Abstract
Introduction:
We tested a combined intervention to reduce children's secondhand smoke exposure (SHSe) and help parents quit smoking.
Methods:
After baseline, mothers who exposed their children younger than 4 years to 10 or more cigarettes/week were randomized to the intervention (n = 76) or usual care control condition (n = 74). Outcomes were assessed at 3, 6, 12, and 18 months. Intervention families were offered 10 in-person at home and 4 telephone counseling sessions over 6 months, and additional pre- and postquit telephone sessions. Counseling procedures included behavioral contracting, self-monitoring, and problem solving.
Results:
Parents’ reports of their smoking and children's exposure showed moderate and significant correlations with children's urine cotinine levels and home air nicotine (r = .40–.78). Thirteen (17.1%) intervention group mothers and 4 (5.4%) controls reported that they quit smoking for 7 days prior to 1 or more study measurements, without biochemical contradiction (p = .024). Results of generalized estimating equations showed significantly greater decrease in reported SHSe and mothers’ smoking in the counseled group compared with controls. Reported indoor smoking and children's urine cotinine decreased, yet group differences for changes were not significant.
Discussion:
Nicotine contamination of the home and resulting thirdhand exposure may have contributed to the failure to obtain a differential decrease in cotinine concentration. Partial exposure to counseling due to dropouts and lack of full participation from all family members and measurement reactivity in both conditions may have constrained intervention effects. Secondhand smoke exposure counseling may have been less powerful when combined with smoking cessation.
Introduction
There is no risk-free level of cumulative secondhand smoke exposure (SHSe; U.S. Department of Health and Human Services [USDHHS], 2006), which threatens almost half of the world's children (Centers for Disease Control and Prevention, 2007; World Health Organization, 1999). Secondhand smoke exposure causes respiratory illness, sudden infant death syndrome, asthma, heart disease, and cancer (California Environmental Protection Agency, 1997; Iscan, Uyanik, Vurgun, Ece, & Yigitoglu, 1996; State of California, 2005; USDHHS; U.S. Environmental Protection Agency, 1992). Secondhand smoke exposure may compromise children's immune systems more than air pollution (Bjorksten, 1999) and is associated with lower cognitive abilities (Yolton, Dietrich, Auinger, Lanphear, & Hornung, 2005), school absence (Mannino, Moorman, Kingsley, Rose, & Repace, 2001), glucose intolerance (Houston et al., 2006), and sick leave as adults (Eriksen, 2004).
Secondhand smoke exposure costs $1 billion in excess medical care for U.S. children and $4.2 billion in annual loss of life costs (Adams & Young, 1999; Aligne & Stoddard, 1997). Healthy People 2010 objectives are to reduce child SHSe prevalence to less than 10% (USDHHS, 2000). National Health Interview Survey data indicate that children's SHSe declined from 36% to 25% between 1992 and 2000 (Soliman, Pollack, & Warner, 2004), yet among some populations of lower socioeconomic status, the proportion exposed is 84% (Cornelius, Goldschmidt, & Dempsey, 2005). Effective interventions are needed to reach the 2010 target, especially for low-income populations.
One strategy for reducing children's SHSe is to help parents quit smoking entirely. Six-month smoking quit rates are as low as 10% for minimal interventions to 26% for high-intensity counseling with nicotine replacement therapy (NRT; Fiore, Smith, Jorenby, & Baker, 1994). Thus, most participants do not quit smoking and therefore do not protect their children from SHSe. Therefore, interventions to help parents smoke away from their children may yield important benefits. Outdoor-only smoking can help protect children from respiratory symptoms (Blizzard, Ponsonby, Dwyer, Venn, & Cochrane, 2003; Johansson, Halling, & Hermansson, 2003) and elevated urine cotinine levels (Blackburn et al., 2003; Johansson, Hermansson, & Ludvigsson, 2004), although not completely (Matt et al., 2004).
Several studies have tested relatively minimal interventions with few or no in-person contacts, which did not provide parents with specific strategies for reducing SHSe and were unsuccessful (Chilmonczyk, Palomaki, Knight, Williams, & Haddow, 1992; Erikson, Sorum, & Bruusgaard, 1996; Irvine et al., 1999; Keintz, Fleisher, & Rimer, 1994; McIntosh, Clark, & Howatt, 1994; Vineis et al., 1993; Wakefield et al., 2002; Woodward, Owen, Gurinovich, Griffith, & Linke, 1987). At least six trials have tested individualized parent counseling for reducing children's SHSe and reported significant effects. Our study reduced SHSe for asthmatic children (Hovell et al., 1994; Wahlgren, Hovell, Meltzer, Hofstetter, & Zakarian, 1997), and our trial with low-income mothers reduced SHSe and prevented increased cotinine (Hovell et al., 2000). Our study of Latino asthmatic children showed reduction in reported SHSe and cotinine (Hovell, Meltzer, et al., 2002). Others have reported decreased SHSe (Greenberg et al., 1994), air nicotine (Emmons et al., 2001), and asthma-related health care (Wilson et al., 2001). Thus, we believe that individualized counseling can reduce children's SHSe for low to middle income and racially mixed families. Frequent home-based contacts appear most effective (Gehrman & Hovell, 2003).
Based on the Behavioral Ecological Model (BEM; Hovell, Wahlgren, & Adams, 2009; Hovell, Wahlgren, & Gehrman, 2002) and our previous studies, we hypothesized that parents may be motivated to quit smoking during SHSe counseling. The BEM suggests that cultural values such as protecting children may motivate smoking cessation, especially during SHSe reduction counseling. Therefore, this study tested the effects of a combined intervention that delivered both SHSe and cessation counseling to high-risk families.
Methods
Design
We used a two-group, repeated measures randomized controlled trial design. Three weekly baseline urine cotinine measures were conducted over 2 weeks to establish a reliable estimate of children's baseline SHSe. The baseline interview was conducted at the third urine collection. Families were randomized to the intervention (n = 76) or control condition (n = 74) after the baseline interview, with outcome assessments for reported and urine cotinine measures at 3 (mid-intervention), 6 (postintervention), 12, and 18 months. A random number list was used to assign pairs of participants matched on child's gender, ethnicity, and recruitment site. Data collection research assistants were blind to group assignment, and control families were unaware of counseling procedures. Investigators were blind to results until all data were collected. Procedures were approved by the San Diego State University Institutional Review Board, and all mothers and other family members who agreed to participate in study measures or the intervention signed informed consent agreements.
Inclusion criteria
Mothers with children younger than 4 years who were exposed to a minimum of 3 of their mothers’ cigarettes per day at telephone screening were recruited. Only those who reported at their baseline interview that their children remained exposed to 10 or more cigarettes/week were randomized, so that the intervention was tested with a sample among whom clinical improvement could be demonstrated. “Exposed” meant the child was in the same room of the home or in the car when any part of a cigarette was smoked. Breast-feeding children were excluded because cotinine in breast milk confounds urine cotinine analyses (Becker et al., 1999; Mascola, Vunakis, Tager, Speizer, & Hanrahan, 1998). Children who did not live with their mothers full time were excluded due to complications with obtaining reported SHSe measures. Families were excluded if they did not plan to reside in San Diego County for the next 19 months, if they had participated in any of our past SHSe intervention studies, or if they were in frequent communication (more than once/month) with other study participants.
Recruitment
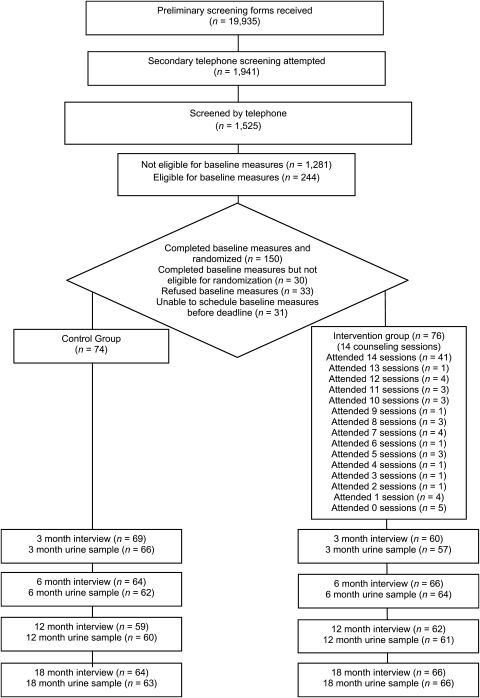
We collected 19,935 preliminary screening forms from seven offices of the Supplemental Nutrition Program for Women, Infants, and Children (WIC) in San Diego County, California. Women, Infants, and Children families are at or below 185% of Federal Poverty Income Guidelines. The flyers advertised a research project designed to improve families’ health. For 24 months beginning February 2001, research assistants completed secondary telephone screening with 1,525 of 1,941 mothers who indicated on preliminary forms that they had a young child and smoked cigarettes in the past month, and recruitment continued until 150 of 244 eligible families were enrolled and randomized (Figure 1). Research assistants explained that the purpose of the study was to learn more about children's and mothers’ health habits including tobacco use and that families would be randomly assigned to one of two free health education programs. Motivation to change smoking behavior was not assessed during the participant screening process, and therefore, mothers with low motivation to quit or reduce children's SHSe were not excluded. Mothers received $10 for completing each of the three baseline urine collections, $20 for measures collected at 3, 6, and 12 months, and $40 for 18-month measures, with gift cards and raffles for continued participation.
Figure 1.
Flow of participants through the trial.
Research assistant supervision and training
Data collection assistants and counselors received 30 hr of group and individual didactic training and role-playing practice from the Project Coordinators. They were provided with detailed manuals and protocols for study measurement and intervention delivery. Weekly training was provided during staff meetings with quality control feedback based on review of digital recordings, questionnaire answers, and standardized counseling progress reports completed for each session. Counselors were masters-level students or graduates of psychology, social work, and public health.
Intervention procedures
Each family in the intervention condition was assigned to a study counselor, who delivered an intervention targeting SHSe reduction for the family's youngest child with a smoking cessation component that was individually tailored to each participant. Treatment consisted of 14 biweekly counseling sessions over 6 months: 10 in-person at home and 4 by telephone (Sessions 6, 10, 12, and 13). Mean length was 23 min (95% CI = 22.3–24.4). At each session, participants were asked if they would like assistance to quit. If so, sessions were divided between the SHSe reduction and the quitting process. Additional telephone support was provided for participants who set a quit date (1 or 2 days pre-quit, 1 or 2 days postquit, and 1 week postquit).
To facilitate counseling, counselors offered flexible scheduling for daytime, evening, and weekend appointments. Counselors gave mothers personalized gifts (e.g., bath products, photo frames) for attendance at Sessions 4, 8, and 14. They provided referrals for assistance with social welfare issues as needed. Counselors invited and encouraged all family members, especially smokers, to participate in as many counseling sessions as possible.
SHSe reduction counseling.
We used SHSe counseling procedures based on Learning Theory that were effective in our previous trials, including behavioral contracting, self-monitoring, and problem solving (Hovell et al., 2000; Hovell, Meltzer, et al., 2002; Wahlgren et al., 1997). At the first session, counselors conducted informal, open-ended clinical interviews to complete a “Where's the Smoke?” worksheet to identify levels and specific times, places, and conditions of SHSe. These worksheets were completed again at Session 9. At each session, counselors contracted with mothers and other participants to achieve short- and long-term goals for reducing children's SHSe (e.g., gradually increasing the proportion of cigarettes smoked outdoors, eliminating indoor smoking, restricting smoking in certain rooms of the home, not smoking in the car when children are present, and/or asking grandparents to smoke outdoors when children are visiting). Over sessions, counselors shaped participants’ smoking behavior to protect children from their own smoking and smoking by other family members, friends, and others. Objectives achieved resulted in positive feedback and prompting to do more.
Smoking cessation counseling.
Counselors provided health education materials to support cessation. All smokers in the counseling group families were offered free nicotine patches and/or gum to assist with quit attempts. Family members were required to participate in at least one counseling session before receiving these products, for instruction on their use. Participants were advised to use the products according to the manufacturer's instructions: 10 weeks of daily use of 2 or 4 mg gum or 8–10 weeks of daily patch use starting with 14 or 21 mg patches and tapering to 7 mg patches depending on smoking rate. Participants were encouraged to select a quit date early enough to allow counselors to assist with preparation, use of NRT, and early maintenance.
Throughout the program, participants who did not already have a long-term quit goal were encouraged to set one. All families were counseled to set SHSe reduction goals, regardless of their interest in or success with quitting. For participants who tried to reduce their smoking rate, counselors episodically probed for willingness to set a quit goal. With successful changes in tobacco use or experimental quits of short duration, counselors provided positive feedback and promoted longer term or new quits. In this fashion, shaping for both SHSe and quitting was implemented.
Usual care control group
The usual care control group received all study measures. They did not receive SHSe or cessation counseling from study counselors. Women, Infants, and Children personnel were unaware of clients’ participation in the study or group assignment. When smoking or SHSe was identified by WIC personnel for any clients, their protocol was to provide a referral to the free California Smoker's Helpline for telephone counseling. These referrals were not tracked for study purposes. At outcome assessments, only one mother in the intervention group and two controls reported they had called the Helpline during their study participation. After their final 18-month study measure, research assistants gave interested mothers a self-help booklet and written materials based on the counseling protocol. Nicotine replacement products were provided to one control mother after her final study measure.
Measures of children's SHSe and parents’ smoking
Parent's reports.
Interviews included demographics and mothers’ health behaviors with emphasis on tobacco use and children's SHSe. Mean length was 31.9 min (95% CI = 31.1–32.7). At each interview, mothers and “other parents” (husbands or partners living in the home) reported their smoking inside the home and their child's SHSe on typical work and nonwork days (or week and weekend days if parents did not work outside the home) during the past 7 days, including exposure from parents, other residents, and visitors, and outside the home, including in the car. If the other parent was unavailable, mothers provided proxy reports. Exposure was defined as the number of cigarettes smoked while the child was in the same indoor room or car. Children’s weekly exposure to mothers’ cigarettes in the home and “total exposure” to all cigarettes in the home, car, and elsewhere were calculated. These measures have shown acceptable test–retest reliability and validity in relation to cotinine and nicotine assays in our past studies (Emerson et al., 1995; Matt et al., 2000). To further examine the test–retest reliability of our measures, smoking and SHSe questions were re-asked of a random sample of 65 mothers 24–48 hr following their 6-month interview.
Children's urine cotinine concentration.
Urine samples were collected at three baselines and each outcome assessment with a standard urine collection cup or by placing two cotton pads (Natracare LLC, Denver, CO) in the diaper, which were expressed into a 5-ml sterile vial. Our previous research showed that cotton rolls do not alter cotinine concentration (Matt et al., 1999). Whenever possible, research assistants collected urine samples at the home interview. Mothers were trained to collect samples and store them in their home freezers until pickup by a research assistant when necessary, and those who moved outside San Diego County were provided with materials to collect and send urine samples to the research office. Samples were frozen at -20 °C and packed in dry ice for shipping to the University of California at San Francisco for analysis of cotinine concentration by liquid chromatography–tandem mass spectrometry with a limit of quantitation of 0.2 ng/ml (Benowitz, 1999; Dempsey et al., 2004). The laboratory was blind to participants’ identity and group assignment.
As in our past studies, a small number of cotinine values were within the range of smokers’ values. These included values as high as 3,070 ng/ml. The nine cotinine values greater than 423 ng/ml were excluded from analyses, as the validity of these assessments was in question. The mean of the three baseline urine cotinine values was used to provide a single, more reliable baseline estimate for each child.
Parents’ smoking status.
Mothers and other parents reported the start date of their latest quit, if any. Adults who reported that they had not smoked within 7 days prior to the interview provided saliva samples for verification, and urine samples if they were concurrently using nicotine replacement products. Saliva was analyzed for cotinine by gas chromatography. Urine was analyzed for anabasine and anatabine by gas chromatography–mass spectrometry (Jacob, Yu, Liang, Shulgin, & Benowitz, 1993). These tobacco-specific alkaloids can be used to validate abstinence in persons using nicotine replacement products (Jacob et al., 2002). Reported quits were confirmed by cotinine concentration <15 ng/ml or anabasine and anatabine levels <2 ng/ml.
Air nicotine.
To provide objective validation of reported smoking, a nicotine dosimeter (37-mm diameter cassette containing a Teflon-coated glass fiber filter; Emfab TX 40h120WW, Pallflex, Putnam, CT) was placed in the room of primary exposure at the second baseline (n = 50) and 1 week before the 6-month interview (n = 36) in randomly selected homes. Dosimeters remained in place for the 1-week period corresponding to reported smoking and SHSe and were removed by research assistants at the interview home visit. To enhance reporting accuracy, inactive dosimeters were placed in all homes in the three rooms where children's greatest SHSe was reported at baseline. Analysis of nicotine concentration by gas chromatography was conducted at the University of California at Berkeley School of Public Health (Leaderer & Hammond, 1991).
Statistical analyses
Analyses were based on intention to treat. To control for non-normal distributions and heterogeneous error variances, we adjusted dependent variables by logarithmic transformation and report geometric means. The test–retest reliability of mothers’ reports was examined by comparing smoking and exposure levels reported at the 6-month interview with their retests using Pearson's correlations. As a validation test, we computed the bivariate correlation coefficient between each pair of our four outcome variables—reported SHSe, reported indoor smoking, children's urine cotinine concentrations, and air nicotine. The computational procedure used data from all five measurement points, controlling for the within-subject correlation of measures repeated over time (Bland & Altman, 1995). Cross-sectional group differences for demographic and other variables at baseline were examined using one-way analysis of variance and Pearson's chi-square tests.
Differential change in exposure outcomes relied on analyses of repeated measures over time. First, we investigated immediate intervention effects based on change from baseline to 6 months postintervention. Next, we investigated change from 6 to 18 months to examine maintenance effects. We used generalized estimating equations (GEE), with linear, quadratic, and cubic components of time, group, and Group × Time interactions as explanatory variables (Stata version 10; Diggle, Liang, & Zeger, 1995). Estimated power to detect differential change between groups, within-subjects change, and for the Group × Time interaction exceeded 0.80 for all dependent variables, for an effect size d ≥ 0.25. Response variables were regressed on explanatory variables using a Gaussian link function and assuming an exchangeable correlation structure. We examined robust models using the Huber–White sandwich estimator of variance, and models using the iteratively reweighted least squares variance estimator. These different analyses yielded the same conclusions, and we present results from the more conservative robust models. Mothers’ smoking cessation was assessed with chi-square tests for group differences. Mothers lost to follow-up and not measured were counted as smokers.
Results
Participant flow and follow-up
Figure 1 shows the number of participants enrolled through completion of measures. The total sample size available for analyses was 130 families (87%) at 6 and 18 months.
Participants and success of random assignment
At baseline, no group differences were found for any of the demographic and theoretical variables shown in Table 1 (all ps > .05). There were no group differences for any of the reported measures of smoking or children's exposure. However, baseline children's urinary cotinine concentration was significantly higher among the controls (p = .005; Table 2), indicating that randomization did not balance groups with respect to cotinine.
Table 1.
Baseline characteristics of study participants
| Variablea | Intervention group (n = 76) | Control group (n = 74) |
| Mothers’ racial/ethnic group | ||
| Non-Hispanic White | 50 (65.8) | 52 (70.3) |
| Hispanic | 11 (14.5) | 7 (9.5) |
| Black | 8 (10.5) | 8 (10.8) |
| Other | 5 (6.6) | 4 (5.4) |
| Asian or Pacific Islander | 2 (2.6) | 3 (4.1) |
| Children’s gender (girls) | 44 (57.9) | 40 (54.1) |
| Single parent families | 25 (32.9) | 26 (35.1) |
| Employed mothers | 25 (32.9) | 28 (37.8) |
| Mothers’ education | ||
| Less than high school or GED | 21 (27.6) | 17 (23.0) |
| GED | 9 (11.8) | 8 (10.8) |
| High school graduate | 17 (22.4) | 17 (23.0) |
| Trade school | 2 (2.6) | 1 (1.4) |
| Some college | 23 (30.3) | 27 (36.5) |
| College graduate | 4 (5.3) | 4 (5.4) |
| Home smoking policy | ||
| No one is allowed to smoke in home | 23 (30.3) | 16 (21.6) |
| Only special guests allowed | 5 (6.6) | 4 (5.4) |
| Only allowed in certain areas | 31 (40.8) | 38 (51.4) |
| Smoking allowed anywhere in home | 17 (22.4) | 16 (21.65) |
| Mother quit smoking 24+ hr in the past year | 25 (33.3) | 30 (40.5) |
| Mothers’ mean age (years) | 30.2 (6.9) | 30.0 (7.4) |
| Children's mean age (months) | 22.7 (13.9) | 23.8 (12.3) |
Note. Values are n (%) or M (SD). GED = generalized equivalency degree.
Pearson's chi-square analyses for categorical variables and analysis of variance for continuous variables showed no statistically significant group differences (all ps > .05).
Table 2.
Children's exposure to secondhand smoke, mothers’ smoking, and indoor smoking at baseline, mid-intervention, postintervention, and follow-up measures
| Variable | Baseline | 3 months (mid-intervention) | 6 months (postintervention) | 12 months (follow-up) | 18 months (follow-up) |
| Children’s reported exposure to secondhand smoke (number of cigarettes/week) | |||||
| Exposure from mothers inside the home | |||||
| Counseled | 8.73 (5.79–12.93) | 3.19 (1.73–5.42) | 1.76 (0.93–2.96) | 2.04 (1.06–3.47) | 1.93 (0.92–3.48) |
| Controls | 10.37 (6.97–15.22) | 6.51 (4.03–10.23) | 4.68 (2.93–7.20) | 4.38 (2.41–7.49) | 6.16 (3.61–10.12) |
| Total exposure from all smokers inside and outside the home | |||||
| Counseled | 33.67 (25.88–43.72) | 11.51 (7.16–18.18) | 4.99 (2.82–8.40) | 6.74 (3.78–11.55) | 5.15 (2.71–9.17) |
| Controls | 40.17 (30.79–52.31) | 23.68 (15.80–35.26) | 17.16 (11.56–25.24) | 15.33 (9.54–24.28) | 22.97 (15.14–34.58) |
| Children’s urine cotinine concentration (ng/ml)a | |||||
| Counseled | 9.74 (7.78–12.15) | 9.98 (7.52–13.15) | 7.27 (5.38–9.72) | 7.38 (5.31–10.14) | 7.63 (5.43–10.58) |
| Controls | 14.70 (12.08–17.84) | 12.70 (9.73–16.50) | 10.95 (8.29–14.37) | 9.70 (7.42–12.58) | 10.06 (7.64–13.14) |
| Mothers’ reported smoking (number of cigarettes/week) | |||||
| Counseled | 89.23 (79.85–98.60) | 77.20 (64.64–89.76) | 58.53 (47.45–69.61) | 69.79 (58.65–80.93) | 77.91 (64.22–91.60) |
| Controls | 93.58 (80.76–106.40) | 89.35 (77.97–100.72) | 88.83 (78.58–99.08) | 90.77 (77.47–104.06) | 92.88 (80.59–105.16) |
| Reported smoking indoors at home (number of cigarettes/week) | |||||
| Mothers | |||||
| Counseled | 15.84 (10.73–23.19) | 6.64 (3.74–11.31) | 3.51 (2.01–5.74) | 4.09 (2.28–6.89) | 3.94 (2.06–6.97) |
| Controls | 18.81 (13.15–26.73) | 10.34 (6.42–16.32) | 8.72 (5.43–13.68) | 9.55 (5.50–16.14) | 10.37 (6.16–17.06) |
| All smokers | |||||
| Counseled | 27.18 (17.78–41.28) | 9.69 (5.24–17.32) | 5.46 (2.96–9.52) | 7.23 (3.76–13.22) | 6.46 (3.16–12.40) |
| Controls | 35.58 (23.38–53.86) | 18.95 (10.93–32.38) | 14.81 (8.90–24.24) | 14.95 (8.10–26.96) | 19.18 (11.15–32.52) |
Note. Values are geometric means and their 95% CIs.
Baseline group differences for children’s urine cotinine concentration were statistically significant (p = .005). Baseline group differences were not statistically significant (all ps > .05) for any reported smoking or exposure variables.
Intervention implementation
Counseling participation.
Figure 1 shows the number of counseling sessions completed. Of the 76 mothers assigned to the experimental condition, 5 (6.6%) did not participate in counseling and 41 (53.9%) completed all 14 sessions. Sixty (84.5%) completed 7 or more sessions, or at least half of those offered. Of the 35 other parents who smoked, 21 (60%) participated in counseling, 10 attended only 1 session, and only 3 attended 7 or more. Only 1 of the 11 nonsmoking other parents participated in counseling (5 sessions). Other residents participated in counseling with 17 mothers; none in more than 4 sessions.
Provision of NRT.
Study counselors provided 18 mothers (23.7%) with nicotine patches only, 16 (21.1%) with nicotine gum only, and 22 (28.9%) with both. Of those who received these products, 16 (44.4%) used the patches daily and only 5 (18.5%) used the gum daily. Nicotine replacement therapy was also provided to 21 other parents and to 1 or more other adult residents in 14 families. At their 3- and 6-month interviews, 45 mothers assigned to counseling (66.2%) and 7 controls (10.8%) reported that they had used NRT (p < .001) during the intervention period. At their 12- and 18-month interviews, 23 counseling group mothers (37.7%) and 7 controls (11.5%) reported NRT use (p < .001).
Reliability and validity of mothers’ reports
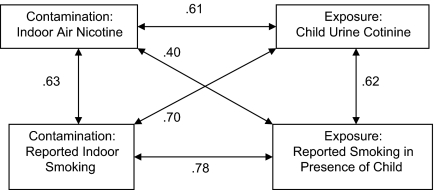
Correlation coefficients for mothers’ reports at the 6-month interview and 24- to 48-hr retest were r = .95 for children’s exposure from mothers inside the home, r = .94 for mothers’ smoking rate inside the home, and r = .99 for mothers’ overall smoking rate (all ps < .001). Validity correlations between outcome variables ranged from .40 to .78 (Figure 2).
Figure 2.
Validity correlations among biochemical, environmental, and reported measures of exposure and contamination.
Intervention effects (baseline to 6 months)
Reported children’s SHSe from mothers’ smoking in the home.
Table 2 shows the means for mothers’ smoking rates and children’s SHSe at each measure. From baseline to 6 months, mothers’ reports of children’s exposure to their smoking inside the home showed a statistically significant group by linear time interaction effect (p = .032) and a quadratic time effect (p = .028). There was a decrease in both groups, which was larger among the counseled families. At 6 months, reported mean SHSe from mothers had decreased 79.8% from baseline among the counseled group and 54.9% among controls.
Reported children’s “total SHSe” from all sources.
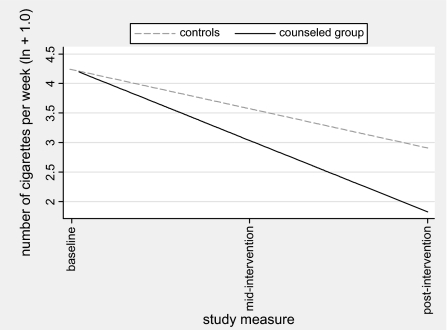
Children’s total SHSe showed a significant group by linear time interaction (p = .012) and a linear time effect (p < .001) from baseline to 6 months. Total SHSe showed a steeper decline among the counseled group (Figure 3). At 6 months, reported total SHSe had decreased 85.2% among the counseled group and 57.3% among controls.
Figure 3.
Children’s reported total secondhand smoke exposure from baseline to postintervention.
Children’s urine cotinine concentration.
For children’s urinary cotinine concentration, only the main effect of time was significant from baseline to 6 months (p = .043). Both groups decreased by 25%.
Mothers’ reported smoking.
The group by linear time interaction (p = .028) and quadratic time effects (p = .029) were statistically significant. At 6 months, mothers’ mean smoking rate had decreased 34.4% among the counseling group and 5.1% among controls.
Mothers’ and all reported indoor smoking.
Mothers’ smoking indoors showed a statistically significant group main effect (p = .008) and linear time effect (p < .001), but the Group × Time interaction did not reach significance. For all indoor smoking, including that by residents and visitors, the group main effect (p = .007) and linear time effect (p < .001) were statistically significant, while the Group × Time interaction was not. Mothers’ and all indoor smoking decreased in both groups.
Maintenance effects (6–18 months)
Reported children’s SHSe from mothers’ smoking in the home.
During the follow-up period, we found a statistically significant group main effect (p = .011) for reported SHSe from mothers at home, suggesting that children’s SHSe from mothers remained lower among the counseled families.
Reported children’s “total SHSe” from all sources.
For total SHSe, the group by quadratic time interaction (p = .006) and the group effect (p < .001) were statistically significant. During follow-up, the intervention group showed an increase and then decrease, while controls showed a slight decrease and then increase. Mean total SHSe increased 3.2% among the intervention group and 33.9% among controls.
Children's urine cotinine concentration.
Only the group main effect was significant for children's urine cotinine concentration from 6 to 18 months (p = .026). Controls showed higher cotinine at baseline and through the follow-up period.
Mothers’ reported smoking.
During follow-up, the group by quadratic time interaction was significant (p = .024). Mothers’ smoking increased and then decreased slightly in the counseled group and decreased and then increased among controls. From 6 to 18 months, mothers’ mean smoking increased 33.1% among the counseled group and 4.6% among controls.
Mothers’ and all reported indoor smoking.
For mothers’ smoking inside the home, only the group main effect was significant during follow-up (p = .010). Similarly, the group main effect was significant (p = .014) for all indoor smoking. Levels remained higher among controls.
Number of counseling sessions completed by mothers.
This measure of “dose” of counseling was not a significant covariate in the GEE analyses of the intervention or follow-up period.
Mothers’ and others’ smoking cessation
Thirteen (17.1%) mothers in the intervention group reported that they had quit smoking for 7 days prior to one or more study measures, without biochemical contradiction, versus four (5.4%) controls (p = .024). Four mothers sustained their smoking cessation for at least 6 months: 7.0 and 11.3 months for two intervention mothers and 6.4 and 8.0 months for two controls. In seven intervention families and two controls, another family member reported quitting smoking for at least 7 days prior to one or more study measures (p = .09). Biochemical testing showed discordance (i.e., non-agreement for cotinine or anabasine/anatabine with reports) for 20% of quits reported by experimental group mothers and 16.7% for controls (p = .860).
Discussion
This was our first study to test an intervention that promoted both SHSe reduction and smoking cessation. It was based on past SHSe trials, where we noticed unprompted higher short-term quit rates for experimental families compared with controls (Hovell et al., 1994). These observations fit the BEM in that SHSe counseling involved shaping parents to reduce their smoking frequency around the child. These shaping and sensitizing procedures, theoretically, could motivate parents to quit (Laraway, Snycerski, Michael, & Poling, 2003). In the present study, the addition of smoking cessation counseling with SHSe counseling resulted in more short-term quits among counseled families than controls, suggesting that SHSe counseling can offer a foundation for promoting experimentation with quitting. Parents’ reports of exposure and smoking levels showed moderate and significant correlations with children’s urine cotinine levels and home air nicotine in the present trial, equivalent to those found in our past studies (Emerson et al., 1995; Matt et al., 2000). These findings confirm our previous observations that parents’ reports of smoking and SHSe rates can be as reliable and valid as cotinine biomarkers or nicotine assays.
Our results confirm previous research demonstrating the efficacy of counseling for children’s SHSe reduction among diverse races/ethnicities. This study demonstrated sustained decreases in SHSe in the counseled group. These results suggest that children and their families would benefit if such services were implemented in clinical or community settings, including WIC programs, which serve over 8 million low-income women, infants, and children nationwide. Three states, including California, pioneered using SHSe as a criterion for determining nutritional risk and provided SHSe counseling with WIC services, although this criterion was eliminated at the recommendation of a scientific review panel (Committee on the Scientific Evaluation of WIC Nutrition Risk Criteria, Institute of Medicine, 1996).
Children’s cotinine concentration showed a decrease in both study conditions that was not significantly different by group over time. This finding is consistent with a number of studies (Gehrman & Hovell, 2003; Greenberg et al., 1994; Roseby et al., 2002). However, it departs from two of our previous trials. One showed stable cotinine levels for the experimental children, while levels increased for controls, suggesting a prevention effect (Hovell et al., 2000). In the other (Hijos Sanos), cotinine concentrations decreased significantly more for the counseled group than for controls by postintervention; but late in the follow-up period, controls decreased to similar levels, suggesting a delayed reactivity effect (Hovell, Meltzer, et al., 2002). These patterns across studies suggest that reduction in children’s cotinine is not yet reliably obtained. This may be due to a number of complications.
The definition of reported “exposure” for our trials has been that a child was in the same indoor room or a car when a cigarette was smoked. A child was not considered “exposed” to cigarettes that were smoked on a porch or balcony with a door open to the home, or anyplace inside the home when the child was not present in the same room. Yet children may be in close proximity to a room where smoking occurs or they may enter a room soon after cigarettes are smoked. Because tobacco smoke disperses quickly throughout a residence, our parent-reported outcome measure was not as inclusive in measuring all sources of SHSe as was urinary cotinine. Evidence to date suggests that SHSe should be defined by any smoking in the home or car, even when children are not present.
Nicotine contamination and thirdhand exposure
Home contamination may also have contributed to the failure to obtain a differential decrease in children’s urine cotinine concentration. Since this trial was conducted, we have learned more about the behavior of nicotine in indoor environments. Volatile SHSe components such as nicotine sorb into surfaces within minutes of emission, contaminating furniture, carpets, walls, clothes, and skin (Daisey, 1999). Subsequently, SHSe components are re-emitted from contaminated surfaces into the air over months (Van Loy, Riley, Daisey, & Nazaroff, 2001). Our research has found that indoor surfaces, dust, and air in smokers’ homes showed nicotine concentrations 5–7 times higher than nonsmokers’ homes, and homes of smokers who reported always smoking outside had intermediate levels of contamination (Matt et al., 2004). Cars of smokers without car smoking bans showed significantly higher levels of nicotine in dust, on surfaces, and in the air compared with nonsmokers’ cars with smoking bans (p < .001), and dust and surface nicotine in cars of smokers who had imposed car smoking bans were at similar levels as for smokers’ cars without bans (Matt et al., 2008). Young children are at high risk of SHSe through dust and surface contamination because they spend more time near floors. They exhibit mouthing (e.g., hand–mouth, toy–mouth) and pica behaviors (i.e., ingesting nonfood objects), increasing risk via ingestion and skin contact with contaminated objects. Thus, parents cannot easily eliminate contamination sources of children’s exposure unless they discontinue all smoking in the home and car. The nonsignificant Group × Time interactions for mothers’ and all indoor smoking in the present trial indicate that the counseling interventions we have tested to date may not have focused sufficiently on this goal.
One of the prerequisite steps in establishing a complete home smoking ban may be to direct counseling to all family members. The planned intervention might have been more effective in helping parents achieve entirely smoke-free homes if more family members had participated. Mothers enrolled their families in the study and counselors encouraged all family members, especially smokers, to participate in counseling sessions. All were eligible to receive free nicotine replacement products. Yet other family members seldom participated.
Measurement and methodological issues
A source of reduced power for the trial may have been measurement reactivity in both conditions. Our first SHSe trial tested limited measures (interviews and air nicotine monitors), full measures (adding diary monitoring of parent smoking and children’s exposure and asthma symptoms), and full measures plus counseling (Wahlgren et al., 1997). That study showed that measurement alone may have accounted for about 50% of the total decrease in SHSe. The current study shows that about two thirds of the experimental effect may be accounted for by measurement reactivity. Results from Hijos Sanos might reflect counseling effects as well as measurement reactivity in the experimental condition and delayed measurement reactivity in the control condition. The decrease in cotinine levels for both groups in our current study raises the possibility that measures alone were partly responsible and that counseling was not powerful enough to cause detectable differential decreases for cotinine outcomes in the context of measurement reactivity.
Partial exposure to counseling
Another source of reduced power may have been incomplete counseling due to dropouts and families that were changing slowly and could not reach substantive reductions in the time available. Almost half of families assigned to the experimental condition did not complete all the 14 offered sessions. At the end of the intervention period, 65% of families in the experimental condition still reported child SHSe. This implies that these counseling procedures provided as a routine clinical service might result in greater reductions in SHSe and a larger proportion and longer duration of quit attempts, as clinical services typically continue as long as there is need and would not be curtailed due to a research timeline. Another possible source of reduced power in the current trial might have been the combination of cessation and SHSe counseling. It is possible that the inclusion of cessation services and parents’ quit attempts distracted the counselors and/or parents from protecting children from SHSe.
High-risk families
Finally, smoking cessation rates following experimental interventions have decreased over time. This may be due to greater recalcitrance in the remaining smokers, who persist in smoking despite increasing social, regulatory, and medical pressures to quit (Irvin & Brandon, 2000). Our participants reported serious life events, including difficulties with sustaining housing (26.7%), employment (27.9%), or telephone service (22.7%); their own or a family member’s incarceration (23.3%) or alcohol or other drug abuse (24.0%); domestic violence (11.6%); and problems with child custody (7.8%). Therefore, these low-income families experienced significant life challenges that may have compounded their difficulties with attending counseling sessions and quitting smoking or reducing children’s SHSe. Such high-risk families may require even more intensive clinical interventions to make larger changes in quit rates and children’s differential cotinine reduction.
Recommendations for future research
Future studies should be directed to removing all smoking from the home, car, and other environments. Studies need to be supported to test more powerful services by continuing counseling and shaping processes until children’s SHSe is eliminated and total smoking bans are enacted. Future interventions should consider additional methods to ensure involvement from all family members, especially smokers, including the possibility of providing incentives for each individual’s participation and/or for confirmed abstinence from smoking (Donatelle et al., 2004). Longer follow-up periods are recommended so that reductions in home contamination might be reflected in children’s cotinine concentration. Future studies should also be supported to test factorial models of no counseling, SHSe counseling, smoking cessation counseling, combined counseling, and measurement reactivity effects. At present, we are unable to determine the comparable efficacy of our past interventions that provided parents only with counseling for reducing children’s SHSe with the combined intervention tested in this study. Similar SHSe counseling procedures should be tested in states that do not yet have well-established tobacco control programs. It is conceivable that they might prove more powerful in these states due to inclusion of families who have not already quit due to local policies. However, it is also plausible that counseling might be synergistic with long-term and intensive statewide efforts to control tobacco, making clinical outcomes more likely for counseled families in states with strong tobacco control programs. The degree and direction of moderation of counseling effects due to statewide tobacco control policies warrant formal analysis. Such studies will inform the need for policies in other states and help highlight moderating effects of such policies for counseling services.
Conclusions
This combined SHSe and smoking cessation intervention demonstrated promising outcomes for reducing children’s SHSe and mothers’ smoking, and increasing mothers’ short-term quits. These results extend previous findings and suggest that adding smoking cessation services to SHSe counseling may increase experimentation with quitting and contribute to subsequent long-term abstinence.
Funding
This study, WIC Families Who Smoke: A Behavioral Counseling Study, was supported by the Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services (Grant numbers R40 MC 00185, R40 MC 02494) and by discretionary funds from the Center for Behavioral Epidemiology and Community Health, San Diego State University. Dr. Benowitz and the analytical chemistry were supported in part by the Flight Attendants Medical Research Institute and the U.S. Public Health Service, National Institute on Drug Abuse (Grant DA12393).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors thank San Diego State University Research Foundation WIC, and Cynthia D. Rich, MS, RD, IBCLC, and San Diego American Red Cross WIC for their assistance. We also thank GlaxoSmithKline and Perrigo Company for their donations of nicotine patches and gum.
References
- Adams EK, Young TL. Costs of smoking: A focus on maternal, childhood, and other short-run costs. Medical Care Research and Review. 1999;56:3–29. doi: 10.1177/107755879905600101. [DOI] [PubMed] [Google Scholar]
- Aligne C, Stoddard J. Tobacco and children: An economic evaluation of the medical effects of parental smoking. Archives of Pediatrics & Adolescent Medicine. 1997;151:648–653. doi: 10.1001/archpedi.1997.02170440010002. [DOI] [PubMed] [Google Scholar]
- Becker AB, Manfreda J, Ferguson AC, Dimich-Ward H, Watson WTA, Chan-Yeung M. Breast-feeding and environmental tobacco smoke exposure. Archives of Pediatrics & Adolescent Medicine. 1999;153:689–691. doi: 10.1001/archpedi.153.7.689. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environmental Health Perspectives. 1999;107(s2):349–355. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorksten B. The environmental influence of childhood asthma. Allergy. 1999;54:17–23. doi: 10.1111/j.1398-9995.1999.tb04383.x. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 2—correlation between subjects. British Medical Journal. 1995;310:633. doi: 10.1136/bmj.310.6980.633. Retrieved 23 March 2009, from http://www.bmj.com/cgi/content/full/310/6980/633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn C, Spencer N, Bonas S, Cow C, Dolan A, Moy R. Effect of strategies to reduce exposure of infants to environmental tobacco smoke in the home: Cross sectional survey. British Medical Journal. 2003;327:257–261. doi: 10.1136/bmj.327.7409.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizzard L, Ponsonby AL, Dwyer T, Venn A, Cochrane JA. Parental smoking and infant respiratory infection: How important is not smoking in the same room with the baby? American Journal of Public Health. 2003;93:482–488. doi: 10.2105/ajph.93.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Environmental Protection Agency. Health effects of exposure to environmental tobacco smoke. 1997. Final report. Office of Environmental Health Hazard Assessment. Retrieved 12 March 2009, from www.oehha.ca.gov/pdf/exec.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Exposure to secondhand smoke among students aged 13-15 years: Worldwide, 2000–2007. Morbidity and Mortality Weekly Report. 2007;56:497–500. [PubMed] [Google Scholar]
- Chilmonczyk B, Palomaki G, Knight G, Williams J, Haddow J. An unsuccessful cotinine-assisted intervention strategy to reduce environmental tobacco smoke exposure during infancy. American Journal of Diseases of Children. 1992;146:357–360. doi: 10.1001/archpedi.1992.02160150097031. [DOI] [PubMed] [Google Scholar]
- Committee on the Scientific Evaluation of WIC Nutrition Risk Criteria, Institute of Medicine. WIC nutrition risk criteria: A scientific assessment. Washington, DC: National Academy of Sciences; 1996. Retrieved March 12, 2009, from http://www.nap.edu/catalog/5071.html. [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, Dempsey DA. Environmental tobacco smoke exposure in low-income 6-year-olds: Parent report and urine cotinine measures. Nicotine & Tobacco Research. 2005;5:333–339. doi: 10.1080/1462220031000094141. [DOI] [PubMed] [Google Scholar]
- Daisey JM. Tracers for assessing exposure to environmental tobacco smoke: what are they tracing? Environmental Health Perspectives. 1999;107(Suppl. 2):319–327. doi: 10.1289/ehp.99107s2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, III, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical Pharmacology & Therapeutics. 2004;76(1):64–72. doi: 10.1016/j.clpt.2004.02.011. doi:10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford, England: Clarendon Press; 1995. [Google Scholar]
- Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: Status of the field and implications for research and practice with pregnant smokers. Nicotine & Tobacco Research. 2004;6(S2):S163–S179. doi: 10.1080/14622200410001669196. [DOI] [PubMed] [Google Scholar]
- Emerson JA, Hovell MF, Meltzer SB, Zakarian JM, Hofstetter CR, Wahlgren DR, et al. The accuracy of environmental tobacco smoke exposure measures among asthmatic children. Journal of Clinical Epidemiology. 1995;48:1251–1259. doi: 10.1016/0895-4356(95)00021-u. [DOI] [PubMed] [Google Scholar]
- Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108:18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- Eriksen W. Do people who were passive smokers during childhood have increased risk of long-term work disability? European Journal of Public Health. 2004;14:296–300. doi: 10.1093/eurpub/14.3.296. [DOI] [PubMed] [Google Scholar]
- Erikson W, Sorum K, Bruusgaard D. Effects of information on smoking behavior in families with preschool children. Acta Paediatrica. 1996;85:209–212. doi: 10.1111/j.1651-2227.1996.tb13994.x. [DOI] [PubMed] [Google Scholar]
- Fiore M, Smith S, Jorenby D, Baker T. The effectiveness of the nicotine patch for smoking cessation: A meta-analysis. Journal of the American Medical Association. 1994;271:1940–1947. [PubMed] [Google Scholar]
- Gehrman CA, Hovell MF. Protecting children from environmental tobacco smoke (ETS) exposure: A critical review. Nicotine & Tobacco Research. 2003;5:289–301. doi: 10.1080/1462220031000094231. [DOI] [PubMed] [Google Scholar]
- Greenberg RA, Strecher VJ, Bauman KE, Boat BW, Fowler MG, Keyes LL, et al. Evaluation of a home-based intervention program to reduce infant passive smoking and lower respiratory illness. Journal of Behavioral Medicine. 1994;17:273–290. doi: 10.1007/BF01857953. [DOI] [PubMed] [Google Scholar]
- Houston TK, Person SD, Pletcher MJ, Liu K, Irabarren C, Kiefe CI. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. British Medical Journal. 2006;332:1064–1069. doi: 10.1136/bmj.38779.584028.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell MF, Meltzer SB, Wahlgren DR, Matt GE, Hofstetter CR, Jones JA, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: A controlled trial. Pediatrics. 2002;110:946–956. doi: 10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- Hovell MF, Meltzer SB, Zakarian JM, Wahlgren DR, Emerson JA, Hofstetter CR, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: A controlled trial [published erratum appears in Chest 1995;107:480] Chest. 1994;106:440–446. doi: 10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- Hovell MF, Wahlgren DR, Adams MA. The logical and empirical basis for the Behavioral Ecological Model. In: DiClemente RJ, Crosby RA, Kegler M, editors. Emerging theories and models in health promotion research and practice. Strategies for enhancing public health. 2nd ed., pp. 415–449. San Francisco: Jossey-Bass; 2009. [Google Scholar]
- Hovell MF, Wahlgren DR, Gehrman CA. The Behavioral Ecological Model: Integrating public health and behavioral science. In: DiClemente RJ, Crosby R, Kegler M, editors. New and emerging models and theories in health promotion and health education. San Francisco: Jossey-Bass Inc; 2002. pp. 47–385. [Google Scholar]
- Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counseling mothers on their children’s exposure to environmental tobacco smoke: Randomized controlled trial. British Medical Journal. 2000;321:337–342. doi: 10.1136/bmj.321.7257.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin JE, Brandon TH. The increasing recalcitrance of smokers in clinical trials. Nicotine & Tobacco Research. 2000;2:79–84. doi: 10.1080/14622200050011330. [DOI] [PubMed] [Google Scholar]
- Irvine L, Crombie I, Clark R, Slane P, Feyerabend C, Goodman K, et al. Advising parents of asthmatic children on passive smoking: Randomized controlled trial. British Medical Journal. 1999;318:1456–1459. doi: 10.1136/bmj.318.7196.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscan A, Uyanik BS, Vurgun N, Ece A, Yigitoglu MR. Effects of passive exposure to tobacco, socioeconomic status and a family history of essential hypertension on lipid profiles in children. Japanese Heart Journal. 1996;37:917–923. doi: 10.1536/ihj.37.917. [DOI] [PubMed] [Google Scholar]
- Jacob P, III, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiology, Biomarkers & Prevention. 2002;II:1668–1673. [PubMed] [Google Scholar]
- Jacob P, III, Yu L, Liang G, Shulgin AT, Benowitz NL. Gas chromatographic-mass spectrometric method for determination of anabasine, anatabine and other tobacco alkaloids in urine of smokers and smokeless tobacco users. Journal of Chromatography. 1993;619:49–61. doi: 10.1016/0378-4347(93)80445-a. [DOI] [PubMed] [Google Scholar]
- Johansson AK, Hermansson G, Ludvigsson J. How should parents protect their children from environmental tobacco-smoke exposure in the home? Pediatrics. 2004;113:e291–e295. doi: 10.1542/peds.113.4.e291. [DOI] [PubMed] [Google Scholar]
- Johansson A, Halling A, Hermansson G. Indoor and outdoor smoking: Impact on children's health. European Journal of Public Health. 2003;13:61–66. doi: 10.1093/eurpub/13.1.61. [DOI] [PubMed] [Google Scholar]
- Keintz M, Fleisher L, Rimer B. Reaching mothers of preschool-aged children with a targeted quit smoking intervention. Journal of Community Health. 1994;19:25–40. doi: 10.1007/BF02260519. [DOI] [PubMed] [Google Scholar]
- Laraway S, Snycerski S, Michael J, Poling A. Motivating operations and terms to describe them: Some further refinements. Journal of Applied Behavior Analysis. 2003;36:407–414. doi: 10.1901/jaba.2003.36-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaderer BP, Hammond SK. Evaluation of vapor-phase nicotine and respirable suspended particle mass as markers for environmental tobacco smoke. Environmental Science & Technology. 1991;25:770–777. [Google Scholar]
- Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States. Date from the Third National health and Nutrition Examination Survey. Archives of Pediatrics & Adolescent Medicine. 2001;155:36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- Mascola MA, Vunakis HV, Tager IB, Speizer FE, Hanrahan JP. Exposure of young infants to environmental tobacco smoke: Breast-feeding among smoking mothers. American Journal of Public Health. 1998;88:893–896. doi: 10.2105/ajph.88.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt GE, Hovell MF, Zakarian JM, Bernert JT, Pirkle JL, Hammond SK. Measuring secondhand smoke exposure in babies: The reliability and validity of mother reports in a sample of low-income families. Health Psychology. 2000;19:232–241. doi: 10.1037//0278-6133.19.3.232. [DOI] [PubMed] [Google Scholar]
- Matt GE, Quintana PJE, Bernert JT, Song S, Navianti N, Juarez T, et al. Households contaminated by environmental tobacco smoke: sources of infant exposure. Tobacco Control. 2004;13:29–37. doi: 10.1136/tc.2003.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt GE, Quintana PJE, Hovell MF, Chatfield D, Ma DS, Romero R, et al. Residual tobacco smoke pollution in used cars for sale: Air, dust, and surfaces. Nicotine & Tobacco Research. 2008;10:1467–1475. doi: 10.1080/14622200802279898. [DOI] [PubMed] [Google Scholar]
- Matt GE, Wahlgren DR, Hovell MF, Zakarian JM, Bernert JT, Meltzer SB, et al. Measuring environmental tobacco smoke exposure in infants and young children through urine cotinine and memory-based parental reports: empirical findings and discussion. Tobacco Control. 1999;8:282–289. doi: 10.1136/tc.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh N, Clark N, Howatt W. Reducing tobacco smoke in the environment of the child with asthma: A cotinine-assisted, minimal-contact intervention. Journal of Asthma. 1994;31:453–462. doi: 10.3109/02770909409089487. [DOI] [PubMed] [Google Scholar]
- Roseby R, Waters E, Polnay A, Campbell R, Webster P, Spencer N. Family and car smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database of Systematic Reviews. 2002;(Issue 3) doi: 10.1002/14651858.CD001746. Art. No: CD001746. doi:10.1002/14651858.DD001746. [DOI] [PubMed] [Google Scholar]
- Soliman S, Pollack HA, Warner KE. Decrease in the prevalence of environmental tobacco smoke exposure in the home during the 1990s in families with children. American Journal of Public Health. 2004;94:314–320. doi: 10.2105/ajph.94.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State of California. Air Resources Board. Proposed identification of environmental tobacco smoke as a toxic air contaminant. As approved by the Scientific Review Panel on June 24, 2005. 2005 Retrieved 12 March 2009, from http://www.arb.ca.gov/toxics/ets/ets.htm. [Google Scholar]
- U.S. Department of Health and Human Services. Healthy People 2010. (2nd ed.). With Understanding and Improving Health and Objectives for Improving Health. 2 vols. Washington, DC: U.S. Government Printing Office; 2000. [Google Scholar]
- U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: A report of the U.S. surgeon general. Technical report. Atlanta, GA: Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- U.S. Environmental Protection Agency. Respiratory health effects of passive smoking: Lung cancer and other disorders. Washington, DC: Office of Health and Environmental Assessment; 1992. EPA/600/6-90/006F. [Google Scholar]
- Van Loy MD, Riley WJ, Daisey JM, Nazaroff WW. Dynamic behavior of semivolatile organic compounds in indoor air. 2. Nicotine and phenanthrene with carpet and wallboard. Environmental Science & Technology. 2001;35:560–567. doi: 10.1021/es001372a. [DOI] [PubMed] [Google Scholar]
- Vineis P, Ronco G, Ciccone G, Vernero E, Troia B, D’Incalci T, et al. Prevention of exposure of young children to parental tobacco smoke: Effectiveness of an educational program. Tumori. 1993;79:183–186. doi: 10.1177/030089169307900304. [DOI] [PubMed] [Google Scholar]
- Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children: A 2-year follow-up. Chest. 1997;111:81–88. doi: 10.1378/chest.111.1.81. [DOI] [PubMed] [Google Scholar]
- Wakefield M, Banham D, McCaul K, Martin J, Ruffin R, Badcock N, et al. Effect of feedback regarding urinary cotinine and brief tailored advice on home smoking restrictions among low-income parents of children with asthma: A controlled trial. Preventive Medicine. 2002;34:58–65. doi: 10.1006/pmed.2001.0953. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Yamada EG, Sudhakar R, Roberto L, Mannino D, Mejia C, et al. A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120:1709–1722. doi: 10.1378/chest.120.5.1709. [DOI] [PubMed] [Google Scholar]
- Woodward A, Owen N, Gurinovich N, Griffith F, Linke H. Trial of an intervention to reduce passive smoking in infancy. Pediatric Pulmonology. 1987;3:173–178. doi: 10.1002/ppul.1950030311. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International consultation on environmental tobacco smoke (ETS) and child health. International Consultation on ETS and Child Health Report. Division of Noncommunicable Diseases, Tobacco Free Initiative. 1999. Retrieved 12 March 2009, from www.who.int/tobacco/research/en/ets_report.pdf. [Google Scholar]
- Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environmental Health Perspectives. 2005;113:98–103. doi: 10.1289/ehp.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.