Abstract
Objective To investigate associations between physical activity at age 12 and subsequent adiposity at age 14.
Design Prospective birth cohort study with data collected between 2003 and 2007.
Setting Original recruitment in 1991-2 of 14 541 pregnant women living in the former County of Avon (United Kingdom).
Participants At age 12, 11 952 children were invited to attend the research clinic. Of these, 7159 attended, and 4150 (1964 boys, 2186 girls) provided sufficient data on exposure, outcome, and confounding variables.
Main outcome measure Fat mass at age 14, measured by dual emission x ray absorptiometry, associated with physical activity at age 12, measured by accelerometry.
Results Prospective associations of fat mass at age 14 (outcome) with physical activity at age 12 (exposure) were strong for both total activity (accelerometer counts/min) and for daily amount of moderate-vigorous physical activity (min/day). An extra 15 minutes of moderate-vigorous physical activity per day at age 12 was associated with lower fat mass at age 14 in boys (by 11.9% (95% confidence interval 9.5% to 14.3%)) and girls (by 9.8% (6.7% to 12.8%)). The proportion of physical activity due to moderate-vigorous physical activity was between 20% and 30% in boys and girls at the two ages.
Conclusions Higher levels of physical activity, in particular activity of moderate to higher intensities, are prospectively associated with lower levels of fat mass in early adolescence. Interventions to raise levels of physical activity in children are likely to be important in the fight against obesity.
Introduction
More than 30% of children and adolescents in the Americas, and about 20% of children in Europe, are overweight or obese.1 The prevalence of obesity in children aged 2-10 years in England increased from 9.9% in 1995 to 13.7% in 2003, and overweight and obese combined rose from 22.7% to 27.7%.2 The highest increases (from 11.2% to 16.5%) were seen in children aged 8-10 years, and levels of obesity persist into adolescence, whereby, at 15 years old, 17.9% of boys and 15.6% of girls are obese.3 Not only is the prevalence of obesity high and increasing, but the annual rates of increase across many countries in Europe are also increasing.4 Risk factors for cardiovascular disease such as hypertension, dyslipidaemia, impaired glucose tolerance, and vascular abnormalities are already present in overweight children,5 6 and overweight during childhood is associated with a higher risk of cardiovascular disease in adulthood.7
Evidence about the determinants of obesity in children is sparse, largely because studies investigating the influence of obesogenic behaviours (such as low physical activity) have been limited by methodological problems including cross sectional designs, lack of power, and imprecise measurements of activity and adiposity. For example, reviews of physical activity and obesity prevention8 9 have reported inconsistent results, with studies often reporting no association, especially when activity was measured with self reported questionnaires. Where associations were observed, they were generally reported as small or weak.
Nevertheless, physical inactivity is regularly postulated as a major contributory factor in the increased prevalence of both adult and child obesity,10 a view based largely on ecological data.9 With the development of more precise, objective measures of physical activity, especially the use of accelerometers, clear associations between children’s levels of physical activity and health risk—most notably obesity and risk factors for cardiovascular disease—have been reported in cross sectional analyses.11 12 13 14 To gain an accurate measure of associations between activity and obesity, prospective studies must use precise instruments in large and representative samples of children.
We have previously reported in a cross sectional analysis a strong, inverse, dose-response relation between physical activity (measured by accelerometry) and risk of obesity (measured by dual emission x ray absorptiometry (DEXA)) in a large group of 12 year old children from the Avon Longitudinal Study of Parents and Children (ALSPAC).13 We can now extend this analysis by assessing prospective associations between activity and obesity through early adolescence.
The aim of this study was to investigate prospective associations of physical activity at age 12 years with subsequent fat mass at age 14 in these well characterised ALSPAC children. We also investigated associations of changes in physical activity with changes in fat mass through ages 12-14 years. The time span between measurements (two years) is relatively short. However, measurements at any two time points gives a measure of the “trajectory” (gain, maintenance, loss) of adiposity and of physical activity. Further, this period of early puberty is one of rapid change in both biological and behavioural factors, and substantial changes would be expected. Our primary hypothesis was that children with higher levels of physical activity at age 12 would have lower levels of fat mass at age 14 compared with children with lower levels of activity. We also hypothesised that changes in physical activity through ages 12-14 years would be inversely associated with changes in fat mass over the same period.
Methods
Detailed descriptions of the study,15 the obesity measurement,16 and the physical activity measures17 have been reported previously. Brief descriptions are included below. Measurements that were made at both 12 and 14 years of age (physical activity, body composition, height, weight, pubertal stage) used essentially identical protocols.
Study population
ALSPAC is a birth cohort study that recruited 14 541 pregnant women living in one of three Bristol based health districts in the former County of Avon with an expected delivery date between April 1991 and December 1992. Posters advertising ALSPAC were displayed in pharmacies, libraries, mother and toddler groups, preschool playgroups, general practices, and antenatal clinics. ALSPAC staff approached eligible mothers when they attended for routine ultrasound examinations. The hospitals sent information to the mothers with their booking information. Community midwives gave newly pregnant women cards to send for further details. There was considerable local and national coverage in the press, radio, and television. ALSPAC staff also approached eligible but non-enrolled mothers after delivery while they were in the maternity hospital. State run maternity care is widespread in the UK, and only 2% of women were cared for by private midwives. Because of the numerous methods of advertising the study, it is unlikely that these mothers using private maternity care were missed.
Detailed information was collected using self administered questionnaires, data extraction from medical notes, linkage to routine information systems, and at research clinic examinations. Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee (approved as an institutional review board) and local research ethics committees.
Anthropometric variables
Fat mass was derived using a Lunar Prodigy DEXA scanner (GE Medical Systems Lunar, Madison, WI, USA). Each scan was manually screened for anomalies, motion, and material artefacts. Subregion edges and nodes were aligned manually according to specified criteria based on bony anatomical landmarks. Fat-free mass was calculated from the sum of lean and bone mass. The sum of fat and fat-free mass (total mass) estimated by DEXA was in strong agreement with measured mass (r2>0.99). We chose DEXA measured fat mass as our primary outcome as DEXA is substantially more precise than most other methods of assessing adiposity. In epidemiological studies, it is important to measure both exposures and outcomes with a high level of accuracy in order to uncover potentially subtle changes and associations. Although body mass index is commonly used, it does not differentiate between fat and lean mass. This may be important as the determinants and health consequences of each may be different in children. However, as body mass index is easily understood and widely used in clinical settings, we have included an analysis with this variable for comparison.
Height was measured with shoes and socks removed using a Harpenden stadiometer (Holtain, Crymych, Pembs, UK). Weight was measured using a Tanita TBF 305 body fat analyser and weighing scales (Tanita, www.tanita.co.uk). Body mass index was calculated as weight (in kilograms) divided by height squared (in metres).
Physical activity variables
Measurement
All ALSPAC children who attended the 11 year and 13 year research clinics were asked to wear an Actigraph AM7164 2.2 accelerometer (Actigraph LLC, Fort Walton Beach, FL, USA) for seven days. As the mean ages of children at these clinics were 11.8 and 13.9 years, we refer to the children as 12 and 14 year olds. The accelerometer is worn around the waist and detects acceleration and deceleration in a vertical plane as a combined function of movement frequency and intensity. Data are recorded as counts, which are averaged over a defined period (one minute in this study). The Actigraph has been validated in both children and adolescents.18 19 20 21 Children wore the accelerometer during waking hours, except for showering, bathing, or any water sports. The accelerometers were returned by post, and data were downloaded with the Actigraph Reader Interface Unit RIU-41A with RIU software (version 2.26B, Actigraph LLC).
Derivation of physical activity variables
Physical activity variables were derived from the raw accelerometer counts using customised software. Data from children who had worn the accelerometer for at least 10 hours a day for at least three days after deletion of missing data were considered valid, a level which we have previously shown achieves greatest power and good reliability.17 Missing data were determined as all periods of >10 consecutive minutes of zeros.17
Two main physical activity variables were calculated—total physical activity and time spent in moderate to vigorous physical activity (usually defined as any activity at least equivalent to the physiological stress of brisk walking for a person of average fitness). Aerobic activities such as dancing, cycling, or swimming that cause increased heart rate and possibly sweating are usually above the threshold for moderate intensity activity. More strenuous activities such as jogging, hill walking, and competitive racket sports would usually be classified as vigorous.
Total physical activity was calculated as the average accelerometer counts per minute over the full period of valid recording. Moderate-vigorous physical activity was calculated as the average minutes of such activity per valid day of measurement. Total physical activity was used because this is the summary measure of total physical activity that has been validated against doubly labelled water.20 Minutes of moderate-vigorous physical activity were used because UK government recommendations for physical activity in children are framed in terms of time spent each day in moderate-vigorous physical activity.22
All minutes of recording with a total of >3600 accelerometer counts were classified as moderate-vigorous physical activity. The threshold of 3600 counts/min was derived from a calibration study conducted in a subsample of 246 ALSPAC children who were asked to perform a series of everyday activities while wearing an accelerometer and a portable metabolic unit (Cosmed K4b2, Cosmed, Rome, Italy).21 This threshold corresponded to four times resting metabolic rate and approximated to brisk walking in these children.
In analytical models, associations with total physical activity were calculated per 100 counts/min as this difference approximates to the difference between boys and girls in activity levels.23 Associations with moderate-vigorous physical activity were calculated per 15 minutes of such activity to enable comparisons with the current recommendations that children participate in at least 60 minutes of moderate-vigorous physical activity per day.22 Both increments represent realistic targets for intervention studies.
Potential confounding variables
We selected possible confounding factors that were available on the whole cohort and that have been shown to be independently associated with obesity in ALSPAC.24
Age was recorded as the age at which the child attended the two research clinics.
The 32 week antenatal questionnaire asked each mother to record her highest education level—categorised into none, CSE (national school exams at age 16), or vocational; O level (national school exams at age 16, higher than CSE); A level (national school exams at age 18); or university degree. The mother also recorded the occupation of both herself and her partner, which were used to allocate them to social class groups (I, II, III manual, III non-manual, IV, V) using the 1991 Office of Population, Censuses and Surveys classification; the lowest class of the mother and her partner was used in analysis.
At enrolment, the mother was asked to record her height and pre-pregnancy weight, which were used to calculate mother’s pre-pregnancy body mass index, which we classified as obese (body mass index >30) or not.
Infant sex was recorded in the delivery room or abstracted from obstetric records or birth notifications.
The mother was asked about her smoking habits on two occasions (the 18 week and 32 week antenatal questionnaires), and a dichotomous variable was created for any smoking during pregnancy.
A puberty questionnaire was filled in by the child’s carer (usually the child’s mother) when the child was about 11 years old and again at age 13, which included questions on pubertal stage.25 Both measures were included in the analysis relative to the appropriate time point. Only children who returned the puberty questionnaire within 16 weeks of the clinic were included.
Statistical analyses
Descriptive statistics
Means and standard deviations were calculated for continuous variables that approximated a normal distribution. Medians and interquartile ranges were calculated for skewed variables (physical activity, fat mass). Log fat mass was used throughout subsequent analyses, because of its skewed distribution. Although both physical activity variables showed some skewness, these variables were not log transformed in order to facilitate comparisons with our previously reported cross sectional analysis.
Main analysis
Multilevel modelling is a powerful method to study change over time and the factors that effect change in repeated measures studies. Such methods allow flexible modelling of covariance of the repeated measures and explicitly model individual change over time. In our analysis all associations with fat mass at age 14 are effectively controlled for the confounding effect of fat mass at age 12. Further, multilevel models can accommodate different numbers of repeated observations, maximising power.
A univariate multilevel model consists of fixed effects and random effects. In the fixed effects, the outcome is related to exposure and confounders using the usual linear regression. In the random effects, each individual is allowed to have different regression coefficients, which vary about the population average. A bivariate multilevel model has two outcomes, each with its own set of fixed effects relating it to the exposure and confounders. Each outcome also has its own set of random effects, which are correlated with each other and with the random effects corresponding to the other outcome.
In our analysis, each multilevel model had two outcomes (fat mass and physical activity (either total activity or moderate-vigorous activity)) and three levels (measurement (fat mass or physical activity), occasion (age 12 or age 14), and person). Each outcome was related to the confounding factors and to age. Additionally, for each outcome, individuals had their own intercept and their own coefficient for age. The multilevel model describes how these four individual coefficients (intercept and change with age for fatness, intercept and change with age for physical activity) are distributed, thus allowing the regression coefficients of any set of exposure and outcome variables to be derived. For example, the adjusted regression coefficient for the association between moderate-vigorous physical activity at 12 years (exposure) and fatness at 14 years (outcome) was calculated from all the random effects associated with moderate-vigorous physical activity and fatness.26 To estimate the standard errors for the derived regression coefficients, we used Markov chain Monte Carlo simulation in WinBUGS with non-informative priors. Fat mass (log transformed) and the measure of physical activity in the model were assumed to have a bivariate normal distribution.
Model fitting
All statistical models were fitted separately for boys and girls, using three stages of adjustment for confounding factors. In the first model (minimally adjusted), log fat mass was adjusted for height and height squared. Adjustment for height was to normalise fat mass for stature, and height squared was used as there is evidence of a non-linear relation between fat mass and height.13 Additionally, all analyses using the variable moderate-vigorous physical activity were further adjusted for the amount of time the accelerometer was worn. This was not done for total activity as the variable counts/min is already time based. In the second model (partially adjusted), maternal education, maternal smoking during pregnancy, and maternal obesity were added to the original model. In the third model (fully adjusted) pubertal stage at ages 12 and 14 were added. Pubertal stage was added separately because this variable introduced a large amount of missing data (when puberty was added to the models, only 1988 boys and 2740 girls had data available for analysis). The derived social class variable could not be added to any models because of collinearity with maternal education.
Finally, in order to ascertain whether total activity or moderate-vigorous physical activity was the stronger predictor variable, we assessed the associations of log fat mass on total activity (per 100 counts/min) and moderate-vigorous physical activity (per 15 min) by adjusting total activity for moderate-vigorous physical activity and vice versa.
All analyses were performed using Stata version 9 (StataCorp, College Station, TX, USA), MLwiN version 2.02,27 and WinBUGS version 1.4.3.28
Results
Final sample
At the initial research clinic, 11 952 children were invited to attend, and 7159 (60%) attended. Of these, we included 4150 (1964 boys, 2186 girls) in the analysis (35% of those invited), after exclusion of children with incomplete data on covariates. At the second research clinic 11 267 children were invited to attend, 6152 (55%) attended, and 2882 (1353 boys, 1529 girls) (26% of those invited) provided sufficient data. Of these children, 2418 (1130 boys, 1288 girls) provided valid data at both time points, 1732 children (834 boys, 898 girls) provided data only at age 12, and 464 (223 boys, 241 girls) only at age 14. Overall, 4614 children provided data at one or both time points, which represents 39% of the original sample invited to attend the first clinic. Tables 1 and 2 contain full descriptive data for this sample of children.
Table 1.
Characteristics of children who participated in study: continuous variables at the first and second assessments (at ages 12 and 14 years)
| All | Boys | Girls | ||||||
|---|---|---|---|---|---|---|---|---|
| No of children | Value | No of children | Value | No of children | Value | |||
| Median (IQR) total physical activity (counts/min): | ||||||||
| At first assessment | 4150 | 576 (472–706) | 1964 | 643 (530–771) | 2186 | 525 (443–632) | ||
| At second assessment | 2882 | 513 (403–645) | 1353 | 573 (457–711) | 1529 | 460 (372–567) | ||
| Median (IQR) moderate-vigorous physical activity (min/day): | ||||||||
| At first assessment | 4150 | 20 (12–31) | 1964 | 26 (16–38) | 2186 | 16 (10–24) | ||
| At second assessment | 2882 | 21 (12–33) | 1353 | 26 (15–39) | 1529 | 17 (9–27) | ||
| Median (IQR) fat mass (kg): | ||||||||
| At first assessment | 4150 | 9.7 (6.7–14.9) | 1964 | 8.2 (5.5–13.1) | 2186 | 11.2 (7.8–16.0) | ||
| At second assessment | 2882 | 12.0 (7.7–17.7) | 1353 | 8.4 (5.6–13.9) | 1529 | 14.7 (10.7–20.0) | ||
| Mean (SD) age (years): | ||||||||
| At first assessment | 4150 | 11.8 (0.24) | 1964 | 11.8 (0.24) | 2186 | 11.8 (0.24) | ||
| At second assessment | 2882 | 13.9 (0.20) | 1353 | 13.9 (0.20) | 1529 | 13.9 (0.19) | ||
| Mean (SD) height (m): | ||||||||
| At first assessment | 4150 | 1.51 (0.072) | 1964 | 1.50 (0.071) | 2186 | 1.51 (0.073) | ||
| At second assessment | 2882 | 1.63 (0.076) | 1353 | 1.65 (0.087) | 1529 | 1.62 (0.063) | ||
IQR=interquartile range. SD=standard deviation.
Table 2.
Characteristics of mothers and children who participated in study: categorical variables. (Values are numbers (percentages) of participants)
| All | Boys | Girls | |
|---|---|---|---|
| Maternal education: | |||
| None, CSE, or vocational | 824 (18) | 389 (18) | 435 (18) |
| O levels | 1285 (28) | 611 (28) | 674 (28) |
| A levels | 1638 (36) | 771 (35) | 867 (36) |
| Degree | 867 (19) | 416 (19) | 451 (19) |
| Social class: | |||
| IV or V | 753 (16) | 340 (16) | 413 (17) |
| III Manual | 1187 (26) | 547 (25) | 640 (26) |
| III Non-manual | 1275 (28) | 617 (28) | 658 (27) |
| I or II | 1399 (30) | 683 (31) | 716 (30) |
| Maternal smoking during pregnancy: | |||
| No | 3752 (81) | 1777 (81) | 1975 (81) |
| Yes | 862 (19) | 410 (19) | 452 (19) |
| Maternal pre-pregnancy obesity: | |||
| No | 4381 (95) | 2074 (95) | 2307 (95) |
| Yes | 233 (5) | 113 (5) | 120 (5) |
| Child’s pubertal stage at 12 years old: | |||
| Stage 1 | 548 (21) | 388 (37) | 160 (10) |
| Stage 2 | 945 (35) | 456 (43) | 489 (30) |
| Stage 3 | 722 (27) | 162 (15) | 560 (35) |
| Stage 4* | 376 (14) | 50 (5) | 326 (20) |
| Stage 5* | 77 (3) | 77 (5) | |
| Child’s pubertal stage at 14 years old: | |||
| Stage 1 | 119 (6) | 105 (11) | 14 (1) |
| Stage 2 | 288 (14) | 197 (21) | 91 (8) |
| Stage 3 | 553 (27) | 272 (29) | 281 (25) |
| Stage 4* | 832 (40) | 358 (38) | 474 (42) |
| Stage 5* | 268 (13) | 268 (24) |
*For boys, stages 4 and 5 have been amalgamated.
At age 12, the children who attended the clinic were more likely to be girls, have a higher birth weight, be from a higher social class, have older and taller mothers, and have mothers with higher levels of education compared with non-attenders.17 There was a similar pattern of differences between clinic attenders who provided valid measures of physical activity and attenders who did not. The differences between attenders and non-attenders were similar at age 14. However, the differences between children who did and did not provide valid accelerometer data were markedly reduced at age 14, with the exception of mother’s level of education.
Main analyses
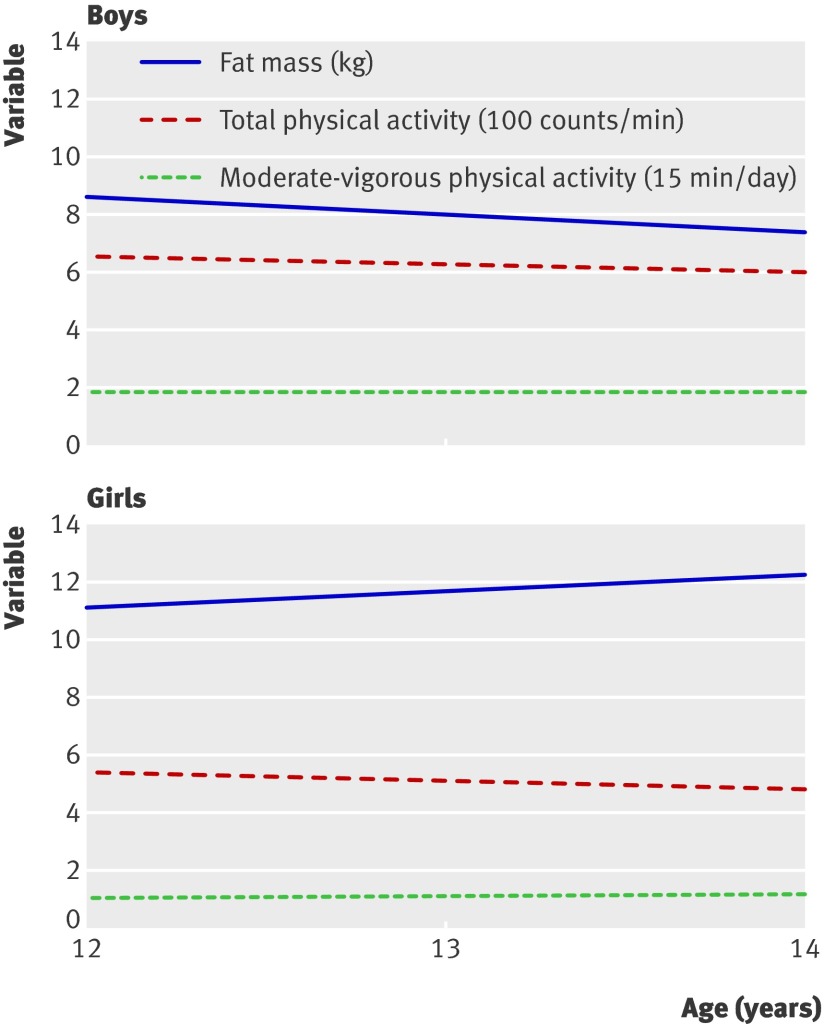
In all analyses, there was little or no attenuation with any level of adjustment; therefore we report results from the minimally adjusted model. The estimates of the fixed effect parts of the associations between age and each of the three outcomes (fat mass, total activity, and moderate-vigorous physical activity) are given in table 3, and these trajectories are also displayed in the figure.
Table 3.
Regression coefficients (95% confidence intervals) showing relations between children’s age and their fat mass and physical activity for the average child (minimally adjusted models)
| Boys | Girls | ||||||
|---|---|---|---|---|---|---|---|
| Fat mass (kg)* | Total physical activity (100 counts/min) | Moderate-vigorous physical activity (15 min/day)† | Fat mass (kg)* | Total physical activity (100 counts/min) | Moderate-vigorous physical activity (15 min/day)† | ||
| Intercept value at age 12 | 8.648 (8.437 to 8.865) | 6.544 (6.468 to 6.620) | 1.799 (1.607 to 1.991) | 11.143 (10.926 to 11.364) | 5.407 (5.348 to 5.466) | 1.108 (0.986 to 1.230) | |
| Linear or % change with age (per year)‡ | −7.4% (−9.3% to −5.5%) | −0.283 (−0.332 to −0.234) | 0.023 (−0.010 to 0.056) | 5.0% (3.7% to 6.3%) | −0.287 (−0.326 to −0.248) | 0.067 (0.043 to 0.091) | |
*Adjusted for height and height squared. These coefficients are taken from the bivariate model with fat mass and total activity as outcomes, but the corresponding coefficients from the model with fat mass and moderate-vigorous physical activity were similar.
†Adjusted for amount of time (minutes) that the accelerometer was worn.
‡For total activity and moderate-vigorous physical activity, the linear change with age is given. Log fat mass was used in the models, so the change in fat mass with age is best expressed as a percentage change.
Average pattern of changes in fat mass, total physical activity, and moderate-vigorous physical activity from ages 12 to 14 years in boys and girls
Total activity was lower at age 14 than at 12 for both boys and girls. Total activity at age 12 was lower for girls than boys, with no difference between the sexes in rate of decrease to age 14. Fat mass was higher at age 14 than at 12 for girls and lower for boys, and fat mass at age 12 was higher for girls than boys. Moderate-vigorous physical activity showed a slight increase between age 12 and age 14 for boys and a greater increase for girls.
Derived regression coefficients
As coefficients for log fat mass are difficult to interpret, we have transformed the coefficients (by using exponentials) to show effect sizes in terms of the percentage change in fat mass (see table 4). A total activity level that is 100 counts/min higher at age 12 is associated with a 4.0% lower fat mass at age 14 in girls and 6.4% lower fat mass in boys. Moderate-vigorous physical activity that is 15 min/day higher at age 12 is associated with 9.8% lower fat mass in girls and a 11.9% lower fat mass in boys at age 14. It can also be seen that children on a rising trajectory of physical activity (positive changes of either total activity or moderate-vigorous physical activity through ages 12-14) tend to be on a reducing trajectory of fat mass between those two ages. For example, an increase in activity of 100 counts/min between ages 12 and 14 is associated with a 1.3% lower fat mass at age 14 in both boys and girls. An increase in moderate-vigorous physical activity of 15 min/day between ages 12 and 14 is associated with 2.4% lower fat mass in boys and 2.3% in girls at age 14 (table 4).
Table 4.
Cross sectional and prospective associations between children’s physical activity and fat mass at ages 12 and 14 years
| Outcome | Exposure | % change in fat mass (95% CI) | |
|---|---|---|---|
| Total physical activity* | Moderate-vigorous physical activity† | ||
| Boys | |||
| Fat mass at age 12 | Activity at age 12 | −8.0 (−9.3 to −6.6) | −14.6 (−16.6 to −12.4) |
| Fat mass at age 14 | Activity at age 12 | −6.4 (−7.8 to −4.9) | −11.9 (−14.3 to −9.5) |
| Fat mass at age 14 | Activity at age 14 | −5.0 (−6.2 to −3.9) | −9.1 (−10.8 to −7.3) |
| Change in fat mass ages 12-14 | Change in activity ages 12-14 | −1.3 (−2.0 to −0.6) | −2.4 (−3.6 to −1.1) |
| Girls | |||
| Fat mass at age 12 | Activity at age 12 | −5.1 (−6.8 to −3.5) | −12.4 (−15.7 to −9.1) |
| Fat mass at age 14 | Activity at age 12 | −4.0 (−5.5 to −2.5) | −9.8 (−12.8 to −6.7) |
| Fat mass at age 14 | Activity at age 14 | −3.8 (−5.0 to −2.7) | −5.2 (−7.1 to −3.2) |
| Change in fat mass age 12-14 | Change in activity ages 12-14 | −1.3 (−1.9 to −0.6) | −2.3 (−3.5 to −1.2) |
*For total physical activity (counts/min), change in fat mass is for additional activity of 100 counts/min.
†For moderate-vigorous physical activity (min/day), change in fat mass is for additional activity of 15 min/day.
When analyses were repeated with mutual adjustment of the two activity variables, the associations with total activity (counts/min) disappeared (effect sizes −6% to 1% for boys, −4% to −2% for girls (95% confidence intervals included zero for boys)), whereas associations with moderate-vigorous physical activity strengthened (effect sizes −12% to −14% for boys, −5% to −7% for girls).
In order to enhance the face validity of our model, we repeated analyses using standard linear regression with baseline adjustment. Results were similar but with wider confidence intervals for all coefficients (results available on request). This is likely due to smaller sample sizes and hence reduced power. The smaller sample size using standard linear regression is because only the children with complete data at both time points (n=2418) can be included in the analysis, whereas the bivariate multilevel models allow information from children with data at only one time point or on only one outcome variable to be used (n=4614).
Body mass index as outcome
We also repeated analyses using body mass index as the outcome in order to assess its clinical utility. Results showed a similar pattern of associations to those found with fat mass but were considerably weaker, by a factor of around four. The percentage change in body mass index at age 14 for an increase of 100 counts/min in total activity at age 12 was −1.5% in boys and −0.8% in girls, compared with changes in fat mass of −6.4% and −4.0% in boys and girls. The equivalent figures for a 15 min/day increase in moderate-vigorous physical activity were −2.9% and −2.2% for body mass index in boys and girls, versus −11.9% and −9.8% for fat mass. The changes in body mass index with incremental changes in total activity through ages 12-14 were −0.2% and −0.4% in boys and girls, versus changes in fat mass of −1.3% and −1.3%. The changes in body mass index with incremental changes in moderate-vigorous physical activity were −0.4% and −0.7% in boys and girls, versus changes in fat mass of −2.4% and −2.3%.
Discussion
In this prospective analysis, we have confirmed our previous findings that higher levels of physical activity are strongly and inversely associated with levels of fat mass in 12 year old children.13 An increase of 15 min/day in moderate-vigorous physical activity at age 12 is associated with around 10% lower fat mass in girls and a 12% lower fat mass in boys at age 14. This percentage difference is equivalent to a reduced fat mass of about 1 kg.
Strengths and limitations of study
The strengths of this study are the large sample size, the well characterised cohort, the longitudinal design, the high levels of measurement precision in both the physical activity and body composition measures, and the availability of an extensive array of possible confounding variables from both the children and their parents.
As with all observational studies, there are some potential sources of error. Residual confounding and cohort attrition can lead to bias. Though the observed associations could be due to confounding, we think this is unlikely as physical activity in this cohort was only weakly (and negatively) associated with higher social position, and the associations were largely unaltered by adjustment for various confounding factors, including social position. Cohort attrition leads to reduced power, which is not a particular problem in a study of this size. However, more importantly, missing data can lead to bias if the association between physical activity and obesity is different in the children who did not take part. While we cannot exclude bias due to missing data, the fact that the associations were not altered by adjustment for factors associated with missing data—such as social position—provides some reassurance.
The study also has only two time points, so even in this longitudinal design, inferring direction of causality must remain tentative.
Finally, the accelerometer cannot be worn for swimming and does not capture cycling activities adequately. However, we have previously shown that when analyses were repeated in children who did not report any swimming in the week of measurement and in children who did not report cycling in the week of measurement, associations were unchanged.13
Comparison with other studies
To our knowledge, only three prospective studies have examined the association between an accelerometer measure of physical activity and obesity in children.29 30 31 These studies were relatively small and measured adiposity by skinfold thicknesses or bioimpedence, which are less accurate measures than the DEXA used in our study. The first study measured physical activity in 103 US children aged 3-5 years for 3-5 days with a Caltrac accelerometer.29 This accelerometer gives only a measure of total activity and cannot measure different intensities of activity. Measurements were taken annually for eight years. Activity levels during the period of measurement were prospectively associated with subsequent sum of skinfold thicknesses. The second study measured 454 American Indian children (average age 7.5 years) using a Tritrac R3D tri-axial accelerometer on one day and followed them up three years later.30 There was a prospective association between total activity and obesity in children who were normal weight at baseline but not in those who were overweight at baseline. The third study measured the extent to which physical activity (measured by Actigraph accelerometer) at the government-recommended intensity (three times resting metabolic rate) is associated with changes in body mass index, fatness (measured by skinfold thickness), and metabolic health in 212 prepubertal children.31 The time spent in activities of sufficient intensity at age 5 was not associated with changes in body mass or fatness in either sex during ages 5-8 years. Some associations were seen with metabolic parameters.
These studies of younger children were smaller than ours. Hence, they had limited ability to compare sexes or dimensions of activity (total activity and moderate-vigorous physical activity). Our study therefore adds to these, as we have precise measures of both physical activity and body composition and greatly increased power to conduct analyses by sex, account for a greater range of confounders, and compare different dimensions of physical activity.
Implications of results
Overweight and obesity are normally defined in terms of body mass index. Although DEXA measurement of fat mass is a much more accurate estimate of adiposity, there are currently no health related criterion levels of fat mass for either adults or children. It is therefore difficult to assess fully the clinical implications of the fat mass levels and changes over time seen in this study. The essential point, however, is that we have observed strong prospective associations between physical activity level and subsequent fat mass over a two year period during early adolescence.
In broad terms, we observed a difference in fat mass of about 10% between active and inactive children. At this early stage of the lifespan, this difference is unlikely to be trivial, given that overweight and obesity track over time and levels of adiposity increase with age. It should also be noted that 12 year old children in this analysis who meet current health related recommendations of 60 minutes of moderate-vigorous physical activity a day would be expected to have around 4.3 kg less fat mass at age 14 than children who do no moderate-vigorous physical activity.
The observed inverse association between changes in activity and changes in fat mass through ages 12-14 years suggests that children who are on a rising trajectory of activity during these years are on a declining trajectory of fat mass. This suggests a dynamic relation between physical activity and adiposity, with relatively acute effects of the behaviour on the physiological response.
In the current study, and in keeping with our previous cross sectional analysis,13 differences in moderate-vigorous physical activity were more strongly associated with difference in fat mass than were differences in total activity. Although we selected arbitrary increments of total activity (100 counts/min) and moderate-vigorous physical activity (15 min), the two are broadly equivalent in the amount of activity involved. A difference of 100 counts/min over a 10 hour day (our minimum threshold) represents 60 000 accelerometer counts. A difference of 15 min/day of moderate-vigorous physical activity at the threshold of 3600 counts/min (again our minimum threshold) represents 54 000 additional accelerometer counts. We can therefore be confident that the stronger association observed for moderate-vigorous physical activity is not a function of the moderate-vigorous physical activity variable representing a larger volume of activity than the variable for total activity. This is supported by the fact that, with mutual adjustment of the two activity variables, the effect sizes for total activity disappeared.
It might be expected that total activity measured in counts/min—which captures the majority of movement and hence might be considered to more closely represent total activity energy expenditure—would exhibit the stronger association with fat mass. Moderate-vigorous physical activity represents a relatively small proportion of a child’s daily energy expenditure. For example, at age 12 years, the proportion of physical activity due to moderate-vigorous physical activity was 26.1% (SD 10.9%) for boys and 20.6% (10.3%) for girls. At age 14, the corresponding figures were 29.7% (13.3%) for boys and 24.8% (13.2%) for girls.
One possible explanation for this is that an accelerometer measures only trunk movement and does not measure peripheral movement such as arm exercise or fidgeting. If these activities—known collectively as non-exercise activity thermogenesis32—contribute substantially to energy expenditure, then the total accelerometer counts may not be that closely related to energy expenditure.
Alternatively, more vigorous activity may stimulate excess post-exercise oxygen consumption—a measurably increased rate of oxygen uptake for a time after strenuous activity. The extra oxygen is used in the homoeostatic processes that restore the body to a resting state including hormonal adjustment, replenishment of fuel stores, and cellular repair. The total energy value of vigorous activity therefore includes both the energy expended during the activity plus additional energy expended after exercise.
At present, however, we are unable to say why more intense exercise is the more important dimension of physical activity with respect to adiposity. Perhaps moderate-vigorous physical activity acts as a better marker of an overall behavioural pattern that is protective against obesity, or perhaps it has a greater effect on appetite regulation. Alternatively, more vigorous activity may lead to the development of more muscle mass and hence more metabolically active tissue. It is also possible that children with higher levels of cardiorespiratory fitness—which has a heritability component—may be more able to perform moderate-vigorous physical activity and hence the association between moderate-vigorous physical activity and adiposity is partially confounded by inherited traits.
The much weaker (but still discernible) associations of physical activity with body mass index indicate that body mass index may have some clinical utility as an indirect proxy for adiposity, but its main attraction may be financial and practical rather than scientific.
Conclusions
We have demonstrated in a large, well characterised group of children that physical activity levels at age 12 are strongly and inversely associated with fat mass two years later. Public health policies to reduce obesity in children should include strategies to promote higher levels of physical activity, particularly activity that is of moderate intensity and above.
What is already known on this topic
The role of physical activity in the prevention of childhood obesity is not well described, because of a lack of large representative studies using precise measures of both physical activity and fat mass
The level and type of activity that is most protective against obesity is also unknown
What this study adds
Higher levels of physical activity, especially activity that is of at least moderate intensity, are strongly associated with lower levels of fat mass two years later
Physical activity should be a primary target in public health initiatives to prevent obesity
We thank all the families who took part in this study, the midwives who helped in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. We also thank Chris Bain for his insightful comments on an earlier draft of this paper.
Contributors: CJR was principal investigator, led the team that obtained funding from the National Heart, Lung and Blood Institute, wrote all drafts of the paper, and is guarantor for the study. SDL performed statistical analyses and helped write all drafts of the paper. ARN was deputy director of ALSPAC and led the team that obtained funding from the Wellcome Trust. SNB advised at all stages of the study. KD organised day to day aspects of data collection and liaison with parents and children. CM led the fieldwork team and participated in the statistical analysis. AG performed statistical analyses and helped write all drafts of the paper. GDS was director of ALSPAC and advised at all stages of the study. KT performed analyses and helped write all drafts of the paper. All authors contributed to all drafts of the paper and approved the final version.
Funding: ALSPAC is supported by the Medical Research Council, Wellcome Trust, UK Department of Health, Department of the Environment, Department of Education and the Environment, National Institutes of Health, and a variety of medical research charities and commercial companies. This research was specifically funded by grants from the US National Heart, Lung and Blood Institute (R01HL071248-01A1) and the Wellcome Trust (GR068049MA).
All researchers are independent of the main funding body. The funding sources had no input to the study design, apart from changes required by external peer reviewers.
Competing interests: None declared.
Ethical approval: Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee (approved as an institutional review board) and local research ethics committees.
Data sharing: ALSPAC is run as a supported access resource. Full details of the collaboration policy can be found at www.bristol.ac.uk/alspac/sci-com/collab-policy/.
Cite this as: BMJ 2009;339:b4544
References
- 1.Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes Rev 2004;5(suppl 1):4-104. [DOI] [PubMed] [Google Scholar]
- 2.Jotangia D, Moody A, Stamatakis E, Wardle J, eds. Obesity among children under 11. National Centre for Social Research, 2006.
- 3.Stamatakis E. Anthropometric measurements, overweight and obesity. In: Sproston K, Primatesta P, eds. Health survey for England 2002. Volume 1: The health of children and young people. Stationery Office, 2003.
- 4.Jackson-Leach R, Lobstein T. Estimated burden of paediatric obesity and co-morbidities in Europe. Part 1. The increase in the prevalence of child obesity in Europe is itself increasing. Int J Pediatr Obes 2006;1(1):26-32. [DOI] [PubMed] [Google Scholar]
- 5.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362-74. [DOI] [PubMed] [Google Scholar]
- 6.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338:1650-6. [DOI] [PubMed] [Google Scholar]
- 7.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 2007;357:2329-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molnar D, Livingstone B. Physical activity in relation to overweight and obesity in children and adolescents. Eur J Pediatr 2000;159(suppl 1):S45-55. [DOI] [PubMed] [Google Scholar]
- 9.Wareham N. Physical activity and obesity prevention. Obes Rev 2007;8(suppl 1):109-14. [DOI] [PubMed] [Google Scholar]
- 10.Foresight Panel. Tackling obesities: future choices—project report. Government Office for Science, 2007.
- 11.Ekelund U, Brage S, Froberg K, Harro M, Anderssen SA, Sardinha LB, et al. TV viewing and physical activity are independently associated with metabolic risk in children: the European Youth Heart Study. PLoS Med 2006;3:e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen LB, Harro M, Sardinha LB, Froberg K, Ekelund U, Brage S, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study). Lancet 2006;368:299-304. [DOI] [PubMed] [Google Scholar]
- 13.Ness AR, Leary SD, Mattocks C, Blair SN, Reilly JJ, Wells J, et al. Objectively measured physical activity and fat mass in a large cohort of children. PLoS Med 2007;4:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brage S, Wedderkopp N, Ekelund U, Franks PW, Wareham NJ, Andersen LB, et al. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European Youth Heart Study (EYHS). Diabetes Care 2004;27:2141-8. [DOI] [PubMed] [Google Scholar]
- 15.Golding J, Pembrey M, Jones R. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 2001;15:74-87. [DOI] [PubMed] [Google Scholar]
- 16.Ness AR. The Avon Longitudinal Study of Parents and Children (ALSPAC)—a resource for the study of the environmental determinants of childhood obesity. Eur J Endocrinol 2004;151(suppl 3):U141-9. [DOI] [PubMed] [Google Scholar]
- 17.Mattocks C, Ness A, Leary S, Tilling K, Blair SN, Shield J, et al. Use of accelerometers in a large field-based study of children: protocols, design issues, and effects on precision. J Phys Act Health 2008;5(suppl 1):S94-107. [DOI] [PubMed] [Google Scholar]
- 18.Melanson EL, Freedson PS. Validity of the Computer Science Applications (CSA) activity monitor. Med Sci Sports Exerc 1995;27:934-40. [PubMed] [Google Scholar]
- 19.Fairweather SC, Reilly JJ, Grant S, Whittaker A, Paton JY. Using the Computer Science and Applications (CSA) activity monitor in preschool children. Pediatr Exerc Sci 1999;11:413-20. [Google Scholar]
- 20.Ekelund U, Sjostrom M, Yngve A, Poortvliet E, Nilsson A, Froberg K, et al. Physical activity assessed by activity monitor and doubly labeled water in children. Med Sci Sports Exerc 2001;33:275-81. [DOI] [PubMed] [Google Scholar]
- 21.Mattocks C, Leary S, Ness A, Deere K, Saunders J, Tilling K, et al. Calibration of an accelerometer during free-living activities in children. Int J Pediatr Obes 2007;2:218-26. [DOI] [PubMed] [Google Scholar]
- 22.Department of Health. At least 5 a week: physical activity and health outcomes: a review of the chief medical officer. DoH, 2004.
- 23.Riddoch CJ, Mattocks C, Deere K, Saunders J, Kirkby J, Tilling K, et al. Objective measurement of levels and patterns of physical activity. Arch Dis Child 2007;92:963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ 2005;330:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanner JM. Normal growth and techniques of growth assessment. Clin Endocrinol Metab 1986;15:411-51. [DOI] [PubMed] [Google Scholar]
- 26.Tilling K, Sterne JAC, Wolfe CDA. Multilevel growth curve models with covariate effects: application to recovery after stroke. Stat Med 2001;20:685-704. [DOI] [PubMed] [Google Scholar]
- 27.Rasbash J, Steele F, Browne W, Prosser B. A user’s guide to mlwin version 2.0. Institute of Education, 2004.
- 28.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Statistics and Computing 2000;10:325-37. [Google Scholar]
- 29.Moore LL, Gao D, Bradlee ML, Cupples LA, Sundarajan-Ramamurti A, Proctor MH, et al. Does early physical activity predict body fat change throughout childhood? Prev Med 2003;37:10-7. [DOI] [PubMed] [Google Scholar]
- 30.Stevens J, Suchindran C, Ring K, Baggett CD, Jobe JB, Story M, et al. Physical activity as a predictor of body composition in American Indian children. Obes Res 2004;12:1974-80. [DOI] [PubMed] [Google Scholar]
- 31.Metcalf BS, Voss LD, Hosking J, Jeffery AN, Wilkin TJ. Physical activity at the government-recommended level and obesity-related health outcomes: a longitudinal study (Early Bird 37). Arch Dis Child 2008;93:772-7. [DOI] [PubMed] [Google Scholar]
- 32.Levine JA. Nonexercise activity thermogenesis (NEAT): environment and biology. Am J Physiol Endocrinol Metab 2004;286:E675-85. [DOI] [PubMed] [Google Scholar]