, as well as increased adiposity which may influence the storage and availability of some nutrients. As the obesity epidemic continues unabated and the popularity of bariatric surgery rises for both severely obese adults and adolescents, medical practitioners must be aware of pre-existing nutritional deficiencies in overweight and obese patients and appropriately recognize and treat both common and rare nutritional deficiencies that may arise or worsen following bariatric surgery. This article will review our current knowledge of nutritional deficits in obese and overweight individuals and those that commonly present after bariatric surgery and summarize current recommendations for screening and supplementation.
Keywords: obesity, pediatric obesity, nutritional deficiencies, bariatric surgery, malabsorption
Introduction
The presence of nutritional deficiencies in overweight and obesity may seem paradoxical in light of the evidence of excess caloric intake, but a growing body of literature has documented that several micronutrient deficiencies may be higher in prevalence in overweight and obese adults and children, particularly in those suffering from extreme obesity (BMI > 40kg/m2 in adults and ≥ 99th percentile in children). Consumption of excess calories does not automatically equate with over-consumption of fruits, vegetables and other unprocessed, high quality nutrient-dense foods. Increased adiposity itself may influence the serum levels of some fat-soluble vitamins, such as vitamin D.[1] Compounding the problem, surgical treatments for severe obesity have also grown in frequency and may exacerbate pre-existing vitamin and mineral deficiencies or produce new ones, depending on dietary intake, adherence to recommended post-operative supplementation and degree of malabsorption associated with the bariatric surgery procedure. Much of the recent data on nutritional deficits in obese individuals are from studies of adults undergoing pre-operative evaluations for bariatric surgery, which indicate that baseline nutritional deficiencies are not negligible in extremely obese patients.
As the obesity epidemic continues unabated and the popularity of bariatric surgery rises for both extremely obese adults and adolescents, clinicians must be aware of pre-existing nutritional deficiencies in overweight and obese patients. To optimize long-term health after bariatric surgery, it is important to screen for and recognize symptoms of deficiency, prescribe appropriate supplementation and treat common and rare nutritional deficiencies that may emerge both in the short term and long-term post-operatively. Though not as common as in adults, the incidence of bariatric surgery has also increased dramatically in adolescents with severe obesity, rising 5-fold between 1997 and 2003.[2] Therefore, pediatric practitioners may well encounter the post-operative bariatric patient in their practice and must be able to screen for and treat predictable nutritional deficiencies. The adolescent bariatric patient may be particularly at risk for non-adherence to recommended supplementation and requires close follow-up, given the longer anticipated life-span with altered digestive physiology.[3] This article will summarize our current knowledge of nutritional deficiencies in obese and overweight individuals, with a particular focus on those that commonly occur after bariatric surgery. Current algorithms for screening and supplementation will also be reviewed.
Mechanisms contributing to nutritional deficiencies in obesity
Though commonly considered a state of “overnutrition”, obesity has increasingly been recognized as a risk factor for several nutrient deficiencies, including lower levels of anti-oxidants and certain fat-soluble vitamins.[4] More recently, studies of extremely obese adults undergoing bariatric surgery have identified a wider array of pre-existing nutritional deficiencies prior to surgery. The cause of these nutritional deficiencies in overweight and obese individuals is not completely known, but in large part is likely due to higher intake of higher-calorie processed foods associated with poor nutritional quality, particularly in highly developed countries in which there is an abundance of relatively cheap, energy-dense, but nutrient-poor food. About 27–30% of the daily caloric intake of American children and adults is comprised of low nutrient-density food, with sweeteners and desserts contributing 18–24% of the total.[5, 6] Unprocessed nutrient-dense foods include fruits and vegetables, dairy products, whole grains, nuts and legumes, and fish and protein sources which contribute the bulk of vitamins and minerals obtained from a non-supplemented diet. As intake of nutrient-poor food increases, the intake of unprocessed, nutrient-dense foods decreases proportionately.[5] Higher fat diets (>30% of total caloric intake) are associated with decreased intake of vitamins A, C and folate.[7] Increased consumption of sweetened beverages is also associated with lower intake of milk, and therefore calcium and fortified vitamin D3.[8] In the case of vitamin D3, additional risk factors for deficiency may include reduced physical activity leading to decreased sun exposure, increased storage in excess adipose tissue, as well as ethnicity and skin tone.[9]
The rising rate of bariatric surgeries in extremely obese adults and adolescents also plays a role in increasing the risk of nutritional deficiencies associated with overweight and obese individuals.[2, 10] The presence of nutritional deficiencies of selected micronutrients and macronutrients after bariatric surgery has been recognized for decades, but varies widely in prevalence and severity depending on type of bariatric surgery (see Figure 1).
Figure 1.
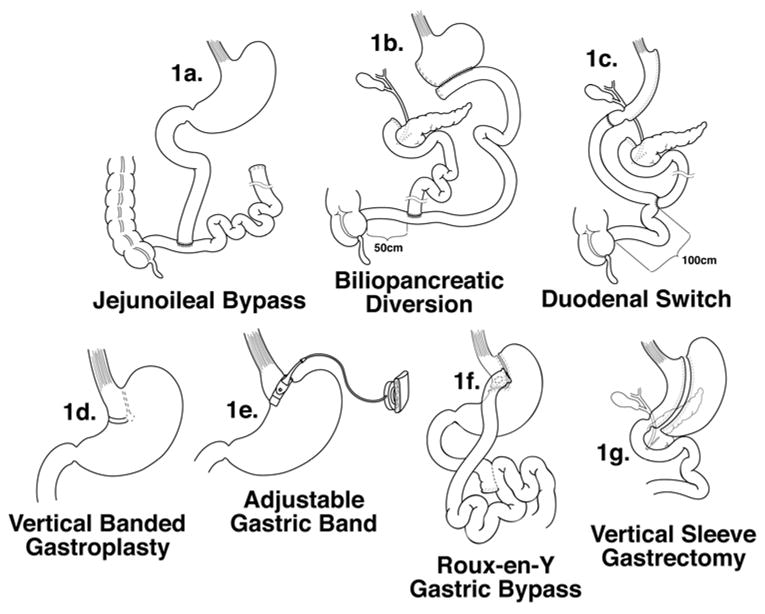
Types of Past and Present Bariatric Surgery Procedures. Jejunoileal bypass (1a) has been largely abandoned due to high risk of malabsorptive complications. Biliopancreatic diversion (1b) is also less commonly performed. The most commonly performed procedures include the Roux-en-y gastric bypass (1f), the biliopancreatic diversion with duodenal switch (1c) and the purely restrictive procedures, the adjustable gastric band (1e), vertical-banded gastroplasty (1d), and the vertical sleeve gastrectomy (1g).[10]
From Xanthakos SA. Bariatric surgery for extreme adolescent obesity: indications, outcome and physiologic effects on the gut-brain axis. Pathophysiology 2008;15:135, with permission.
Surgical weight loss procedures are generally classified as restrictive, malabsorptive, or a combination of both. Of the purely restrictive procedures, the vertical banded gastroplasty (VBG, figure 1d) and adjustable gastric band (AGB, figure 1e) create a 30–50ml gastric pouch in the proximal stomach just under the gastroesophageal junction. The AGB is currently more commonly performed than the VBG. The vertical sleeve gastrectomy (VSG, figure 1g) is also a purely restrictive procedure, but does remove the greater curvature of the stomach, leaving a narrow gastric sleeve in continuity with the remainder of the small intestine. Initially performed as the first stage in a biliopancreatic diversion with duodenal switch procedure, it is gaining increased interest as a stand-alone procedure for surgical weight loss.
The purely malabsorptive jejunoileal bypass (JIB, figure 1a) that involved bypassing the majority of the small intestine.has largely been abandoned due to numerous metabolic and nutritional complications. The biliopancreatic diversion (BPD, figure 1b) and biliopancreatic diversion with duodenal switch (BPD-DS, figure 1c) both involve restriction and malabsorption. In the BPD up to 60% of the stomach is resected. The duodenal stump is closed and the proximal ileum transected and anastomosed to the distal small bowel about 50 cm proximal to the ileocecal valve. The distal ileal segment is anastomosed to the remaining stomach. The BPD-DS variant involves a gastric sleeve resection of the greater curvature of the stomach, but a small cuff of duodenum is preserved and anastomosed to the distal ileum. The proximal ileum is anastomosed to the distal small bowel about 100cm from the ileocecal valve. This reduces the degree of micronutrient malabsorption in comparison with the BPD. The Roux-en-Y gastric bypass (RYGB, figure 1f) which currently is the most commonly performed procedure in both adults and children is mainly restrictive, creating a 20–30ml gastric pouch which is then anastomosed to a Roux limb of proximal jejunum. However, it also bypasses the body of the stomach and the duodenum and delays the mixing of biliary and pancreatic secretions with food in the proximal small bowel, which can contribute to poor digestion and absorption of several nutrients.
Procedures that bypass a portion of the small intestine, including JIB, BPD, BPD-DS, and RYGB carry the greatest risk of nutritional deficiencies. The proximal small intestine is the primary site of vitamin D, calcium, copper and iron absorption. Not surprisingly, the risk of malabsorption and nutrient deficiencies increases proportionally with the length of bypassed proximal intestine, which tends to be greatest in BPD and JIB.[11] Gastric resection or bypass of the body of the stomach also reduces mechanical digestion and acid secretion, which impairs digestion and absorption of iron, vitamin B12 and other protein-bound nutrients, and diminishes or abrogates the secretion of intrinsic factor, further impairing the absorption of vitamin B12.
Fortunately, among the most malabsorptive procedures, the JIB has been abandoned and BPD is much less commonly performed today. The majority of bariatric surgeries performed in adults and children is the RYGB which bypasses a much shorter length of proximal small intestine (typically 100–150cm) and lessens the risk of severe protein-calorie malnutrition, but still carries a significant risk of micro-nutrient deficiency, particularly with non-adherence to recommended vitamin supplementation. However, even the purely restrictive procedures, including AGB, VSG, and VBG can lead to nutritional deficiencies resulting from restricted dietary intake, particularly within the first few months of surgeries, but also over long-term follow up.[12] Excessive postoperative nausea and vomiting, though rare, can also contribute to and exacerbate nutritional deficits in both restrictive and restrictive-malabsorptive procedures.
Therefore, some form of multi-vitamin supplementation is recommended after all bariatric procedure. Often the number of supplements is dictated by the type of procedure and the potential for malabsorption. After AGB, it is often typical to prescribe a single multi-vitamin and recommend additional elemental calcium supplementation, but single multi-vitamin supplementation does not appear to be sufficient to prevent iron and vitamin B12 deficiencies and anemia following the restrictive-malabsorptive procedures.[13] Recent comprehensive allied health nutritional guidelines from the American Society for Metabolic and Bariatric Surgery suggest laboratory measures to monitor pre-existing and post-procedure deficiencies, as well as suggested post-operative vitamin and mineral supplementation, including dosing and administration specifications.[14] All recommendations for supplementation are based on expert opinion and observational research demonstrating the emergence of predictable nutrient deficiencies after specific types of bariatric surgery. Prospectively controlled randomized trials to determine optimal types and amounts of supplementation are largely lacking. Therefore, available guidelines for supplementation are likely to change as new evidence becomes available. Supplementation practices currently vary widely across programs, but with emergence of guidelines, clinical practice may become more standardized, which will make it easier to compare nutritional outcomes across different programs and further refine guidelines.[15, 16] A synopsis of screening and supplementation recommendations based on reported expert opinion and the current practice at the author’s adolescent bariatric program is presented in Table 1.[14, 17] However, despite supplementation practices already in place in the vast majority of bariatric programs, the prevalence of nutritional deficiencies among bariatric patients is still quite high as reviewed in the following sections, perhaps due to difficulties with adherence or because supplementation needs may vary among patients depending on adequacy of dietary intake and type of surgery. Evidence of poor adherence to medications and supplementation pre-operatively might also prompt choice of a purely restrictive bariatric procedure to reduce postoperative risk of significant nutritional deficits.
Table 1.
Recommended Nutritional Screening and Supplementation After Bariatric Surgery
| Baseline, 6 month and annual screening after bariatric surgery | ||
|---|---|---|
| Nutrient | Biomarker(s) | Primary symptoms of deficiency |
| Vitamin B1 | Serum thiamin | Ophthalmoplegia, nystagmus, ataxia, encephalopathy, rapid visual loss (Wernicke encephalopathy) Isolated peripheral neuropathy |
| Vitamin B12 | Serum vitamin B12 | Anemia, neurological dysfunction, visual loss |
| Folate | Red blood cell folate Consider plasma homocysteine |
Anemia |
| Iron | Serum, ferritin, total iron binding capacity, complete blood count with differential | Microcytic anemia |
| Vitamin D | Serum 25(OH) vitamin D, calcium, phosphorus, parathyroid hormone | Decreased bone mineral density Secondary hyperparathyroidism |
| Protein | Serum albumin | Edema, excessive alopecia, poor wound- healing |
| Additional annual screening after BPD and BPD-DS | ||
| Vitamin A | Plasma retinol | Reduced night vision, visual impairment |
| Vitamin E | Plasma alpha-tocopherol | Neuropathy, ataxia |
| Vitamin K | Prothrombin time | Bleeding, easy bruising |
| Screen after any bariatric procedure if suggestive symptoms | ||
| B6 (pyridoxine) | Plasma pyridoxal-5′-phosphate | Anemia, neurological symptoms |
| Copper | Serum copper | Anemia, neuropathy |
| Zinc | Plasma zinc | Acrodermatitis enteropathica-like rash, taste alterations |
| General supplementation recommendations | ||
| Supplement | Daily Recommendations | |
| Multivitamin (contains folic acid) | AGB/VSG RYGB BPD-DS |
One daily One to two daily Two daily |
| Calcium citrate with vitamin D3 | AGB RYGB and BPD-DS |
1200–1500mg/day 1800 mg/day |
| Vitamin D3 | RYGB BPD-DS |
consider 1000 IU/day 2000 IU/day |
| Vitamin B12 | RYGB BPD-DS |
crystalline 500 μg/day oral or 1000 μg/month intramuscularly monitor and start if needed. |
| Elemental iron | RYGB and BDP-DS | 65 mg elemental iron in menstruating females |
| Vitamin B1 | All procedures | consider once daily in first 6 months |
| Vitamin A, K | BPD-DS | 10,000 IU vitamin A and 300 μg/vitamin K |
Recommend in most cases that routine supplementation begin at discharge from hospital so that the patient develops a routine early. The author’s program begins a multi-vitamin supplement and B complex pre-operatively during preparatory weight-management phase in all patients, and adds 1000 IU vitamin D3, if pre-operative vitamin D deficiency is found.
Key: RBC: red blood cell
TIBC: total iron binding capacity
25 (OH) D: 25 hydroxy-vitamin D
PTH: parathyroid hormone
AGB: adjustable gastric band
VSG: vertical sleeve gastrectomy
RYGB: Roux-en-Y gastric bypass
BPD-DS: biliopancreatic diversion with duodenal switch
Prevalence and clinical significance of nutritional deficiencies in obesity and after bariatric surgery Macronutrient deficiencies
All types of bariatric surgery lead to very reduced total calorie intake, especially in the first 6 postoperative months, typically ranging from 700 to 900 kcalories per day following RYGB.[18] This can contribute to the decreased intake of all macronutrients, especially protein, as patients may have difficulty achieving recommended protein intakes in the face of severely restricted caloric intake and in some cases temporary intolerance of protein-rich or dairy foods. Although patients are advised to consume at least 1 to 1.5 gm of protein per kg of ideal body weight (a minimum of 60 gm of protein per day), some studies have indicated that protein intake in the first year after surgery may be much lower than recommended, often closer to 0.5 gm/kg.[18, 19] Baseline hypoalbuminemia appears to be uncommon in the United States, but was reported in up to 15.6% of subjects undergoing RYGB in Brazil.[18] Certainly, any pre-existing hypoalbuminemia could be worsened if adequate protein intake is not achieved during the periods of highest caloric restriction. Though the purely restrictive procedures and RYGB with 75–150cm Roux limb lengths rarely cause hypoalbuminemia, BPD is more likely to lead to hypoalbuminemia, with reported prevalence of hypoalbuminemia ranging from 3–11%. [20–22] Longer Roux limb lengths in RYGB can however increase the risk of hypoalbuminemia.[23] Low protein intake can lead to increased hair loss and contribute to poor wound-healing, which is especially critical if body-contouring surgeries are pursued by the patient at a later time.[24] Fortunately, significant protein-calorie malnutrition or kwashiorkor is rarely seen, except in cases of extreme non-adherence to dietary recommendations.[25]
Micronutrient deficiencies
Micronutrient deficiencies are the most likely long-term adverse events after bariatric surgery and can lead to wide-ranging symptoms, most commonly anemia (10% to 74%) and neurological dysfunction (5–9%) (Table 1).[26, 27] Determining the true risk of developing micronutrient deficiencies is challenging as there has been no consensus on the appropriate type and amount of vitamin and mineral supplementation across bariatric surgery programs, and therefore supplementation practices vary widely.[15] Further, varying levels of adherence and dietary intake make it difficult to estimate the true risk of developing nutritional deficiencies. Nonetheless, it is clear that micronutrient deficiencies are relatively common in patients before and after all types of bariatric surgery and therefore it is important to screen patients at baseline, and at a minimum, annually for deficiencies. In women who become pregnant after bariatric surgery, it is critical to screen and treat nutritional deficits before and during pregnancies. A recommended screening and supplementation algorithm is presented in Table 1, but it is again important to emphasize that these suggestions are based on expert opinion and reported recommendations, rather than results of randomized-controlled trials. Importantly supplements should not be enteric-coated or time-release formulations, as most bariatric patients have altered and diminished gastric phase digestion, particularly after RYGB, BPD and VSG. Supplements and medications should readily dissolve in a glass of room temperature water within at most 30 minutes; liquid, suspension or chewable preparations are ideal. In the early post-operative period (first month), liquid or chewable preparations are often better tolerated.
Iron
Iron deficiency is perhaps the most common and earliest nutritional deficiency to occur following bariatric surgery, occurring in up to 12 to 47% of patients, particularly after RYGB and BPD.[15, 18, 28] Menstruating and pregnant females are at greatest risk.[28, 29] Anemia can also be exacerbated by chronic inflammation secondary to obesity.[30] While often asymptomatic, iron deficiency can lead to anemia and fatigue and in severe cases, can present with pica.[28, 31] However, it is important to emphasize that baseline iron deficiency has also been reported in up to 44% of adults prior to bariatric surgery which may contribute to iron deficiency post-operatively if not identified and treated.[32] In a cohort study of 379 consecutive patients presenting for RYGB, iron deficiency was also more prevalent in younger subjects <25 years old versus older subjects >60 years in age (79% versus 42% respectively).[32] Routine multi-vitamin supplementation does not appear to be sufficient to prevent iron deficiency after RYGB, and in most cases supplemental iron is necessary.[28] However, it is not clear if this is because pre-operative deficiencies were not adequately identified and corrected prior to surgery, as baseline studies are often lacking in most retrospective studies. If refractory to oral iron supplementation and correction, parenteral iron therapy or even blood transfusions may be necessary, especially in menstruating and pregnant women.[29, 33]
Calcium and vitamin D
Deficiency of vitamin D has gained widespread attention as the hormone has been increasingly recognized as a risk factor for a multitude of diseases beyond development of rickets in childhood and osteomalacia in adulthood. In addition to reducing dietary calcium absorption, vitamin D deficiency may contribute to dysfunction of the innate immune system, and therefore play a role in increasing risk of cancers, diabetes mellitus, autoimmune diseases and cardiovascular disease.[34] The optimal levels of 25-hydroxyvitamin D [25-OH D], the primary circulating form of the vitamin D, are debated, but deficiency is commonly defined as 25-OH D level less than 20ng/mL (50nmol/L) and insufficiency as 21–29 ng/mL (50 to 80 nmol/L). Widespread deficiency (40 to 100%) of 25-OH D has been noted in population-based studies in North America and many other countries [34–37], in part due to seasonal variation in sunlight exposure, increased use of sunscreens to prevent skin cancer, and changes in lifestyle favoring more indoor time.[34] Mean levels of 25-OH D have declined in national surveys of adults in the United States between 1988–94 and 2000–2004.[38]
Accordingly, the current 2008 American Academy of Pediatrics supplementation recommendations for vitamin D in childhood now include a recommendation that all infants, children and adolescents have a minimum daily intake of 400 IU of vitamin D beginning soon after birth.[39] Expert opinion suggests that the intake be increased to 800 to 1000 IU for children and adults receiving inadequate sun exposure.[34] Adequate sun exposure is defined as twice-a-week exposure of arms and legs for 5 to 30 minutes between the hours of 10 a.m. and 3 p.m., depending on the latitude, season and skin pigmentation. Optimal supplementation for overweight and obese adults and children has not been determined, and current recommendations for the general population should be followed. Notably, vitamin D2 which is more commonly found in over-the-counter multivitamins is 70% less effective than vitamin D3 in increasing and maintaining sufficient 25-OH D levels. [40]
Dietary intake may also play a role, though the predominant source of vitamin D remains synthesis in the skin after ultraviolet radiation exposure, with less than 10% of vitamin D originating from dietary intake.[41] Intake of vitamin D-fortified milk, which is a large source of vitamin D, declines as children age.[42] Further, milk intake declines as consumption of larger quantities of sweetened beverages increases, as observed in young African-American and Caucasian girls followed longitudinally between ages 9–10 and age 19 years.[43] Some studies have noted disparities in vitamin D intake in certain racial and ethnic groups, with Mexican American and African-American adults consuming lower levels of vitamin D, potentially due to higher rates of lactose intolerance.[44] Due to frequently darker skin tones, these groups may also make lower levels of vitamin D after sun exposure.
Overweight and obese individuals tend to have lower mean levels of 25-OH D compared with lean subjects.[45] Potential explanations include decreased dietary intake of fortified milk products, more sedentary lifestyle and reduced exposure to bright sunlight as well as sequestration of the lipid-soluble vitamin in increased adipose tissue stores. Low serum 25-OH D appears to be inversely proportional to increasing fat mass.[46, 47] Marked seasonal variation in 25-OH D levels may also be present in adult obese subjects, independent of the degree of obesity. In one cross-sectional study of 248 obese subjects with BMI ranging from 30.1 to 68.9 kg/m2, prevalence of 25-OH D deficiency was 3.8 fold higher during the winter when compared to summer months (91.2 vs. 24.3%, p<.001).[48] In contrast, comparison of 41 age, sex, race-ethnicity and seasonally matched pre-operative obese adult patients with non-obese controls demonstrated that the prevalence of vitamin D deficiency (61%) and insufficiency (90%) was substantially higher than in controls (12% and 32% respectively), even after controlling for sunlight exposure and dietary intake of calcium and vitamin D.[49]
Accordingly, 25 to 80% of adult pre-bariatric patients may have baseline vitamin D deficiency.[32, 50] Hispanic and African-American patients undergoing bariatric surgery may have even higher rates of vitamin D deficiency (~78%) than their Caucasian counterparts (36%).[51, 52] In a study of 70 adult patients presenting for RYGB, mean 25-OH D levels were also inversely correlated with BMI, lending support to the theory that fat mass may influence bio-availability.[53] Of concern is that 45% of these individuals continued to have insufficient vitamin D levels, despite recommended post-operative supplementation. Optimal dosing of vitamin D after various types of bariatric surgery remains unclear. A recent expert guideline suggests supplementation of 2000 IU per day for patients after BPD-DS, with no additional vitamin D3 for patients after RYGB or AGB, above that associated with the elemental calcium supplement.[14] Based on the experience at the author’s adolescent bariatric surgery program, vitamin D deficiency appears to be very prevalent both before and after RYGB, necessitating additional vitamin D3 supplementation, up to 1000 IU orally daily or 50,000 IU monthly in cases of severe deficiency (unpublished data). One recent prospective randomized clinical study has indicated that doses up to 5,000 IU appear to be safe and necessary in some adults patients after RYGB to maintain vitamin D sufficiency, yet not enough to prevent vitamin D insufficiency for others.[54] Supplementation of calcium with vitamin D at recommended levels for 6 months did not appear to suppress secondary hyperparathyroidism or bone resorption in 44 women 3 or more years after RYGB.[55] Further prospective studies will be necessary to determine the true prevalence and risk factors for vitamin D3 deficiency before and after different types of bariatric surgery in extremely obese adults and adolescents and optimal dosing strategies.
Despite high prevalence of vitamin D deficiency after bariatic surgery, serum calcium levels are frequently maintained in normal ranges. Elevated parathyroid hormone levels are far more common (up to 29% in RYGB and 63% after BPD), but it is unclear whether prevalence differs significantly from baseline.[56–58] Reported sequelae of vitamin D and resultant calcium deficiency after bariatric surgery, particularly in surgeries that include a malabsorptive component, include high bone turnover and decreased bone mass.[57, 59, 60] Concomitant risk factors may lead to earlier presentation. Osteomalacia has been reported in a 42-year-old patient with a history of corticosteroid-dependent asthma, within 6 ½ years of RYGB.[59] Up to 70% of patients develop secondary hyperparathyroidism after BPD[61]
A routine multi-vitamin typically contains 400 IU of vitamin D (most often ergocalciferol or D2) and 100–200 mg of calcium carbonate. Calcium with vitamin D supplements vary considerably in the amount and type of calcium and vitamin D. A typical calcium with vitamin D supplement often contains 500–600 mg per of calcium (carbonate or citrate) and 400–500 IU of vitamin D (ergocalciferol (D2) or cholecalciferol(D3)). Calcium citrate is easier to absorb, particularly in conditions with reduced stomach acid, and is therefore preferable for patients after bariatric surgery. Taken three times a day, calcium citrate + vitamin D supplements can provide up to1500 –1800 mg of calcium and 1200–1500 IU of vitamin D. While these amounts of calcium and vitamin D are likely to be sufficient for patients after the purely restrictive procedures, patients after RYGB may require additional supplemental vitamin D3 and patients after BPD-DS should be prescribed supplemental vitamin D3, as outlined in the table.
Other fat-soluble vitamins: Vitamin A, K and E
Several large cross-sectional studies of obese and overweight children indicate that they may have lower concentrations of antioxidant vitamins, retinol and beta-carotene (vitamin A), as well as alpha-tocopherol (vitamin E).[4, 62] Studies of baseline nutritional deficits in adults presenting for bariatric surgery also indicate potential vitamin A and E deficiencies in the extremely obese. Upto a 12.5% prevalence of low levels of retinols and beta- carotene has recently been described pre-operatively in adults undergoing bariatric surgery, with increased severity post-operatively.[63, 64] In cases of very severe deficiency, this can cause xerophthalmia and nyctalopia, with visual deterioration reported in one 39-year-old woman 3 years following RYGB.[65] Pre-existing vitamin E deficiency has been reported in up to 23 % of RYGB patients.[66] Though less common, significant declines in vitamin E have also been reported after RYGB.[67] Vitamin E may be clinically significant due to its antioxidant function. Neuropathy has been reported in association with vitamin E deficiency after gastrectomy for gastric cancer, but reports of symptomatic deficiencies after bariatric surgery are lacking.[68] In general however, fat-soluble vitamin deficiencies, including vitamin A (69%), E (7.1%) and K (68%), appear to be more common after BPD due to significant fat malabsorption.[69, 70] Routine annual monitoring is therefore currently suggested only after BPD or BPD-DS procedures, but should be considered after any bariatric surgery procedure if symptoms develop (see Table 1).
Vitamin B12, B1 (thiamin), folate and vitamin B6
The B vitamins are generally important for neurological and hematological function. Further, low folate and B12 levels can also be associated with elevated plasma homocysteine levels, which may be a potential independent risk factor for oxidative stress and cardiovascular disease.[71] However, a recent meta-analysis of supplemental folate therapy did not show any benefit in reducing cardiovascular disease, so evidence is still inconclusive.[72]
Overweight has been associated with a greater risk of decreased intake of folate in adolescents.[73] Prevalence of pre-operative folic acid deficiency (up to 54%) has been noted in international studies prior to bariatric surgery. However, recent American studies report very low prevalence of folate deficiency (0–6%) at baseline, perhaps due to widespread folate fortification of foods in the United States.[51, 70, 74] Similarly, risk of folate deficiency appears to be very low after bariatric surgery in the United States, and addition of a routine multi-vitamin nearly always corrects any deficiencies.[28] This is likely due to additional dietary sources from fortified foods, the absorption of folate along the entire length of the small intestine and bacterial synthesis of folate in the intestine.[75] Low folate levels after bariatric surgery therefore can indicate lack of adherence to multivitamin supplementation.[74]
In contrast with the low baseline risk of folate deficiency, low vitamin B12 levels have been reported prior to bariatric surgery in up to 18% of severely obese adults.[51] Vitamin B12 deficiency post-operatively is more commonly associated with RYGB (up to one third of patients),[28] but the rate is significantly reduced to approximately 4% of patients with vitamin B12 supplementation.[64, 76] Multivitamin supplementation alone is not sufficient to prevent vitamin B12 deficiency.[28] Daily oral vitamin B12 (350 – 600 ug per day) is effective in correcting deficiency in 81 to 95% of patients.[77, 78], and intramuscular monthly vitamin B12 injections are another option in patients who have trouble adhering to daily oral supplements.
Baseline vitamin B1 or thiamin deficiency has been reported in up to 29% of patients undergoing bariatric surgery.[32] One study of 378 adults found that pre-existing thiamin deficiency may be highest in African American (31%) and Hispanic patients (47%), compared to Caucasians (7%). Thiamin deficiency is more common after procedures which involve gastric bypass due to decreased acidification of food and impaired absorption, but has also been reported in isolated cases after purely restrictive procedures.[79, 80] Asymptomatic thiamin deficiency has been reported in up to 18% of patients 1 year post RYGB. [64] Severe thiamin deficiency leading to peripheral neuropathy, and in some cases Wernicke encephalopathy, has been reported in both adults and adolescents after bariatric surgery.[81, 82] Typically this occurs around 6 weeks to 3 months after surgery, but has been reported to occur as early as 2 weeks post-operatively.[83] Risk factors include excessive post-operative vomiting leading to reduced intake and non-adherence to multi-vitamin supplementation.[84] Rapid identification and treatment with intravenous supplementation can rapidly improve visual loss and promote resolution of neurologic sequelae, while delay in repletion can cause permanent disability.[81]
Vitamin B6 (17.6%) and vitamin B2 (13.6%) deficiencies have also been reported in patients 1 year post-RYGB, but the true prevalence of pre-existing B6 and B2 deficiencies in obese patients is unknown as these are not commonly screened for.[64]
Vitamin C (ascorbic acid)
Ascorbic acid or vitamin C deficiency has been noted in up to 36 % of adult patients aged 20 to 66 years prior to bariatric surgery.[85] Ascorbic acid deficiency correlated with higher BMI, younger age, decreased intake of fruit and vegetables, and lack of vitamin supplementation. More recently, ascorbic acid deficiency has been reported in 34.5% of post-RYGB patients at 1 year post-operatively.[64] Like vitamin E, ascorbic acid has important antioxidant functions. Preliminary studies suggest that vitamin C and E supplementation lowers markers of inflammation and may improve insulin sensitivity, but demonstration of significant adverse effects of vitamin C deficiency on clinical outcome after bariatric surgery are still lacking.[86] Routine screening is not currently recommended and standard multivitamin supplementation should be sufficient to prevent deficiency postoperatively.
Rare but clinically significant nutritional deficiencies after bariatric surgery
Low serum zinc levels have been reported in both pre-operative (up to 28%) and post-operative bariatric patients (36–51%).[69, 87] In most cases, these zinc deficiencies have been asymptomatic, but zinc deficiency with an acrodermatitis enteropathica-like rash has been reported, in a patient who was completely non-adherent to recommended multivitamin supplementation after a distal RYGB procedure.[25] Cardiomyopathy presumably secondary to selenium deficiency has been reported 9 months following BPD.[88] Selenium also has antioxidant function. Copper deficiency following bariatric surgery has gained increased attention in recent years. Copper deficiency causing anemia and neurological impairment has been reported in two patients following RYGB and copper deficiency has also been shown to increase in prevalence after BPD.[70, 89] If unexplained anemia persists in patients post-bariatric surgery, copper deficiency should be excluded. Zinc supplementation in high doses (50mg/day or more) can interfere with intestinal absorption and patients receiving prolonged zinc supplementation should be monitored for copper deficiency. Routine screening for these less common deficiencies is probably not cost-effective given their low incidence but should be triggered by the onset of symptoms of unexplained anemia, neurological impairment, unusual skin manifestations or cardiac dysfunction.
Nutritional deficiencies and pregnancy post-bariatric surgery
The importance of routine nutritional screening and supplementation is heightened in pregnancy following bariatric surgery. In general, pregnancy after bariatric surgery appears to be very safe after the rapid weight loss phase has ended and a stable weight has been achieved.[90, 91] Anemia is the most common problem during pregnancy after bariatric surgery, however, rare but severe fetal and maternal complications due to nutrient deficiencies have been reported. A fetal cerebral hemorrhage was reported due to maternal vitamin K deficiency following vomiting after gastric band slippage.[92] Infantile visual impairment has also occurred secondary to maternal, and subsequently fetal, vitamin A deficiency.[93] Therefore, all nutrient deficiencies should be identified before pregnancy if possible and corrected prior to pregnancy. Additional vitamin and calcium supplementation is often necessary. In some cases, parenteral iron therapy or blood transfusions have been necessary to correct anemia.[29]
Summary
Overweight and obese individuals are at risk for deficiencies in several micronutrients, including iron, and vitamins D, B12, E and C. Risk factors include predominantly nutrient poor diets. However, additional factors may play a role in vitamin D deficiency, including reduced sun exposure and increased adipose stores. Bariatric surgery, an increasingly acceptable treatment for severe obesity in both adults and adolescents can also lead to several predictable nutritional deficiencies and can worsen pre-existing ones. It is critical to screen for nutritional deficiencies in obese patients prior to bariatric surgery and at regular intervals after bariatric surgery, and encourage adherence to supplementation.
At present there is a lack of evidence or expert recommendation to screen all overweight and obese children for nutritional deficiencies. However given the emerging data on the high prevalence of nutritional deficiencies associated with overweight and obesity, practice recommendations may evolve as further information becomes available. If signs or symptoms suggest a nutritional deficiency, screening for specific micronutrient deficits should be performed, independent of bariatric surgery status. Despite a very high prevalence of vitamin D deficiency in the general population, universal 25-OH D screening has not been recommended. Rather, supplemental vitamin D (400 IU/day) should be recommended for those children and adolescents who do not obtain at least 400 IU/day through fortified milk, cereals and other foods. Given the high prevalence of baseline nutritional deficiencies, the quality of the diet should be screened in all overweight and obese children and a routine multi-vitamin and focused dietary consultation should be considered, if the child’s diet is not adequately balanced.
Acknowledgments
This work was supported by Grant No.K23DK080888 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gillis L, Gillis A. Nutrient inadequacy in obese and non-obese youth. Can J Diet Pract Res. 2005;66:237–42. doi: 10.3148/66.4.2005.237. [DOI] [PubMed] [Google Scholar]
- 2.Schilling PL, Davis MM, Albanese CT, et al. National trends in adolescent bariatric surgical procedures and implications for surgical centers of excellence. J Am Coll Surg. 2008;206:1–12. doi: 10.1016/j.jamcollsurg.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 3.Rand CS, Macgregor AM. Adolescents having obesity surgery: a 6-year follow-up. South Med J. 1994;87:1208–13. doi: 10.1097/00007611-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Strauss RS. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. J Pediatr. 1999;134:160–5. doi: 10.1016/s0022-3476(99)70409-9. [DOI] [PubMed] [Google Scholar]
- 5.Kant AK. Reported consumption of low-nutrient-density foods by American children and adolescents: nutritional and health correlates, NHANES III, 1988 to 1994. Arch Pediatr Adolesc Med. 2003;157:789–96. doi: 10.1001/archpedi.157.8.789. [DOI] [PubMed] [Google Scholar]
- 6.Kant AK. Consumption of energy-dense, nutrient-poor foods by adult Americans: nutritional and health implications. The third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2000;72:929–36. doi: 10.1093/ajcn/72.4.929. [DOI] [PubMed] [Google Scholar]
- 7.Hampl JS, Betts NM. Comparisons of dietary intake and sources of fat in low- and high-fat diets of 18- to 24-year-olds. J Am Diet Assoc. 1995;95:893–7. doi: 10.1016/s0002-8223(95)00247-2. [DOI] [PubMed] [Google Scholar]
- 8.Keller KL, Kirzner J, Pietrobelli A, et al. Increased sweetened beverage intake is associated with reduced milk and calcium intake in 3- to 7-year-old children at multi-item laboratory lunches. J Am Diet Assoc. 2009;109:497–501. doi: 10.1016/j.jada.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wachs TD. Multiple influences on children’s nutritional deficiencies: a systems perspective. Physiol Behav. 2008;94:48–60. doi: 10.1016/j.physbeh.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. Jama. 2005;294:1909–17. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 11.Gracia JA, Martinez M, Aguilella V, et al. Postoperative morbidity of biliopancreatic diversion depending on common limb length. Obes Surg. 2007;17:1306–11. doi: 10.1007/s11695-007-9233-9. [DOI] [PubMed] [Google Scholar]
- 12.Nadler EP, Youn HA, Ren CJ, et al. An update on 73 US obese pediatric patients treated with laparoscopic adjustable gastric banding: comorbidity resolution and compliance data. J Pediatr Surg. 2008;43:141–6. doi: 10.1016/j.jpedsurg.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Vargas-Ruiz AG, Hernandez-Rivera G, Herrera MF. Prevalence of iron, folate, and vitamin B12 deficiency anemia after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18:288–93. doi: 10.1007/s11695-007-9310-0. [DOI] [PubMed] [Google Scholar]
- 14.Aills L, Blankenship J, Buffington C, et al. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4:S73–108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Brolin RE, Leung M. Survey of vitamin and mineral supplementation after gastric bypass and biliopancreatic diversion for morbid obesity. Obes Surg. 1999;9:150–4. doi: 10.1381/096089299765553395. [DOI] [PubMed] [Google Scholar]
- 16.Pournaras DJ, le Roux CW. After bariatric surgery what vitamins should be measured and what supplements should be given? Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2009.03564.x. [DOI] [PubMed] [Google Scholar]
- 17.Xanthakos SA, Inge TH. Nutritional consequences of bariatric surgery. Curr Opin Clin Nutr Metab Care. 2006;9:489–96. doi: 10.1097/01.mco.0000232913.07355.cf. [DOI] [PubMed] [Google Scholar]
- 18.Bavaresco M, Paganini S, Lima TP, et al. Nutritional Course of Patients Submitted to Bariatric Surgery. Obes Surg. 2008 doi: 10.1007/s11695-008-9721-6. [DOI] [PubMed] [Google Scholar]
- 19.Moize V, Geliebter A, Gluck ME, et al. Obese patients have inadequate protein intake related to protein intolerance up to 1 year following Roux-en-Y gastric bypass. Obes Surg. 2003;13:23–8. doi: 10.1381/096089203321136548. [DOI] [PubMed] [Google Scholar]
- 20.Kalfarentzos F, Papadoulas S, Skroubis G, et al. Prospective evaluation of biliopancreatic diversion with Roux-en-Y gastric bypass in the super obese. J Gastrointest Surg. 2004;8:479–88. doi: 10.1016/j.gassur.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Wylezol M, Gluck M, Zubik R, et al. Biliopancreatic diversion in Poland. J Physiol Pharmacol. 2005;56 (Suppl 6):117–26. [PubMed] [Google Scholar]
- 22.Skroubis G, Anesidis S, Kehagias I, et al. Roux-en-Y gastric bypass versus a variant of biliopancreatic diversion in a non-superobese population: prospective comparison of the efficacy and the incidence of metabolic deficiencies. Obes Surg. 2006;16:488–95. doi: 10.1381/096089206776327251. [DOI] [PubMed] [Google Scholar]
- 23.Kalfarentzos F, Dimakopoulos A, Kehagias I, et al. Vertical banded gastroplasty versus standard or distal Roux-en-Y gastric bypass based on specific selection criteria in the morbidly obese: preliminary results. Obes Surg. 1999;9:433–42. doi: 10.1381/096089299765552701. [DOI] [PubMed] [Google Scholar]
- 24.Agha-Mohammadi S, Hurwitz DJ. Nutritional deficiency of post-bariatric surgery body contouring patients: what every plastic surgeon should know. Plast Reconstr Surg. 2008;122:604–13. doi: 10.1097/PRS.0b013e31817d6023. [DOI] [PubMed] [Google Scholar]
- 25.Lewandowski H, Breen TL, Huang EY. Kwashiorkor and an acrodermatitis enteropathica-like eruption after a distal gastric bypass surgical procedure. Endocr Pract. 2007;13:277–82. doi: 10.4158/EP.13.3.277. [DOI] [PubMed] [Google Scholar]
- 26.Brolin RE, LaMarca LB, Kenler HA, et al. J Gastrointest Surg. Vol. 6. 2002. Malabsorptive gastric bypass in patients with superobesity; pp. 195–203. discussion 04–5. [DOI] [PubMed] [Google Scholar]
- 27.Berger JR. The neurological complications of bariatric surgery. Arch Neurol. 2004;61:1185–9. doi: 10.1001/archneur.61.8.1185. [DOI] [PubMed] [Google Scholar]
- 28.Brolin RE, Gorman JH, Gorman RC, et al. Are vitamin B12 and folate deficiency clinically important after roux-en-Y gastric bypass? J Gastrointest Surg. 1998;2:436–42. doi: 10.1016/s1091-255x(98)80034-6. [DOI] [PubMed] [Google Scholar]
- 29.Gurewitsch ED, Smith-Levitin M, Mack J. Pregnancy following gastric bypass surgery for morbid obesity. Obstet Gynecol. 1996;88:658–61. doi: 10.1016/0029-7844(96)00187-1. [DOI] [PubMed] [Google Scholar]
- 30.von Drygalski A, Andris DA. Anemia after bariatric surgery: more than just iron deficiency. Nutr Clin Pract. 2009;24:217–26. doi: 10.1177/0884533609332174. [DOI] [PubMed] [Google Scholar]
- 31.Kushner RF, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104:1393–7. doi: 10.1016/j.jada.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Flancbaum L, Belsley S, Drake V, et al. Preoperative nutritional status of patients undergoing Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg. 2006;10:1033–7. doi: 10.1016/j.gassur.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Varma S, Baz W, Badine E, et al. Need for parenteral iron therapy after bariatric surgery. Surg Obes Relat Dis. 2008;4:715–9. doi: 10.1016/j.soard.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 35.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 36.Hintzpeter B, Mensink GB, Thierfelder W, et al. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–89. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 37.Siddiqui AM, Kamfar HZ. Prevalence of vitamin D deficiency rickets in adolescent school girls in Western region, Saudi Arabia. Saudi Med J. 2007;28:441–4. [PubMed] [Google Scholar]
- 38.Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988– 1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–27. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 40.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 41.Misra M, Pacaud D, Petryk A, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 42.Bowman SA. Beverage choices of young females: changes and impact on nutrient intakes. J Am Diet Assoc. 2002;102:1234–9. doi: 10.1016/s0002-8223(02)90273-7. [DOI] [PubMed] [Google Scholar]
- 43.Striegel-Moore RH, Thompson D, Affenito SG, et al. Correlates of beverage intake in adolescent girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2006;148:183–7. doi: 10.1016/j.jpeds.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 44.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135:2478–85. doi: 10.1093/jn/135.10.2478. [DOI] [PubMed] [Google Scholar]
- 45.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S–64S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 46.Buffington C, Walker B, Cowan GS, Jr, et al. Vitamin D Deficiency in the Morbidly Obese. Obes Surg. 1993;3:421–24. doi: 10.1381/096089293765559142. [DOI] [PubMed] [Google Scholar]
- 47.Vilarrasa N, Maravall J, Estepa A, et al. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J Endocrinol Invest. 2007;30:653–8. doi: 10.1007/BF03347445. [DOI] [PubMed] [Google Scholar]
- 48.Ernst B, Thurnheer M, Schmid SM, et al. Seasonal variation in the deficiency of 25-hydroxyvitamin D(3) in mildly to extremely obese subjects. Obes Surg. 2009;19:180–3. doi: 10.1007/s11695-008-9636-2. [DOI] [PubMed] [Google Scholar]
- 49.Goldner WS, Stoner JA, Thompson J, et al. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008;18:145–50. doi: 10.1007/s11695-007-9315-8. [DOI] [PubMed] [Google Scholar]
- 50.Ernst B, Thurnheer M, Schmid SM, et al. Evidence for the necessity to systematically assess micronutrient status prior to bariatric surgery. Obes Surg. 2009;19:66–73. doi: 10.1007/s11695-008-9545-4. [DOI] [PubMed] [Google Scholar]
- 51.Gemmel K, Santry HP, Prachand VN, et al. Vitamin D deficiency in preoperative bariatric surgery patients. Surg Obes Relat Dis. 2009;5:54–9. doi: 10.1016/j.soard.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Carlin AM, Rao DS, Meslemani AM, et al. Prevalence of vitamin D depletion among morbidly obese patients seeking gastric bypass surgery. Surg Obes Relat Dis. 2006;2:98–103. doi: 10.1016/j.soard.2005.12.001. discussion 04. [DOI] [PubMed] [Google Scholar]
- 53.Mahlay NF, Verka LG, Thomsen K, et al. Vitamin D Status Before Roux-en-Y and Efficacy of Prophylactic and Therapeutic Doses of Vitamin D in Patients After Roux-en-Y Gastric Bypass Surgery. Obes Surg. 2008 doi: 10.1007/s11695-008-9698-1. [DOI] [PubMed] [Google Scholar]
- 54.Goldner WS, Stoner JA, Lyden E, et al. Finding the optimal dose of vitamin D following Roux-en-Y gastric bypass: a prospective, randomized pilot clinical trial. Obes Surg. 2009;19:173–9. doi: 10.1007/s11695-008-9680-y. [DOI] [PubMed] [Google Scholar]
- 55.Goode LR, Brolin RE, Chowdhury HA, et al. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12:40–7. doi: 10.1038/oby.2004.7. [DOI] [PubMed] [Google Scholar]
- 56.Ybarra J, Sanchez-Hernandez J, Gich I, et al. Unchanged hypovitaminosis D and secondary hyperparathyroidism in morbid obesity after bariatric surgery. Obes Surg. 2005;15:330–5. doi: 10.1381/0960892053576758. [DOI] [PubMed] [Google Scholar]
- 57.Hamoui N, Kim K, Anthone G, et al. The significance of elevated levels of parathyroid hormone in patients with morbid obesity before and after bariatric surgery. Arch Surg. 2003;138:891–7. doi: 10.1001/archsurg.138.8.891. [DOI] [PubMed] [Google Scholar]
- 58.Newbury L, Dolan K, Hatzifotis M, et al. Calcium and vitamin D depletion and elevated parathyroid hormone following biliopancreatic diversion. Obes Surg. 2003;13:893–5. doi: 10.1381/096089203322618722. [DOI] [PubMed] [Google Scholar]
- 59.Collazo-Clavell ML, Jimenez A, Hodgson SF, et al. Osteomalacia after Roux-en-Y gastric bypass. Endocr Pract. 2004;10:195–8. doi: 10.4158/EP.10.3.195. [DOI] [PubMed] [Google Scholar]
- 60.Compher CW, Badellino KO, Boullata JI. Vitamin D and the bariatric surgical patient: a review. Obes Surg. 2008;18:220–4. doi: 10.1007/s11695-007-9289-6. [DOI] [PubMed] [Google Scholar]
- 61.Balsa JA, Botella-Carretero JI, Peromingo R, et al. Role of calcium malabsorption in the development of secondary hyperparathyroidism after biliopancreatic diversion. J Endocrinol Invest. 2008;31:845–50. doi: 10.1007/BF03346429. [DOI] [PubMed] [Google Scholar]
- 62.de Souza Valente da Silva L, Valeria da Veiga G, Ramalho RA. Association of serum concentrations of retinol and carotenoids with overweight in children and adolescents. Nutrition. 2007;23:392–7. doi: 10.1016/j.nut.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Pereira S, Saboya C, Chaves G, et al. Class III Obesity and its Relationship with the Nutritional Status of Vitamin A in Pre- and Postoperative Gastric Bypass. Obes Surg. 2008 doi: 10.1007/s11695-008-9478-y. [DOI] [PubMed] [Google Scholar]
- 64.Clements RH, Katasani VG, Palepu R, et al. Incidence of vitamin deficiency after laparoscopic Roux-en-Y gastric bypass in a university hospital setting. Am Surg. 2006;72:1196–202. doi: 10.1177/000313480607201209. discussion 203–4. [DOI] [PubMed] [Google Scholar]
- 65.Lee WB, Hamilton SM, Harris JP, et al. Ocular complications of hypovitaminosis a after bariatric surgery. Ophthalmology. 2005;112:1031–4. doi: 10.1016/j.ophtha.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 66.Boylan LM, Sugerman HJ, Driskell JA. Vitamin E, vitamin B-6, vitamin B-12, and folate status of gastric bypass surgery patients. J Am Diet Assoc. 1988;88:579–85. [PubMed] [Google Scholar]
- 67.Coupaye M, Puchaux K, Bogard C, et al. Nutritional consequences of adjustable gastric banding and gastric bypass: a 1-year prospective study. Obes Surg. 2009;19:56–65. doi: 10.1007/s11695-008-9571-2. [DOI] [PubMed] [Google Scholar]
- 68.Rino Y, Suzuki Y, Kuroiwa Y, et al. Vitamin E malabsorption and neurological consequences after gastrectomy for gastric cancer. Hepatogastroenterology. 2007;54:1858–61. [PubMed] [Google Scholar]
- 69.Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8:48–55. doi: 10.1016/j.gassur.2003.09.020. discussion 54–5. [DOI] [PubMed] [Google Scholar]
- 70.de Luis DA, Pacheco D, Izaola O, et al. Clinical results and nutritional consequences of biliopancreatic diversion: three years of follow-up. Ann Nutr Metab. 2008;53:234–9. doi: 10.1159/000185641. [DOI] [PubMed] [Google Scholar]
- 71.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Bmj. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bazzano LA, Reynolds K, Holder KN, et al. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. Jama. 2006;296:2720–6. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 73.Vitolo MR, Canal Q, Campagnolo PD, et al. Factors associated with risk of low folate intake among adolescents. J Pediatr (Rio J) 2006;82:121–6. doi: 10.2223/JPED.1449. [DOI] [PubMed] [Google Scholar]
- 74.Mallory GN, Macgregor AM. Folate Status Following Gastric Bypass Surgery (The Great Folate Mystery) Obes Surg. 1991;1:69–72. doi: 10.1381/096089291765561493. [DOI] [PubMed] [Google Scholar]
- 75.Russell RM, Dhar GJ, Dutta SK, et al. Influence of intraluminal pH on folate absorption: studies in control subjects and in patients with pancreatic insufficiency. J Lab Clin Med. 1979;93:428–36. [PubMed] [Google Scholar]
- 76.Kalfarentzos F, Skroubis G, Kehagias I, et al. A prospective comparison of vertical banded gastroplasty and Roux-en-Y gastric bypass in a non-superobese population. Obes Surg. 2006;16:151–8. doi: 10.1381/096089206775565096. [DOI] [PubMed] [Google Scholar]
- 77.Schilling RF, Gohdes PN, Hardie GH. Vitamin B12 deficiency after gastric bypass surgery for obesity. Ann Intern Med. 1984;101:501–2. doi: 10.7326/0003-4819-101-4-501. [DOI] [PubMed] [Google Scholar]
- 78.Rhode BM, Tamin H, Gilfix BM, et al. Treatment of Vitamin B12 Deficiency after Gastric Surgery for Severe Obesity. Obes Surg. 1995;5:154–58. doi: 10.1381/096089295765557953. [DOI] [PubMed] [Google Scholar]
- 79.Sola E, Morillas C, Garzon S, et al. Rapid onset of Wernicke’s encephalopathy following gastric restrictive surgery. Obes Surg. 2003;13:661–2. doi: 10.1381/096089203322190934. [DOI] [PubMed] [Google Scholar]
- 80.Bozbora A, Coskun H, Ozarmagan S, et al. A rare complication of adjustable gastric banding: Wernicke’s encephalopathy. Obes Surg. 2000;10:274–5. doi: 10.1381/096089200321643610. [DOI] [PubMed] [Google Scholar]
- 81.Serra A, Sechi G, Singh S, et al. Wernicke encephalopathy after obesity surgery: a systematic review. Neurology. 2007;69:615. doi: 10.1212/01.wnl.0000278895.59835.68. author reply 15–6. [DOI] [PubMed] [Google Scholar]
- 82.Towbin A, Inge TH, Garcia VF, et al. Beriberi after gastric bypass surgery in adolescence. J Pediatr. 2004;145:263–7. doi: 10.1016/j.jpeds.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 83.Al-Fahad T, Ismael A, Soliman MO, et al. Very early onset of Wernicke’s encephalopathy after gastric bypass. Obes Surg. 2006;16:671–2. doi: 10.1381/096089206776945075. [DOI] [PubMed] [Google Scholar]
- 84.Aasheim ET, Hofso D, Hjelmesaeth J, et al. Peripheral neuropathy and severe malnutrition following duodenal switch. Obes Surg. 2008;18:1640–3. doi: 10.1007/s11695-008-9539-2. [DOI] [PubMed] [Google Scholar]
- 85.Riess KP, Farnen JP, Lambert PJ, et al. Ascorbic acid deficiency in bariatric surgical population. Surg Obes Relat Dis. 2009;5:81–6. doi: 10.1016/j.soard.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 86.Rizzo MR, Abbatecola AM, Barbieri M, et al. Evidence for anti-inflammatory effects of combined administration of vitamin E and C in older persons with impaired fasting glucose: impact on insulin action. J Am Coll Nutr. 2008;27:505–11. doi: 10.1080/07315724.2008.10719732. [DOI] [PubMed] [Google Scholar]
- 87.Madan AK, Orth WS, Tichansky DS, et al. Vitamin and trace mineral levels after laparoscopic gastric bypass. Obes Surg. 2006;16:603–6. doi: 10.1381/096089206776945057. [DOI] [PubMed] [Google Scholar]
- 88.Boldery R, Fielding G, Rafter T, et al. Nutritional deficiency of selenium secondary to weight loss (bariatric) surgery associated with life-threatening cardiomyopathy. Heart Lung Circ. 2007;16:123–6. doi: 10.1016/j.hlc.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 89.Griffith DP, Liff DA, Ziegler TR, et al. Acquired copper deficiency: a potentially serious and preventable complication following gastric bypass surgery. Obesity (Silver Spring) 2009;17:827–31. doi: 10.1038/oby.2008.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guelinckx I, Devlieger R, Vansant G. Reproductive outcome after bariatric surgery: a critical review. Hum Reprod Update. 2009;15:189–201. doi: 10.1093/humupd/dmn057. [DOI] [PubMed] [Google Scholar]
- 91.Roehrig HR, Xanthakos SA, Sweeney J, et al. Pregnancy after gastric bypass surgery in adolescents. Obes Surg. 2007;17:873–7. doi: 10.1007/s11695-007-9162-7. [DOI] [PubMed] [Google Scholar]
- 92.Van Mieghem T, Van Schoubroeck D, Depiere M, et al. Fetal cerebral hemorrhage caused by vitamin K deficiency after complicated bariatric surgery. Obstet Gynecol. 2008;112:434–6. doi: 10.1097/AOG.0b013e3181649e7b. [DOI] [PubMed] [Google Scholar]
- 93.Huerta S, Rogers LM, Li Z, et al. Vitamin A deficiency in a newborn resulting from maternal hypovitaminosis A after biliopancreatic diversion for the treatment of morbid obesity. Am J Clin Nutr. 2002;76:426–9. doi: 10.1093/ajcn/76.2.426. [DOI] [PubMed] [Google Scholar]


