Abstract
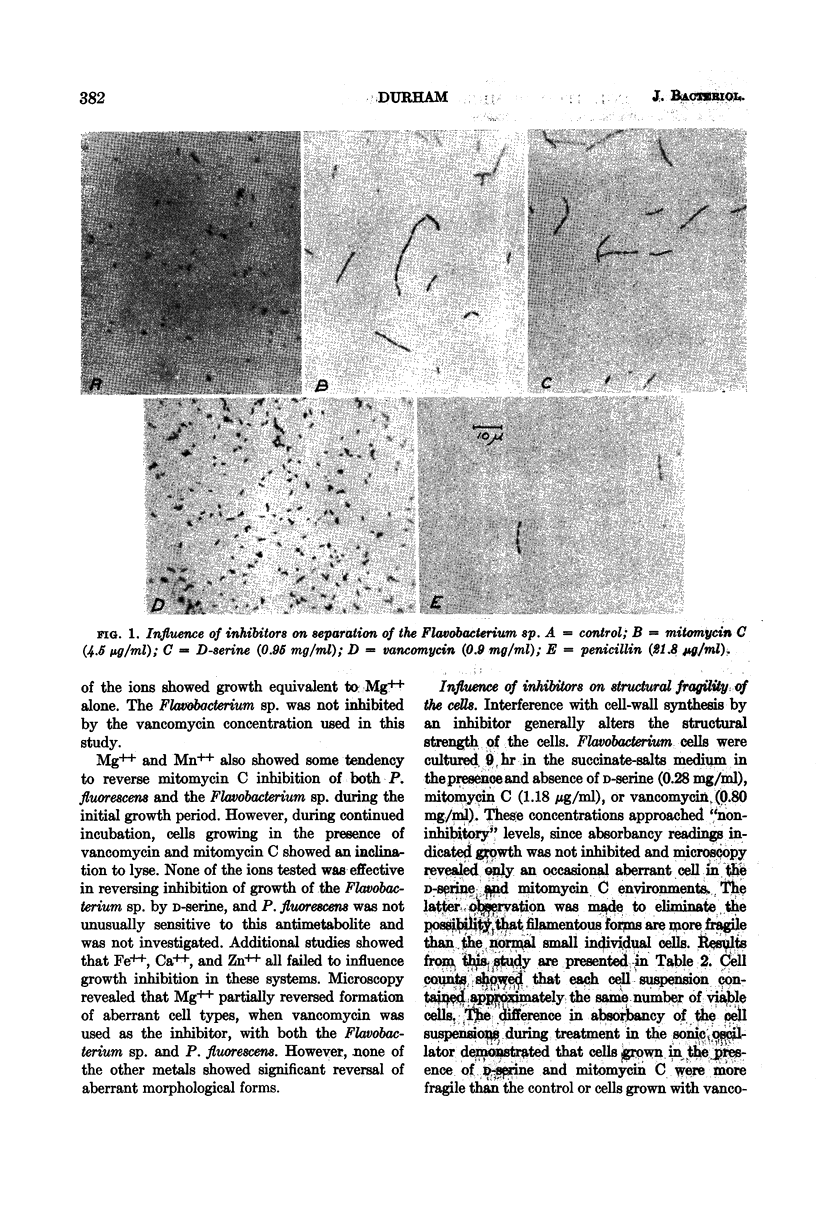
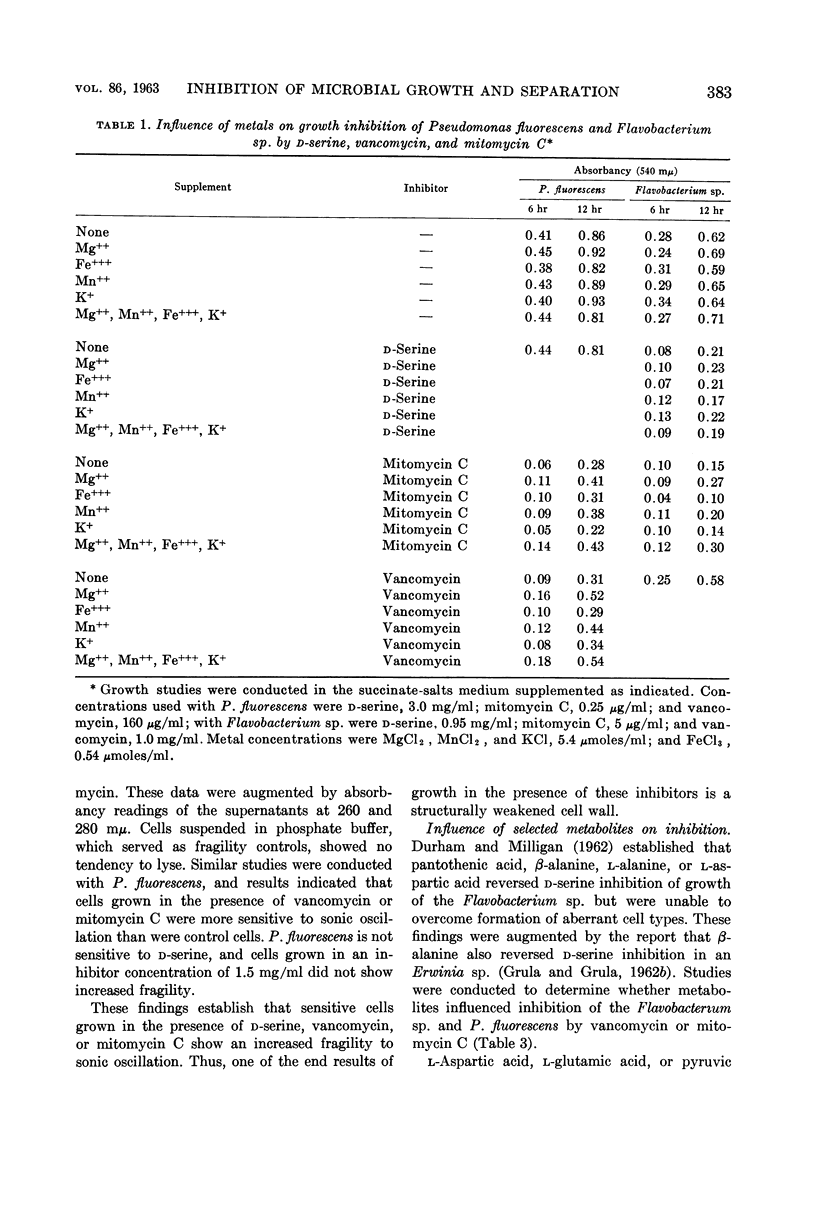
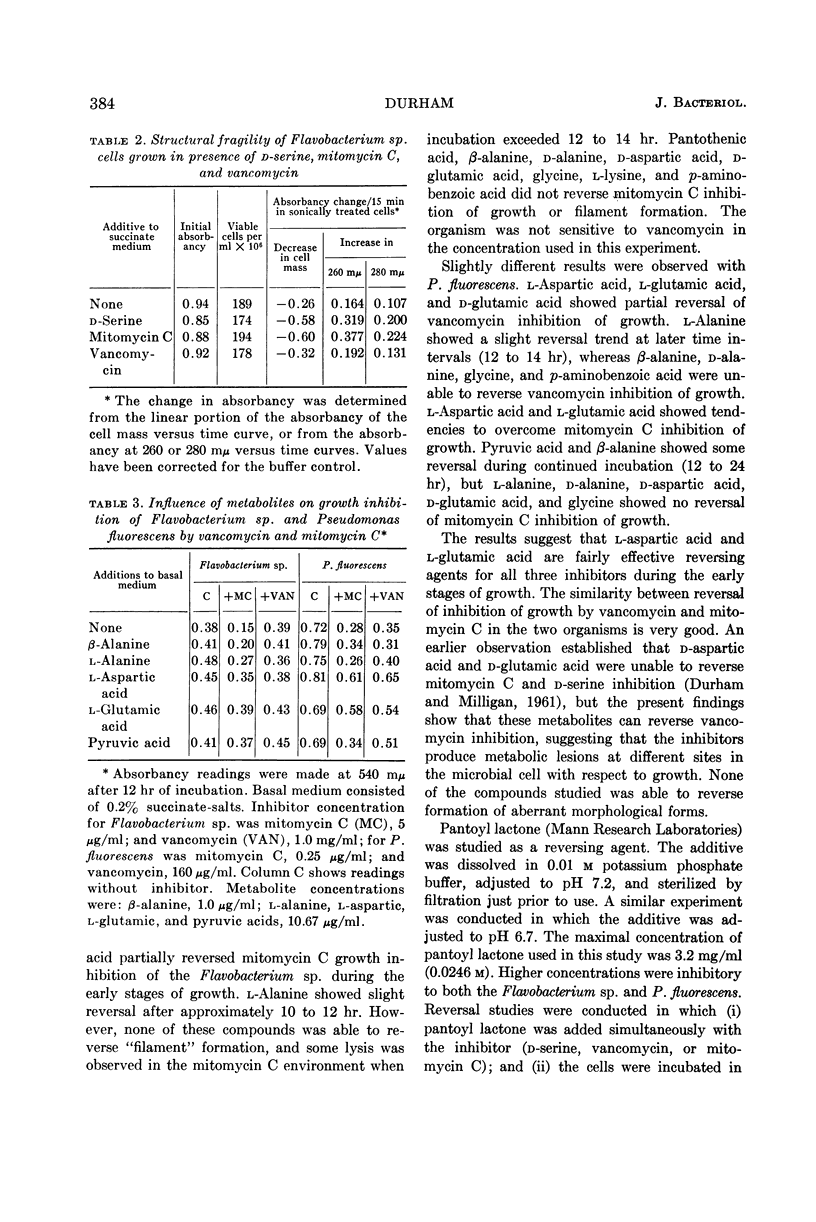
Durham, Norman N. (Oklahoma State University, Stillwater). Inhibition of microbial growth and separation by d-serine, vancomycin, and mitomycin C. J. Bacteriol. 86:380–386. 1963.—A study was made of the growth and separation inhibition of Pseudomonas fluorescens by vancomycin and mitomycin C and of a Flavobacterium sp. sensitive to mitomycin C and d-serine. Aberrant morphological forms (primarily “boat-shaped” cells and “chain-like” filaments) were observed during growth of the sensitive organism in the presence of the inhibitors. Reversal studies indicated that Mg++, and to a lesser extent Mn++, partially reversed vancomycin and mitomycin C growth inhibition during the early stages of growth but did not influence d-serine inhibition. Selected metabolites also showed a tendency to partially reverse mitomycin C and vancomycin inhibition of growth during the initial growth phase, and the differences in reversal trends suggested that d-serine, vancomycin, and mitomycin C produce metabolic lesions at different sites in the cell with respect to growth. Cultivation of the sensitive organism in low concentrations of the inhibitors resulted in a structurally fragile population, indicating formation of a defective cell structure. Mg++ partially reversed the vancomycin inhibition of separation in these organisms. None of the other metals or metabolites, either singly or in varied combinations, was able to reverse inhibition of separation by the various inhibitors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN A. D. The peripheral structures of gram-negative bacteria. III. Effects of cations of proteolytic degradation of the cell envelope of a marine pseudomonad. Biochim Biophys Acta. 1962 Jul 30;62:132–144. doi: 10.1016/0006-3002(62)90498-5. [DOI] [PubMed] [Google Scholar]
- DESREUX V., HACHA R., FREDERICQ E. Activation of deoxyribonucleases by divalent cations. J Gen Physiol. 1962 Mar;45(4):93–102. doi: 10.1085/jgp.45.4.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURHAM N. N., MILLIGAN R. A mechanism of growth inhibition by D-serine in a Flavobacterium. Biochem Biophys Res Commun. 1962 May 11;7:342–345. doi: 10.1016/0006-291x(62)90311-x. [DOI] [PubMed] [Google Scholar]
- DURHAM N. N., MILLIGAN R. Reversal of the D-serine inhibition of growth and division in a Flavobacterium. Biochem Biophys Res Commun. 1961 Jun 2;5:144–147. doi: 10.1016/0006-291x(61)90028-6. [DOI] [PubMed] [Google Scholar]
- GRULA E. A., GRULA M. M. Cell division in a species of Erwinia III. Reversal of inhibition of cell division caused by D-amino acids, penicillin, and ultraviolet light. J Bacteriol. 1962 May;83:981–988. doi: 10.1128/jb.83.5.981-988.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULA M. M., GRULA E. A. Cell division in a species of Erwinia IV. Metabolic blocks in panothenate biosynthesis and their relationship to inhibition of cell division. J Bacteriol. 1962 May;83:989–997. doi: 10.1128/jb.83.5.989-997.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHKO A., AMOZ S., MAGER J. Effect of polyamines and divalent metals on in vitro incorporation of amino acids into ribonucleoprotein particles. Biochem Biophys Res Commun. 1961 May 15;5:46–51. doi: 10.1016/0006-291x(61)90078-x. [DOI] [PubMed] [Google Scholar]
- JORDAN D. C. Effect of vancomycin on the synthesis of the cell wall mucopeptide of Staphylococcus aureus. Biochem Biophys Res Commun. 1961 Nov 20;6:167–170. doi: 10.1016/0006-291x(61)90122-x. [DOI] [PubMed] [Google Scholar]
- KERSTEN H., RAUEN H. M. Degradation of deoxyribonucleic acid in Escherichia coli cells treated with mitomycin C. Nature. 1961 Jun 24;190:1195–1196. doi: 10.1038/1901195a0. [DOI] [PubMed] [Google Scholar]
- LARK C., LARK K. G. The effects of D-amino acids on Alcaligenes fecalis. Can J Microbiol. 1959 Aug;5:369–379. doi: 10.1139/m59-046. [DOI] [PubMed] [Google Scholar]
- NEUHAUS F. C. The enzymatic synthesis of D-alanyl-D-alanine. I. Purification and properties of D-alanyl-D-alanine synthetase. J Biol Chem. 1962 Mar;237:778–786. [PubMed] [Google Scholar]
- REICH E., SHATKIN A. J., TATUM E. L. Bacteriocidal action of mitomycin C. Biochim Biophys Acta. 1961 Oct 14;53:132–149. doi: 10.1016/0006-3002(61)90800-9. [DOI] [PubMed] [Google Scholar]
- TUTTLE A. L., GEST H. Induction of morphological aberrations in Rhodospirillum rubrum by D-amino acids. J Bacteriol. 1960 Feb;79:213–216. doi: 10.1128/jb.79.2.213-216.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYLIE E. B., JOHNSON M. J. Effect of penicillin on the cell wall of Escherichia coli. Biochim Biophys Acta. 1962 May 21;59:450–457. doi: 10.1016/0006-3002(62)90195-6. [DOI] [PubMed] [Google Scholar]




