Abstract
Introduction:
Smokeless tobacco (ST) use is associated with adverse health consequences, and effective treatments are needed. Pilot data suggest that 4-mg nicotine lozenge decreases tobacco craving and nicotine withdrawal symptoms among ST users.
Methods:
We conducted a randomized, placebo-controlled multicenter clinical trial to evaluate the efficacy of 12 weeks of 4-mg nicotine lozenge for ST use.
Results:
We randomized 270 participants (136 active lozenge, 134 placebo). No significant differences were observed between the groups in biochemically confirmed all tobacco abstinence rates at Week 12 (36% lozenge vs. 27.6% placebo; odds ratio [OR] 1.5, 95% CI 0.7–2.1; p = .138). However, the 4-mg nicotine lozenge increased self-reported all tobacco abstinence (44.1% vs. 29.1%; OR 1.9, 95% CI 1.2–3.2; p = .011) and self-reported ST abstinence (50.7% vs. 34.3%; OR 2.0, 95% CI 1.2–3.2; p = .013) compared with placebo at the end of treatment (Week 12). Following target quit date (TQD), nicotine withdrawal symptoms decreased significantly with time (time effect = −.022 per day, SE = .003; p < .001) and was significantly lower for the active lozenge (treatment effect = −.213, SE = .071; p = .003). Tobacco craving also decreased significantly following TQD (time effect = −.071, SE = .006; p < .001) and was lower for the active nicotine lozenge (treatment effect = −.452, SE = .164; p = .006).
Discussion:
The 4-mg nicotine lozenge increased self-reported but not biochemically confirmed tobacco abstinence rates at 3 months. The use of the 4-mg nicotine lozenge is associated with decreased nicotine withdrawal symptoms and tobacco craving.
Introduction
Smokeless tobacco (ST) is tobacco used orally and not burned. The prevalence of ST use among Americans aged 12 years and older has increased significantly from 2004 to 2006 (3.0% vs. 3.3%; p < .05; Substance Abuse and Mental Health Services Administration, 2007). Recently, cigarette manufacturers have gained a significant foothold in the ST market through the acquisition of ST companies and the introduction of new ST products with flagship cigarette brands (e.g., Marlboro, Camel) designed to appeal to smokers (Stepanov, Jensen, Hatsukami, & Hecht, 2008). Although the impact of these new ST marketing initiatives on ST use prevalence remains uncertain, the changing tobacco landscape suggests a need for developing effective interventions for ST users.
ST has significant adverse health consequences. Among the U.S. populations, long-term ST use has been associated with periodontal disease (Fisher, Taylor, & Tilashalski, 2005). Long-term ST use may increase the risk for oral (Stockwell & Lyman, 1986), kidney (Muscat, Hoffmann, & Wynder, 1995), and pancreatic (Muscat, Stellman, Hoffmann, & Wynder, 1997) cancer. Long-term risk ST use is associated with death from coronary heart disease and stroke (Henley, Thun, Connell, & Calle, 2005).
A need for efficacious interventions exists, as 64% of ST users report the desire to quit (Severson, 1992). Behavioral interventions are effective for increasing ST abstinence rates (Severson, 2003). Nicotine patch increases ST abstinence rates at 10 and 15 weeks when given for 10 weeks (Hatsukami et al., 2000) and at 3 months when given for 6 weeks (Howard-Pitney, Killen, & Fortmann, 1999). Both the nicotine gum and the patch are effective for decreasing withdrawal symptoms (Hatsukami, Jensen, Allen, Grillo, & Bliss, 1996; Hatsukami et al., 2000).
Nicotine lozenge is the newest form of nicotine replacement therapy (NTR) with demonstrated efficacy for increasing tobacco abstinence rates and decreasing withdrawal symptoms among cigarette smokers (Shiffman et al., 2002). Compared with the nicotine gum, the nicotine lozenge delivers 8%–10% higher maximal plasma concentrations and 25%–27% higher area under the curve nicotine concentration values in single-dose studies (Choi, Dresler, Norton, & Strahs, 2003). This may translate into important clinical benefits for ST users who have high levels of nicotine exposure (Benowitz, Porchet, Sheiner, & Jacob, 1988). In our preliminary work, use of 4-mg nicotine lozenge was effective for decreasing craving and withdrawal symptoms among ST users (Ebbert, Dale, et al., 2007).
To explore the effectiveness of the nicotine lozenge for increasing tobacco abstinence rates and decreasing nicotine withdrawal symptoms and tobacco craving in ST users, we conducted a clinical trial.
Methods
Study design
Our study was a randomized, blinded, placebo-controlled, parallel group multicenter clinical trial with a 12-week medication phase and follow-up through 6 months. The study was conducted at the Mayo Clinic in Rochester, Minnesota, and the Research Institute in Eugene, Oregon. Enrollment took place between January 2007 and April 2008. The institutional review boards at each study site approved the study protocol before participant recruitment. This study was overseen by a data and safety monitoring board, which met annually.
Study population
ST users were recruited through press releases and advertising. Individuals were eligible for inclusion if they were 18 years or older, reported ST use as their primary tobacco of use (i.e., could use other forms of tobacco), wanted to quit, had used ST daily for at least 6 months, and were in good general health. Individuals were excluded if they (a) were currently using treatments for ST use; (b) had unstable angina, myocardial infarction, or coronary angioplasty in the previous 2 weeks; (c) had a severe untreated cardiac dysrhythmia; (d) were lactating; (e) had uncontrolled hypertension; (f) had another household member participating in the study; or (g) had phenylketonuria. All female participants of childbearing potential were required to have a negative pregnancy test before enrollment and to have agreed to use contraception during study participation.
Screening and recruitment
Potential participants were screened by telephone. If participants passed the phone screen, they were invited to attend an information session at which the study was explained and informed consent obtained. Participants returned for a baseline visit for medical screening and randomization. Medical screening included a history and physical examination. Baseline measures included the Fagerström Test for Nicotine Dependence-Smokeless Tobacco (FTND-ST) (Ebbert, Patten, & Schroeder, 2006) and the Severson Smokeless Tobacco Dependence Scale (Severson, Akers, Boles, Andrews, & Yovanoff, 2004). Participants were also assessed for depression at baseline with the self-administered Center for Epidemiological Studies Depressed Mood Scale (Radloff, 1977).
Assignment of participants to condition
A computer-generated randomization sequence assigned participants in a 1:1 ratio to treatment condition with a block size of four stratified by site. Using this randomization schedule, study personnel who did not have any participant contact dispensed the appropriate study medication into containers labeled according to study identification number. Potential subject participants who passed all screening visits and provided written informed consent were assigned the next sequential subject identification number. Study participants, investigators, and all other study staff were blinded to treatment assignment.
Treatment and control conditions
At the baseline visit (randomization), enrolled participants were assigned to the 4-mg mint-flavored nicotine lozenge or matching placebo. The 4-mg nicotine lozenge dose was selected based on our previous experience with this dose (Ebbert, Dale, et al., 2007). Medication was given for 12 weeks. All participants were instructed to quit all tobacco products and start using lozenges the following day. For Weeks 1–6, participants were instructed to use one lozenge orally every 1–2 hr, with a maximum of 16 lozenges per day. After 6 weeks, lozenges were tapered. For Weeks 7–9, participants were instructed to use eight lozenges per day or one every 2–4 hr. For Weeks 10–12, participants were instructed to use four lozenges per day or one every 4–8 hr.
All participants received a self-help quitting guide developed specifically for ST users (Severson, 1999). Participants were provided with brief behavioral counseling at each study visit tailored to participant quitting status. Counseling included best practice topics such as the health effects of ST, preparing for quit day, dealing with withdrawal, avoiding relapse, stress and time management, weight management, and wellness and exercise. Counseling was typically 10 min in duration. Participants attended study visits at Weeks 2, 4, 6, and 12 at which time research staff assessed vital signs, tobacco use status, medication compliance, adverse events, and concomitant medication use. At the end of Visits 2, 4, and 6, participants also received a supply of medication.
Participants who completed the 12-week medication phase were followed up for 6 months after randomization. Serious adverse events and concomitant medications used for tobacco cessation were assessed.
A urine specimen was collected for biochemical confirmation of tobacco use status at 12 weeks (end of treatment) and 6 months after randomization (end of study) among participants self-reporting tobacco abstinence.
Measures
Tobacco abstinence was determined by self-report at each visit. Biochemical confirmation of self-reported abstinence was obtained at the end of treatment (Week 12) and end of study (6 months after randomization). Although cotinine is the traditional measurement for adjudication of self-reported tobacco abstinence (Benowitz et al., 2002), cotinine cannot be used to biochemically validate tobacco abstinence during use of NRT. Since nicotine replacement products do not contain the tobacco alkaloid anabasine, while tobacco does, urinary anabasine has been proposed as a biomarker of tobacco consumption that could differentiate tobacco users and nonusers who use NRT (Jacob, Yu, Shulgin, & Benowitz, 1999). We used a urine anabasine concentration of less than 2 ng/ml as our cutoff concentration to indicate tobacco abstinence in participants reporting use of NRT only, since this is below the level of detection and we would not expect to detect anabasine in individuals not exposed to tobacco. This cutoff has been used in previous investigations and has been validated by our own lab (Hatsukami et al., 2000; Moyer et al., 2002).
Study endpoints
The primary endpoint was the biochemically confirmed 7-day point-prevalence all-tobacco abstinence rate at the end of treatment (Week 12), defined as self-reported all-tobacco abstinence in the past 7 days confirmed by a urine anabasine concentration of less than 2 ng/ml. ST abstinence was a secondary endpoint. Individuals biochemically confirmed abstinent from all tobacco are, by definition, abstinent from ST. In addition, participants self-reporting use of a tobacco product other than ST were considered abstinent from ST. Prolonged abstinence from ST was also assessed. Participants were classified as failing criteria for prolonged ST abstinence if they reported using ST on 7 consecutive days or at least once per week for 2 consecutive weeks following a 2-week grace period after target quit date (TQD; Hughes et al., 2003). Point-prevalence and prolonged abstinence rates were analyzed at 6 months after randomization.
Withdrawal and craving
Participants were asked to keep a daily diary to record symptoms of nicotine withdrawal and medication use. Daily diaries were collected for 6 weeks starting at the information session. The daily diary included the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes, 1998; Hughes & Hatsukami, 1986). The MNWS is an eight-item measure consisting of the following symptoms rated on a 5-point Likert scale ranging from 0 (not present) to 4 (severe): desire to smoke (i.e., craving); anger, irritability, or frustration; anxiety or nervousness; difficulty concentrating; impatience or restlessness; hunger; awakening at night; and depression. The MNWS was modified for ST users by replacing “desire to smoke” with “desire to use tobacco.”
Adverse events
All observed and self-reported adverse events were documented on case report forms and followed up according to a safety management protocol until the adverse events were resolved or the participant completed the study.
Statistical analyses
The sample size for the current study was determined for the primary endpoint of 7-day point-prevalence tobacco abstinence for Week 12 (end of treatment). Based on previous research, we hypothesized that the end-of-treatment abstinence rate for individuals receiving placebo would be 35%, and the abstinence rate for those receiving the 4-mg nicotine lozenge would be 53%. A resulting power calculation indicated that 270 participants (135 per group) were required to have an 85% power to detect a significant difference (two sided, α = .05 level test).
For tobacco abstinence endpoints, we used an intent-to-treat imputation in which participants missing a visit were considered to be using tobacco. Tobacco abstinence endpoints were analyzed using logistic regression. The dependent variable for the primary analysis was the biochemically confirmed 7-day point-prevalence tobacco abstinence at the end of treatment (Week 12), and the independent variables were treatment (active lozenge vs. placebo) and study site. An initial analysis was carried out that included the appropriate interaction terms to verify the assumption that the effect of the active lozenge was not study site dependent. After confirming the absence of a significant treatment by site interaction, all subsequent analyses were carried out with main effect terms, with study site as a covariate. Odds ratios (ORs) and 95% CIs for the active lozenge versus placebo were calculated using the parameter estimates from this logistic regression model. Self-reported point-prevalence and prolonged abstinence endpoints at end of treatment and end of study were also analyzed using similar logistic regression models. The duration of prolonged abstinence was further analyzed using survival methods. For participants who reported a relapse, the date of relapse was obtained via self-report. Relapse was defined as using tobacco on 7 consecutive days or at least 1 day a week on 2 consecutive weeks following the grace period. For participants who dropped out of the study without previously meeting relapse criteria, we assumed that the individuals relapsed at the midpoint between the date of the study visit at which they were last known to meet prolonged abstinence criteria and the first study visit at which they were assumed to be using tobacco. Relapse curves were constructed separately for each treatment group and compared between groups using the log rank test.
Withdrawal symptoms and craving were assessed daily using the MNWS modified for ST users. For analysis purposes, a composite withdrawal score was computed as the mean of the ratings assigned to each of seven symptoms. Craving was analyzed separately. The average score for the 7 days before TQD was used as baseline, and scores obtained following TQD were analyzed as change from baseline. The repeated measures of withdrawal and craving for the first 2 weeks following TQD were analyzed using generalized estimating equations. For these models, the explanatory variables were treatment group (nicotine lozenge vs. placebo) and time in days following TQD. The time by treatment interaction effect was included to assess whether changes in withdrawal symptoms or craving over time differed by treatment group. Initial analyses were carried out using all available data regardless of tobacco use status. Analyses were repeated using data only for the period of initial abstinence for each participant. To supplement the repeated measures analyses, daily scores were compared between groups using the two-sample t test.
Average daily lozenge use was obtained using drug dispensation logs and calculated by dividing the total number of lozenges used between study visits divided by the interval, in days, between visits. Lozenge use was compared between groups using the rank sum test. Adverse events judged to be possibly, probably, or definitely related to study drug were summarized and compared between groups using Fisher's exact test. At the end of study visit, participants were asked to rate perceived effectiveness and acceptability of the lozenge using a 5-point Likert scale. Effectiveness and acceptability ratings were compared between groups using the chi-square test.
Results
Participants
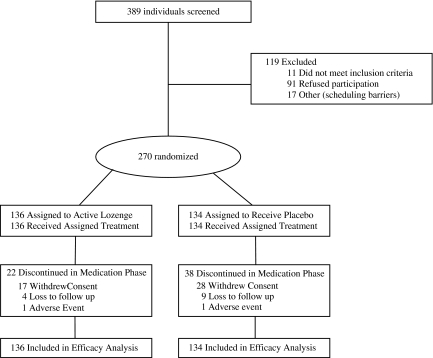
Of 389 individuals screened, 270 were eligible and randomized to receive treatment (136 active lozenge, 134 placebo) and included in the final analysis (Figure 1). All the women in the study (n = 6) were randomly assigned to placebo, but individuals were otherwise similar at baseline as shown in Table 1. In keeping with the intent-to-treat approach, all randomized participants were included in the primary analysis. Analyses were repeated after excluding the six female participants to assess the comparability of findings.
Figure 1.
CONSORT study flow diagram.
Table 1.
Baseline demographics of smokeless tobacco users in a randomized clinical trial of the 4-mg nicotine lozenge (N = 270)a
| Characteristic | 4-mg nicotine lozenge (n = 136) | Placebo (n = 134b) |
| Age, years | 36.6 ± 10.7 | 36.5 ± 11.0 |
| Male, n (%) | 136 (100.0) | 128 (95.5) |
| Caucasian, n (%) | 127 (93.4) | 133 (99.3) |
| Marital status, n (%) | ||
| Married/living as married | 88 (64.7) | 91 (67.9) |
| Never married | 30 (22.1) | 29 (21.6) |
| Separated/divorced | 18 (13.2) | 13 (9.7) |
| Other | 0 (0.0) | 1 (0.7) |
| Highest level of education, n (%) | ||
| Less than high school graduate | 2 (1.5) | 5 (3.7) |
| High school graduate | 24 (17.6) | 33 (24.6) |
| Some college | 80 (58.8) | 73 (54.5) |
| College graduate | 30 (22.1) | 23 (17.2) |
| FTND-ST | 5.0 ± 2.0 | 5.1 ± 2.0 |
| Current type of smokeless tobacco used, n (%) | ||
| Snuff only | 134 (98.5) | 131 (97.8) |
| Chewing tobacco only | 1 (0.7) | 3 (2.2) |
| Both snuff and chewing tobacco | 1 (0.7) | 0 (0.0) |
| Smokeless tobacco used per week, cans/pouches | 4.3 ± 2.6 | 4.2 ± 2.6 |
| Years of regular smokeless tobacco use | 16.7 ± 9.9 | 15.8 ± 10.6 |
| Current use of other tobacco productsc, n (%) | 15 (11.0) | 14 (10.4) |
| Other users of tobacco in household, n (%) | 29 (21.3) | 28 (20.9) |
| Number of serious stop attempts, n (%) | ||
| 0 | 19 (14.0) | 20 (15.0) |
| 1–2 | 63 (46.3) | 67 (50.4) |
| 3–4 | 34 (25.0) | 20 (15.0) |
| 5+ | 20 (14.7) | 26 (19.5) |
| Longest time off tobacco, n (%) | ||
| <24 hr | 14 (10.3) | 10 (7.6) |
| 1–7 days | 24 (17.6) | 27 (20.5) |
| 2–8 weeks | 36 (26.5) | 35 (26.5) |
| 9 weeks to 6 months | 32 (23.5) | 39 (29.5) |
| >6 months | 30 (22.1) | 21 (15.9) |
Note. FTND-ST = Fagerström Test for Nicotine Dependence–Smokeless Tobacco.
Data are presented as mean ± SD or n (%) as indicated.
Number of serious stop attempts was missing for one participant in the placebo group and longest time off tobacco was missing for two participants in the placebo group.
In addition to smokeless tobacco, current use of other tobacco products was reported by 14 participants in the placebo group (11 cigarettes only, 3 cigars only) and 15 participants in the nicotine group (8 cigarettes only, 4 cigars only, 3 both cigarettes and cigars).
A total of 60 participants (22 active lozenge and 38 placebo; p = .016), representing 25% of the original study group, discontinued study participation during the medication phase. Of the 38 placebo participants who discontinued study participation, 33 (87%) reported using tobacco at the last study visit they attended.
Abstinence
No differences were observed in the biochemically confirmed all-tobacco abstinence at Week 12 (36% lozenge vs. 27.6% placebo; OR 1.5, 95% CI 0.7–2.1; p = .138) as shown in Table 2. A total of 13 participants (11 nicotine vs. 2 placebo; p = .071) self-reported abstinence from all tobacco but failed biochemical confirmation at Week 12. Of these, six participants in the active lozenge group failed unexpectedly because of assay interference due to high concentrations of cotinine in the sample. When the analysis of the Week 12 biochemically confirmed all-tobacco abstinence endpoint was repeated with these six participants excluded, the treatment effect was not statistically significant (OR = 1.6, 95% CI 0.9–2.7; p = .083). The biochemically confirmed abstinence rates did not change when we excluded the six female participants (data not shown).
Table 2.
Tobacco abstinence outcomes in a randomized clinical trial of the 4-mg nicotine lozenge for smokeless tobacco users
| Abstinence definitiona | 4-mg nicotine lozenge (n = 136) |
Placebo (n = 134) |
Logistic regression resultsb |
||||
| n | % | n | % | Odds ratio | 95% CI | p value | |
| Week 12 (end of medication) | |||||||
| Point prevalence | |||||||
| Self-report | |||||||
| Smokeless tobacco abstinence | 69 | 50.7 | 46 | 34.3 | 2.0 | 1.2–3.2 | .013 |
| All tobacco abstinence | 60 | 44.1 | 39 | 29.1 | 1.9 | 1.2–3.2 | .011 |
| Biochemically confirmedc | |||||||
| All tobacco abstinence | 49 | 36.0 | 37 | 27.6 | 1.5 | 0.7–2.1 | .138 |
| Prolonged | |||||||
| Smokeless tobacco abstinence | 65 | 47.8 | 41 | 30.6 | 2.1 | 1.3–3.4 | .004 |
| Week 24 | |||||||
| Point prevalence | |||||||
| Self-report | |||||||
| Smokeless tobacco abstinence | 43 | 31.6 | 35 | 26.1 | 1.3 | 0.8–2.2 | .319 |
| All tobacco abstinence | 36 | 26.5 | 29 | 21.6 | 1.3 | 0.7–2.3 | .345 |
| Biochemically confirmed | |||||||
| All tobacco abstinence | 34 | 25.0 | 24 | 17.9 | 1.5 | 0.8–2.8 | .158 |
| Prolonged | |||||||
| Smokeless tobacco abstinence | 41 | 30.2 | 31 | 23.1 | 1.4 | 0.8–2.5 | .194 |
Note. aPoint-prevalence abstinence is defined as no use within the past 7 days. Participants were classified as failing criteria for prolonged smokeless tobacco abstinence if they reported using smokeless tobacco on 7 consecutive days or at least once per week for 2 consecutive weeks following a 2-week grace period after the target quit date (Hughes et al., 2003). In all cases, participants who missed a visit were assumed to be using tobacco.
In addition to treatment (nicotine vs. placebo), the logistic regression analysis included a covariate for study site. Odds ratios >1.0 indicate an increased likelihood of abstinence for active nicotine lozenge compared with placebo.
Of the 11 participants in the 4-mg nicotine lozenge group who failed biochemical confirmation at Week 12, 6 failed because of assay interference due to high concentrations of cotinine in the sample.
The 4-mg nicotine lozenge increased self-reported all-tobacco abstinence and self-reported ST abstinence compared with placebo at the end of treatment (Week 12) as shown in Table 2. When these analyses were repeated excluding the six enrolled female participants, the self-reported abstinence findings did not change. No significant differences were observed between groups in the self-reported ST or all-tobacco point-prevalence or prolonged abstinence rates at 24 weeks.
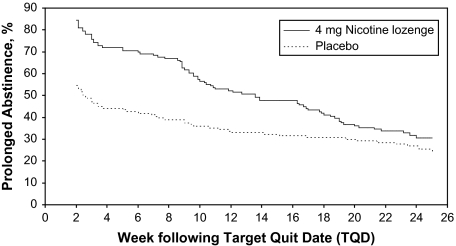
Kaplan–Meier curves for the endpoint of prolonged ST abstinence are presented in Figure 2. The curves start 2 weeks following the TQD, which corresponds to the end of the grace period used for the definition of prolonged abstinence. At Week 2, 21 of 136 participants (15.4%) in the active lozenge group and 61 of 134 (45.5%) in the placebo group were classified as failing prolonged abstinence criteria because these participants did not achieve abstinence from ST prior to the end of the grace period. The duration of prolonged ST abstinence was increased for the active lozenge group compared with placebo (log rank test, p = .013). Further inspection of the survival curves reveal clear advantages for participants in the active lozenge group. However, the active lozenge group shows a steeper relapse slope over time such that the relative advantage compared with the placebo lozenge condition decreased over time.
Figure 2.
Prolonged abstinence from smokeless tobacco assessed using a time-to-event analysis with curves constructed using the Kaplan–Meier method (log rank p = .013).
Withdrawal and desire to use tobacco
For each participant, we calculated the proportion of study days that they completed a diary from the start of the baseline period until 28 days following TQD, or until the date that the participant dropped out of the study if before 28 days following TQD. From this, the median (interquartile range) adherence with diary reporting was 89% (72%–94%) for the nicotine group and 86% (71%–94%) for the placebo group. Using all available data, withdrawal following TQD decreased significantly with time (time effect = −.022 per day, SE =.003; p < .001) and was significantly lower for the active lozenge group (treatment effect = −.213, SE = .071; p = .003). Desire to use tobacco (i.e., “craving”) also decreased significantly following TQD (time effect = −.071, SE = .006; p < .001) and was lower for the active lozenge group (treatment effect = −.452, SE = .164; p = .006). When the analysis was repeated using only data for period of initial abstinence, similar findings were obtained for both withdrawal symptoms (time effect = −.028 per day, SE = .004; p < .001; treatment effect = −.258, SE = .078; p < .001) and desire to use tobacco (time effect = −.089 per day, SE = .009; p < .001; treatment effect = −.586, SE =.195; p = .006). When these analyses were repeated excluding the six female participants enrolled, the withdrawal findings did not change.
Lozenge use
Individuals in the active lozenge group had higher median lozenge use than the placebo group for Weeks 3–4 (6.9 vs. 5.1, rank sum test p = .013), Weeks 5–6 (7.1 vs. 3.4; p < .001), and Weeks 7–12 (4.9 vs. 1.9; p < .001), as depicted in Table 3. Among individuals completing the medication phase, 89% in the active lozenge group and 66% in the placebo group (p < .001) used lozenges between Weeks 7 and 12. In the active lozenge group, only 5% of the participants in Weeks 1–2, 9% in Weeks 3–4, and 7% in Weeks 5–6 used 16 or more lozenges per day. The corresponding rates in the placebo group were 10%, 4%, and 3%.
Table 3.
Average daily lozenge use in a randomized clinical trial of the 4-mg nicotine lozenge for smokeless tobacco usersa
| Study weeks | Placebo |
Nicotine |
pb | ||
| n | Median (25th, 75th) | n | Median (25th, 75th) | ||
| 1–2 | 112 | 6.9 (3.4, 12.0) | 122 | 7.4 (4.2, 10.3) | .510 |
| 3–4 | 97 | 5.1 (1.7, 10.3) | 118 | 6.9 (3.4, 10.7) | .013 |
| 5–6 | 90 | 3.4 (0.0, 8.8) | 119 | 7.1 (3.4, 12.0) | <.001 |
| 7–12 | 76 | 1.9 (0.0, 5.0) | 94 | 4.9 (1.9, 6.3) | <.001 |
Note. aAverage daily lozenge use was determined at each study visit during the treatment phase.
Rank sum test.
Adverse events
During the 12-week medication phase, the following adverse events were reported by 5% or more of the participants in either treatment group: gastroesophageal reflux (11% active vs. 1% placebo; p = .002), sleep disturbance (6% active vs. 7% placebo; p = ns), and headache (7% active vs. 5% placebo; p = ns).
One death occurred in the active lozenge group related to an assault that occurred 3 months after study medication was stopped. One participant in the active lozenge group was hospitalized for neck spasms but had not used medication for 2 months. In the placebo group, one participant was diagnosed with breast cancer and one with rectal cancer, and one participant had a motor vehicle accident.
Perceived effectiveness
At the end of study, a higher proportion of participants in the active lozenge group rated the lozenge as “extremely helpful” (41% vs. 9%; p < .001). Participants in the active lozenge group were more likely to report that the lozenge would be acceptable to other ST users trying to quit (76% vs. 61%, p = .023). When asked whether they thought they were on active or placebo, 55% of the participants in the active lozenge group correctly guessed that they were on active medication and 53% of those in the placebo group correctly guessed that they were on placebo. The proportion of participants guessing group allocation correctly did not differ significantly between groups (p = .80).
Discussion
Based on biochemical confirmation, tobacco abstinence rates did not differ between the two groups at 3 months. However, we observed that the 4-mg nicotine lozenge significantly increased both self-reported all-tobacco abstinence and ST abstinence at 3 months compared with placebo. However, no differences were observed in the biochemically confirmed tobacco abstinence rates at 12 weeks. The 4-mg nicotine lozenge significantly decreased nicotine withdrawal symptoms and tobacco cravings compared with placebo, and the nicotine lozenge was well tolerated.
In contrast to observations in cigarette smokers (Shiffman et al., 2002), we did not detect significant differences in tobacco abstinence rates between treatment groups at 6 months. Although the current trial may have been underpowered for this between-group analysis, previous larger trials of pharmacotherapeutic interventions for ST users have also failed to detect improvements in long-term (>6 months) tobacco abstinence rates among ST users (Boyle, 1992; Dale et al., 2007; Hatsukami et al., 1996, 2000; Howard-Pitney et al., 1999).
We postulate that the observed lack of efficacy of traditional pharmacotherapies for increasing long-term (≥6 months) ST abstinence may relate to (a) under-replacement of nicotine with standard NRT dosing, (b) similarities between ST and certain nicotine replacement products (i.e., nicotine gum), (c) treatment-naive ST users, and (d) high control condition abstinence rates in clinical trials of ST users.
Traditional pharmacotherapies may result in under-replacement of nicotine in ST users. In a previous study evaluating the efficacy of the 2-mg nicotine gum for ST users, serum cotinine concentrations achieved after 4 weeks of nicotine gum use were about 32% of the concentrations attained during ad lib ST use (Hatsukami et al., 1996). In our own studies with the nicotine patch, we observed that the mean nicotine percentage replacement (nicotine percentage replacement = nicotine concentrations on nicotine patch/nicotine concentrations with ad lib ST use × 100%) among ST users who used 3 or more cans/pouches per week was 53.3% ± 17.1 with the 21 mg/day nicotine patch. Higher doses of NRT or combination NRT (i.e., nicotine patch with the nicotine lozenge) may be needed to increase abstinence rates among ST users.
Similarities between ST and particular nicotine replacement products may negatively impact abstinence rates. The rate of absorption, pharmacokinetic pattern similarities, and behavioral similarities may cause a “priming effect” predisposing to relapse, an observation that has been observed across different drugs of abuse (Hatsukami et al., 1996, 2000). Products like the nicotine lozenge may enhance this effect, whereas the nicotine patch may overcome this priming effect in ST users.
We also postulate that ST users are highly responsive to tobacco use interventions, as they are relatively treatment “naive” because treatments for ST users are not widely available and ST users tend to be younger and healthier (Ebbert, Carr, & Dale, 2004) and less likely to seek medical attention. This may create a “ceiling effect” with behavioral treatment against which it is difficult to detect an effect of pharmacotherapy.
Another possible explanation of why traditional pharmacotherapies do not have demonstrated efficacy for ST users may relate to high abstinence rates in control conditions in ST intervention trials. Studies of pharmacotherapies for the treatment of ST users have observed high end-of-treatment point-prevalence tobacco abstinence rates in control groups (i.e., 40%–58%; Boyle, 1992; Dale et al., 2007; Hatsukami et al., 1996, 2000; Howard-Pitney et al., 1999). Behavioral interventions for ST users have clearly been shown to be effective for increasing ST abstinence rates (Ebbert, Montori, et al., 2007; Severson, 2003). We postulate that these large control group effects may be due, in large part, to the number and intensity of the behavioral counseling delivered to both study groups in these previous ST intervention trials (Boyle; Dale et al.; Hatsukami et al., 1996, 2000). In the current study, we provided minimal counseling, aiming to optimize and limit the amount of behavioral counseling. However, the nature of a multivisit treatment protocol may accentuate the impact of behavioral counseling. Although the point-prevalence ST abstinence rate (34.3%) was lower than in previous studies, relapse rates among the active nicotine lozenge group were high, resulting in no detected differences between groups at 6 months.
Our data suggest that future work with the lozenge and ST users should focus on lozenge dosing and duration of therapy. On average, participants in the active nicotine lozenge group used only one half as many lozenges as were allocated for the first 6 weeks. In the active lozenge group, only 5% of the participants in Weeks 1–2, 9% in Weeks 3–4, and 7% in Weeks 5–6 used 16 or more lozenges per day. When we analyzed active lozenge use among heavier ST users in this study (i.e., participants who used ≥3 cans per week), median (interquartile range) lozenge use was 7.5 (5.1–10.3) lozenges for the first 2 weeks. In our previous studies with ST users using 3 or more cans per week, mean serum nicotine concentration at steady state was 38 ng/ml (median 37.0; range 8–95; n = 40; Ebbert, Post, et al., 2007). In multidose studies of the 4-mg nicotine lozenge, dosing every 90 min (approximately nine lozenges over 14 hr) achieved maximal serum nicotine concentrations of 26 ng/ml. In combination, these data suggest that ST users may be “under-replacing” the amount of nicotine to which they are exposed when using ST ad lib. Unlike the previous nicotine lozenge study for cigarette smokers (Shiffman, 2005), we did not provide lozenges to be used ad lib from Weeks 12 to 24. In the lozenges for smoking cessation study, the continuous abstinence rates among the low-dependency group fell by 10% in the active lozenge group from Week 12 to Week 24 and 7% in the placebo. Among the high-dependency smokers, continuous abstinence rates dropped by 12% in the active lozenge group and 4% in placebo. We observed a substantial decline in abstinence rates in the active lozenge group from Week 12 to Week 24 (i.e., 19% decline for point-prevalence ST abstinence). Providing ad lib lozenges to users after 12 weeks may provide ST users with additional support to prevent relapse to tobacco use.
Interpretation of our results is limited by a technical difficulty we encountered in accomplishing the biochemical cotinine and anabasine assays. High urinary concentrations of cotinine in the setting of ongoing nicotine lozenge use interfered with the ability of the laboratory to detect anabasine. This is supported by the high rate of biochemical disconfirmation in the active lozenge group in the current study compared with the lower rates of disconfirmation in the placebo group and in previous studies. Among participants in the current study who self-reported abstinence from tobacco at the end of medication, 18.3% (11 of 60) failed biochemical confirmation in the active lozenge group and 5.1% (2 of 39) failed in placebo. In our previous trial of bupropion SR for ST users, the corresponding proportions of biochemical disconfirmation of individuals who self-reported tobacco abstinence at the end of medication were 3.2% (2 of 62) for bupropion and 7.1% (4 of 56) for placebo (Dale et al., 2007). Anabasine is present in tobacco but not in NRT and can distinguish between exposure to NRT and tobacco. Previous investigators have suggested that the simultaneous use of anabasine and anatabine to differentiate between NRT and tobacco may increase specificity (Jacob et al., 2002). Our laboratory could not measure anatabine, but we suggest that future research with NRT in ST users should use both biomarkers to avoid the problems we encountered.
We conclude that the 4-mg nicotine lozenge is well tolerated and acceptable to ST users and can be used to decrease craving and withdrawal symptoms associated with tobacco abstinence. Future studies should investigate ways to enhance dosing of the nicotine lozenge, since some ST users may be under-replacing nicotine with this product. Possible ways to enhance nicotine dosing may include recommending the use of combination NRTs (i.e., nicotine patch and lozenge), educating patients on symptom-triggered dosing, and developing new nicotine delivery devices.
Funding
This project was supported by the National Cancer Institute award (CA121165). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
We thank the individuals who participated in this research and the staff of the Mayo Clinic Nicotine Research Program and the Oregon Research Institute without whom this project would not have been possible. We thank the members of our data and safety monitoring board, comprising Drs. Scott Tomar, Timothy Lineberry, Scott Leischow, and Raymond Boyle. We also thank Dr. Saul Shiffman for his expertise and leadership in the area of tobacco treatment and for his comments and scientific input on the manuscript.
References
- Benowitz NL, Ahijevych K, Hall S, Hansson A, Henningfield J, Hurt RD, et al. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Sheiner L, Jacob P., 3rd Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clinical Pharmacology and Therapeutics. 1988;44:23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Boyle RG. Smokeless tobacco cessation with nicotine replacement: A randomized clinical trial. Dissertation Abstracts International. 1992;54(3-A):825. [Google Scholar]
- Choi JH, Dresler CM, Norton MR, Strahs KR. Pharmacokinetics of a nicotine polacrilex lozenge. Nicotine & Tobacco Research. 2003;5:635–644. doi: 10.1080/1462220031000158690. [DOI] [PubMed] [Google Scholar]
- Dale LC, Ebbert JO, Glover ED, Croghan IT, Schroeder DR, Severson HH, et al. Bupropion SR for the treatment of smokeless tobacco use. Drug and Alcohol Dependence. 2007;90:56–63. doi: 10.1016/j.drugalcdep.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Carr AB, Dale LC. Smokeless tobacco: An emerging addiction. Medical Clinics of North America. 2004;88:1593–1605. doi: 10.1016/j.mcna.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Dale LC, Severson HH, Croghan IT, Rasmussen DF, Schroeder DR, et al. Nicotine lozenges for the treatment of smokeless tobacco use. Nicotine & Tobacco Research. 2007;9:233–240. doi: 10.1080/14622200601080349. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Montori V, Vickers KS, Erwin PC, Dale LC, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systemic Reviews. 2007 doi: 10.1002/14651858.CD004306.pub3. (4), CD004306. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Patten CA, Schroeder DR. The Fagerström Test for Nicotine Dependence-Smokeless Tobacco (FTND-ST. Addictive Behaviors. 2006;31(9):1716–1721. doi: 10.1016/j.addbeh.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Post JA, Moyer TP, Dale LC, Schroeder DR, Hurt RD. Nicotine percentage replacement among smokeless tobacco users with nicotine patch. Drug and Alcohol Dependence. 2007;89(2-3):223–226. doi: 10.1016/j.drugalcdep.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MA, Taylor GW, Tilashalski KR. Smokeless tobacco and severe active periodontal disease, NHANES III. Journal of Dental Research. 2005;84:705–710. doi: 10.1177/154405910508400804. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Grillo M, Boyle R, Allen S, Jensen J, Bliss R, et al. Treatment of spit tobacco users with transdermal nicotine system and mint snuff. Journal of Consulting and Clinical Psychology. 2000;68:241–249. [PubMed] [Google Scholar]
- Hatsukami DK, Jensen J, Allen S, Grillo M, Bliss R. Effects of behavioral and pharmacological treatment on smokeless tobacco users. Journal of Consulting and Clinical Psychology. 1996;64:153–161. doi: 10.1037//0022-006x.64.1.153. [DOI] [PubMed] [Google Scholar]
- Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States) Cancer Causes and Control. 2005;16:347–358. doi: 10.1007/s10552-004-5519-6. [DOI] [PubMed] [Google Scholar]
- Howard-Pitney B, Killen JD, Fortmann SP. Quitting chew: Results from a randomized trail using nicotine patches. Experimental and Clinical Psychopharmacology. 1999;7:362–371. doi: 10.1037//1064-1297.7.4.362. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Errors in using tobacco withdrawal scale [Letter to editor] Tobacco Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Jacob P, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiology, Biomarkers and Prevention. 2002;11:1668–1673. [PubMed] [Google Scholar]
- Jacob P, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: Comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. American Journal of Public Health. 1999;89:731–736. doi: 10.2105/ajph.89.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer TP, Charlson JR, Enger RJ, Dale LC, Ebbert JO, Schroeder DR, et al. Simultaneous analysis of nicotine, nicotine metabolites, and tobacco alkaloids in serum or urine by tandem mass spectrometry, with clinically relevant metabolic profiles. Clinical Chemistry. 2002;48:1460–1471. [PubMed] [Google Scholar]
- Muscat JE, Hoffmann D, Wynder EL. The epidemiology of renal cell carcinoma. A second look. Cancer. 1995;75:2552–2557. doi: 10.1002/1097-0142(19950515)75:10<2552::aid-cncr2820751023>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Stellman SD, Hoffmann D, Wynder EL. Smoking and pancreatic cancer in men and women. Cancer Epidemiology, Biomarkers and Prevention. 1997;6:15–19. [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Severson HH. Enough snuff: ST cessation from the behavioral, clinical, and public health perspectives. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, September 1992.: Bethesda, MD; 1992. (NIH Publication No. 93–3461) [Google Scholar]
- Severson HH. Enough snuff: A guide for quitting smokeless tobacco. 5th ed. Eugene, OR: Applied Behavior Science Press; 1999. [Google Scholar]
- Severson HH. What have we learned from 20 years of research on smokeless tobacco cessation? American Journal of the Medical Sciences. 2003;326:206–211. doi: 10.1097/00000441-200310000-00011. [DOI] [PubMed] [Google Scholar]
- Severson HH, Akers L, Boles SM, Andrews JA, Yovanoff P. Development of a brief smokeless tobacco dependence scale. 2004. Paper presented at the Society for Nicotine and Tobacco Research, Prague. [Google Scholar]
- Shiffman S. Nicotine lozenge efficacy in light smokers. Drug and Alcohol Dependence. 2005;77:311–314. doi: 10.1016/j.drugalcdep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Archives of Internal Medicine. 2002;162:1267–1276. doi: 10.1001/archinte.162.11.1267. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. Nicotine & Tobacco Research. 2008;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell HG, Lyman GH. Impact of smoking and smokeless tobacco on the risk of cancer of the head and neck. Head and Neck Surgery. 1986;9:104–110. doi: 10.1002/hed.2890090206. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National findings. 2007. (NSDUH Series H-32, DHHS Publication No. SMA 07–4293). Rockville, MD: Office of Applied Studies. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.