Abstract
Acute cholecystitis consists of various morbid conditions, ranging from mild cases that are relieved by the oral administration of antimicrobial drugs or that resolve even without antimicrobials to severe cases complicated by biliary peritonitis. Microbial cultures should be performed by collecting bile at all available opportunities to identify both aerobic and anaerobic organisms. Empirically selected antimicrobials should be administered. Antimicrobial activity against potential causative organisms, the severity of the cholecystitis, the patient’s past history of antimicrobial therapy, and local susceptibility patterns (antibiogram) must be taken into consideration in the choice of antimicrobial drugs. In mild cases which closely mimic biliary colic, the administration of nonsteroidal anti-inflammatory drugs (NSAIDs) is recommended to prevent the progression of inflammation (recommendation grade A). When causative organisms are identified, the antimicrobial drug should be changed for a narrower-spectrum antimicrobial agent on the basis of the species and their susceptibility testing results.
Key words: Acute cholecystitis, Anti-infective agents, Guidelines, Infection, Biliary
Introduction
Acute cholecystitis consists of various morbid conditions, ranging from mild cases that are relieved by the oral administration of antimicrobial drugs or that resolve even without antimicrobials to severe cases complicated by biliary peritonitis, each of which requires a different treatment strategy. Decisions on antimicrobial therapy must be based upon knowledge of the likely infecting microorganisms, the pharmacokinetics/pharmacodynamics and adverse reactions/effects of available agents, and the results of local antimicrobial susceptibility testing (local antibiogram). The severity of illness and history of exposure to antimicrobials are also key factors in determining appropriate therapy. Once presumptive antimicrobial agents are selected and administered, they should be changed for more appropriate agents, based on the organisms identified and their susceptibility testing results. Continuous use of unnecessarily broader-spectrum agents should be avoided to prevent the emergence of antimicrobial resistance. Furthermore, the duration of therapy should be strictly evaluated periodically to avoid unnecessarily prolonged use of antimicrobial agents.
In this article we discuss the medical treatment strategy, including antimicrobial therapy, for acute cholecystitis. In an extensive literature search, we were faced with the fact that there were very few, if any, randomized controlled trials (RCTs) of antimicrobial therapy for acute cholecystitis. Therefore, we propose consensus-based and in vitro activities-based guidelines for empirical antimicrobial therapy for acute cholecystitis. The text is organized in a question and recommendation format.
Q1. What microbiological studies should be performed in acute cholecystitis?
Bile and blood culture should be performed at all available opportunities, especially in severe cases (recommendation B).
The clinical significance of microbial examination in acute cholecystitis depends on the severity of the disease. Although most mild and moderate cases are curable without microbial information, biliary infection is associated with postoperative complications and higher mortality rates in patients with severe cases or biliary stones. A positive bile culture is correlated with the progression of the cholecystitis to a severe form (level 2b–3b).1,2 Therefore, especially in severe cases, gallbladder bile should be collected at the time of operative, laparoscopic, or percutaneous intervention for culture and susceptibility testing. A sample of the gallbladder wall should be sent separately for culture, and for histopathology if needed. Aerobic cultures only should be obtained. Positive rates for bacterial culture in acute cholecystitis and other biliary diseases are listed in Table 1 (level 2b–3b).3–8
Table 1.
Bacterial culture positive rates in bile (%) in various biliary diseases
| Bile | Non-biliary disease | Cholelithiasis | Acute cholecystitis | Choledocholithiasis (+cholangitis) | Hepatolithiasis (+cholangitis) | |
|---|---|---|---|---|---|---|
| Chang (2002)3 | Gallbladder | 17.0 | 47.0 | 63.0 | 70.0 | |
| Csendes (1996)4, 5 | Gallbladder | 0 | 22.2 | 46.1 | ||
| Csendes (1994)6 | Gallbladder | 0 | 32.0 | 41.0 | 58.0 | |
| Maluenda (1989)2 | Gallbladder | 0 | 43.0 | |||
| Csendes (1975)7 | (Gallbladder wall) | 47.0 (Chronic; 33) | ||||
| Kune (1974)8 | Gallbladder | 0 | 13.0 | 54.0 | 59.0 |
The importance of blood culture results is relatively limited in acute cholecystitis and the presence of positive blood cultures does not alter the agents to be used or the duration of treatment.
Q2. How should antimicrobial agents be used in patients with acute cholecystitis?
Antimicrobial agents should be administered to patients diagnosed with acute cholecystitis, except for those with mild cases (recommendation A).
Patients with mild case of disease, with little abdominal pain and mild inflammatory findings, (closely mimicking biliary colic), may be observed and treated with oral antimicrobial drugs or even observed without antimicrobials. In these patients, the administration of nonsteroidal anti-inflammatory drugs (NSAIDs) is recommended, as described below.
When early cholecystectomy is performed, antimicrobial therapy may be considered prophylactic in that the infection itself is surgically removed.
Q3. Is the administration of NSAIDs to patients suffering from an attack of biliary colic effective to prevent the development of acute cholecystitis?
Administration of NSAIDs to patients with an attack of biliary colic is recommended, to prevent the onset of acute cholecystitis (recommendation A).
NSAIDs such as diclofenac or indomethacin should be used in the medical treatment for their analgesic effects and their inhibition of prostaglandin release from the gallbladder wall. An RCT of NSAID administration (75 mg diclofenac; intramuscular injection) in patients with biliary colic attack showed that the NSAID had the effect of relieving the patients’ pain and preventing the progression of the disease to acute cholecystitis (level 1b).9 Although it has been reported that NSAIDs effectively improve gallbladder function in patients with chronic cholangitis (level 3a),10 there is no report to date showing that NSAID administration after the onset of acute cholecystitis alleviates the disease.
Q4. What are the important factors for consideration in antimicrobial drug selection?
Antimicrobial activity against causative bacteria
Severity of acute cholecystitis
Presence/absence of renal and hepatic dysfunction
Patient’s past history of antimicrobial administration
Local susceptibility patterns (antibiogram)
The dose of antimicrobial agents should be reduced for patients with reduced renal function. Because most cephalosporins, penicillins, aminoglycosides, and carbapenems are excreted by the kidneys, the dose is to be reduced for patients with decreased renal function. The Sanford guide to antimicrobial therapy (2006)11 and Goodman and Gilman’s The pharmacological basis of therapeutics12 recommend that renal function be estimated by the following formula:
Predicted creatinine clearance from serum creatinine (×0.85 for females) = (140 − age)(optimum body weight kg)/(72 × serum creatinine mg/dl)
where male optimum body weight is 50.0 kg + 0.9 kg/cm (150 cm and taller) and female optimum body weight is 45.5 kg + 0.91 kg/cm (150 cm and taller)
Drug dosage should be adjusted in patients with decreased renal function. The Sanford guide to antimicrobial therapy and Goodman and Gilman’s the pharmacological basis of therapeutics should be consulted (recommendation A).
Drug dosage adjustment for ceftriaxone is not necessary in patients with renal dysfunction. By contrast, dose adjustment of ceftriaxone may be indicated in patients with severe hepatic impairment.11
If patients have a biliary obstruction that blocks the enterohepatic circulation of bile, in view of the fact that the administration of wider-spectrum antimicrobials such as third- and fourth-generation cephalosporins may replace intestinal microorganisms and disturb vitamin K absorption, which could lead to hemorrhage, vitamin K is administered intravenously as required.
Q5. Should penetration into the bile or gallbladder wall be considered important in the selection of therapeutic antimicrobials in acute cholecystitis?
There is a common belief, particularly in Japan, that antimicrobial agents with excellent penetration into the gallbladder wall should be chosen for antimicrobial therapy. There was some debate on whether penetration into the gallbladder should be considered in choosing antimicrobial agents. However, there are no clinical or experimental data to support this. For reference, Table 2 shows antimicrobial agents with good penetration of the gallbladder wall (level 3b–4).13–16
Table 2.
Intravenous antimicrobial drugs with good penetration into the gallbladder wall5
| Penicillins | Ampicillin, piperacillin, piperacillin/tazobactam |
|---|---|
| Cephalosporins | |
| 1st generation | Cefazoline |
| 2nd generation | Cefmetazole, flomoxef, cefotiam, |
| 3rd, 4th generation | Cefoperazone/sulbactam,13 ceftriaxone,14 ceftazidime, cefpirome, cefozopran |
| Fluoroquinolones | Ciprofloxacin,13 pazufloxacin |
| Monobactams | Aztreonam15 |
| Carbapenems | Meropenem, panipenem/betamipron |
| Lincosamides | Clindamycin16 |
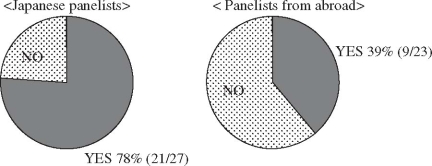
The usefulness of biliary wall penetration for the selection of therapeutic antimicrobials in acute cholecystitis is still controversial. At the Tokyo International Consensus Meeting, consensus was not obtained on this question (Fig. 1). See “Discussion” for details.
Fig. 1.
Clinical question, “Should the biliary penetration of antimicrobial agents be considered important in their selection in moderate or severe acute cholecystitis?” Responses at the International Consensus Meeting. Responses from Japanese panelists and panelists from abroad showed that 78% (21/27) and 39% (9/23), respectively, answered “Yes” to the question
Q6. What are the results of clinical trials regarding antimicrobial therapy for acute cholecystitis?
Three RCTs have evaluated the effect of antimicrobial agents in patients with acute cholecystitis (Table 3) (level 2b),17–19 and all of them demonstrated that recently developed antimicrobial drugs had effectiveness and usefulness equivalent to that of ampicillin and an aminoglycoside, which was regarded as a standard regimen for cholecystitis in the 1980s (level 4–5).20,21 Therefore, according to the clinical trials available so far, piperacillin, ampicillin and an aminoglycoside, as well as several cephalosporins, are recommended for the treatment of acute cholecystitis (recommendation A).
Table 3.
Comparative clinical tests of antimicrobial drugs in cholecystitis
| Authors | Subjects | Antimicrobial | Clinical cure rate | Significant difference |
|---|---|---|---|---|
| Muller (1987)17 | Cholecystitis | ABPC+TOB | 11/13 (85%) | |
| Piperacillin | 18/19 (95%) | NS | ||
| Cefoperazone | 19/20 (95%) | NS | ||
| Chacon (1990)18 | Cholecystitis + | Pefloxacin | 49/50 (98%) | NS |
| cholangitis | ABPC+GM | 45/47(95.7%) | ||
| Thompson (1993)19 | Cholecystitis + | Cefepime | 78/80 (97.5%) | NS |
| cholangitis | Mezlocillin+GM | 40/40 (100%) |
ABPC, ampicillin; TOB, tobramycin; GM, gentamicin
However, only one RCT focused only on acute cholecystitis. In addition, the antimicrobial agents widely used at present for acute cholecystitis, including penicillin/β-lactamase inhibitors, carbapenems, and the third- and fourth-generation cephalosporins, were not tested in these RCTs. In this regard, in the Tokyo Guidelines, we recommend alternative regimens of antimicrobial agents, as given below. A consensus on these recommendations was reacted at the International Consensus Meeting.
Q7. What are the current recommendations for antimicrobial therapy in acute cholecystitis?
Antimicrobial drugs should be selected according to the severity assessment.
Empirically administered antimicrobial drugs should be changed for more appropriate agents, according to the identified causative microorganisms and their susceptibility testing results.
Antimicrobial drugs should be selected on the basis of the severity assessment, according to the Infectious Diseases Society of America (IDSA) guidelines (level 4)22 for complicated intraabdominal infections. But there is very little evidence that supports this notion. Adequate dosages of antimicrobial drugs should be determined in each country; the issue of cost is not addressed in the Tokyo Guidelines. See “Discussion at the Tokyo International Consensus Meeting” for details.
Empirically administered antimicrobial drugs should be changed for more appropriate agents according to the identified causative microorganisms and their susceptibility testing results. There is very little evidence that supports this notion; however, at the Tokyo International Consensus Meeting, consensus in support of this notion was obtained with Japanese and overseas panelists (Fig. 2).
Fig. 2.
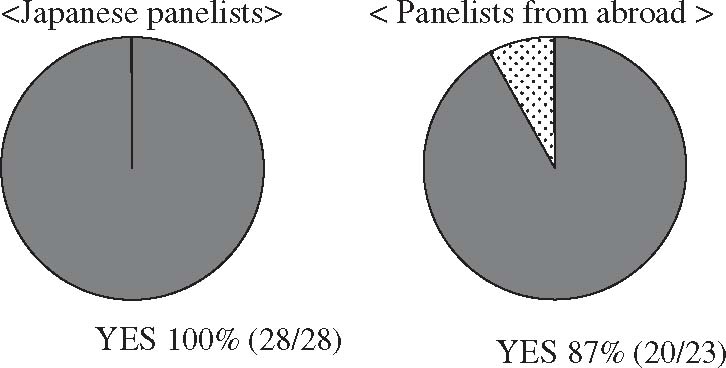
Clinical question: “Should empirically administered antimicrobial drugs be changed for more appropriate agents according to the identified causative microorganisms and their sensitivity to antimicrobials?” Responses at the International Consensus Meeting. Responses from Japanese panelists and panelists from abroad showed that 100% (28/28) and 39% (20/23), respectively, answered “Yes” to the question
Mild (grade I) acute cholecystitis
Mild (Grade I) acute cholecystitis is often caused by a single intestinal organism, such as Escherichia coli, and therefore monotherapy with one of the antimicrobial drugs listed in Table 4 is recommended. Because intestinal organisms producing β-lactamase, which are resistant to penicillins, and cefazoline, are likely to be detected, the use of penicillin/β-lactamase inhibitors, such as piperacillin/tazobactam,23 or ampicillin/sulbacta m is recommended.
Table 4.
Antibacterials for mild (grade I) acute cholecystitis
| Oral fluoroquinolones | Levofloxacin, ciprofloxacin |
| Oral cephalosporins | Cefotiam, cefcapene cefazolin |
| First-generation cephalosporins | |
| Wide-spectrum penicillin/β-lactamase inhibitor | Ampicillin/sulbactam |
Patients with mild acute cholecystitis, with relatively mild abdominal pain and mild inflammatory findings on laboratory data and imaging studies, closely mimicking biliary colic, may be observed with oral antimicrobial drugs, or even without antimicrobials.
Moderate (grade II) and severe (grade III) acute cholecystitis (Table 5)
Table 5.
Antibacterials for moderate (grade II) and severe (grade III) acute cholecystitis
| First options for moderate cases | |
| Wide-spectrum penicillin/β-lactamase inhibitors | Piperacillin/tazobactam, ampicillin/sulbactam |
| Second-generation cephalosporins | Cefmetazole, cefotiam, oxacephem, flomoxef |
| First options for severe cases | |
| Third- and fourth-generation cephalosporins | Cefoperazon/sulbactam, ceftriaxone, ceftazidime, cefepime, cefozopran |
| Monobactams | Aztreonam |
| One of above + metronidazole (when anaerobic bacteria are detected or are expected to co-exist) | |
| Second options for severe cases | |
| Fluoroquinolones | Ciprofloxacin, levofloxacin, pazufloxacin + metronidazole (when anaerobic bacteria are detected or are expected to co-exist) |
| Carbapanems | Meropenem, impenem/cilastatin, panipenem/betamipron |
For moderate (grade II) acute cholecystitis, wider-spectrum penicillins, second-generation cephalosporins, and oxacephems are recommended empirically as the drug of first choice. For patients with severe (grade III) acute cholecystitis, who are often infected with multiple and/or resistant organisms (level 2b–3b),24–26 third- and fourth-generation cephalosporins with a wider antimicrobial spectrum are recommended as the drug of first choice. Depending on the local susceptibility patterns (antibiogram), if the drug of first choice is ineffective, fluoroquinolones and carbapenems can be used.
It should be emphasized that the inappropriate use or overuse of third- and fourth-generation cephalosporins and carbapenems would likely result in the emergence of resistant bacteria.
Of note, the ratio of piperacillin to tazobactam in Japan (4 : 1) is different vs. from that used in the United States (8 : 1).
In each country, antimicrobials should be chosen from the available agents that meet the concepts and criteria discussed above and agreed on at this Consensus Meeting.
Q8. What is the appropriate antimicrobial dosing regimen?
On the basis of pharmacokinetics and pharmacodynamics, there is a significant difference between the United States and Japan in antimicrobial dosing regimens. To provide practical recommendations on antimicrobial therapy, this issue, even though it is a domestic one in Japan, needed to be discussed here as well as at the Consensus Meeting.
Regarding Japanese domestic issues about antimicrobial dosing regimens, the basic principles of antimicrobial therapy will be discussed first.
Antimicrobial therapy is classified into three types according to the purpose of the antimicrobial use. These are, presumptive or empirical therapy, definitive or specific therapy, and prophylaxis. Presumptive therapy is the antimicrobial usage when infection is suspected and causative organisms are not yet identified or when the results of microbiological studies are pending. After the microbiological testing results come back, therapy should be changed accordingly, to what is called “definitive therapy or specific therapy.” Lastly, “prophylaxis” includes either primary or secondary prevention for expected infections in the future. Of note, in our Guidelines, presumptive or empirical therapy for biliary tract infection is discussed and provided.
After the appropriate antimicrobial agents are selected, dosing regimens should be determined, on the basis of their pharmacokinetics and pharmacodynamics, to achieve the best clinical outcomes and to avoid the emergence of antimicrobial resistance. Andes et al.27 classified antimicrobial agents by their bactericidal patterns. These are either time-dependent or concentratio ndependent. Time-dependent agents are the ones whose bactericidal activities are affected by the time above the minimum inhibitory concentration (time > MIC), i.e., the duration of time that bacteria are exposed to an antimicrobial concentration above the MIC. For these agents, the time intervals of antimicrobial administration are critical for gaining an appropriate clinical response. On the other hand, for concentration-dependent agents, the peak concentration at the site of infection is important for achieving an appropriate clinical response or bactericidal activity. Time-dependent agents include β-lactams, and concentration-dependent agents with a prolonged persistent effect include fluoroquinolones, ketolides, and aminoglycosides. Concentration-dependent agents with a moderate to prolonged persistent effect include the macrolides.
In Japan, in general, for most of the available agents, significantly fewer doses per day are approved compared with practice in the Unite States. Morever, in Japan, the time intervals of administration of time-dependent agents such as β-lactams do not seem to be determined by their half-life.
These facts suggest that, in Japan, antimicrobial dosing regimens should be re-considered and/or reexamined to provide the best available therapy to achieve the best clinical outcomes for the patients. To provide appropriate dosing regimens, body size differences among Asians, Caucasians, and other ethnic groups should be addressed. At the Consensus Meeting, it was agreed that doses per kilogram (body size) should be provided, instead of the absolute number of total doses of each agent. For details see “Discussion at the Tokyo International Consensus Meeting.”
Discussion at the Tokyo International Consensus Meeting
Community-acquired or hospital-acquired biliary infections
Henry Pitt (USA): We should talk about community-acquired or hospital-acquired infections. The bacteriology of a de novo cholecystitis/cholangitis patient with the latter, most likely to have stones, is one spectrum that is typically the E. coli, Klebsiella Enterococcus, Enterobacter, but it was clear that that subset of patients had a different bacteriology with much more resistant organisms, more yeast and more methicillin-resistant Staphylococcus aureus (MRSA) and the kinds of things that are like vancomycin-resistant Enterococcus (VRE) now, that are likely to cause trouble. So we have to take that into account here as well; that there may be de novo cholangitis/cholecystitis, and then there is another group of patients that have hospital-acquired type infections.
Duration of antimicrobial therapy
Henry Pitt: I think in particular, for the acute cholecystitis, if you do a cholecystectomy, that you can often get away with a very short course, especially if it is a mild case, which meant most of the cases.
Joseph S. Solomkin (USA): The other point I will make, just to relay our experience in North America, is that there is increasing emphasis on shortened duration of therapy and the i.v. to oral switch is very helpful if you are interested in sending patients home more rapidly; it does reduce, also, the problem of needing to have an i.v. in place, and a nurse to give the infusion.
Biliary penetration
At the International Consensus Meeting, 78% (21/27) and 39% (9/23), respectively, of the Japanese panelists and the panelists from abroad answered “yes” to the clinical question: “Should the biliary penetration of antimicrobial agents be considered to be important in their selection in moderate or severe acute cholecystitis?” (see Fig. 1).
Steven Strasberg (USA): The reason why the importance of biliary penetration in Japan and overseas (especially the United States) is significantly different seems to be derived from treatment strategies in both countries. In the United States, cholecystectomy tends to be performed after diagnosis immediately, so that you can often get away with a very short course, and biliary penetration is not so important for them.
Nagai (Japan): With acute cholecystitis, the good penetration of the antimicrobial selection is nonsense; that is what I am telling to my residents.
Drug selection on the basis of severity assessment
Joseph S. Solomkin: The notion that more seriously ill patients should get different antibiotics, and that is the notion that we put into the IDSA guidelines, and I think is suggested here also; one has to realize that there is very little real evidence that works. I think it is very important to know that there really is very little, if any, evidence that that is the case. It is very reasonable to simply take an approach that targets the organism separate from the severity of illness, and I think that is one issue that might be put to this group to respond to whether these guidelines should be based on severity or not.
Drug dosage and cost
Joseph S. Solomkin: If you are making a recommendation that these guidelines should include what I would call North American dosing; or what would be your recommendation? Also, realizing the regulatory issues about drug dosing.
Harumi Gomi (Japan): That is the critical point; dosing regimens are the critical point of microbial therapy and I personally think that doses should be included in the Guidelines. Then for legal issues, I think that this is our domestic issue, and we need to ask the government or we need to make some actions to make those antimicrobial agents available for Japanese patients.
Sheung-Tat Fan (China): I wondered if dosage should be expressed in terms of the body weight — kilograms — rather than absolute amount. I see that there is a difference in body size between Asians and Americans, so there may be a difference. We have to be more realistic; that means we have to talk about the body weight, rather than absolute amount.
Harumi Gomi: For example, 30 kg — for these low-body-weight patients, we may have to use FDA-approved pediatric dosage.
Henry Pitt: I think that the issue of cost should be in our guidelines as well.
Harumi Gomi: The major reasons for proposals on appropriate dosing regimens are as follows:
Best available medical treatment with appropriate antimicrobial dosing regimens should be provided to patients, when possibile, to avoid inadequate clinical response
Overuse or unnecessary use of broader-spectrum antimicrobial agents, such as carbapenems, should be avoided in Japan.
Medical professionals in Japan should be aware of scientifically sound or appropriate antimicrobial dosing regimens on the basis of pharmacokinetics and pharmacodynamics of the agents
To make the appropriate dosing regimens available in Japan, legal action or policy-making is required.
Acknowledgment
We would like to express our deep gratitude to the Japanese Society for Abdominal Emergency Medicine, the Japan Biliary Association, and the Japanese Society of Hepato-Biliary-Pancreatic Surgery, who provided us with great support and guidance in the preparation of the Guidelines. This process was conducted as part of the Project on the Preparation and Diffusion of Guidelines for the Management of Acute Cholangitis (H-15-Medicine-30), with a research subsidy for fiscal 2003 and 2004 (Integrated Research Project for Assessing Medical Technology) sponsored by the Japanese Ministry of Health, Labour, and Welfare.
We also truly appreciate the panelists who cooperated with and contributed significantly to the International Consensus Meeting, held on April 1 and 2, 2006.
References
- 1.Pitt H, Postier R, Cameron J. Consequences of preoperative cholangitis and its treatment on the outcome of operation for choledocholithiasis. Surgery. 1983;94:447–52. [PubMed] [Google Scholar]
- 2.Maluenda F, Csendes A, Burdiles P, Diaz J. Bacteriological study of choledochal bile in patients with common bile duct stones, with or without acute suppurative cholangitis. Hepatogastroenterology. 1989;36:132–5. [PubMed] [Google Scholar]
- 3.Chang W, Lee K, Wang S, Chuang S, Kuo K, Chen J, et al. Bacteriology and antimicrobial susceptibility in biliary tract disease: an audit of 10-year’s experience. Kaohsiung J Med Sci. 2002;18:221–8. [PubMed] [Google Scholar]
- 4.Csendes A, Burdiles P, Maluenda F, Diaz J, Csendes P, Mitru N. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch Surg. 1996;131:389–94. doi: 10.1001/archsurg.1996.01430160047008. [DOI] [PubMed] [Google Scholar]
- 5.Csendes A, Mitru N, Maluenda F, Diaz J, Burdiles P, Csendes P, et al. Counts of bacteria and pyocites of choledochal bile in controls and in patients with gallstones or common bile duct stones with or without acute cholangitis. Hepatogastroenterology. 1996;43:800–6. [PubMed] [Google Scholar]
- 6.Csendes A, Becerra M, Burdiles P, Demian I, Bancalari K, Csendes P. Bacteriological studies of bile from the gallbladder in patients with carcinoma of the gallbladder, cholelithiasis, common bile duct stones and no gallstones disease. Eur J Surg. 1994;160:363–7. [PubMed] [Google Scholar]
- 7.Csendes A, Fernandez M, Uribe P. Bacteriology of the gallbladder bile in normal subjects. Am J Surg. 1975;129:629–31. doi: 10.1016/0002-9610(75)90334-7. [DOI] [PubMed] [Google Scholar]
- 8.Kune G, Schutz E. Bacteria in the billary tract. A study of their frequency and type. Med J Aust. 1974;1:255–8. [PubMed] [Google Scholar]
- 9.Akriviadis E, Hatzigavriel M, Kapnias D, Kirimlidis J, Markantas A, Garyfallos A. Treatment of biliary colic with diclofenac: a randomized, double-blind, placebo-controlled study. Gastroenterology. 1997;113:225–31. doi: 10.1016/S0016-5085(97)70099-4. [DOI] [PubMed] [Google Scholar]
- 10.Goldman G, Kahn P, Alon R, Wiznitzer T. Biliary colic treatment and acute cholecystitis prevention by prostaglandin inhibitor. Dig Dis Sci. 1989;34:809–11. doi: 10.1007/BF01540262. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert D, Moellering R, Jr, Sande M. The Sanford guide to antimicrobial therapy. 36th ed. Hyde Park, VT: Antimicrobial Therapy; 2006. [Google Scholar]
- 12.Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill; 2006. [Google Scholar]
- 13.Leung J, Ling T, Chan R, Cheung S, Lai C, Sung J, et al. Antibiotics, biliary sepsis, and bile duct stones. Gastrointest Endosc. 1994;40:716–21. [PubMed] [Google Scholar]
- 14.Orda R, Berger S, Levy Y, Shnaker A, Gorea A. Penetration of ceftriaxone and cefoperazone into bile and gallbladder tissue in patients with acute cholecystitis. Dig Dis Sci. 1992;37:1691–3. doi: 10.1007/BF01299860. [DOI] [PubMed] [Google Scholar]
- 15.Martinez O, Levi J, Devlin R. Biliary excretion of aztreonam in patients with biliary tract disease. Antimicrob Agents Chemother. 1984;25:358–61. doi: 10.1128/aac.25.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sales J, Sutcliffe M, O’Grady F. Excretion of clindamycin in the bile of patients with biliary tract disease. Chemotherapy. 1973;19:11–5. doi: 10.1159/000221434. [DOI] [PubMed] [Google Scholar]
- 17.Muller E, Pitt H, Thompson JJ, Doty J, Mann L, Manchester B. Antibiotics in infections of the biliary tract. Surg Gynecol Obstet. 1987;165:285–92. [PubMed] [Google Scholar]
- 18.Chacon J, Criscuolo P, Kobata C, Ferraro J, Saad S, Reis C. Prospective randomized comparison of pefloxacin and ampicillin plus gentamicin in the treatment of bacteriologically proven biliary tract infections. J Antimicrob Chemother. 1990;26(Suppl B):167–72. doi: 10.1093/jac/26.suppl_b.167. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JJ, Bennion R, Roettger R, Lally K, Hopkins J, Wilson S. Cefepime for infections of the biliary tract. Surg Gynecol Obstet. 1993;177(Suppl):30–4. [PubMed] [Google Scholar]
- 20.Boey J, Way L. Acute cholangitis. Ann Surg. 1980;191:264–70. doi: 10.1097/00000658-198003000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson JJ, Tompkins R, Longmire WJ. Factors in management of acute cholangitis. Ann Surg. 1982;195:137–45. doi: 10.1097/00000658-198202000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomkin J, Mazuski J, Baron E, Sawyer R, Nathens A, DiPiro J, et al. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis. 2003;37:997–1005. doi: 10.1086/378702. [DOI] [PubMed] [Google Scholar]
- 23.Investigators of the Piperacillin/Tazobactam Intra-abdominal Infection Study Group Results of the North American trial of piperacillin/tazobactam compared with clindamycin and gentamicin in the treatment of severe intra-abdominal infections. Eur J Surg. 1994;573(Suppl):61–6. [PubMed] [Google Scholar]
- 24.Marne C, Pallares R, Martin R, Sitges-Serra A. Gangrenous cholecystitis and acute cholangitis associated with anaerobic bacteria in bile. Eur J Clin Microbiol. 1986;5:35–9. doi: 10.1007/BF02013458. [DOI] [PubMed] [Google Scholar]
- 25.Claesson B, Holmlund D, Matzsch T. Microflora of the gallbladder related to duration of acute cholecystitis. Surg Gynecol Obstet. 1986;162:531–5. [PubMed] [Google Scholar]
- 26.Nielsen M, Justesen T. Anaerobic and aerobic bacteriological studies in biliary tract disease. Scand J Gastroenterol. 1976;11:437–46. [PubMed] [Google Scholar]
- 27.Andes D, Anon J, Jacobs MR, Craig WA. Application of pharmacokinetics and pharmacodynamics to antimicrobial therapy of respiratory tract infections. Clin Lab Med. 2004;24:477–502. doi: 10.1016/j.cll.2004.03.009. [DOI] [PubMed] [Google Scholar]