Abstract
Background
Interferon-gamma (IFN-γ) release assays (IGRAs) were designed to detect latent tuberculosis infection (LTBI). However, discrepancies were found between the tuberculin skin test (TST) and IGRAs results that cannot be attributed to prior Bacille Calmètte Guerin vaccinations. The aim of this study was to evaluate tools for improving LTBI diagnosis by analyzing the IFN-γ response to RD1 proteins in prolonged (long-term response) whole blood tests in those subjects resulting negative to assays such as QuantiFERON-TB Gold In tube (QFT-IT).
Methods
The study population included 106 healthy TST+ individuals with suspected LTBI (recent contact of smear-positive TB and homeless) consecutively enrolled. As controls, 13 healthy subjects unexposed to M. tuberculosis (TST-, QFT-IT-) and 29 subjects with cured pulmonary TB were enrolled. IFN-γ whole blood response to RD1 proteins and QFT-IT were evaluated at day 1 post-culture. A prolonged test evaluating long-term IFN-γ response (7-day) to RD1 proteins in diluted whole blood was performed.
Results
Among the enrolled TST+ subjects with suspected LTBI, 70/106 (66.0%) responded to QFT-IT and 64/106 (60.3%) to RD1 proteins at day 1. To evaluate whether a prolonged test could improve the detection of LTBI, we set up the test using cured TB patients (with a microbiologically diagnosed past pulmonary disease) who resulted QFT-IT-negative and healthy controls as comparator groups. Using this assay, a statistically significant difference was found between IFN-γ levels in cured TB patients compared to healthy controls (p < 0.006). Based on these data, we constructed a receiver operating characteristic (ROC) curve and we calculated a cut-off. Based on the cut-off value, we found that among the 36 enrolled TST+ subjects with suspected LTBI not responding to QFT-IT, a long term response to RD1 proteins was detected in 11 subjects (30.6%).
Conclusion
These results indicate that IFN-γ long-term response to M. tuberculosis RD1 antigens may be used to detect past infection with M. tuberculosis and may help to identify additional individuals with LTBI who resulted negative in the short-term tests. These data may provide useful information for improving immunodiagnostic tests for tuberculosis infection, especially in individuals at high risk for active TB.
Background
In recent years, several immunodiagnostic assays have been developed for diagnosing Mycobacterium tuberculosis (M. tuberculosis) infection [1,2]. These assays, referred as interferon (IFN)-γ release assays (IGRAs), have been specifically designed to overcome the problem of low specificity of the tuberculin skin test (TST). In fact, they detect cellular immune response to antigens which are absent in Bacille Calmètte Guerin (BCG) and most environmental mycobacteria, and specifically present in M. tuberculosis. Two such antigens, early-secreted antigenic target (ESAT)-6 and culture filtrate protein (CFP)-10, encoded in the mycobacterial genomic region of difference (RD)-1 were first evaluated in a 6-day lymphocyte stimulation test (LST) and found to be sensitive and specific for diagnosing tuberculosis (TB) [3-7]. Subsequently, other IGRAs were developed that differed from the classical LST with respect to the in vitro incubation period, the type of cells cultured [whole blood, frozen or fresh peripheral blood mononuclear cells (PBMC)] and the way that the IFN-γ response is detected [(by enzyme-linked immunosorbent assay (ELISA) or enzyme-linked immunospot assay (ELISPOT)].
A number of clinical studies found discrepancies between TST and IGRAs that were mostly attributed to TST positivity associated with prior BCG vaccination [8-10]. However, data from recent studies [11-13] indicate that this explanation may not account for all discrepant results, as a substantial group of BCG-unvaccinated subjects with TST indurations of >15 mm had negative results by commercially available IGRAs, the QuantiFERON-TB Gold In-tube (QFT-IT) test and/or the T-SPOT. TB test (Oxford Immunotec, Abingdon, United Kingdom) [11-13]. These data suggest that IGRAs may have a suboptimal sensitivity, and indicate the need to develop new tools to increase their accuracy.
Prolonged whole blood stimulation has been used to detect memory response in vaccine trials [14]. Using RD1 proteins, it has also been employed to identify subjects with LTBI comparing the results obtained with those found by short-term ELISPOT [15].
The aim of this study was to evaluate if IFN-γ response to RD1 antigens by prolonged (long-term response, 7-day) whole blood stimulation could improve identification of LTBI in subjects resulting negative to QFT-IT. The study population included 106 healthy Human Immunodefciency Vius (HIV)-negative TST+ individuals exposed to M. tuberculosis (recent contacts of smear positive pulmonary TB patients) or at higher likelihood to have LTBI, such as the homeless population, as they are at higher risk to develop active TB disease compared to the general population [16]. Controls included 13 TST- QFT-IT-unexposed individuals and 29 cured TB subjects. All the subjects were consecutively enrolled.
Methods
Study population
At the "L. Spallanzani" National Institute for Infectious Diseases (INMI) we consecutively enrolled in this study TST+ individuals with risk factors for TB infection including close contacts in the last 3 months of patients with a positive sputum culture for M. tuberculosis and a group of TST+ homeless people known to have been exposed to M. tuberculosis in the past. Chemoprophylaxis was offered to all subjects, thereafter referred to as suspected LTBI, but none of them was under chemoprophylaxis at the time of the study. As control groups, we enrolled: 1) "controls", individuals with no risk of M. tuberculosis infection who tested TST-negative and QFT-IT-negative; 2) "cured tuberculosis", individuals with culture-positive pulmonary tuberculosis at the time of diagnosis and who were culture-negative upon treatment completion when the draw blood was performed.
Individuals who tested positive to HIV-antibody assay or who were on immunosuppressive drugs were not included in the study. Upon enrolment, demographic and epidemiological information was collected by a physician through a structured questionnaire, including information about BCG vaccination.
The study was approved by the INMI ethical committee, and all enrolled individuals provided written consent.
TST
Participants underwent a TST on the day of blood sampling (see below). The TST was administered by the Mantoux procedure using 5 IU of tuberculin (Chiron, Siena, Italy). Results were read after 72 hrs. Induration of at least 5 mm was considered a positive response for the group of close contacts and a reaction of ≥ 10 mm was considered positive for homeless people and controls [17].
Commercially available IGRA
QuantiFERON TB-Gold In tube (Cellestis Limited) was used. The tube containing TB antigen uses overlapping peptides from CFP-10 and ESAT-6 [18] and TB7.7. The assay was performed and the results were scored as indicated by the manufacturer (cut-off value for a positive test was 0.35 IU/ml). For IFN-γ values above 10 IU/ml serial dilutions of plasma were performed.
Whole blood cultures with M. tuberculosis antigens and mitogen
1-day (short-term) response
0.5 ml per well of heparinized whole blood was seeded in a 48-well plate (Corning Costar, Corning Incorporated, New York, NY, USA) and treated with RD1 intact proteins (ESAT-6 and CFP-10) at 0.2 μg/ml each protein (Lionex, Braunschweig, Germany) and the mitogen phytohaemagglutinin (PHA) at 5 μg/ml (Sigma, St Louis, MO, USA). Samples were incubated for 24 hrs. At day 1, plasma was harvested and cold stored until tested (+4°C or if used after 15 days at -20°C).
7-day (long-term) response
We used the described methodology [14,15]. Briefly at the day of sampling, an aliquot of blood was diluted 5-fold using RPMI 1640 supplemented with penicillin, streptomycin and 2 mM L-glutamine (all four products are from Euroclone Ltd, United Kingdom) and was plated into 48-well plates (Corning) and stimulated as described above. The day-7 diluted plasma was harvested following incubation at 37°C and cold stored until tested (+4°C or if used after 15 days at -20°C).
IFN-γ determination
IFN-γ from day-1 and day-7 plasma were evaluated by a commercial ELISA (CMI, Cellestis Limited, Carnegie, Victoria, Australia) and are presented as IU/ml after subtraction of the appropriate control. For day-1 analysis, a cut-off value was previously determined by constructing a Receiver Operator Characteristic (ROC) curve by means of LABROC-1 software and was 0.7 IU/mL for all stimuli [19,20]
Statistical analysis
The main outcome of the study was the evaluation of IFN-γ production in response to the mitogen PHA and antigenic stimulation in the QFT-IT and whole blood assays (WBA), expressed as dichotomous (positive/negative) and continuous (IU/ml) measures. Median and interquirtile range (IQR) of IFN-γ production were calculated. The Mann-Whitney U test was used to compare continuous variables and Chi square or McNemar tests were used for categorical variables. The cut-off value for definition of positivity of the WBA based on RD1 proteins at day 7 was defined by ROC analysis. SPSS v 14 for Windows (SPSS Italia srl, Bologna, Italy) and Prism 4 software (Graphpad Software 4.0, San Diego, CA, USA) were used in the analysis.
Results
Characteristics of the population
We studied a population of TST+ subjects with risk factors for LTBI, 54 healthy contacts of patients with a positive pulmonary TB smear and 52 homeless people. No differences were found in terms of the characteristics reported and therefore the data were pooled together. The population is referred to as suspected LTBI. As controls, we used cured TB subjects and healthy individuals unexposed to M. tuberculosis who resulted negative to TST and QFT-IT. The characteristics of the groups are reported in table 1. Note that among the suspected LTBI subjects, the TST median was high (20 mm).
Table 1.
Characteristics of study subjects.
| Controls N (%) 13 (8.8) |
TST+, suspected LTBI N (%) 106 (71.6) |
Cured TB N (%) 29 (19.6) |
Total N (%) 148 (100) |
|
|---|---|---|---|---|
| Age Median (IQR) | 33 (29.5-35) | 35 (21-48) | 31.5 (25-49) | 36 (28-50) |
| Female Gender | 8 (61.5) | 33 (31.1) | 15 (51.7) | 56 (37.8) |
| Origin | ||||
| Italy | 12 (92.3) | 49 (46.3) | 9 (31.1) | 70 (47.3) |
| Abroad | 1 (7.7) | 57 (53.7) | 20 (68.9) | 78 (52.7) |
| TST Median in mm (IQR) | 0 (0) | 20 (15-45) | ND | 20* (15-30) |
| TST+ | 0 | 106 (100) | ND | 106/119** (89) |
| BCG | ||||
| Vaccinated | 4 (30.8) | 37 (34.9) | 19 (65.5) | 60 (40.6) |
|
unvaccinated unknown |
9 (69.2) 0 |
50 (47.2) 19 (17.9 |
10 (34.5) 0 |
69 (46.6) 19 (12.8) |
Footnotes: BCG: Bacillus Calmètte et Guerin; LTBI: latent tuberculosis infection; N: number; TST: tuberculin skin test.
*among TST+
** among those tested by TST
Responses to QFT-IT and RD1 proteins by short-term stimulation
All the enrolled subjects responded to PHA (data not shown). As for the selection performed, none of the controls responded to the QFT-IT and to RD1 proteins. The median of IFN-γ production in response to QFT-IT was 0 IU/mL (IQR: 0-0) and to RD1 proteins 0 IU/mL (IQR: 0-0.1).
Among the enrolled TST+ subjects with suspected LTBI, the response to QFT-IT was found in 66.0% (70/106), whereas to RD1 proteins in 60.3% (64/106) (table 2). Among all of the subjects of this group, the median of IFN-γ production in response to QFT-IT was 2.3 IU/mL (IQR: 0-15.1) and to RD1 proteins 1.4 IU/mL (IQR: 0.2-8.9). Among the responders, the median of IFN-γ production in response to QFT-IT was 8.1 IU/mL (IQR: 2.4-22.6) and to RD1 proteins 7.6 IU/mL (IQR: 2.3-20.3).
Table 2.
In vitro responses to M. tuberculosis antigens in the study group populations.
|
In vitro response to: |
Controls N (%) |
TST+, suspected LTBI N (%) |
Cured TB N (%) |
|
|---|---|---|---|---|
| 13 (100) | 106 (100) | 29 (100) | ||
| QFT-IT | ||||
| Positive N.(%) | 0 | 70 (66.03) | 19 (63.3) | |
|
IFN-γ (IU/ml) median (IQR) Among all |
0 (0-0.1) | 2.3 (0-15.1) | 1.1 (0.1-4.2) | |
|
IFN-γ (IU/ml) median (IQR) Among the positive responders |
NA | 8.1 (2.4-22.6) | 3.8 (1.5-5.0) | |
| RD1 proteins | ||||
| Positive N.(%) | 0 | 64 (60.3) | 19 (65.5) | |
|
IFN-γ (IU/ml) median (IQR) Among all |
0 (0-0.2) | 1.4 (0.2-8.9) | 1.6 (0.4-2.9) | |
|
IFN-γ (IU/ml) median (IQR) Among the positive responders |
NA | 7.6 (2.3-20.3) | 2.6 (1.6-6.2) |
Footnotes: IQR: inter quartile range; LTBI: latent tuberculosis infection; N: number; QFT-IT: QuantiFERON-TB Gold In-tube; RD: region of difference, TB: tuberculosis.
Among the cured TB subjects, the response to QFT-IT and RD1 proteins was found for both tests in 65.5% (19/29). The median of IFN-γ production in response to QFT-IT was 1.1 IU/mL (IQR: 0.1-4.2) and to RD1 proteins 1.6 IU/mL (IQR: 0.4-2.9). Among the responders, the median of IFN-γ production in response to QFT-IT was 3.8 IU/mL (IQR: 1.5-5.0) and to RD1 proteins 2.6 IU/mL (IQR: 1.6-6.2).
No statistical difference was found between the enrolled subjects with suspected LTBI and cured TB in response to QFT-IT and RD1 proteins, in terms of both qualitative and quantitative response (p > 0.05). However, a significant statistical difference was found between controls and both groups of suspected LTBI and cured TB in response to QFT-IT and RD1 proteins, in terms of both qualitative and quantitative response (p < 0.0001 for all comparisons).
Long-term response to RD1 proteins
In an effort to find new tools for detecting RD1-specific response in those resulting negative to QFT-IT and to RD1 proteins at day-1 post stimulation, we evaluated long-term-specific IFN-γ production. We used the RD1 proteins as antigenic stimulus because previously used for prolonged WBA [15], because we could plate the diluted blood and also because the results obtained by RD1 proteins stimulation were similar to those obtained by QFT-IT test, as reported above (Table 2) and in previous studies [19,20].
As reported in the literature, IGRA scoring may become negative after successful tuberculosis therapy in diseased patients [21-23]. Therefore, we evaluated whether long-term stimulation could be a good approach for detecting a memory response in cured TB subjects at the time of therapy completion that resulted negative to QFT-IT. Within the 29 subjects with cured TB enrolled in the study, 10 (34.4%) resulted QFT-IT-negative. In this subgroup of subjects, the long-term IFN-γ response to RD1 proteins was significantly higher (p < 0.006; median: 2.1; IQR: 0.050-4.3) compared to healthy controls (median: 0; IQR: 0-0.15) (Figure 1A). Based on these data, we performed a ROC analysis using the QFT-IT-negative cured TB subjects and the healthy controls as comparator groups (Figure 1B). Significant results for area under the curve (AUC) analysis were obtained [AUC, 0.84; confidence interval (CI), 0.65-1.02, p < 0.005]. For scoring purposes we chose a cut-off to maximize the sum of sensitivity and specificity. We found that a cut-off of 0.75 (IU/ml) predicted a long term response in cured TB with 70.0% sensitivity (95% CI, 34.75%-93.33%) and 100% specificity (95% CI, 75.29%-100.0%).
Figure 1.
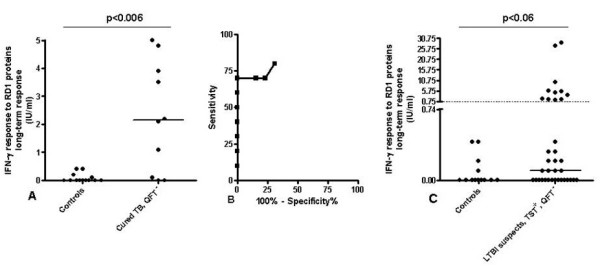
A-B-C. Long-term response to RD1 proteins in cured TB subjects and suspect of LTBI resulted negative to QFT-IT compared to healthy controls. IFN-γ production in response to RD1 proteins was evaluated at day 7 in diluted whole blood of subjects who resulted QFT-IT-negative as described in the material and method section. A) IFN-γ release was significantly higher in cured TB patients than healthy controls (p < 0.006). Horizontal lines indicate the median IFN-γ production. The data are presented as IU/mL. B). A ROC analysis was performed using the established cured TB group and the healthy controls group as comparator groups. C) IFN-γ production in response to RD1 proteins was higher, although not significantly, compared to controls (p < 0.06). Eleven out of thirty-six (30.6%) responded to RD1 proteins. Horizontal lines indicate the median IFN-γ production. The dotted line indicates the cut-off. The data are presented as IU/mL.
Based on this cut-off value we scored the results as negative and positive. None of the controls resulted positive, whereas among the cured TB subjects not responding to QFT-IT, 7/10 (70%) scored positive (Figure 1A). Among those with suspected LTBI who did not respond to QFT-IT 11/36 (30.6%) responded to the long-term test (Figure 1C). Moreover, in this subgroup, a higher, although not significant level of IFN-γ production (p < 0.06) was found in response to RD1 proteins (median: 0.1, IQR 0-1.65) compared to controls.
We then evaluated the impact of BCG vaccination in the response to the prolonged test in the suspected LTBI subjects not responding to QFT-IT (Table 3). Among those BCG-unvaccinated 5/14 (35.7%) responded to the prolonged test, among those BCG-vaccinated 3/15 (20.0%) responded and among those with BCG status unknown 3/7 (42.9%) responded. Based on these data, no clear association was found between BCG vaccination and lack of response to the long-term test.
Table 3.
Impact of BCG vaccination on the results obtained by the prolonged test.
| TST+, suspected LTBI, not responding to QFT-IT | ||||
|---|---|---|---|---|
| BCG | ||||
| Response to the prolonged test |
Unvaccinated N. 14 (%) |
Vaccinated N. 15 (%) |
Unknown N.7 (%) |
Total N. 36 (%) |
| No responders N. 25 | 9 (64.3) | 12 (80.0) | 4 (57.1) | 25 (69.4) |
| Responders N. 11 | 5 (35.7) | 3 (20.0) | 3 (42.9) | 11 (30.6) |
Footnotes: BCG: Calmette et Guerin; LTBI: latent tuberculosis infection; N: number; QFT-IT: QuantiFERON TB-Gold-In tube; TST: tuberculin skin test.
Discussion
In this study we demonstrated that IFN-γ long-term response to M. tuberculosis RD1 proteins may be detected in TST+ individuals who, although with a high likelihood of having LTBI, resulted negative to a commercial IGRA. Among these individuals, we found a specific-IFN-γ long-term response in 11/36 (30.6%). A higher proportion of positive responses (7/10, 70.0%) was found among patients with cured TB, while no positive responses were detected among low risk controls. These observations suggest that an IFN-γ long-term response may indeed be associated with TB infection. These results provide useful information on the immunity against M. tuberculosis and may be the preliminary basis for further improvement of immunodiagnostic tests for LTBI. In particular it may be hypothesized that a long-term test can be used in a series with a "conventional IGRA" when a high sensitivity for LTBI detection is required, such as in the screening of candidates for an immunosuppressive treatment.
In absence of a gold standard for diagnosing LTBI, identifying a population of individuals with this condition is not straightforward. However, we believe that the TST+ individuals enrolled in the present study were very likely to have been infected with M. tuberculosis for two reasons: first, they all had high risk factors for TB infection and second, they had a great TST response which is most likely associated to a past infection, even among those who are BCG-vaccinated [24]. Thus, the observation that almost 34% of the these TST+ individuals had negative results by QFT-IT may reflect, at least in part, a less than perfect sensitivity to this test for detecting LTBI. This observation is consistent with previous reports [10,15,25,26]. In particular, in two cross-sectional studies performed in South Africa, approximately one-third of adults with a TST induration higher than 15 mm had a negative QFT-IT test result and 38% had a negative T-SPOT.TB test result [25,27]. Another study reported that in a mostly BCG-vaccinated Korean control population, 51% of the subjects were TST+ and only 4% were QFT-IT positive, while the expected prevalence of M. tuberculosis infection was 33% [26].
We performed short and prolonged tests using identical M. tuberculosis-specific proteins (RD1) that were found to be highly specific for M. tuberculosis [1,2,11,25] to provide consistent results. By this approach, one third of the TST+ individuals who had negative results by QFT-IT or RD1 proteins-based short-term assay resulted positive. Among the participants who scored negative on short test, high levels of IFN-γ could be detected by the prolonged test, indicating that the observed discrepancy was not simply explained by differences in the levels of detection of IFN-γ. Another plausible explanation for the difference in sensitivity would be the diversities in the in vitro incubation periods for the QFT-IT and RD1 proteins-based short test and prolonged assay. Probably the long term incubation leads to higher likelihood of IFN-γ production since the protein processing may be performed by a higher number of T cell populations with various T-cell receptors types with consequently a better antigen presentation than in short term culture (1-day). Moreover, we hypothesize that after 24 hours of incubation, only circulating effector memory T-cells had sufficient time to produce IFN-γ, while central memory T-cells or T cells in resting state first started producing IFN-γ after a more prolonged incubation needed to allow them to differentiate. In individuals who had been infected with M. tuberculosis in the past, the number of circulating effector cells could be low, causing negative results in a short-incubation assay but positive responses after a prolonged incubation, as reported by our studies and by others [11,28].
In accordance with this line of thought, findings from a hepatitis C virus study show that short-term ELISPOT responses were not influenced by depletion of lymphotropic chemokine receptor 7-positive T-cells, representing memory cells, while the depletion of these memory cells did decrease the antigen-specific responses after prolonged culture [29].
There is a lack of knowledge of the performance of IGRAs with a short-term incubation period compared to those with a more prolonged incubation time for detecting M. tuberculosis infection. To our knowledge, there are few studies comparing the overnight ELISPOT or WBA with either the 6-day LST or the 7-day whole blood incubation test and the results indicate that the prolonged tests performed better [11,15,30]. This is also in agreement with our previous data in which we reported that negative responses to a panel of RD1 peptides in an overnight ELISPOT became positive responses in a cultured ELISPOT [28].
In this study, 69.4% (25/36) of the enrolled TST+ subjects with suspected LTBI who did not respond to QFT-IT, did not respond to the long-term assay either. This was also reported in the cured TB subjects enrolled, although in a lower proportion (3/10, 30%). Further research is needed to elucidate this lack of specific response.
This is a pilot study which presents limitations that will require additional work to be overcome. Above all, the study was performed on a relatively small number of individuals and certainly the research would benefit from a larger study. Other limitations include the self-reported BCG vaccination which may account for potential mistakes and imprecise information, the potential exposure to environmental mycobacteria which may have caused a cross-reaction with the tuberculin preparation present in the TST and finally the amount of the refusal rate among the homeless [refusal rate was 4.3% (13/300) at recruitment, 10.1% (29/287) for the TST reading, and 11.8% (7/59) to undergo further investigation for those resulted TST+] that may have accounted for a selection of the subjects tested.
Conclusion
In conclusion, IFN-γ long-term response to M. tuberculosis specific antigens among TST+ individuals who resulted negative to QFT-IT can potentially be considered to be an additional tool for detecting LTBI. Further studies are needed to confirm these data in a larger number of patients and to evaluate the possible implications in terms of TB risk, on the positivity of short vs. prolonged tests.
Abbreviations
AUC: area under the curve; BCG: Bacillus Calmette-Guérin; CFP-10: culture filtrate protein-10 kDa; CI: confidence interval; ELISA: Enzyme Linked ImmunoSorbent Assay; ELISPOT: enzyme-linked immunospot assay; ESAT-6: early secretory antigenic target-6; HIV: human immunodeficiency virus; IFN: interferon; IGRAs: Interferon gamma release assay; INMI: National Institute for Infectious Diseases; IQR: interquartile range; LST: lymphocyte stimulation test; LTBI: latent tuberculosis infection; M. tuberculosis: Mycobacterium tuberculosis; PBMC: peripheral blood mononuclear cells; PHA: phytohaemagglutinin; QFT-IT: QuantiFERON TB-Gold In tube; RD1: region of difference 1; ROC: Receiver operating characteristic; TB: Tuberculosis; TST: Tuberculin skin test; WBA: Whole blood assays.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
OB carried out the experimental data and analysis, drafted and revised the manuscript; TC, and VV carried out the Quantiferon -TB-Gold In tube assay; SM, GGNC, FD, SC, RC were responsible for the experimental data analysis; MS provided RD1 reagents; EG and DG were responsible for the statistical analysis; MV, FNL, AM, PL, DG were responsible for patient selection and enrolment; DG and EG were responsible for the conception of the study design and the preparation of the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Ornella Butera, Email: ornella.butera@inmi.it.
Teresa Chiacchio, Email: chiacchio@inmi.it.
Stefania Carrara, Email: carrara@inmi.it.
Rita Casetti, Email: casetti@inmi.it.
Valentina Vanini, Email: valentina.vanini@inmi.it.
Serena Meraviglia, Email: meraviglia@unipa.it.
Giuliana Guggino, Email: guggino@unipa.it.
Francesco Dieli, Email: dieli@unipa.it.
Marco Vecchi, Email: vecchi@inmi.it.
Francesco N Lauria, Email: lauria@inmi.it.
Almerico Marruchella, Email: marruchella@inmi.it.
Patrizia Laurenti, Email: plaurenti@rm.unicatt.it.
Mahavir Singh, Email: info@lionex.de.
Nadia Caccamo, Email: caccamo@unipa.it.
Enrico Girardi, Email: girardi@inmi.it.
Delia Goletti, Email: delia.goletti@inmi.it.
Acknowledgements
The authors are grateful to all the patients and nurses (in particular Cristina Copertino and Gilda Cuzzi) who took part to the study. We are grateful to Ms Andrea Baker for the editing.
Financial support: The paper was supported by grants from Ricerca Corrente and Ricerca Finalizzata 06.76.1.
References
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: An update. Ann Intern Med. 2008;149:177–84. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goletti D, Carrara S, Butera O, Amicosante M, Ernst M, Sauzullo I, Vullo V, Cirillo D, Borroni E, Markova R, Drenska R, Dominguez J, Latorre I, Angeletti C, Navarra A, Petrosillo N, Lauria FN, Ippolito G, Migliori GB, Lange C, Girardi E. Accuracy of immunodiagnostic tests for active tuberculosis using single and combined results: a multicenter TBNET-Study. PLoS ONE. 2008;3:e3417. doi: 10.1371/journal.pone.0003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend SM, Andersen P, van Meijgaarden KE, Skjot RL, Subronto YW, van Dissel JT, Ottenhoff TH. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J Infect Dis. 2000;181:1850–1854. doi: 10.1086/315448. [DOI] [PubMed] [Google Scholar]
- Munk ME, Arend SM, Brock I, Ottenhoff TH, Andersen P. Use of ESAT-6 and CFP-10 antigens for diagnosis of extrapulmonary tuberculosis. J Infect Dis. 2001;183:175–176. doi: 10.1086/317663. [DOI] [PubMed] [Google Scholar]
- Ravn P, Demissie A, Eguale T, Wondwosson H, Lein D, Amoudy HA, Mustafa AS, Jensen AK, Holm A, Rosenkrands I, Oftung F, Olobo J, von Reyn F, Andersen P. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–645. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- Wu-Hsieh BA, Chen CK, Chang JH, Lai SY, Wu CH, Cheng WC, Andersen P, Doherty TM. Long-lived immune response to early secretory antigenic target 6 in individuals who had recovered from tuberculosis. Clin Infect Dis. 2001;33:1336–1340. doi: 10.1086/323044. [DOI] [PubMed] [Google Scholar]
- Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–82. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, Monk P, Lalvani A. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361:1168–1173. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- Lalvani A, Pathan AA, Durkan H, Wilkinson KA, Whelan A, Deeks JJ, Reece WH, Latif M, Pasvol G, Hill AV. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001;357:2017–2021. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- Shams H, Weis SE, Klucar P, Lalvani A, Moonan PK, Pogoda JM, Ewer K, Barnes PF. Enzyme-linked immunospot and tuberculin skin testing to detect latent tuberculosis infection. Am J Respir Crit Care Med. 2005;172:1161–1168. doi: 10.1164/rccm.200505-748OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyten EM, Arend SM, Prins C, Cobelens FG, Ottenhoff TH, van Dissel JT. Discrepancy between Mycobacterium tuberculosis-specific gamma interferon release assays using short and prolonged in vitro incubation. Clin Vaccine Immunol. 2007;14:880–5. doi: 10.1128/CVI.00132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend SM, Thijsen SF, Leyten EM, Bouwman JJ, Franken WP, Koster BF, Cobelens FG, van Houte AJ, Bossink AW. Comparison of two interferon-gamma assays and tuberculin skin test for tracing TB contacts. Am J Respir Crit Care Med. 2007;175:618–627. doi: 10.1164/rccm.200608-1099OC. [DOI] [PubMed] [Google Scholar]
- Leyten EM, Prins C, Bossink AW, Thijsen S, Ottenhoff TH, van Dissel JT, Arend SM. Effect of tuberculin skin testing on a Mycobacterium tuberculosis-specific IFN-γ assay. Eur Respir J. 2007;29:1212–6. doi: 10.1183/09031936.00117506. [DOI] [PubMed] [Google Scholar]
- Black GF, Dockrell HM, Crampin AC, Floyd S, Weir RE, Bliss L, Sichali L, Mwaungulu L, Kanyongoloka H, Ngwira B, Warndorff DK, Fine PE. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J Infect Dis. 2001;1(184):322–9. doi: 10.1086/322042. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- McAdam JM, Bucher SJ, Brickner PW, Vincent RL, Lascher S. Latent tuberculosis and active tuberculosis disease rates among the homeless. Emerg Infect Dis. 2009;15:1109–11. doi: 10.3201/eid1507.080410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Bangani N, Vogt M, Bekker LG, Badri M, Ntobongwana M, Dockrell HM, Wilkinson RJ, Wood R. Utility of interferon-gamma ELISPOT assay responses in highly tuberculosis-exposed patients with advanced HIV infection in South Africa. BMC Infect Dis. 2007;7:99. doi: 10.1186/1471-2334-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, Shigeto E, Harada N, Mitarai S, Okada M, Suzuki K, Inoue Y, Tsuyuguchi K, Sasaki Y, Mazurek GH, Tsuyuguchi I. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med. 2004;170:59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- Goletti D, Parracino MP, Butera O, Bizzoni F, Casetti R, Dainotto D, Anzidei G, Nisii C, Ippolito G, Poccia F, Girardi E. I.soniazid prophylaxis differently modulates T-cell responses to RD1-epitopes in contacts recently exposed to Mycobacterium tuberculosis a pilot study. Respir Res. 2007;8:5. doi: 10.1186/1465-9921-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goletti D, Carrara S, Vincenti D, Saltini C, Rizzi EB, Schininà V, Ippolito G, Amicosante M, Girardi E. Accuracy of an immune diagnostic assay based on RD1 selected epitopes for active tuberculosis in a clinical setting: a pilot study. Clin Microbiol Infect. 2006;12:544–50. doi: 10.1111/j.1469-0691.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- Aiken AM, Hill PC, Fox A, McAdam KP, Jackson-Sillah D, Lugos MD, Donkor SA, Adegbola RA, Brookes RH. Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect Dis. 2006;30:6–66. doi: 10.1186/1471-2334-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis. 2004;38:754–6. doi: 10.1086/381754. [DOI] [PubMed] [Google Scholar]
- Sauzullo I, Mengoni F, Lichtner M, Massetti AP, Rossi R, Iannetta M, Marocco R, Del Borgo C, Soscia F, Vullo V, Mastroianni CM. In vivo and in vitro effects of antituberculosis treatment on mycobacterial Interferon-gamma T cell response. PLoS One. 2009;4:e5187. doi: 10.1371/journal.pone.0005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192–204. [PubMed] [Google Scholar]
- Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni C, Mouton P, Diwakar L, Connell TG, Maartens G, Wilkinson RJ. The effect of HIV-1 infection on T cell based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007;175:514–520. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, Yim JJ. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005;293:2756–2761. doi: 10.1001/jama.293.22.2756. [DOI] [PubMed] [Google Scholar]
- Mahomed H, Hughes EJ, Hawkridge T, Minnies D, Simon E, Little F, Hanekom WA, Geiter L, Hussey GD. Comparison of Mantoux skin test with three generations of a whole blood IFN-gamma assay for tuberculosis infection. Int J Tuberc Lung Dis. 2006;10:310–316. [PubMed] [Google Scholar]
- Goletti D, Butera O, Bizzoni F, Casetti R, Girardi E, Poccia F. Region of difference 1 antigen-specific CD4_ memory T cells correlate with a favorable outcome of tuberculosis. J Infect Dis. 2006;194:984–992. doi: 10.1086/507427. [DOI] [PubMed] [Google Scholar]
- Godkin AJ, Thomas HC, Openshaw PJ. Evolution of epitope-specific memory CD4_ T cells after clearance of hepatitis C virus. J Immunol. 2002;169:2210–2214. doi: 10.4049/jimmunol.169.4.2210. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–61. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]


