Cyclosporin neurotoxicity may result from interaction with chemotherapy used for transplant conditioning1,2 and to reverse multidrug resistance.3 We describe a case of neurotoxicity after chemotherapy for post-transplantation lymphoproliferative disease.
A 9 year old boy presented with monoclonal post-transplantation lymphoproliferative disease six years after cardiac transplantation for congenital heart disease. He was taking azathioprine and cyclosporin (Neoral) 90 mg twice daily (6 mg/kg/day) with whole blood trough concentrations between 29 μg/l and 288 μg/l (Behring enzymatic assay) in the six months before diagnosis. Azathioprine treatment was discontinued, cyclosporin was reduced to 70 mg twice daily, and three courses of low dose chemotherapy were given: vincristine 1.5 mg/m2, cyclophosphamide 300 mg/m2, and 7 days of prednisolone 60 mg/m2. Five triple intrathecal injections of methotrexate and hydrocortisone 15 mg and cytarabine 30 mg were also given. This was followed by high dose treatment: methotrexate 1 g/m2, cyclophosphamide 2 g/m2, vincristine, prednisolone, and one triple intrathecal injection as before.
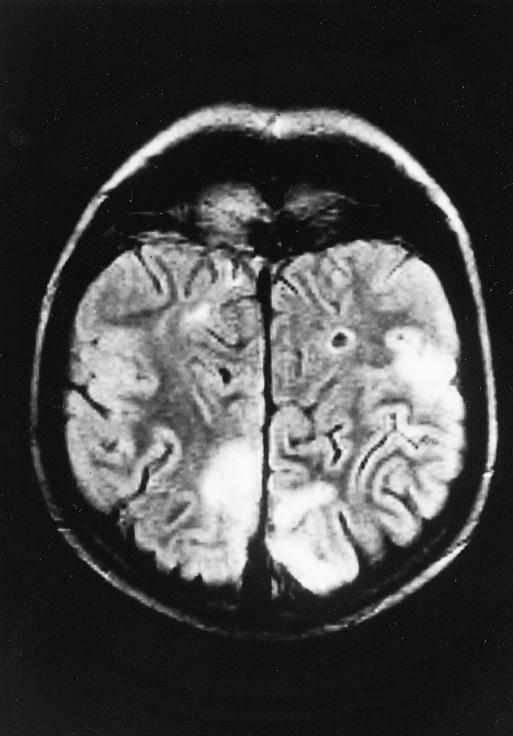
Six days after starting high dose chemotherapy he presented with headache, fever (38°C), seizures, and visual agnosia. Abnormal serum concentrations included urea 11.9 mmol/l (2.5-6.4 mmol/l), magnesium 0.56 mmol/l (0.6-1.0 mmol/l), alanine aminotransferase 156 IU/l) (<31 IU/l), and bilirubin 23 μmol/l (3-19 μmol/l). His cyclosporin concentration was 250 μg/l and his methotrexate concentration was 0.1 μmol/l (<0.1 μmol/l). An electroencephalogram showed diffuse slow wave activity, but a cranial contrast enhanced computed tomogram and results of lumbar puncture were both normal. Cyclosporin was discontinued and intravenous magnesium, broad spectrum antibiotics, aciclovir, phenobarbital, and diazepam were given. His condition improved, but concern over the possibility of rejection led to reintroduction of cyclosporin at the previous dose. Three days later he became confused, with auditory and visual hallucinations, hypertension, a parkinsonian-type tremor, and rigidity. His cyclosporin concentration was 219 μg/l and serum magnesium was 0.7 mmol/l. Magnetic resonance imaging of his brain showed abnormal, symmetrical non-enhancing signals throughout the parietal and occipital lobes (figure). Cyclosporin neurotoxicity was diagnosed.1–3 The drug was withdrawn and his neurological abnormalities resolved. Three further courses of chemotherapy were given with no clinical evidence of rejection.
To our knowledge, neurotoxicity has not been reported for cyclosporin when given with chemotherapy for post-transplantation lymphoproliferative disease. The temporal association between chemotherapy and the onset of symptoms implies that neurotoxicity was related to an interaction between cyclosporin and high doses of cyclophosphamide,1 perhaps through inhibition of cytochrome P-450 enzymes4, and possibly to prednisolone,2 vincristine,3 and methotrexate.5 We advise clinicians to be cautious if continuing cyclosporin during chemotherapy as normal concentrations do not preclude toxicity.
Figure.
Magnetic resonance scan of head showing abnormal signal throughout cortex and white matter of the parietal and occipital lobes
References
- 1.Ghany AM, Tutschka PJ, McGhee RB, Avalos BR, Cunningham I, Kapoor N, et al. Cyclosporine-associated seizures in bone marrow transplant recipients given busulphan and cyclophosphamide preparative therapy. Transplantation. 1991;52:310–315. doi: 10.1097/00007890-199108000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Reece DE, Frei-Lahr DA, Shepherd JD, Dorovini-Zis K, Gascoyne RD, Graeb DA, et al. Neurologic complications in allogeneic bone marrow transplant patients receiving cyclosporin. Bone Marrow Transplant. 1991;8:393–401. [PubMed] [Google Scholar]
- 3.Bertrano Y, Capdeville R, Balduck N, Phillipe N. Cyclosporin A used to reverse drug resistance increases vincristine neurotoxicity. Am J Hematol. 1992;40:158–159. doi: 10.1002/ajh.2830400222. [DOI] [PubMed] [Google Scholar]
- 4.Leblanc GA, Waxman DJ. Mechanisms of cyclophosphamide action on hepatic P-450 expression. Cancer Res. 1990;50:5720–5726. [PubMed] [Google Scholar]
- 5.O’Sullivan DP. Convulsions associated with cyclosporin A. BMJ. 1985;290:858. doi: 10.1136/bmj.290.6471.858-a. [DOI] [PMC free article] [PubMed] [Google Scholar]