Abstract
A mechanism of how polyanions influence the channel formed by Staphylococcus aureus α-hemolysin is described. We demonstrate that the probability of several types of polyanions to block the ion channel depends on the presence of divalent cations and the polyanion molecular weight and concentration. For heparins, a 10-fold increase in molecular weight decreases the half-maximal inhibitory concentration, IC50, nearly 104-fold. Dextran sulfates were less effective at blocking the channel. The polyanions are significantly more effective at reducing the conductance when added to the trans side of this channel. Lastly, the effectiveness of heparins on the channel conductance correlated with their influence on the ζ-potential of liposomes. A model that includes the binding of polyanions to the channel-membrane complex via Ca2+-bridges and the asymmetry of the channel structure describes the data adequately. Analysis of the single channel current noise of wild-type and site-directed mutant versions of α-hemolysin channels suggests that a single polyanion enters the pore due to electrostatic forces and physically blocks the ion conduction path. The results might be of interest for pharmacology, biomedicine, and research aiming to design mesoscopic pore blockers.
Introduction
Glycosaminoglycans, structurally heterogeneous polyanions (PAs), contribute to a wide range of physiological processes including recognition, adhesion, membrane transport, antibacterial defense, and virus entry (1,2). They interact with phosphatidylcholine in the presence of Ca2+ (3,4) and are implicated in ion channel and receptor regulation (e.g., (5,6)). Moreover, there are reports indicating a size-dependent interaction of PAs with ion channels and membranes (7,8). However, the detailed mechanism of PA influence on ion channel function is poorly understood. To address this issue, we studied the effects of two types of differently sized PAs on the ionic conductance of a mesoscopic ion channel formed by Staphylococcus aureus α-hemolysin (αHL) in planar lipid bilayer membranes. The αHL channel is ideally suited for these studies because its three-dimensional structure, determined by x-ray crystallography (9), is consistent with many different experimental techniques (10,11).
We show here that the ability of the PA to block the αHL channel conductance depended upon the PA properties (i.e., structure, size, and type). The interaction appears to occur in two steps.
First, PA most likely interacts with the αHL channel-membrane complex via Ca2+-bridges, resulting in a nonlinear gradient of PA near the complex. Because of the difference in number of charges and molecular weight, the PA distribution, close to the channel entrances, depends upon its size.
Second, upon occasion, a polyanion, approaching a channel entrance by diffusion, is forced either into or away from the pore by the transmembrane electric field.
Linear heparins, which are more flexible (12) than branched dextran sulfates (DS), were found significantly more effective than DS of the same molecular weight. The differences in both the distance between the membrane surface and the two pore entrances, and the asymmetric distribution of charges in the two halves of the αHL channel, determine the strongly pronounced side-dependent difference in PA effectiveness.
Materials and Methods
αHL proteins
Wild-type αHL, which contains no cysteines (13), was purchased from Calbiochem (San Diego, CA). Point cysteine-substitution αHL mutants were prepared as previously described (14).
Chemicals
5,5′-Dithio-bis-(2-nitrobenzoic acid) (DTNB); dithiothreitol (DTT); phosphatidylcholine (PC, Type V-E); poly(ethylene glycol) with average molecular mass of 35,000 g/mol; bovine serum albumin; cholesterol; heparin-albumin (H-0403; HepAlb, 4.8 mol heparin coupled to 1 mol bovine serum albumin); heparins (as Na-salt) from porcine intestinal mucosa with average molecular mass of 18,000 g/mol (most chains in the range 17,000–19,000 Da, H-9399, Hep); 6000 g/mol (H-5284, Hep6000); 3000 g/mol (H-3400, Hep3000); heparin disaccharides III-S with molecular mass of 563 g/mol (H9392, HepDi); dextran sulfates with average molecular mass of 500,000 g/mol (D-6001, DS500); 10,000 g/mol (D-6924, DS10); and 5000 g/mol (D-7037, DS5); and agarose, CaCl2, EDTA, TRIS, and HCl, were purchased from Sigma (St. Louis, MO). 1,2-Diphytanoyl-sn-glycero-3-phosphatidylcholine (DiPhyPC) was purchased from Avanti Polar Lipids (Alabaster, AL). 2-(trimethylammonium) ethyl methanethiosulfonate (MTSET) was purchased from Toronto Research Chemicals (North York, Ontario, Canada). All reagents were used as purchased. Milli-Q plus 18 MΩ cm deionized water (Millipore, Bedford, MA) was used to prepare all aqueous buffer solutions. Unless stated otherwise, the solution contained 50 mM CaCl2, 1 mM EDTA, 10 mM TRIS-HCl, at pH 7.5. Such composition of the solution was chosen to make the effects of PAs more expressive.
Electrophysiological measurements
Planar lipid bilayer membranes for multi-channel experiments were formed via the painting technique (15) from a mixture of PC/Chol (1:1, w/w) across a hole (∼0.3 mm diameter) in a 25-μm-thick Teflon partition in a Teflon cell. In single channel experiments, the bilayers were formed from DiPhyPC by the solvent-free method (16). The ionic current was measured at room temperature (25 ± 2°C) using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) in the voltage-clamp mode. The current was converted to voltage, filtered by a low-pass eight-pole Butterworth filter, digitized with a sampling frequency of 0.5 kHz (for multichannel experiments) or 50 kHz (for single channel recording), stored on a computer and analyzed off-line with the Whole Cell Electrophysiology Program (WCP V1.7b, J. Dempster, University of Strathclyde, Glasgow, UK) and/or the Electrophysiology software developed by Dr. M. A. Pustovoit (Petersburg Nuclear Physics Institute, Gatchina, Russia).
Channels were formed by adding several microliters of the αHL stock solution (5–50 μg/mL) to one side of the chamber (herein defined as cis). Each step in current corresponds to the formation of a single, slightly anion-selective channel (17). The mean value of single-channel insertion current was 4.8 ± 0.3 pA for 50 mM CaCl2 and 40 mV applied potential. The potential was defined as positive when it was greater at the side of the protein addition. At pH 7.5, αHL channels are usually in a high conductance state and rarely switch to low conductance states (18).
For multichannel experiments, the contents of the cis side were replaced by αHL-free solution to keep the number of channels constant. To test the PA blocking effect, the transmembrane voltage was then increased stepwise from 0 to ± 100 mV. Control current traces (i.e., with no PA in the bath) showed no significant channel closure (Fig. 1, C and D). However, in the presence of PA, 100 mV applied potential caused relatively rapid current reduction. The decrease in current is described by a single exponential function (Fig. 1, C and D). The characteristic time constant of the current decay, τ, was used to estimate the action of the PA on the channel. The half-maximal inhibitory polyanion concentration was defined as IC50.
Figure 1.
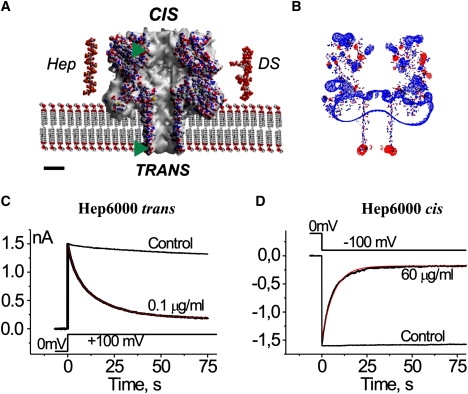
Representations of (A) the αHL channel, heparin, and DS used in this work, (B) the charge distribution at the αHL channel, and (C and D) the side-dependent heparin effect. (A) Cross section through the αHL channel (Protein Data Bank, 7AHL.pdb) embedded in membrane. Structure of the dodecameric heparin (Protein Data Bank, 1HPN.pdb; molecular mass of 3453.64 g/mol) and eventual structure of the hexadecameric DS (molecular mass of 5009.56 g/mol) were built with Chem3D (CambridgeSoft). (Abbreviations: Hep, heparin; DS, dextran sulfate.) The atoms are represented using the following color codes: O (which possesses a negative charge) in red, C in gray, H in white, N (which possesses a positive charge) in blue, and S in yellow. (Green triangles) Levels where the novel Cys and its charged derivatives are located in the channel structure. Scale bar is 2 nm. (B) The charge distribution was calculated using Coulomb calculation method (Swiss-PDBViewer, Ver. 3.7) assuming solvent ionic strength of 0.15 mol dm−3, equal to that of the 50 mM CaCl2 solution mainly used in this study. The isopotential contouring values, equal to 1.8 kT/e, are shown (red, positive potentials; blue, negative potentials). (C and D) The current decays after application of 100 mV steps to multichannel bilayers in the presence of Hep6000 at the trans (C) and cis (D) compartments of the experimental chamber. (Red lines) Best-fit of a single exponential function. Control traces (no PA) are shown for comparison. Concentrations of Hep6000, voltage protocols, current, and timescales are given in the figure. Note that the current inhibition is not total. All other conditions for the experiment are described in Materials and Methods.
Liposomes
Liposomes were formed by following a standard procedure (19). Briefly, 2 mg PC dissolved in Folch solution was transferred to a round-bottom glass flask, and subsequently dried with a nitrogen stream to form a thin film. One milliliter of the aqueous solution was added to the flask and vigorously shaken to remove the lipid film from the flask wall. Finally, the mixture was sonicated (Mini-som; Thornton INPEC Eletrónica, Vinhedo, Brazil) to yield an aqueous vesicle suspension (2 mg/mL), which was used the same day. The size distribution of the phospholipid vesicles (580 ± 50 nm mean diameter) was measured using a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, Worcestershire, UK).
The binding of charged species to the membrane alters the surface and the ζ-potentials; therefore, measurement of changes in the liposome ζ-potential provides a simple and sensitive method to quantitate the binding of inorganic and organic compounds to the membrane (4,20). The ζ-potential was determined directly from the measured electrophoretic mobility with the Zetasizer Nano ZS90 (Malvern Instruments). The experiments were performed in the absence of αHL to determine the degree to which PAs bind to the membrane, and at a low-ionic-strength aqueous solution (3 mM CaCl2, 1 mM Tris-HCl, at pH 7.5) to enhance the measurement sensitivity.
Chemical modification
In this set of experiments, the standard solution also included 1–2 mM DTT. The sulfhydryl-specific reagents used in this study are small water-soluble substances with fixed positive or negative charges. They react with cysteine side chains exposed to the aqueous phase. The reagents were usually applied to both compartments of the planar bilayer chamber at 0.5 mM (DTNB) or 1.0 mM (MTSET) greater than the concentration of SH-groups of DTT. The reaction of these reagents with a reduced cysteine side chain converts the −SH group to −SS-R, where R is the charged moiety, −C6H3NO2COO- (DTNB) or −CH2CH2N+(CH3)3 (MTSET).
Artwork
The longitudinal sections of the αHL channel (Protein Data Bank 7AHL.pdb) were visualized with Swiss-PDBViewer, Ver. 3.7 (21). CS Chem3D Pro (CambridgeSoft, Cambridge, MA) was used to build the heparin (1HPN.pdb) and DS structures.
Statistical analysis
Data are reported as the mean ± SD obtained in 3–7 independent experiments.
Results and Discussion
Structural properties of the channel and polyanions
The αHL channel is ∼10-nm long (Fig. 1 A). It is comprised of both a large cap domain that contains a vestibule with a maximum diameter of ∼4.6 nm and an anti-parallel β-barrel stem region that spans the membrane (9). Both ends of the stem region contain a ring of acidic or basic residues separated by a 4-nm section of neutral amino acids. The cap and stem are separated by a constriction with a diameter of 1.2–1.4 nm (9,11,22). Both entrances of the channel are relatively large (diameter ∼2.6 nm). The cis pore entrance extends 4–5 nm from the membrane surface, whereas the trans entrance is adjacent to the membrane-solution interface. The channel possesses an asymmetric electrostatic potential distribution (Fig. 1 B) with excess negative charge at the trans entrance and positive charge at the cis entrance.
Heparin polymers are composed of disaccharide units that each contain ∼3.5 negative charges (23). Nuclear magnetic resonance suggests that heparin is a relaxed polymer with a diameter of ∼1.3 nm (24), which is less than that of both pore entrances to the αHL channel (Fig. 1 A). Thus, heparin should be able to enter the pore.
Dextran sulfates contain up to three sulfate groups per glucosyl residue. It is composed of ∼95% α-D-(1,6) linkages. The remaining (1,3) linkages cause branching of the polymer. Estimates for the branch length vary from <3 (25) to >50 glucose units (26). In low-ionic-strength solutions, electrostatic repulsion of the negatively charged sulfate groups will cause the branches to be extended. The diameter of a given branch suggests that it could enter the pore. However, the branched structure of DS (Fig. 1 A) precludes the entry of the entire molecule in the pore.
Multiple channel experiments: sidedness
We previously demonstrated that heparin (Na-salt; Hoffmann-La Roche, Nutley, NJ, with a molecular mass between 10,000 and 80,000 g/mol) blockade of the αHL channel is side- and voltage-dependent, and requires divalent cations (27). Specifically, the heparin blocked the pore only when the sign of the applied potential drives negative ions into the pore. We further explored these findings and found that it is true for all PAs reported here.
There was a significant difference in PA effectiveness, depending on the side to which they were added. Fig. 1, C and D, shows that PA added to the trans side were more effective at reducing the channel conductance. The time courses for the current decay were closely similar for 0.1 μg/mL and 60 μg/mL Hep6000 added to the trans and cis sides, respectively. Similar sidedness effects were obtained with the other used PAs.
Conceivably, the sidedness effect may be due to an asymmetry in the fixed charge distribution in the channel (Fig. 1 B). The more positively charged cis entrance and the more negatively charged trans side, respectively, should be more attractive or repulsive, respectively, to PA. Thus, one would conclude that PA should be more effective in blocking the αHL channel when added to the cis side. However, the data suggests otherwise.
There are several possibilities to explain the sidedness. Because heparin was only effective against the αHL channel in the presence of divalent cations, we previously suggested that those cations form bridges between the sulfate groups of PA and negatively charged side chains in the αHL channel (27). In this case, the negative electrostatic charges at the trans entrance may serve as the point of interaction with PA, making this entrance more sensitive to PA than the positively charged cis one. Alternatively, the difference in the distance of the entrances of the αHL channel from the membrane surface (Fig. 1 A) might be the cause of the sidedness effect (the trans entrance is much closer to the membrane than is the cis entrance). The membranes used in our study were made from the zwitterionic lipid, phosphatidylcholine, and their surface potential is nearly zero over a large pH range (28). However, divalent cations (e.g., Ca2+) interact with the negatively charged phosphate groups of phospholipids and introduce a positive charge at the membrane surface (29). This action could provide a Ca2+ bridge formation between phosphate groups of PC and the sulfate groups on the GAG molecules (30) as well as an increase in concentration of negatively charged PA at membrane-solution interfaces. In turn, this negatively charged PA concentration-increase should increase the probability of the channel block by PAs.
Differently sized heparins
To clarify the mechanism of PA influence on αHL channels, experiments with differently sized heparins and DS were performed. All PAs used demonstrated potent inhibitory activities against αHL channel (Fig. 2). The ability of heparins to block the channel depended on the side to which they were added, and such ability increased with their concentration and size (Fig. 2, A, B, and D and Table S1, which is summarizing the results and published as Supporting Material).
Figure 2.
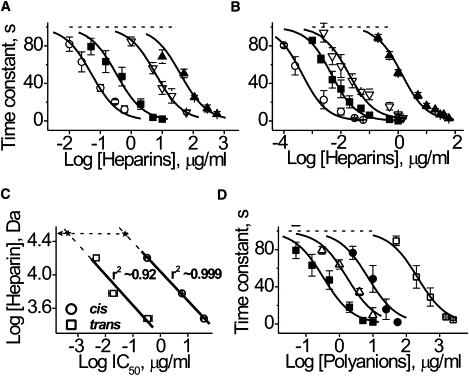
Influence of polyanions on αHL channel current reduction. αHL channel blockage as a function of heparin's concentration in the (A) cis or (B) trans compartment of the chamber. (Solid line) Best-fit of a one-site-binding equation. (Symbols: ○, HepAlb; ■, Hep; ▿, Hep6000; and ▴, Hep3000.) (C) The interrelation between molecular weight and IC50 of heparin. (Solid line) First-order regression through the points. (Dashed lines) Extrapolations of the dependences to experimentally established values of IC50 to HepAlb (x). (Arrow) Apparent molecular weight of heparin in HepAlb. The value r is a correlation coefficient. (D) The influence of DS (presented in the cis compartment of the chamber) on αHL channel blockage. Effect of the Hep is shown for comparison. (Solid line) Best-fit of a one-site-binding equation. (Symbols: ■, Hep; ▵, DS500; ●, DS10; and □, DS5.) Data are reported as means ± SD obtained in 5–7 independent experiments like those shown in Fig. 1, C and D.
The IC50 values were: 38,600 ± 6800 and 1350 ± 150 ng/mL (Hep3000); 6100 ± 400 and 19.1 ± 6.2 ng/mL (Hep6000); 330 ± 50 and 4.6 ± 0.3 ng/mL (Hep); and 52 ± 6 and 0.44 ± 0.07 ng/mL (HepAlb), added to the cis and the trans sides, respectively. The smallest heparin, HepDi, had virtually no effect on the αHL channel current, even at 2 mg/mL. Thus, despite their lesser diffusion coefficients, the larger heparins are more effective and caused the channel current to decay more rapidly. The heparins used in this study ranked as follows in order of decreasing effectiveness: HepAlb > Hep > Hep6000 > Hep3000 ≫ HepDi.
The dependence of the IC50 on heparin molecular weight was linear in log-log plot (Fig. 2 C). A 10-fold increase in the polyanion size increased the polyanion's effectiveness >1000-fold. Extrapolation of a linear regression, obtained at the cis and trans polyanion additions to the experimental value of IC50 of the HepAlb complex (symbolized as a star in Fig. 2 C), suggests that HepAlb acts as a polyanion with an effective molecular mass of 31,000 ± 2000 g/mol. This is approximately twofold greater than the average molecular mass of a single Hep bound to Alb, but less than the sum all of 4–5 Hep molecules linked to Alb (i.e., 72,000–90,000 g/mol, assuming an average Hep molecular mass of 18,000 g/mol).
The high effectiveness of HepAlb (IC50 = 52 ± 6 ng/ml; added to cis) warranted further study. To better understand its interaction with the channel, additional experiments were performed in which Hep was not chemically coupled to Alb but only mixed with it at the same molar ratio. The results demonstrate that the Hep-Alb mixture is approximately eight times less effective in blocking the αHL channel (IC50 = 2500 ± 500 ng/mL) than Hep itself (IC50 = 330 ± 50 ng/mL), which in turn, is approximately six times less potent than HepAlb. Thus, Alb when mixed with Hep even inhibits the latter's action. This effect of Alb might be due to its ability to bind heparin electrostatically (31) in a proportion corresponding to 3–4 Alb bound per Hep molecule, based on a molecular mass of 18,000 g/mol. Alb could bind calcium ions, most likely, via ionized carboxyl groups (32), and then absorb at lipid bilayers, possibly via hydrophobic interactions (33). These hydrophobic interactions may result in covering of the membrane surface, thereby preventing its interaction with Hep—which, in turn, leads to decreased PA concentration near the membrane surface and decreased effectiveness. Thus, Hep chemically coupled to Alb does not act as a simple mixture but as a one-piece heparin with a high molecular weight. The size of the Alb (5 × 8 × 9 nm, 1BM0.pdb) is considerably larger than the size of the αHL channel entrances. Hence, HepAlb need not be transported completely through the pore to elicit its effect.
Differently sized dextran sulfates
Other PAs (e.g., DS) also cause the αHL channel current to decrease (Fig. 2 D). The effectiveness of DS species increases with their size: the IC50 values were 220.0 ± 30.0 μg/mL (DS5), 7.2 ± 1.5 μg/mL (DS10), and 1.7 ± 0.3 μg/mL (DS500). DS have a larger negative charge density than the heparins, but they were significantly less effective against the αHL channel. The difference between PAs topologies most likely accounts for this result. Specifically, DS are branched polymers, whereas heparins are linear. In addition, heparins are presumably highly flexible because of the iduronate residues in their structure, unlike other pyranose rings, can adopt a range of different ring conformations such as chair, 1C4 or 4C1, or skew-boat, 2S0, depending on the nature of adjacent residues and the microenvironment (34).
Single channel experiments
As it was shown above, all PAs decreased the current through a membrane containing multiple channels. To better understand this effect, we performed experiments with single channels. As an example, Hep3000 reduces the pore conductance to one much lower conductance state (Fig. 3, A and C). As expected from the multichannel recordings, the time constant for PA to block the single channel conductance is inversely proportional to the applied potential (Fig. 3 B) and is dependent on PA concentration (i.e., the greater the concentration, the more likely the channel is in the blocked state). The amplitude of the current reduction caused by Hep3000 is side-dependent (Fig. 3 C). Specifically, the conductance reduction is greater for heparin added to the trans side. This result is consistent with the channel structure: the trans region of the pore is narrower than the cis region. The other PAs used act similarly (data not shown).
Figure 3.
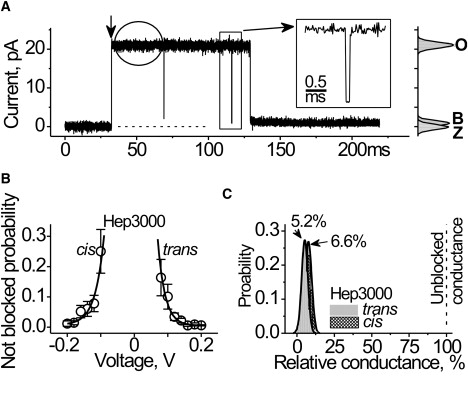
Heparin effects at the single channel level. (A) Single αHL channel. The representative current trace was recorded at 140 mV. Hep3000 (390 μg/mL) was placed at the trans side. Recording was performed at 0.02-ms resolution. (Vertical arrow) Channel insertion. (Rectangle) A single short-blockage-event shown in higher time-resolution (inset). (Circle) Example of the trace used in current noise analysis. The respective all-point histograms are shown at the right, where Z denotes the current through unmodified lipid bilayer, and O and B correspond to the open and blocked channel levels, respectively. (B) Probability to find the channel in fully open state in the presence of Hep3000 (130 μM) at the cis or at the trans compartment. (Solid lines) Best-fit of a first-order exponential function. An e-fold change in the probability of being open was observed for change in every 24 mV. (C) Conductance histograms of the residual conductance of the channel under influence of Hep3000. The histograms were comprised from the observation of the approximately 100 blockage events shown in panel A. Bin width was 4 pS. (Solid line) Best-fit of a single normal distribution to the most probable conductance values for Hep3000 acting from the trans and the cis entrances of the αHL channel.
The PA-induced αHL channel conductance block resembles the channel gating observed at low pH and higher applied potential (18). However, there is a significant difference. The gating caused by pH and high voltages exhibits several low conductance states. In contrast, the blocked conductance distribution caused by heparin is unimodal (Fig. 3 C). The results above are not consistent with a structural rearrangement of the channel structure caused by the transmembrane potential and facilitated by PA. On the contrary, the results suggest that heparins reduce the channel conductance by blocking the ion conduction pathway in the pore.
The data shown in Fig. 3 A demonstrate that heparin molecules interact with the pore at different timescales. Specifically, there are transient and long-lived current blockades. We performed a spectral analysis of the current noise (35) to estimate the dynamics of the more rapid polyanion-channel interactions. The source of useful signals in this case would be the random fluctuations in the number of heparin molecules in immediate proximity to the channel entrance, i.e., the access resistance (36) and/or in the channel pore (35). The background and PA signals were the current of the fully open αHL channel in the absence and presence of heparins (i.e., excluding the large conductance transients), respectively (e.g., see the circle in Fig. 3 A). Heparin increases the αHL current noise (Fig. S1 in the Supporting Material) and the increase is side- and voltage-dependent. The effect, albeit not great, suggests that heparin molecules passed several times in the immediate proximity of the channel entrance before entering and plugging the pore.
Cysteine-substitution mutants
Our objective was to determine the significance of electrostatic force in a polyanion's effective ability to block the αHL channel. Genetically engineered versions of αHL with novel charges located at the channel entrances were used for this purpose. Because the primary sequence of wild-type αHL has no cysteines, we used two point cysteine mutants (I7C and T129C, with cysteine located close to the cis and trans pore entrances, respectively; green triangles in Fig. 1 A). We subsequently chemically modified these cysteine side chains with small water-soluble sulfhydryl-specific reagents to add either a full positive or a negative charge at each cysteine side chain. Because the αHL channel is a heptamer, these agents added up to seven positive or negative charges in the pore lumen.
Theoretical considerations suppose that the conversion of the weak negative charge on cysteine to a strong negative or positive value, causes significant changes in the electrostatic potential distribution that is spread evenly at the opposite channel entrance (Fig. S2 in the Supporting Material). Such changes in the potential might affect the polyanion concentration near both channel entrances. As a result, the change in the effectiveness of PA could be expected not only at the side of the charge addition but at the opposite side as well.
Experiments showed that when full fixed charges are added to the channel entrances, the effect of PA on the channel conductance is markedly altered (Fig. 4). Moreover, the effect of added charges on the trans side is felt on the other (cis) side of the channel, and vice versa. For example, adding a single novel negative charge per monomer at position 7 significantly increased the time constant of heparin-induced channel blockade Hep6000 at the cis side and −100 mV applied potential (Fig. 4 A). A small, but visible increase in the time constant was found with Hep6000 added to the trans side and +100 mV applied potential (Fig. 4 B). A novel positive charge at the position causes the opposite effect, i.e., an increase in heparin's effectiveness (a decrease in the time constant) when it was added to the either side (Fig. 4, A and B). Qualitatively similar, but stronger effects, were observed for charges added at the trans entrance, where the added charges greatly affect the heparin effectiveness from the trans side and weakly affect them from the cis (Fig. 4, C and D). Thus, in agreement with the estimated electrostatic potential distribution (Fig. S2 in the Supporting Material), the novel charges alter the current blocking effect of PA not only at the modified αHL channel entrance but also at the opposite entrance (Fig. 4). The results are not consistent with Ca2+ bridge formation between the negatively charged residues of the pore lumen and sulfate groups of PA. Instead, they suggest the electrostatic potential, generated by these charged residues, modifies the effective concentration of heparin near the pore entrances and that the last step of the polyanion-channel interaction (blocking) is electrostatic in nature. Qualitatively similar results were observed with other PA used (data not shown).
Figure 4.
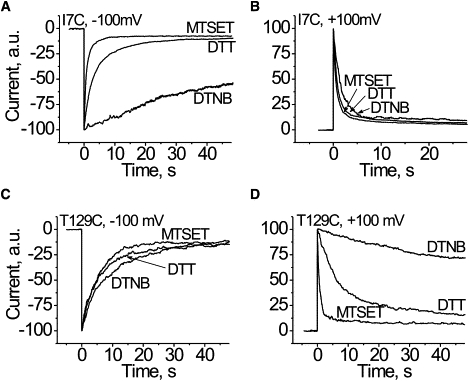
Influence of the strong positive or negative charge at the lumen of the channel close to the cis (A and B; I7C) or the trans (C and D; T129C) αHL channel entrance on the heparin effects. Multichannel lipid bilayers were used. Maximal value of the current, seen soon after potential shift from zero mV, was taken as 100 arbitrary units. Hep6000 was used at concentration of ∼4 μg/mL at the cis compartment (A and C) when voltage was switched from 0 to −100 mV. In experiments with 0–100 mV voltage switch, Hep6000 was used at a concentration of ∼0.4 μg/mL at the trans compartment (B and D). The traces labeled DTT are the Cys mutants kept in a reduced form. The reagents used for derivatization of cysteine (DTNB, MTSET) are given in the figure.
Liposomes
PA appears to enter and occlude the αHL channel. However, the question of why the larger molecular weight PA is so effective at reducing the channel conductance (Fig. 2) remains. It was conceivable that PA accumulates in the channel access resistance region in a voltage-dependent manner (37). Because of the difference in diffusion coefficient and electrophoteric mobility, this Ansatz predicts that the smaller PAs accumulate more rapidly close to the pore entrance than do the larger ones. Thus, one could expect the smaller PA to be more effective in blocking the αHL channel, which is not consistent with the data.
To explain these results, we determined the interaction between the PAs and the membrane surface. Phospholipids, including PC, interact with PA (e.g., (4,8)). Binding PA with phospholipids does not occur in the absence of divalent cations. These cations form bridges between negatively charged phosphate groups of the phospholipids and the sulfate groups on PA (8). Phospholipids and PA interact with divalent cations individually (38,39). This apparently does not impair the Ca2+ bridge formation between phospholipids and PA, most likely because both are in dynamic equilibrium with free ions in the bulk. Moreover, the data suggest that PA, and heparin, in particular, continue to possess a negative net charge even in 100 mM Ca2+ solution (27). Because heparins also require the presence of divalent cations in the bulk to affect the channel (27), we considered the possibility that heparins bind to the membrane, in agreement with the calcium-bridge mechanism suggested in the literature (8,30,41).
Polyanion-binding to liposomes causes a negative ζ-potential (4). In particular, the effect of DS is increased with concentration and molecular weight (8). To verify whether the same is true for heparins and to quantify the binding of these PAs to the membrane, we measured the ζ-potential of PC liposomes in the absence and presence of differently sized heparins. This effort might help us in understanding the mechanism of PA action, and, in particular, its sidedness effect at the αHL channel.
We found that heparins decreased the PC liposome ζ-potential from slightly positive value (in the absence of PA) up to ∼−24 mV (Fig. 5). The larger heparins were more active. The dependence of liposome ζ-potential (Fig. 5) on heparin concentration resembles that for heparin-induced αHL channel current blockade (Fig. 2). There is a strong correlation between the effectiveness of heparins (presented as the IC50) in these two systems (Fig. 5, inset). These results suggest that the binding of PA with the membrane might account for the strong dependence of the channel blockade on PA molecular weight. It appears that at the first step of PA-ion channel interaction, PA binds to membrane via Ca2+-bridges, resulting in higher local concentrations on and near the membrane and thus around the αHL pore entrance area. This proximity to the pore entrance increases the probability that, at the right polarity, the pore-ion flux would be inhibited by the polyanion.
Figure 5.
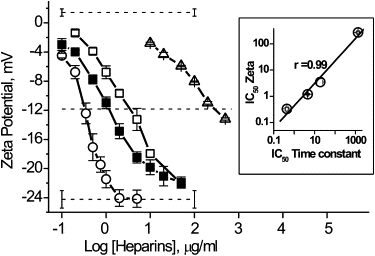
ζ-Potential of liposomes in the presence of growing concentrations of heparins and the correlation between equi-effective concentration of heparins against liposomes and αHL channels (inset). (Symbols: ○, HepAlb; ■, Hep; □, Hep6000; and ▵, Hep3000.) The value r is the correlation coefficient.
The results account for the side-differential effectiveness of PA. The steady-state polyanion concentration decreases nonlinearly (41) with distance from the surface, down to their bulk value. As a result, at the same bulk concentration, the effective steady-state polyanion concentration should be much higher at the trans (nearly to the membrane surface, but negatively charged) than cis (far from the membrane surface, but positively charged) entrance of the channel. The latter clarifies how the difference in the distance between the membrane surface and the entrances of the channel may determine the sidedness in PA effectiveness. Unbound polymer can occasionally approach the channel entrance by diffusion and then (at the second step of PA/ion channel interaction) enter the pore in a voltage-dependent manner and block it.
The lipid bilayer binds PA in a divalent cation-mediated manner. This leads to two consequences:
First, the binding increases the PA concentration close to the membrane surface in a molecular-weight-dependent manner—which, in turn, increases the probability of its blocking the αHL channel, as demonstrated in this study.
Second, the binding might alter the packing and the phase transition of lipid chains of membrane phospholipids (40), which can modify the αHL-membrane interaction (42) and, most likely, the channel transition between voltage-dependent states of different conductances. This then raises a question about the role of the physical state of membranes in the mechanism of PA action against ion channels—including those formed by αHL.
Theoretical model
Assuming that the distribution of PA adjacent to the membrane-solution interface follows the Boltzmann law (41), the effective polyanion concentration at the channel entrance, Ci, is
| (1) |
where Co is the polyanion concentration in the bulk, z is its valence, and ψi is the dimensionless potential at a channel entrance (ψCis or ψTrans).
We assume that PAs have the same concentration at the channel entrance, C′i = C″i, to cause the same current blocking effect. In that case, the ratio of bulk concentrations of two PA can be estimated as
| (2) |
or
| (3) |
where C′o and z′ and C″o and z″ are the bulk concentration and valence of two different PAs in the contact with the same (cis or trans) channel entrance in separate experiments.
The fit of Eqs. 2 and 3 to the dependence of the relative effectiveness of different heparins in the channel-block on their charge difference is shown in Fig. 6. The good agreement supports our assumption that the PA concentration close to surface of the membrane-αHL channel-complex obeys the Boltzmann law and that, at equal bulk concentration, the larger PAs have a greater concentration near the channel entrances. The numerical value of the potential responsible for the difference in effectiveness of differently sized PAs could be obtained by extrapolating the experimental dependencies shown in Fig. 6 (inset) to z′− z″ = 1. For the experiments with heparins added to the cis side or the trans side, values of ψi were ∼1.55 and ∼4.45. The result indicates that the effective potential, which is responsible for the difference in PA concentration at the αHL channel entrances, is approximately three times smaller at the cis than at the trans side.
Figure 6.
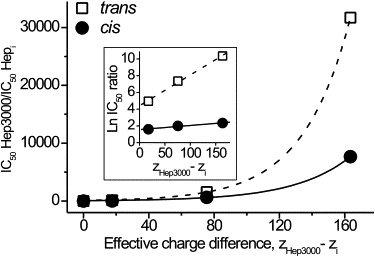
Relative effectiveness of heparins at the cis and the trans applications against their charge difference is presented in linear and semi-logarithmic (inset) plot. To build the plots, values of IC50 were taken in nM. The ratio was defined as . The number of charges per a heparin molecule, z, was taken equal to the number of sulfate groups. The effective charge difference was defined as ZHep3000–ZHepi. The correlation coefficient between experimental data and theory is >0.99.
To clarify the participation of the membrane in determination of this potential, we used the Debye-Hückel approximation, ψi = ψoe−κχ, where κ−1 is the Debye screening length, and χ is the distance between potential at the membrane surface, ψo, and potential at the some distance from it, ψi. Comparing the Debye-Hückel equations for the cis and trans entrances of the channel, we estimate the ratio of potentials at the cis and trans αHL channel entrances to be , where Δχ represents the apparent difference in distance between the cis and trans channel entrances from the membrane surface. Substituting Δχ for 4 nm (based on the available data about αHL channel position in lipid bilayer), and taking κ−1 = 0.8 nm (ionic strength ∼0.15), we found of 0.007, which is ∼50 times less than the experimental value. The result indicates that, in the absence of electrostatic potential generated by the protein part of the αHL channel, the sidedness effect of PA should be remarkably more pronounced. Thus, potential of the membrane surface alone explains the experimental side-dependence of polyanion action qualitatively. The protein channel attenuates the potential generated by lipid membrane. The resulting effective potential (responsible for the polyanion concentration at the entrances) is the result of both constituents of the αHL channel-membrane complex. The influence of the charged regions of the αHL channel is electrostatic in nature. The transmembrane potential does not determine PA concentration close to the channel entrances (see Supporting Material for a detailed discussion). The transmembrane potential acts at the second step, where a polyanion, occasionally approaching a channel entrance by diffusion, is forced either into or away from the pore by the transmembrane electric field. Ca2+-bridges mediate the interaction of PA with lipid membranes only.
Conclusion
Here, we demonstrate that the ability of heparins and DS to block αHL channels correlates with their binding to a phospholipid membrane and significantly increases with polyanion size. The effectiveness of PA reported here depends strongly on the side to which they were added and on the sign of transmembrane voltage applied. In summary, the results obtained with wild-type and genetically engineered channels together with a simplified mathematical analysis of the data suggested particular mechanisms by which PAs affect the αHL channel, and perhaps other mesoscopic channels.
The interaction between the channel and PA requires two steps. First, PAs bind reversibly to the membrane surface via Ca2+-bridges. The distribution of PAs close to the surface is determined by two factors: an attraction to membrane (which retains PA at the surface via Ca2+-bridges) and Brownian diffusion (which tends to equalize their surface and the bulk concentrations). At equilibrium, the polyanion concentration distribution is greatest close to the surface and decreases with distance in an exponential manner (41). At the same bulk concentration, the larger PA is at the larger concentration in proximity to the surface. Because the two entrances of the αHL channel are at different distances from the membrane surface, the effective concentration of PA at the cis entrance is much smaller than at the trans. This qualitatively explains the difference in their effectiveness from the cis and the trans side.
PAs have a negative net charge even in 100 mM solution of CaCl2 (27). The visible interaction of PAs with αHL channels occurs at the second step when a polyanion, which occasionally diffuses to the access zone on the front of the channel entrance, will fall under the influence of the transmembrane electric field, preventing or facilitating its entrance into the channel lumen to plug it. It appears that, at this step, the interaction of PA with the channel is not mediated by Ca2+-bridges, and that long-range electrostatic interactions are important. The conclusion is consistent with the earlier observed (27) decrease in heparin effectiveness under the increase in ionic strength of the bulk solution and the theoretical estimations of blocking of an ion channel by a highly charged drug (43).
Findings provide a noncontradictory molecular explanation for polyanion/protein-ion channel interactions that combines all data known to date including the necessity of divalent cations, voltage-, side-, and size-dependencies of PA effect on the αHL channel. The results shown here might be of interest for pharmacology and biomedicine, where heparins and other PAs are widely used, and are of importance for understanding the ubiquitous role of PA in cellular activity. We expect that the results obtained here will be applicable to other mesoscopic (proteinaceous and synthetic) water-filled nanopores, and should be helpful in research to design drugs that might block mesoscopic pores formed by channel-forming toxins (e.g., (44)).
Supporting Material
Two figures and one table are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(09)01465-9.
Supporting Material
Acknowledgments
The authors are grateful to Dr. C. P. de Melo and Prof. C. A. S. Andrade for use of the Zetasizer, and C. G. dos Santos (all from Physics Department of Federal University of Pernambuco) for technical assistance. The authors also thank Dr. John Kasianowicz (National Institute of Standards and Technology, Gaithersburg, MD) for editing the manuscript.
This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, a Rede de Nanotecnologia Molecular e de Interfaces, and Instituto do Milênio de Materiais Complexos (Brazil) and by the Deutsche Forschungsgemeinschaft Grant SFB 490, project D3.
References
- 1.Olenina L.V., Kuzmina T.I., Sobolev B.N., Kuraeva T.E., Kolesanova E.F. Identification of glycosaminoglycan-binding sites within hepatitis C virus envelope glycoprotein E2∗. J. Viral Hepat. 2005;12:584–593. doi: 10.1111/j.1365-2893.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 2.Hurst R.E., Moldwin R.M., Mulholland S.G. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology. 2007;69:S17–S23. doi: 10.1016/j.urology.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 3.Vannucchi S., Ruggiero M., Chiarugi V. Complexing of heparin with phosphatidylcholine—a possible supramolecular assembly of plasma heparin. Biochem. J. 1985;227:57–65. doi: 10.1042/bj2270057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sagrista M.L., Mora M., De Madariaga M.A. Surface modified liposomes by coating with charged hydrophilic molecules. Cell. Mol. Biol. Lett. 2000;5:19–33. [Google Scholar]
- 5.Suppiramaniam V., Vaithianathan T., Manivannan K., Dhanasekaran M., Parameshwaran K. Modulatory effects of dextran sulfate and fucoidan on binding and channel properties of AMPA receptors isolated from rat brain. Synapse. 2006;60:456–464. doi: 10.1002/syn.20319. [DOI] [PubMed] [Google Scholar]
- 6.Van V.D., Wall D.P., Johnson K.G. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr. Opin. Neurobiol. 2006;16:40–51. doi: 10.1016/j.conb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Chicoine L.M., Suppiramaniam V., Vaithianathan T., Gianutsos G., Bahr B.A. Sulfate- and size-dependent polysaccharide modulation of AMPA receptor properties. J. Neurosci. Res. 2004;75:408–416. doi: 10.1002/jnr.10871. [DOI] [PubMed] [Google Scholar]
- 8.Zschornig O., Richter W., Paasche G., Arnold K. Cation-mediated interaction of dextran sulfate with phospholipid vesicles: binding, vesicle surface polarity, leakage and fusion. Colloid Polym. Sci. 2000;278:637–646. [Google Scholar]
- 9.Song L., Hobaugh M.R., Shustak C., Cheley S., Bayley H. Structure of staphylococcal α–hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 10.Krasilnikov O.V., Merzlyak P.G., Yuldasheva L.N., Rodrigues C.G., Bhakdi S. Electrophysiological evidence for heptameric stoichiometry of ion channels formed by Staphylococcus aureus α-toxin in planar lipid bilayers. Mol. Microbiol. 2000;37:1372–1378. doi: 10.1046/j.1365-2958.2000.02080.x. [DOI] [PubMed] [Google Scholar]
- 11.Movileanu L., Cheley S., Howorka S., Braha O., Bayley H. Location of a constriction in the lumen of a transmembrane pore by targeted covalent attachment of polymer molecules. J. Gen. Physiol. 2001;117:239–251. doi: 10.1085/jgp.117.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst S., Venkataraman G., Sasisekharan V., Langer R., Cooney C.L. Pyranose ring flexibility. Mapping of physical data for iduronate in continuous conformational space. J. Am. Chem. Soc. 1998;120:2099–2107. [Google Scholar]
- 13.Gray G.S., Kehoe M. Primary sequence of the α-toxin gene from Staphylococcus aureus wood 46. Infect. Immun. 1984;46:615–618. doi: 10.1128/iai.46.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valeva A., Pongs J., Bhakdi S., Palmer M. Staphylococcal α-toxin: the role of the N-terminus in formation of the heptameric pore—a fluorescence study. Biochim. Biophys. Acta. 1997;1325:281–286. doi: 10.1016/s0005-2736(96)00266-0. [DOI] [PubMed] [Google Scholar]
- 15.Mueller P., Rudin D.O., Tien H.T., Wescot W.C. Methods for formation of single bimolecular lipid membranes in aqueous solution. J. Phys. Chem. 1963;67:534–535. [Google Scholar]
- 16.Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krasilnikov O.V., Ternovsky V.I., Sabirov R.Z., Zaripova R.K., Tashmukhamedov B.A. Cation-anion selectivity of staphylotoxin channels in lipid bilayer. Biofizika. 1986;31:606–610. [PubMed] [Google Scholar]
- 18.Krasilnikov O.V., Merzlyak P.G., Sabirov R.Z., Ternovsky V.I., Zaripova R.K. Influence of pH on the potential-dependence of staphylococcal toxin channels functioning in phosphatidylcholine bilayer. Ukr. Biokhim. Zh. 1988;60:60–66. [PubMed] [Google Scholar]
- 19.Szoka F., Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes) Annu. Rev. Biophys. Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- 20.Ruso J.M., Besada L., Martinez-Landeira P., Seoane L., Prieto G. Interactions between liposomes and cations in aqueous solution. J. Liposome Res. 2003;13:131–145. doi: 10.1081/lpr-120020316. [DOI] [PubMed] [Google Scholar]
- 21.Guex N., Peitsch M.C. SWISS-MODEL and the Swiss-PDBViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 22.Krasilnikov O.V., Merzlyak P.G., Yuldasheva L.N., Capistrano M.F. Protein electrostriction: a possibility of elastic deformation of the α-hemolysin channel by the applied field. Eur. Biophys. J. 2005;34:997–1006. doi: 10.1007/s00249-005-0485-9. [DOI] [PubMed] [Google Scholar]
- 23.Rabenstein D.L. Heparin and heparan sulfate: structure and function. Nat. Prod. Rep. 2002;19:312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- 24.Mulloy B., Forster M.J., Jones C., Davies D.B. NMR and molecular-modeling studies of the solution conformation of heparin. Biochem. J. 1993;293:849–858. doi: 10.1042/bj2930849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larm O., Lindberg B., Svensson S. Studies on length of side chains of dextran elaborated by Leuconostoc mesenteroides Nrrl B-512. Carbohydr. Res. 1971;20:39–48. doi: 10.1016/s0008-6215(00)84947-2. [DOI] [PubMed] [Google Scholar]
- 26.Bovey F.A. Enzymatic polymerization. 1. Molecular weight and branching during the formation of dextran. J. Polym. Sci. 1959;35:167–182. [Google Scholar]
- 27.Krasilnikov O.V., Merzlyak P.G., Yuldasheva L.N., Rodrigues C.G., Nogueira R.A. Heparin influence on α-staphylotoxin formed channel. Biochim. Biophys. Acta. 1999;1417:167–182. doi: 10.1016/s0005-2736(98)00244-2. [DOI] [PubMed] [Google Scholar]
- 28.Bangham A.D. Membrane models with phospholipids. Prog. Biophys. Mol. Biol. 1968;18:29–95. doi: 10.1016/0079-6107(68)90019-9. [DOI] [PubMed] [Google Scholar]
- 29.Davenport J.B. Physical chemistry of lipids. In: Johnson A.R., Davenport J.B., editors. Biochemistry and Methodology of Lipids. Wiley-Interscience; New York: 1971. pp. 47–83. [Google Scholar]
- 30.Huster D., Arnold K. Ca2+-mediated interaction between dextran sulfate and dimyristoyl-sn-glycero-3-phosphocholine surfaces studied by H-2 nuclear magnetic resonance. Biophys. J. 1998;75:909–916. doi: 10.1016/S0006-3495(98)77579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hattori T., Kimura K., Seyrek E., Dubin P.L. Binding of bovine serum albumin to heparin determined by turbidimetric titration and frontal analysis continuous capillary electrophoresis. Anal. Biochem. 2001;295:158–167. doi: 10.1006/abio.2001.5129. [DOI] [PubMed] [Google Scholar]
- 32.Kragh-Hansen U., Vorum H. Quantitative analyses of the interaction between calcium ions and human serum-albumin. Clin. Chem. 1993;39:202–208. [PubMed] [Google Scholar]
- 33.Yokouchi Y., Tsunoda T., Imura T., Yamauchi H., Yokoyama S. Effect of adsorption of bovine serum albumin on liposomal membrane characteristics. Colloids Surf. B Biointerfaces. 2001;20:95–103. doi: 10.1016/s0927-7765(00)00176-4. [DOI] [PubMed] [Google Scholar]
- 34.Mulloy B., Forster M.J. Conformation and dynamics of heparin and heparan sulfate. Glycobiology. 2000;10:1147–1156. doi: 10.1093/glycob/10.11.1147. [DOI] [PubMed] [Google Scholar]
- 35.De Felice L.J. Plenum Press; New York: 1981. Introduction to Membrane Noise. [Google Scholar]
- 36.Hall J.E. Access resistance of a small circular pore. J. Gen. Physiol. 1975;66:531–532. doi: 10.1085/jgp.66.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangan P.S., Colombini M. Ultrasteep voltage dependence in a membrane channel. Proc. Natl. Acad. Sci. USA. 1987;84:4896–4900. doi: 10.1073/pnas.84.14.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burger K., Gaizer F., Pekli M., Nagy G.T., Siemroth J. The effect of cations on the calcium-ion coordination of heparin. Inorg. Chim. Acta Bioinorg. Chem. 1984;92:173–176. [Google Scholar]
- 39.Seelig J. Interaction of phospholipids with Ca2+ ions. On the role of the phospholipid head groups. Cell Biol. Int. Rep. 1990;14:353–360. doi: 10.1016/0309-1651(90)91204-h. [DOI] [PubMed] [Google Scholar]
- 40.Steffan G., Wulff S., Galla H.J. Divalent cation-dependent interaction of sulfated polysaccharides with phosphatidylcholine and mixed phosphatidylcholine phosphatidylglycerol liposomes. Chem. Phys. Lipids. 1994;74:141–150. doi: 10.1016/0009-3084(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin S. Electrostatic potentials at membrane-solution interfaces. In: Bronner F., Kleinzeller A., editors. Current Topics in Membranes and Transport. Academic Press; New York-London: 1977. pp. 71–144. [Google Scholar]
- 42.Valeva A., Hellmann N., Walev I., Strand D., Plate M. Evidence that clustered phosphocholine head groups serve as sites for binding and assembly of an oligomeric protein pore. J. Biol. Chem. 2006;281:26014–26021. doi: 10.1074/jbc.M601960200. [DOI] [PubMed] [Google Scholar]
- 43.Aguilella-Arzo M., Cervera J., Ramirez P., Mafe S. Blocking of an ion channel by a highly charged drug: modeling the effects of applied voltage, electrolyte concentration, and drug concentration. Phys. Rev. 2006;73:041914. doi: 10.1103/PhysRevE.73.041914. [DOI] [PubMed] [Google Scholar]
- 44.Karginov V.A., Nestorovich E.M., Schmidtmann F., Robinson T.M., Yohannes A. Inhibition of S-aureus α-hemolysin and B. anthracis lethal toxin by β-cyclodextrin derivatives. Bioorg. Med. Chem. 2007;15:5424–5431. doi: 10.1016/j.bmc.2007.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


