Abstract
Introduction:
This study explored predictors of smoking quit attempts in a sample of low-income smoking mothers who participated in a randomized trial of a 6-month, 14-session counseling intervention to decrease their children's secondhand smoke exposure (SHSe) and eliminate smoking.
Methods:
Measures were taken at baseline and at 3, 6, 12, and 18 months on 150 mothers who exposed their children (aged <4 years) to ≥10 cigarettes/week in the home. Reported 7-day quits were verified by saliva cotinine or urine anabasine and anatabine levels.
Results:
There were few quits longer than 6 months. Mothers in the counseling group reported more 24-hr quits (p = .019) and more 7-day quits (p = .029) than controls. Multivariate modeling revealed that having quit for at least 24 hr in the year prior to baseline and the number of alternative cessation methods ever tried were predictive of the longest quit attempt during the 18-month study. Mothers in the counseling group who at baseline felt SHSe posed a health risk for their children or who at baseline had more permissive home smoking policies had longer quit attempts.
Discussion:
Results confirm that attempts to quit smoking predict additional quit attempts. This suggests that practice may be necessary for many people to quit smoking permanently. Findings of interaction analyses suggest that participant factors may alter the effects of treatment procedures. Failure to account for or employ such factors in the analysis or design of community trials could confound the results of intervention trials.
Introduction
Health effects of secondhand smoke exposure
The deleterious effects of secondhand smoke exposure (SHSe) have been reported by the U.S. National Research Council (1986), the U.S. Surgeon General (U.S. Department of Health and Human Services [USDHHS], 2006), the U.S. Environmental Protection Agency (1992), and the International Agency for Research on Cancer (2002). The California Air Resources Board identified environmental tobacco smoke as a toxic air contaminant to which infants and children may be especially susceptible (Office of Environmental Health Hazard Assessment, 2006). Children's SHSe has been associated with increased respiratory symptoms following surgery (Drongowski et al., 2003), dental caries (Aligne, Moss, Auinger, & Weitzman, 2003), lower cognitive test scores (Yolton, Dietrich, Auinger, Lanphear, & Hornung, 2005), nocturnal asthma symptoms (Morkjaroenpong et al., 2002), atopic eczema (Kramer et al., 2004), inpatient sickle cell crises (West et al., 2003), and glucose intolerance among young adults (Houston et al., 2006). Nearly 60% of U.S. children are exposed to secondhand smoke, and the home is the location where children are most exposed (USDHHS).
Health effects of smoking
The dangers of cigarette smoking are more strongly established than those of SHSe. Accounting for 20% of U.S. deaths annually (i.e., 430,000), cigarette smoking ranks as the leading preventable cause of death. Smoking is responsible for 87% of lung cancer, 21% of coronary heart disease, and 82% of chronic obstructive pulmonary deaths in this country, as well as increasing risks for cardiovascular disease, chronic lung disease, lung and other cancers, and complications for babies exposed in utero (Centers for Disease Control and Prevention [CDC], 1993; USDHHS, 1999, 2000).
The challenge of smoking cessation
About 21.6% of U.S. adults were current smokers in 2003 (CDC, 2005). With smoking prevalence rates declining (CDC; Cooper et al., 2005), the remaining pool of smokers is likely to be more resistant to cessation. Further reductions may require more comprehensive and sustained interventions among more challenging populations (CDC; Cooper et al.). Studies conducted with smokers recruited explicitly to receive assistance with quitting smoking may miss the most challenging smokers—those not actively seeking help to quit. Some of these smokers may be reached for assistance with cessation by enlisting them in an effort to help protect their children from SHSe.
Linking SHSe reduction and smoking cessation programs
Interventions to help parents cease smoking also may yield important SHSe benefits beyond those obtained by encouraging parents to smoke away from their children. Smoking in the home has been associated with high levels of tobacco toxins that persist well beyond the period of active smoking (California Environmental Protection Agency, 1997; Singer, Hodgson, & Nazaroff, 2003). These toxins are deposited onto household surfaces and loose household dust in the form of particulate matter and as volatile toxic compounds that off gas into the air over days, weeks, and months (Matt et al., 2004; Singer, Hodgson, Guevarra, Hawley, & Nazaroff, 2002). When parents quit, children's SHSe may be lessened due to reduction in exposure to these toxins, which has been termed “third-hand smoke exposure.”
Previous SHSe reduction and smoking cessation trials
Most SHSe research has tested unsuccessful minimal interventions (Chilmonczyk, Palomaki, Knight, Williams, & Haddow, 1992; Erikson, Sorum, & Bruusgaard, 1996; Irvine et al., 1999; Keintz, Fleisher, & Rimer, 1994; McIntosh, Clark, & Howatt, 1994; Vineis et al., 1993; Wakefield et al., 2002; Woodward, Owen, Gurinovich, Griffith, & Linke, 1987). Some have not measured children's exposure, but focused on parent smoking cessation (Groner, Ahijevych, Grossman, & Rich, 2000; Vineis et al.; Wall, Severson, Andrews, Lichtenstein, & Zoref, 1995). The intervention by Wall et al. showed significant cessation effects at 6-month posttest, though not sustained at 12 months (Severson, Andrews, Lichtenstein, Wall, & Akers, 1997). Groner et al. found no difference in quit rates between controls and mothers who received a cessation intervention emphasizing children's SHSe health risks. These “minimal interventions”—generally involving only brief advice or a few sessions in a group setting—have not demonstrated decreased SHSe or sustained cessation.
Several trials have tested more intensive individualized parent counseling, involving a larger number of sessions that are typically conducted at the participant's residence, and reported statistically significant reductions in children's SHSe. Our clinic-based study decreased SHSe for asthmatic children (Hovell et al., 1994). The home-based study of healthy infants by Greenberg et al. (1994) and our trial with low-income mothers and healthy babies showed reduced SHSe and prevented increases in cotinine (Hovell et al., 2000). Emmons et al. (2001) found decreased air nicotine following counseling for low-income families. Our study of Latino asthmatic children obtained therapeutic effects based on reported SHSe and cotinine assays (Hovell, Meltzer, et al., 2002). These results indicate that intensive individualized counseling can be efficacious for reducing healthy and asthmatic children's SHSe for low- to middle-income and racially mixed families (Gehrman & Hovell, 2003).
Rationale, design, and purpose of the current study
Many mothers in our previous SHSe trials expressed an interest in cessation counseling, and a few quit during the trials (Hovell et al., 2000; Wahlgren, Hovell, Meltzer, Hofstetter, & Zakarian, 1997; Zakarian et al., 2004). The current study added a specific emphasis on smoking cessation to the SHSe reduction counseling of our prior studies. This included making nicotine replacement therapy (NRT) freely available, as both counseling and NRT have been shown to be effective (Fiore, Kenford, et al., 1994; Fiore, Smith, Jorenby, & Baker, 1994). We are aware of no other study that has included formal shaping (Cooper, Timothy, & Heward, 2007; Honig & Staddon, 1977) procedures to promote cessation as part of SHSe counseling. Success at temporarily forgoing smoking in the interests of SHSe reduction can help smokers acquire the experience and skill needed to achieve cessation. The design of the present study enabled us to explore the association of both the counseling intervention and participant factors with the duration of attempts to quit smoking.
The current analyses were designed to determine differences by experimental condition in the length of smoking quit attempts and to identify baseline predictors of length of quit in a sample of low-income women who had a child under age 4. Additionally, theoretically predicted baseline motivating variables were explored as potential moderators of counseling effects on length of quits.
Methods
Design
Data were from a community trial, more fully described elsewhere (please see our companion article, “Counseling to Reduce Children's Secondhand Smoke Exposure and Help Parents Quit Smoking: A Controlled Trial”), of a counseling intervention aimed at lowering SHSe in young children of low-income smoking mothers and at reducing or eliminating smoking by those mothers. After collection of baseline data, mothers qualifying to participate were randomly assigned to the counseling intervention or a treatment-as-usual control group. Interviews and biochemical and environmental assessments were conducted at baseline and at 3, 6, 12, and 18 months.
Recruitment
One hundred and fifty mothers were recruited from seven Women, Infants, and Children Supplemental Food and Nutrition Program (WIC) offices in San Diego County. WIC families must be at or below 185% of the Federal Poverty Income Guidelines. Recruitment flyers at the WIC sites asked women to participate in a general health study. The Institutional Review Board of San Diego State University approved recruitment and study protocols. To qualify for the trial, a mother had to speak English and report that she had exposed her child (<4 years old) to at least 10 cigarettes/week at home indoors or in a car during the week prior to the baseline assessment.
Intervention
Counselors delivered an intervention targeting SHSe reduction for the participant's youngest child, which also included a smoking cessation component that was individually tailored to each mother. Treatment consisted of 14 sessions over 6 months—10 in person at the participant's home and 4 by phone. At each SHSe reduction counseling session, the mother was asked if she would like assistance to quit. If so, sessions were divided between SHSe reduction and the quitting process.
For cessation, topics discussed included social support, addiction, withdrawal, NRT, triggers, smoking place, cost, coping skills, urge control, DEADS (Delay, Escape, Avoid, Distract, Substitute), cigarette fading, how to plan to quit, why smoke is harmful, stress and mood, lifestyle balance, lapse–relapse prevention, weight connection, diet and exercise, and maintenance. Counselors encouraged mothers to consider setting a long-term goal of quitting or reducing smoking at the very first counseling session. Mothers who were interested were encouraged to select a quit date toward the middle of the intervention so that counselor assistance would be available during the quit preparation and early maintenance stages. Mothers received extra contacts by phone from their counselor 1 day prior, the day after, and a week after their quit attempt. Mothers setting tobacco reduction or cessation goals were encouraged to set SHSe reduction goals to protect their child, whether or not they quit smoking. Throughout the program, mothers who did not already have a long-term quit goal were encouraged to set one. Participants in the counseling group were offered free nicotine patches and/or nicotine gum to assist with achieving smoking reduction and cessation goals. With successful changes in tobacco use or experimental quits of short duration, counselors provided positive feedback and promoted longer term or new quits. In this method, shaping for both SHSe and quitting was implemented.
Measures
Environmental and biochemical markers.
Dosimeters to measure nicotine in the air (Hammond & Leaderer, 1987; Leaderer & Hammond, 1991) were assigned to a randomly selected 40% of residences in each experimental group, for the week prior to the baseline interview and again for the week prior to the 6-month interview. Inactive versions of the dosimeters (Matt et al., 1999; Murray, O'Connell, Schmid, & Perry, 1987) were placed in all homes for the duration of the study to increase the validity of reported smoking and SHSe. Self-reported abstinence from smoking during the 7 days prior to the interview was confirmed biochemically by a saliva cotinine level of <15 ng/ml if the participant was not using NRT (SRNT Subcommittee on Biochemical Verification, 2002) or by urine anabasine and anatabine levels <2 ng/ml for those participants using NRT (Jacob, Yu, Liang, Shulgin, & Benowitz, 1993; Jacob et al., 2002).
Reported measures.
Structured interviews were conducted by staff members who were blind to participants’ assignment to condition. Responses were recorded by digital audio recorder as well as by hand to provide a quality control check on interviewer adherence to protocol and as an aid in resolving data collection/entry errors. Table 1 provides data on demographics, baseline measures potentially related to smoking, and the ordinal measure of cumulative quit attempts across the four postbaseline assessments.
Table 1.
Participating mothers by experimental group: demographics; baseline behaviors, attitudes, and social contexts potentially related to smoking; quit attempts across 18 months
| Control |
Counseling |
Total |
||||
| n—sizes other than 74, 76, 150 are given in parentheses |
n = 74 |
n = 76 |
n = 150 |
|||
| Ethnicity | n | % | n | % | n | % |
| Hispanic | 7 | 9.5 | 11 | 14.5 | 18 | 12 |
| Black | 8 | 10.8 | 8 | 10.5 | 16 | 10.7 |
| White | 52 | 70.3 | 50 | 65.8 | 102 | 68 |
| Other | 7 | 9.5 | 7 | 9.2 | 14 | 9.3 |
| Education | ||||||
| Less than high school or GED | 13 | 17.6 | 17 | 22.4 | 30 | 20 |
| High school or GED | 29 | 39.2 | 30 | 39.5 | 59 | 39.3 |
| Trade school | 1 | 1.4 | 2 | 2.6 | 3 | 2 |
| Some college | 29 | 39.2 | 25 | 32.9 | 54 | 36 |
| College graduate | 2 | 2.7 | 2 | 2.6 | 4 | 2.7 |
| Employed | 28 | 37.8 | 25 | 32.9 | 53 | 35.3 |
| Single parent | 26 | 35.1 | 25 | 32.9 | 51 | 34 |
| Partner or boyfriend smoked in the home, past 7 days | 29 | 39.2 | 35 | 46.1 | 64 | 42.7 |
| Thinks smoking can harm own health a lot (vs. not a lot) | 65 | 87.8 | 60 | 78.9 | 125 | 83.3 |
| Thinks SHSe can harm kid's health a lot (vs. not a lot) | 49 | 66.2 | 51 | 67.1 | 100 | 66.7 |
| Seriously considering quitting, next 6 mo. (72, 72, 144) | 38 | 52.8 | 39 | 54.2 | 77 | 53.5 |
| Quit smoking for more than 24 hr, in year prior to baseline | 30 | 40.5 | 25 | 32.9 | 55 | 36.7 |
| M | SD | M | SD | M | SD | |
| Age, years | 30 | 7.4 | 30.2 | 6.9 | 30.1 | 7.1 |
| Permissiveness of home smoking rules, 1 = low, 4 = high | 2.7 | 1 | 2.6 | 1.1 | 2.6 | 1.1 |
| Fagerström Heaviness of Smoking Index | 2.4 | 1.5 | 2.4 | 1.3 | 2.4 | 1.4 |
| Number of types of people who recommended quitting smoking | 3.7 | 1.7 | 3.8 | 1.8 | 3.8 | 1.7 |
| Number of close friends and family who smoke (73, 76, 149) | 4.7 | 1.9 | 4.7 | 1.7 | 4.7 | 1.8 |
| Desire to stop smoking, 1 = none, 4 = a lot | 2.9 | 0.8 | 3 | 0.9 | 3 | 0.8 |
| Quit support by family, friends, coworkers, 1 = none, 4 = a lot | 2.6 | 0.8 | 2.5 | 0.8 | 2.5 | 0.8 |
| Years since began smoking regularly | 13.7 | 7.4 | 14.7 | 7.0 | 14.2 | 7.2 |
| GeoM | Median | GeoM | Median | GeoM | Median | |
| Number of cigarettes smoked/week | 80 | 70 | 76 | 70 | 78 | 70 |
| Number of days since last quit attempt (57, 69, 126) | 303.1 | 402 | 569.7 | 719 | 428.3 | 516 |
| Number of different methods tried for quitting smoking | 1.1 | 1 | 1.4 | 1 | 1.3 | 1 |
| Number of other residents in home who smoked in past 7 days | 0.7 | 1 | 0.7 | 1 | 0.7 | 1 |
| n | % | n | % | n | % | |
| Quit, any duration ≥1 hra (p = .003)b | 37 | 50.0 | 55 | 72.4 | 92 | 61.3 |
| Quit for ≥24 hra (p = .019)b | 32 | 43.2 | 47 | 61.8 | 79 | 52.7 |
| Quit for ≥7 daysc (p = .029)b | 5 | 6.8 | 15 | 19.7 | 20 | 13.3 |
Note. The geometric mean and median are provided as indicators of central tendency for skewed variables. GED = generalized equivalency degree; SHSe = secondhand smoke exposure.
Assessed as an intentional quit since the last interview.
Significance of difference in quits by group is by Fisher's exact test (two-sided).
Assessed as not having smoked during 7 days prior to interview.
Smoking dependence.
As an indicator of smoking dependence, we used the Fagerström Heaviness of Smoking Index (HSI; Heatherton, Kozlowski, Frecker, & Fagerström, 1991; John et al., 2004), a two-question version of the six-question Fagerström Test for Nicotine Dependence (FTND). The HSI is a 0–6 scale that sums responses on two items: (a) the average number of cigarettes the mother smokes in a day, scored from 0 (1–10 cigarettes) to 3 (more than 30 cigarettes); and (b) how soon after awakening she usually smokes her first cigarette, scored from 3 (0–5 min) to 0 (more than 60 min). These two items accounted for the majority of variance in total scores for the Fagerström Tolerance Questionnaire, the earliest version of the FTND.
Home smoking rules.
To measure permissiveness of home smoking rules, we asked the mother to choose among four possible descriptions of how cigarette smoking in her home is handled: (a) No one is allowed to smoke in your home; (b) only special guests are allowed to smoke in your home; (c) people are allowed to smoke only in certain areas in your home; (d) people are allowed to smoke anywhere in your home. This measure has demonstrated correlations with SHSe and cessation in our studies with Mexican Americans (Martinez-Donate et al., 2007) and Korean Americans (Ji et al., 2005).
Other reported variables.
The number of types of people who had recommended the mother quit smoking was a count of the following categories: her partner, her children, other family member, doctor or nurse, dentist, WIC staff member, friend, anyone else.
The response choices 1 (none), 2 (not a lot), 3 (some), and 4 (a lot) were used to assess how much the mother wanted to stop smoking, and the amount of support or understanding for quitting the mother thought she would get from family, friends, and coworkers. The same 4-point scale was used to assess how much the mother thought cigarette smoking could harm her health and how much SHSe could harm children's health. Because few respondents replied none or not much to either question about harm, responses to both questions were dichotomized as 0 (not a lot), 1 (a lot). The question “How old were you when you first started smoking cigarettes regularly?” and the mother's age were used to compute the number of years since she began smoking regularly. We computed mother's weekly smoking as the number of days she had smoked cigarettes in the last 7 days times the average number of cigarettes smoked on the days she had smoked.
The number of other residents who smoked inside the home in the past 7 days was assessed by asking the mother for the names of household residents who had done so. The 4-point scale, 1 (none), 2 (a few), 3 (some), and 4 (most), was used to assess the number of the mother's close friends and family who smoke.
The mother was queried about her use of 14 specific cessation methods (e.g., nicotine gum, group classes, acupuncture), and “yes” responses were summed to get the number of different methods tried. The date of the last time she intentionally quit or tried to quit smoking was used to calculate the number of days since that date.
The following items had “yes/no” responses: whether her partner or boyfriend smoked in the home during the past 7 days; whether she was seriously considering quitting smoking in the next 6 months; and whether she had quit smoking for more than 24 hr in the past year (i.e., the year prior to baseline interview).
Quit attempts.
At each of the 3-, 6-, 12-, and 18-month interviews, three types of quit attempts since the prior interview were assessed: (a) quits of any length ≥1 hr; (b) quits of 24 hr or more; and (c) quits of 7 days or more. The dependent variable was constructed from the values of these three variables to provide an ordinal measure of the mother's longest duration quit during the study:
0 = no quit attempts during the 18 months,
1 = at least one quit attempt of less than 24 hr duration,
2 = at least one quit attempt of 24 hr duration, but less than 7 days,
3 = at least one quit attempt of 7 days duration or longer.
Only 7-day quits confirmed by lab test of saliva or urine samples were used in computing the ordinal dependent variable. Evidence for construct validity of the dependent variable (which cumulated quits across the postbaseline measures) was provided by its negative correlation with postbaseline measures of the child's urine cotinine (r = −.312), reported indoor smoking (r = −.381), reported SHSe (r = −.423), and air nicotine concentrations (r = −.192). Bivariate correlations were computed between the dependent variable and each of the other outcome variables, utilizing data obtained at four measurement points—Month 3 to Month 18—and controlling for the within-subjects correlation of measures repeated over time. The exception was air nicotine, for which there were postbaseline data only at Month 6.
Statistical analyses
All potential predictor variables were screened for outliers and for bivariate associations with the ordinal dependent variable. To uncover any instances of variables for which interaction with group might obscure associations for the entire sample, bivariate associations were also examined within each treatment group. Only variables associated with the dependent variable (p < .10), or of essential theoretical importance, were included in regression analyses in order to limit the number of independent variables. Predictor variables were in all instances baseline measures, in order to avoid conceptual confusion between possible moderating and mediating functions of variables.
The ordinal logistic regression procedure (ologit) in Stata 9.1 for an ordinal dependent variable was used to model smoking quit attempts, using as predictors the variables that had passed the bivariate screening and selected demographics. Skewed variables were adjusted by logarithmic transformation to approximate normality. All quantitative variables were centered to reduce multicollinearity and to facilitate interpretation of regression coefficients. All independent variables were entered simultaneously.
We tested the proportional odds assumption (aka the parallel regression assumption) for the model, using the Brant test procedure. The test did not reach significance (p = .158), providing evidence that the assumption had not been violated and thus that the odds estimated for each predictor were not significantly different across the three cutpoints of the dependent variable.
Analyses were based on intention to treat. Each mother assigned to the experimental group was treated as if she had received the full intervention regardless of her compliance with counseling or the number of missed sessions. Mothers in either group who failed to complete a measure were counted as smokers at that measure. Analyses were performed using SPSS version 14.0 (SPSS Inc., 2005) and Stata version 9.1 (StataCorp, 2005) for Windows.
Results
Data collection
The percentage of postbaseline interviews completed was 83.2% for the counseling group (253 of 304 of possible interviews) and 86.8% for the control group (257 of 296). Despite incentives for completing measures and persistent retention efforts, a number of participants missed interviews. This meant that at the next interview, a participant would have a longer recall period for questions about events since the previous interview. Fortunately, approximately the same percentage of postbaseline interviews were completed in each group, suggesting limited differential bias due to missing data.
Characteristics of participants
Counseling and control groups were equivalent at baseline on almost all variables tested to assess success of random assignment (see illustrations in Table 1). However, participants in the control group reported significantly (F = 8.544, p = .004) fewer days since the last intentional quit attempt compared with the counseling group.
Participating mothers were low-income WIC clients, with moderate levels of education, a mean age of 30, and primarily White, Hispanic, or Black. About 33% were single parents. On average, they had started smoking regularly approximately 14 years ago and were consuming about half a pack of cigarettes a day.
Cessation
There were few long-term quits by participants in either group. During the 18-month period, only four mothers (two in each group) reported a continuous abstinence of longer than 6 months. By chi-square analysis of self-reported postbaseline data, mothers in the counseling group, compared with controls, had more 24-hr quits (p = .019), more 7-day quits (p = .029), and a higher mean rank on the constructed ordinal outcome measure of longest quit duration (Mann–Whitney U = 2,071, p = .003).
In the initial ordinal regression model with all independent variables, group assignment was the strongest predictor of longest quit attempt (p = .005). Interaction terms with group were created for each baseline predictor. Statistically nonsignificant terms (i.e., p ≥ .05) were dropped from the regression model, starting with the term having the least predictive value, and models were reestimated. This was repeated to produce the most parsimonious model. Ordinal regression results for the resulting final (i.e., most parsimonious) model are presented in Table 2.
Table 2.
Final (most parsimonious) ordinal regression model of longest quit attempt
| B | SE | z | p | Odds ratio | 95% CI | ||
| Quit smoking ≥24 hr, past year, 0 = “no”, 1 = “yes” | 1.38 | 0.38 | 3.63 | <.001 | 3.99 | 1.89 | 8.42 |
| Number of different methods tried for quitting, ever | 0.75 | 0.38 | 1.96 | .05 | 2.12 | 1.00 | 4.50 |
| Permissiveness of home smoking rules, 1 = low, 4 = high | −0.83 | 0.23 | −3.63 | <.001 | 0.44 | 0.28 | 0.68 |
| Thinks SHSe can harm kids’ health, 0 = not a lot, 1 = a lot | −0.51 | 0.52 | −0.97 | .332 | 0.60 | 0.22 | 1.68 |
| Group, 0 = control, 1 = counseling | −0.20 | 0.59 | −0.34 | .735 | 0.82 | 0.26 | 2.61 |
| Group × Harm | 1.87 | 0.76 | 2.47 | .014 | 6.50 | 1.47 | 28.74 |
| Group × Rules | 0.85 | 0.31 | 2.69 | .007 | 2.33 | 1.26 | 4.31 |
Note. Model χ2 (7) = 51.11; pseudo R2 = .15; SHSe = secondhand smoke exposure.
None of the demographic factors were retained in the final model. Having made a quit attempt of at least 24 hr duration in the year prior to baseline was a significant predictor of longest quit attempt, with an odds ratio of 3.99. That odds ratio is the same across all three cutpoints of the ordinal outcome variable. This means that those mothers with a history of a 24-hr quit in the previous year were four times as likely to report longer quit durations as mothers without that history. The odds ratio for the number of different quit methods tried is 2.12. This indicates that for each additional method the mother had tried prior to randomization, the odds of her being in a longer quit category more than doubled.
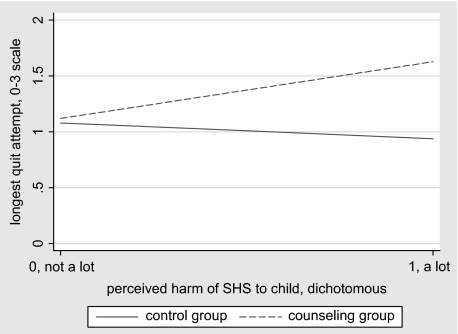
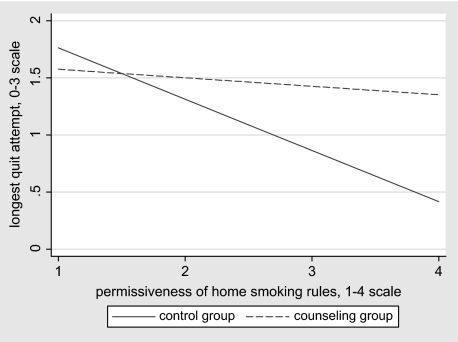
Two variables were found to moderate the effects of counseling as demonstrated by their significant interactions with group: (a) For mothers who at baseline reported that SHSe seriously harmed children, those in the counseling group were 6.50 times more likely than those in the control group to report longer quits; (b) for each point higher on the scale of permissiveness of their baseline home smoking rules, mothers in the counseling group were 2.33 times more likely to have longer quits, relative to those in the control group. As an aid to interpreting these moderating effects, Figures 1 and 2 show best fit lines for the bivariate plots of each of the independent variables with the outcome variable, by group.
Figure 1.
Interaction of group with rated harm of secondhand smoke (SHS) exposure. Lines represent best linear fit of the bivariate data, by group.
Figure 2.
Interaction of group with rules about smoking in the home. Lines represent best linear fit of the bivariate data, by group.
Figure 1 illustrates that among mothers who at baseline felt that SHSe posed little risk to the health of their child, members of control and counseling groups had similar quit lengths; but of mothers who felt SHSe was a serious threat, those in the counseling group had on average longer quits. Figure 2 illustrates that among mothers whose baseline rules against smoking in the home were relatively restrictive, members of the two treatment groups differed little on longest quit attempt; but for mothers with more permissive rules at baseline, those in the counseling group had on average longer quits.
Contrary to expectation, education, heaviness of smoking, and partner smoking status were not predictive of longest quit attempt.
Discussion
This study focused on the duration of smoking cessation episodes and the predictors of duration of the longest of these episodes, to inform future smoking cessation interventions. It was found that prior behaviors—the number of different methods ever tried for quitting smoking, and having successfully quit for at least 24 hr in the previous year—were predictive of the length of the longest attempt at quitting during the 18-month trial by mothers in both groups. Only a few mothers in either group quit for 6 months or longer. However, more mothers in the counseling group reported quits of at least 24 hr and of at least 7 days than did controls. Group assignment interacted with two baseline measures to affect length of longest quit attempt: Mothers in the counseling group who felt SHSe posed a greater risk for their children or who had more permissive home smoking policies reported longer quit attempts during the subsequent 18 months.
Sustained cessation
Counseling to reduce SHSe and promote cessation did not result in long-term or permanent cessation of smoking. This result raises concerns about the design, strength, duration, and fidelity of the intervention procedures. However, the failure may reflect the relatively low smoking rates in California. It is possible that the remaining smokers in the state, having resisted increasingly severe social and economic pressures to quit, are similarly resistant to formal cessation programs (Irvin & Brandon, 2000; Irvin, Hendricks, & Brandon, 2003).
Short-term quits
Compared with control smokers, counseled smokers had more 24-hr quits, and more 7-day quits. This suggests that future studies should explore whether extending the duration or dose of counseling services might contribute to complete cessation. These results also raise the possibility that smokers in SHSe reduction services might be referred to formal smoking cessation services with the expectation that short-term quits have prepared them for longer term cessation, with formal services to this end. This possibility warrants formal evaluation.
Predictors of duration of quitting
Our analyses used baseline data to predict duration of quit across an 18-month period. In accord with evidence that a history of quit attempts is one of the strongest predictors of quitting (Ferguson et al., 2003; Heil, Alessi, Lussier, Badger, & Higgins, 2004; Hymowitz et al., 1997), we found that quit attempts prior to baseline and number of different quit methods tried were predictive of longest quit attempt. This suggests construct validity for our measure of duration of quits, adding to the science concerning measures of quit attempts. This also suggests that the newly established quit history due to the counseling intervention may have provided mothers in the experimental condition with experience that will contribute to complete cessation later in life.
Two additional predictors were identified, each interacting with experimental condition to moderate quitting. First, mothers’ baseline opinions about the degree of harm that SHSe poses for their children were more predictive of longest quit attempt for the counseling group than for controls (see Figure 1). Counseling emphasized the potential harm to children caused by SHSe. So the finding suggests that counselor efforts to motivate mothers to abstain from smoking may work most effectively on mothers with a preexisting concern about the health effects of children's SHSe. This is consistent with the results of Blackburn et al. (2005), who found that fathers’ knowledge about the risks of SHSe for infants predicted quit attempts. If more research confirms this association, counseling mothers to fully understand the acute and cumulative risks that SHSe poses to their children should be tested as a possible means of increasing the efficacy of the counseling program.
Second, a greater differential between-group effect on quitting during the 18-month study was found among those mothers who at baseline reported more permissive home smoking rules and thus stood to benefit most from counseling efforts to promote restrictive rules (see Figure 2). We interpret this to mean that for those who already had fairly restrictive policies in place, there was a ceiling on the degree of rule change that counseling could promote and thus on the effect this factor might have on quitting. Supporting our finding of the importance of initial smoking policies, a longitudinal study showed restrictions in the home were predictive of quit attempts 7 months later (Borland et al., 2006). It would be worth investigating change over time in home smoking policies as a potential mediator of counseling effects on cessation.
The overall negative association of permissiveness with quitting suggests that in the absence of a personalized counseling program to help mothers quit smoking, stronger home smoking restrictions might facilitate quitting. Broader interventions targeting low-income women with young children, such as media campaigns, might therefore effectively focus on messages about home smoking policies. There is certainly ample evidence from recent research that quitting and attempting to quit are more likely in homes with tighter smoking restrictions (Borland et al., 2006; Farkas, Gilpin, Distefan, & Pierce, 1999; Gilpin, White, Farkas, & Pierce, 1999; Ji et al., 2005; Kegler & Malcoe, 2002; Okah, Choi, Okuyemi, & Ahluwalia, 2002; Pizacani et al., 2004; Siahpush, Borland, & Scollo, 2003). Relevant to our sample of low-income mothers, one study found that home smoking policy mediated the relationship between socioeconomic status and cessation (Honjo, Tsutsumi, Kawachi, & Kawakami, 2006).
Null predictors of duration of quitting
For several likely predictors of duration of cessation, no significant relationships were observed. HSI also failed to show a significant bivariate correlation with the dependent variable or with any of its components. Though included in the initial regression model due to its theoretical importance, neither HSI nor its interaction with group was a significant predictor in the context of the other independent variables. This is contrary to results from studies in which high levels of dependence predicted failure to quit (Chandola, Head, & Bartley, 2004; Chatkin, 2004; Ferguson et al., 2003; Grandes, Cortada, Arrazola, & Laka, 2003; Hymowitz et al., 1997; Ji et al., 2005).
Though data for calculating HSI were collected at each interview, HSI is not entirely distinct from the cessation outcome measure. In order to avoid part–whole confounding and to determine the role that mother's addiction level at entrance to the study might have on experimental effects, we considered mother's HSI only at baseline. That measure, on a 0–6 scale, had a distribution sufficiently normal not to require transformation, though with few participants scoring a 5 or 6. But only one variable, of the many we investigated, was significantly correlated with baseline HSI: whether mom smoked after becoming aware of her pregnancy with the target child. This association provides evidence that HSI did function as a measure of level of addiction in our sample.
Partner smoking status also failed to predict quit attempts in spite of evidence in the literature to the contrary (Garvey et al., 2000; McBride et al., 2004; Pisinger, Vestbo, Borch-Johnsen, & Jørgensen, 2005; Torrent et al., 2004). One difficulty with this measure was that 37% of mothers did not have a husband or domestic partner. However, regardless how we interpreted and recoded the data, the predictive value of this variable was not sustained in the regression analyses. It is possible that partner baseline smoking status was not an important determinant of mothers’ short-term quits for either group because of the frequency with which mothers lost or changed partners during the postbaseline quit assessment period.
Education has been associated with abstinence in other studies (Broms, Silventoinen, Lahelma, Koskenvuo, & Kaprio, 2004; Wetter, 2005), but failed to correlate with the outcome measure in the current study. This might be explained by attenuated variance in income among our participants, who all met low-income criteria to qualify for WIC assistance, despite their approximately normal distribution across educational levels.
Implications
The Behavioral Ecological Model posits additive and synergistic interactions among contingencies of reinforcement (Adams et al., 2006; Hovell, Wahlgren, & Adams, in press; Hovell, Wahlgren, & Gehrman, 2002; Hovell & Hughes, 2009). For instance, many parents may restrict smoking in their home but allow smoking when a senior family member, such as a mother-in-law, visits. These exceptions are presumably due to the potential penalties incurred if the special guest is not allowed to smoke. Interacting contingencies often define moderating processes, where the presence of a given contingency (e.g., mother-in-law criticism) may alter the power of a preexisting contingency (e.g., home policy). Due to the complexity of disentangling such relationships, few studies systematically explore potential moderators (Kraemer, Wilson, Fairburn, & Agras, 2002).
Exploring moderating functions is critical for understanding variance in formal prevention or treatment programs, possibly defining the contexts in which they work and the contexts in which they may not work. A nonsignificant difference between control and treatment groups in a community trial could be due to a treatment effect that is restricted to participants having a precondition that sensitizes them to the intervention.
In our earlier smoking prevention study of youth obtaining clinical services from orthodontists (Wahlgren, Hovell, Slymen, et al., 1997), we demonstrated a moderating function for peer pressure to smoke. The incidence of smoking in the group counseled not to start smoking was lower than that in the controls, if they had friends who thought smoking was “cool.” Without consideration of this interaction, the counseling program showed no significant effect. Thus, Type II errors in testing theoretically justified interventions may occur if moderating factors are not included in planned analyses.
Our current findings show that counseling results in longer quit duration for those mothers who considered SHSe damaging to their child's health and for those who had relatively few restrictions on smoking in the home. These moderating relationships suggest subgroups of the population most sensitive to the counseling provided, and they suggest that counseling might be strengthened to effect change in those smokers who do not agree that SHSe is harmful to the health of children or to those who already have relatively strict smoking restrictions in the home. Given the importance of risk reduction and the expense of large-scale community trials, failing to consider moderating factors may be costly in terms of human suffering as well as financial expenditure.
Limitations
Since only 85% of all planned follow-up interviews were completed by the baseline cohort, the analyses are susceptible to missing data bias. However, this may have compromised prevalence or incidence rates more than associations (Hovell et al., 1996).
The intervention included NRT, behavioral smoking cessation treatment, and counseling to reduce secondhand smoking by children. It is not possible to isolate the effects of these components of treatment on the cessation outcomes for the treatment group. Future studies should be promoted that have sufficient funding and power to disentangle the effects of these common components of counseling interventions.
Due to the cost of laboratory analyses, only 40% of homes had functional nicotine air dosimeters installed, and not all installed dosimeters could be collected for analysis, further reducing the available sample of nicotine concentrations. This may have resulted in a less reliable estimate of the correlation (r = −.192) of the ordinal quit variable with air nicotine than with the other outcome measures.
Finally, despite face validity, correlational evidence of construct validity, and the predictive validity provided by the regression analyses, the ordinal outcome measure created for this study might be improved by development of a continuous measure. Nonetheless, our constructed outcome variable captures behaviors that indicate levels of progress toward smoking cessation, unlike the simple assessment of 7-day quits. Davis, Sechrest, and Shapiro (2005) developed a similar ordinal scale, arguing that in the absence of clear-cut abstinence, failing to assess progress toward cessation could miss important intervention effects. Further research using this type of assessment might inform procedures that could gradually shape smokers to quit for longer and longer periods, ultimately leading to permanent abstinence.
Conclusions
Overall, this study showed that blending smoking cessation counseling with SHSe reduction counseling can increase the quit attempts made by low-income mothers with young children. These may be smokers at high risk of continuing to smoke and in need of help but least able to afford services. The small number of long-term quits in the present study suggests that further research is needed on the intensity or type of intervention that might yield more frequent quit attempts and ultimately permanent cessation. Among the directions that warrant special consideration are the effects of community-wide policies, such as bans on public tobacco smoking or media campaigns to increase the public's awareness of the harms of SHSe, and the moderating effect of such awareness on clinical interventions.
Funding
This work was supported by the Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services (grant numbers R40 MC 00185 and R40 MC 02494), and by discretionary funds from the Center for Behavioral Epidemiology and Community Health (CBEACH), San Diego State University. Dr. N. L. Benowitz and the analytical chemistry were supported in part by the Flight Attendants Medical Research Institute and the U.S. Public Health Service, National Institute on Drug Abuse (grant number DA12393).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors thank Sarah Nordahl Larson, M.S., R.D.; the San Diego State University Research Foundation WIC; Cynthia D. Rich, M.S., R.D., IBCLC; and the San Diego American Red Cross WIC for their assistance. The authors also thank GlaxoSmithKline and Perrigo Company for their donations of nicotine patches and gum. Research for this article was conducted at the Center for Behavioral Epidemiology and Community Health Graduate School of Public Health, San Diego State University.
References
- Adams MA, Hovell MF, Irvin V, Sallis JF, Coleman KJ, Liles S. Promoting stair use by modeling: An experimental application of the Behavioral Ecological Model. American Journal of Health Promotion. 2006;21:101–109. doi: 10.4278/0890-1171-21.2.101. [DOI] [PubMed] [Google Scholar]
- Aligne CA, Moss ME, Auinger P, Weitzman M. Association of pediatric dental caries with passive smoking. Journal of the American Medical Association. 2003;289:1258–1264. doi: 10.1001/jama.289.10.1258. [DOI] [PubMed] [Google Scholar]
- Blackburn C, Bonas S, Spencer N, Dolan A, Coe C, Moy R. Smoking behaviour change among fathers of new infants. Social Science & Medicine. 2005;61:517–526. doi: 10.1016/j.socscimed.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Borland R, Yong HH, Cummings KM, Hyland A, Anderson S, Fong GT. Determinants and consequences of smoke-free homes: Findings from the International Tobacco Control (ITC) Four Country Survey. Tobacco Control. 2006;15(Suppl. 3):iii42–iii50. doi: 10.1136/tc.2005.012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Silventoinen K, Lahelma E, Koskenvuo M, Kaprio J. Smoking cessation by socioeconomic status and marital status: The contribution of smoking behavior and family background. Nicotine & Tobacco Research. 2004;6:447–455. doi: 10.1080/14622200410001696637. [DOI] [PubMed] [Google Scholar]
- California Environmental Protection Agency. Health effects of exposure to environmental tobacco smoke. Final report. Sacramento: Author; 1997. Office of Environmental Health Hazard Assessment. Retrieved 25 April 2009, from www.oehha.ca.gov/pdf/exec.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking–attributable mortality and years of potential life lost—United States 1990. Morbidity and Mortality Weekly Report. 1993;42:645–649. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2003. Morbidity & Mortality Weekly Report. 2005;54:509–513. [PubMed] [Google Scholar]
- Chandola T, Head J, Bartley M. Socio-demographic predictors of quitting smoking: How important are household factors? Addiction. 2004;99:770–777. doi: 10.1111/j.1360-0443.2004.00756.x. [DOI] [PubMed] [Google Scholar]
- Chatkin JM. Abstinence rates and predictors of outcome for smoking cessation: Do Brazilian smokers need special strategies? Addiction. 2004;99:778–784. doi: 10.1111/j.1360-0443.2004.00755.x. [DOI] [PubMed] [Google Scholar]
- Chilmonczyk B, Palomaki G, Knight G, Williams J, Haddow J. An unsuccessful cotinine-assisted intervention strategy to reduce environmental tobacco smoke exposure during infancy. American Journal of Diseases in Children. 1992;146:357–360. doi: 10.1001/archpedi.1992.02160150097031. [DOI] [PubMed] [Google Scholar]
- Cooper JOH, Timothy E, Heward WL. Applied behavior analysis. 2nd ed. Upper Saddle River, NJ: Pearson/Merrill-Prentice Hall; 2007. [Google Scholar]
- Cooper TV, Klesges RC, Debon MW, Zbikowski SM, Johnson KC, Clemens LH. A placebo controlled randomized trial of the effects of phenylpropanolamine and nicotine gum on cessation rates and postcessation weight gain in women. Addictive Behaviors. 2005;30:61–75. doi: 10.1016/j.addbeh.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Davis MF, Sechrest LB, Shapiro D. Measuring progress toward smoking cessation. Journal of Applied Measurement. 2005;6:164–172. [PubMed] [Google Scholar]
- Drongowski RA, Lee D, Reynolds PI, Malviya S, Harmon CM, Geiger J, et al. Increased respiratory symptoms following surgery in children exposed to environmental tobacco smoke. Paediatric Anaesthesia. 2003;13:304–310. doi: 10.1046/j.1460-9592.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108:18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- Erikson W, Sorum K, Bruusgaard D. Effects of information on smoking behavior in families with preschool children. Acta Paediatrica. 1996;85:209–212. doi: 10.1111/j.1651-2227.1996.tb13994.x. [DOI] [PubMed] [Google Scholar]
- Farkas AJ, Gilpin EA, Distefan JM, Pierce JP. The effects of household and workplace smoking restrictions on quitting behaviours. Tobacco Control. 1999;8:261–265. doi: 10.1136/tc.8.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JA, Patten CA, Schroeder DR, Offord KP, Eberman KM, Hurt RD. Predictors of 6-month tobacco abstinence among 1224 cigarette smokers treated for nicotine dependence. Addictive Behaviors. 2003;28:1203–1218. doi: 10.1016/s0306-4603(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Fiore M, Kenford S, Jorenby D, Wetter D, Smith S, Baker T. Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest. 1994;105:524–533. doi: 10.1378/chest.105.2.524. [DOI] [PubMed] [Google Scholar]
- Fiore M, Smith S, Jorenby D, Baker T. The effectiveness of the nicotine patch for smoking cessation: A meta-analysis. Journal of the American Medical Association. 1994;271:1940–1947. [PubMed] [Google Scholar]
- Garvey AJ, Kinnunen T, Nordstrom BL, Utman CH, Doherty K, Rosner B, et al. Effects of nicotine gum dose by level of nicotine dependence. Nicotine & Tobacco Research. 2000;2:53–63. doi: 10.1080/14622200050011303. [DOI] [PubMed] [Google Scholar]
- Gehrman CA, Hovell MF. Protecting children from environmental tobacco smoke (ETS) exposure: A critical review. Nicotine & Tobacco Research. 2003;5:289–301. doi: 10.1080/1462220031000094231. [DOI] [PubMed] [Google Scholar]
- Gilpin EA, White MM, Farkas AJ, Pierce JP. Home smoking restrictions: Which smokers have them and how they are associated with smoking behavior. Nicotine & Tobacco Research. 1999;1:153–162. doi: 10.1080/14622299050011261. [DOI] [PubMed] [Google Scholar]
- Grandes G, Cortada JM, Arrazola A, Laka JP. Predictors of long-term outcome of a smoking cessation programme in primary care. British Journal Of General Practice. 2003;53:101–107. [PMC free article] [PubMed] [Google Scholar]
- Greenberg RA, Strecher VJ, Bauman KE, Boat BW, Fowler MG, Keyes LL, et al. Evaluation of a home-based intervention program to reduce infant passive smoking and lower respiratory illness. Journal of Behavioral Medicine. 1994;17:273–290. doi: 10.1007/BF01857953. [DOI] [PubMed] [Google Scholar]
- Groner JA, Ahijevych K, Grossman LK, Rich LN. The impact of a brief intervention on maternal smoking behavior. Pediatrics. 2000;105:267–271. [PubMed] [Google Scholar]
- Hammond SK, Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environmental Science & Technology. 1987;21:494–497. doi: 10.1021/es00159a012. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerström K-O. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heil SH, Alessi SM, Lussier JP, Badger GJ, Higgins ST. An experimental test of the influence of prior cigarette smoking abstinence on future abstinence. Nicotine & Tobacco Research. 2004;6:471–479. doi: 10.1080/14622200410001696619. [DOI] [PubMed] [Google Scholar]
- Honig W, Staddon JER. Handbook of operant behavior. Englewood Cliffs, NJ: Prentice Hall; 1977. [Google Scholar]
- Honjo K, Tsutsumi A, Kawachi I, Kawakami N. What accounts for the relationship between social class and smoking cessation? Results of a path analysis. Social Science & Medicine. 2006;62:317–328. doi: 10.1016/j.socscimed.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Houston TK, Person SD, Pletcher MJ, Liu K, Irabarren C, Kiefe CI. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. British Medical Journal. 2006;332:1064–1069. doi: 10.1136/bmj.38779.584028.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell MF, Hughes SC. The behavioral ecology of secondhand smoke exposure: A pathway to complete tobacco control. Nicotine & Tobacco Research. 2009 doi: 10.1093/ntr/ntp133. Advance Access published September 23; doi:10.1093/ntr/ntp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell MF, Meltzer SB, Wahlgren DR, Matt GE, Hofstetter CR, Jones JA, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: A controlled trial. Pediatrics. 2002;110:946–956. doi: 10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- Hovell MF, Meltzer SB, Zakarian JM, Wahlgren DR, Emerson JA, Hofstetter CR, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: A controlled trial [published erratum appears in Chest 1995, 107, 480] Chest. 1994;106:440–446. doi: 10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- Hovell MF, Slymen DJ, Keating KJ, Jones JA, Burkham-Kreitner S, Hofstetter CR, et al. Tobacco use prevalence and correlates among adolescents in a clinician initiated tobacco prevention trial in California, USA. Journal of Epidemiology and Community Health. 1996;50:340–346. doi: 10.1136/jech.50.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell MF, Wahlgren DR, Adams M. The logical and empirical basis for the Behavioral Ecological Model. In: DiClemente RJ, Crosby RA, Kegler M, editors. Emerging theories and models in health promotion research and practice: Strategies for enhancing public health. 2nd ed. San Francisco: Jossey-Bass; in press. [Google Scholar]
- Hovell MF, Wahlgren DR, Gehrman CA. The Behavioral Ecological Model: Integrating public health and behavioral science. In: DiClemente RJ, Crosby R, Kegler M, editors. New and emerging models and theories in health promotion and health education. San Francisco: Jossey-Bass; 2002. [Google Scholar]
- Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counseling mothers on their children's exposure to environmental tobacco smoke: Randomized controlled trial. British Medical Journal. 2000;321:337–342. doi: 10.1136/bmj.321.7257.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tobacco Control. 1997;6(Suppl. 2):S57–S62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. Summary of data reported and evaluation. 2002;Vol. 83 Retrieved 25 October 2006, from http://monographs.iarc.fr/ENG/Monographs/vol83/volume83.pdf. [PMC free article] [PubMed] [Google Scholar]
- Irvin JE, Brandon TH. The increasing recalcitrance of smokers in clinical trials. Nicotine & Tobacco Research. 2000;2:79–84. doi: 10.1080/14622200050011330. [DOI] [PubMed] [Google Scholar]
- Irvin JE, Hendricks PS, Brandon TH. The increasing recalcitrance of smokers in clinical trials II: Pharmacotherapy trials. Nicotine & Tobacco Research. 2003;5:27–35. doi: 10.1080/1462220031000070534. [DOI] [PubMed] [Google Scholar]
- Irvine L, Crombie I, Clark R, Slane P, Feyerabend C, Goodman K, et al. Advising parents of asthmatic children on passive smoking: Randomized controlled trial. British Medical Journal. 1999;318:1456–1459. doi: 10.1136/bmj.318.7196.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, III, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiology, Biomarkers & Prevention. 2002;II:1668–1673. [PubMed] [Google Scholar]
- Jacob P, III, Yu L, Liang G, Shulgin AT, Benowitz NL. Gas chromatographic-mass spectrometric method for determination of anabasine, anatabine and other tobacco alkaloids in urine of smokers and smokeless tobacco users. Journal of Chromatography. 1993;619:49–61. doi: 10.1016/0378-4347(93)80445-a. [DOI] [PubMed] [Google Scholar]
- Ji M, Hofstetter CR, Hovell M, Irvin V, Song YJ, Lee J, et al. Smoking cessation patterns and predictors among adult Californians of Korean descent. Nicotine & Tobacco Research. 2005;7:59–69. doi: 10.1080/14622200412331328493. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Schumann A, Hapke U, Rumpf H-J, Adam C, et al. A short form of the Fagerström Test for Nicotine Dependence and the Heaviness of Smoking Index in two adult population samples. Addictive Behaviors. 2004;29:1207–1212. doi: 10.1016/j.addbeh.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Kegler MC, Malcoe LH. Smoking restrictions in the home and car among rural Native American and white families with young children. Preventive Medicine. 2002;35:334–342. doi: 10.1006/pmed.2002.1091. [DOI] [PubMed] [Google Scholar]
- Keintz M, Fleisher L, Rimer B. Reaching mothers of preschool-aged children with a targeted quit smoking intervention. Journal of Community Health. 1994;19:25–40. doi: 10.1007/BF02260519. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Kramer U, Lemmen CH, Behrendt H, Link E, Shafer T, Gostomzyk J, et al. The effect of environmental tobacco smoke on eczema and allergic sensitization in children. British Journal of Dermatology. 2004;150:111–118. doi: 10.1111/j.1365-2133.2004.05710.x. [DOI] [PubMed] [Google Scholar]
- Leaderer BP, Hammond SK. Evaluation of vapor-phase nicotine and respirable suspended particle mass as markers for environmental tobacco smoke. Environmental Science & Technology. 1991;25:770–777. [Google Scholar]
- Martinez-Donate AP, Hovell MF, Hofstetter CR, González-Pérez GJ, Adams MA, Kotay A. Correlates of home smoking bans among Mexican-Americans. American Journal of Health Promotion. 2007;21:229–236. doi: 10.4278/0890-1171-21.4.229. [DOI] [PubMed] [Google Scholar]
- Matt GE, Quintana PJE, Bernert JT, Song S, Navianti N, Juarez T, et al. Households contaminated by environmental tobacco smoke: Sources of infant exposure. Tobacco Control. 2004;13:29–37. doi: 10.1136/tc.2003.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt GE, Wahlgren DR, Hovell MF, Zakarian JM, Bernert JT, Meltzer SB, et al. Measuring environmental tobacco smoke exposure in infants and young children through urine cotinine and memory-based parental reports: Empirical findings and discussion. Tobacco Control. 1999;8:282–289. doi: 10.1136/tc.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CM, Baucom DH, Peterson BL, Pollak KI, Palmer C, Westman E, et al. Prenatal and postpartum smoking abstinence a partner-assisted approach. American Journal of Preventive Medicine. 2004;27:232–238. doi: 10.1016/j.amepre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- McIntosh N, Clark N, Howatt W. Reducing tobacco smoke in the environment of the child with asthma: A cotinine-assisted, minimal-contact intervention. Journal of Asthma. 1994;31:453–462. doi: 10.3109/02770909409089487. [DOI] [PubMed] [Google Scholar]
- Morkjaroenpong V, Rand CS, Butz AM, Huss H, Eggleston P, Malveaux FJ, et al. Environmental tobacco smoke exposure and nocturnal symptoms among inner-city children with asthma. Journal of Allergy and Clinical Immunology. 2002;110:147–153. doi: 10.1067/mai.2002.125832. [DOI] [PubMed] [Google Scholar]
- Murray DM, O'Connell CM, Schmid LA, Perry CL. The validity of smoking self-reports by adolescents: A reexamination of the bogus pipeline procedure. Addictive Behavior. 1987;12:7–15. doi: 10.1016/0306-4603(87)90003-7. [DOI] [PubMed] [Google Scholar]
- Office of Environmental Health Hazard Assessment. Secondhand smoke & children's health. 2006. Retrieved 25 April 2009, from http://www.oehha.ca.gov/air/environmental_tobacco/ [Google Scholar]
- Okah FA, Choi WS, Okuyemi KS, Ahluwalia JS. Effect of children on home smoking restriction by inner-city smokers. Pediatrics. 2002;109:244–249. doi: 10.1542/peds.109.2.244. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Vestbo J, Borch-Johnsen K, Jørgensen T. It is possible to help smokers in early motivational stages to quit. The Inter99 study. Preventive Medicine. 2005;40:278–284. doi: 10.1016/j.ypmed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Pizacani BA, Martin DP, Stark MJ, Koepsell TD, Thompson B, Diehr P. A prospective study of household smoking bans and subsequent cessation related behaviour: The role of stage of change. Tobacco Control. 2004;13:23–28. doi: 10.1136/tc.2003.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson HH, Andrews JA, Lichtenstein E, Wall M, Akers L. Reducing maternal smoking and relapse: Long-term evaluation of a pediatric intervention. Preventive Medicine. 1997;26:120–130. doi: 10.1006/pmed.1996.9983. [DOI] [PubMed] [Google Scholar]
- Siahpush M, Borland R, Scollo M. Factors associated with smoking cessation in a national sample of Australians. Nicotine & Tobacco Research. 2003;5:597–602. doi: 10.1080/1462220031000118711. [DOI] [PubMed] [Google Scholar]
- Singer BC, Hodgson AT, Guevarra KS, Hawley EL, Nazaroff WW. Gas-phase organics in environmental tobacco smoke: 1—Effects of smoking rate, ventilation, and furnishing level on emission factors. Environmental Science & Technology. 2002;36:846–853. doi: 10.1021/es011058w. [DOI] [PubMed] [Google Scholar]
- Singer BC, Hodgson AT, Nazaroff WW. Gas-phase organics in environmental tobacco smoke: 2—Exposure-relevant emission factors and indirect exposures from habitual smoking. Atmospheric Environment. 2003;37:5551–5561. [Google Scholar]
- SPSS Inc. SPSS for Windows, release 14.0. Chicago: SPSS; 2005. [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software, release 9.1. College Station, TX: Stata; 2005. [Google Scholar]
- Torrent M, Sunyer J, Cullinan P, Basagaña X, Harris J, García O, et al. Smoking cessation and associated factors during pregnancy. Gaceta Sanitaria/S.E.S.P.A.S. 2004;18:184–189. doi: 10.1016/s0213-9111(04)71831-2. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Healthy People 2000 review, 1998–1999 (national health promotion and disease prevention objectives) 1999. Centers for Disease Control and Prevention, National Center for Health Statistics (DHHS Publication No. PHS 99-1256). Hyattsville, MD. [Google Scholar]
- U.S. Department of Health and Human Services. Healthy People 2010. With understanding and improving health and objectives for improving health. 2nd ed. 2 vols. Washington, DC: U.S. Government Printing Office; 2000. [Google Scholar]
- U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: A report of the U.S. Surgeon General. Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. Retrieved 25 April 2009, from http://www.cdc.gov/tobacco/data_statistics/sgr/sgr_2006/ [Google Scholar]
- U.S. Environmental Protection Agency. Respiratory health effects of passive smoking: Lung cancer and other disorders. EPA/600/6-90/006F. Washington, DC: Office of Health and Environmental Assessment; 1992. [Google Scholar]
- U.S. National Research Council. Environmental tobacco smoke: Measuring exposures and assessing health effects. Washington, DC: National Academy Press; 1986. Committee on Passive Smoking, Board on Environmental Studies and Toxicology. [PubMed] [Google Scholar]
- Vineis P, Ronco G, Ciccone G, Vernero E, Troia B, D'Incalci T, et al. Prevention of exposure of young children to parental tobacco smoke: Effectiveness of an educational program. Tumori. 1993;79:183–186. doi: 10.1177/030089169307900304. [DOI] [PubMed] [Google Scholar]
- Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children: A 2-year follow-up. Chest. 1997;111:81–88. doi: 10.1378/chest.111.1.81. [DOI] [PubMed] [Google Scholar]
- Wahlgren DR, Hovell MF, Slymen DJ, Conway TL, Hofstetter CR, Jones JA. Predictors of tobacco use initiation in adolescents: A two-year prospective study and theoretical discussion. Tobacco Control. 1997;6:95–103. doi: 10.1136/tc.6.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield M, Banham D, McCaul K, Martin J, Ruffin R, Badcock N, et al. Effect of feedback regarding urinary cotinine and brief tailored advice on home smoking restrictions among low-income parents of children with asthma: A controlled trial. Preventive Medicine. 2002;34:58–65. doi: 10.1006/pmed.2001.0953. [DOI] [PubMed] [Google Scholar]
- Wall MA, Severson HH, Andrews JA, Lichtenstein E, Zoref L. Pediatric office-based smoking intervention: Impact on maternal smoking and relapse. Pediatrics. 1995;96:622–628. [PubMed] [Google Scholar]
- West DC, Romano PS, Azari R, Rudominer A, Holman M, Sandhu S. Impact of environmental tobacco smoke on children with sickle cell disease. Archives of Pediatrics & Adolescent Medicine. 2003;157:1197–1201. doi: 10.1001/archpedi.157.12.1197. [DOI] [PubMed] [Google Scholar]
- Wetter DW. What accounts for the association of education and smoking cessation? Preventive Medicine. 2005;40:452–460. doi: 10.1016/j.ypmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Woodward A, Owen N, Gurinovich N, Griffith F, Linke H. Trial of an intervention to reduce passive smoking in infancy. Pediatric Pulmonology. 1987;3:173–178. doi: 10.1002/ppul.1950030311. [DOI] [PubMed] [Google Scholar]
- Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environmental Health Perspectives. 2005;113:98–103. doi: 10.1289/ehp.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakarian JM, Hovell MF, Sandweiss RD, Hofstetter CR, Matt GE, Bernert JT, et al. Behavioral counseling for reducing children’s ETS exposure: Implementation in community clinics. Nicotine & Tobacco Research. 2004;6:1061–1074. doi: 10.1080/1462220412331324820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.