Abstract
For studies of remyelination in demyelinating diseases, the cuprizone model of CC (corpus callosum) demyelination has experimental advantages that include overall size, proximity to neural stem cells of the subventricular zone, and correlation with a lesion predilection site in multiple sclerosis. In addition, cuprizone treatment can be ended to allow more direct analysis of remyelination than with viral or autoimmune models. However, CC demyelination lacks a useful functional correlate in rodents for longitudinal analysis throughout the course of demyelination and remyelination. In the present study, we tested two distinct behavioural measurements in mice fed 0.2% cuprizone. Running on a ‘complex' wheel with varied rung intervals requires integration between cerebral hemispheres for rapid bilateral sensorimotor coordination. Maximum running velocity on the ‘complex' wheel decreased during acute (6 week) and chronic (12 week) cuprizone demyelination. Running velocity on the complex wheel distinguished treated (for 6 weeks) from non-treated mice, even after a 6-week recovery period for spontaneous remyelination. A second behavioural assessment was a resident–intruder test of social interaction. The frequency of interactive behaviours increased among resident mice after acute or chronic demyelination. Differences in both sensorimotor coordination and social interaction correlated with demonstrated CC demyelination. The wheel assay is applicable for longitudinal studies. The resident–intruder assay provides a complementary assessment of a distinct modality at a specific time point. These behavioural measurements are sufficiently robust for small cohorts as a non-invasive assessment of demyelination to facilitate analysis of subsequent remyelination. These measurements may also identify CC involvement in other mouse models of central nervous system injuries and disorders.
Keywords: corpus callosum, cuprizone, demyelination, remyelination, rodent behaviour, wheel activity
Abbreviations: CC, corpus callosum; CNS, central nervous system; LFB, Luxol Fast Blue; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; PLP, proteolipid protein; Vmax, maximum running velocity
INTRODUCTION
Demyelination of axons leads to impaired signal conduction and can leave axons vulnerable to injury. MS (multiple sclerosis) is the most prevalent cause of demyelination in the CNS (central nervous system). Although MS results in focal lesions throughout the CNS, lesions are found in the CC (corpus callosum) in over 90% of MS cases (Gean-Marton et al., 1991). Additionally, at all stages of MS there is significant CC atrophy, which is not attributable to normal aging (Martola et al., 2007). In rodent experimental models, the CC is often used to examine cellular and molecular mechanisms involved in demyelination and remyelination (Armstrong et al., 2002; Jean et al., 2003; Adamo et al., 2006; Armstrong et al., 2006; Lindner et al., 2008). Extensive demyelination of the CC can be induced in mice by ingestion of the neurotoxicant cuprizone (Matsushima and Morell, 2001), and this model is widely used for studies of remyelination since, in contrast with viral and autoimmune demyelination models, cuprizone can be removed from the chow to examine remyelination without continued active demyelination. However, a limitation of the CC as a model in these studies is the lack of accepted functional measures to non-invasively monitor the effect of demyelination, especially for longitudinal studies. The present study tested behavioural measurements targeted for likely involvement of the CC to determine: (i) applicability in longitudinal studies, especially as relevant to recovery of function and remyelination; (ii) sensitivity for use among small cohorts, as is feasible for remyelination studies; (iii) ability to detect continued effects during chronic demyelination; and (iv) assessment of motor and non-motor modalities, preferably without interference between measurements to allow evaluation using both assays in a given mouse.
The CC is important for transfer and integration of information between the two hemispheres of the brain. Humans with agenesis of the CC have normal intelligence, but present with subtle cognitive deficits, especially in language ability (Paul et al., 2007). Similar deficits are also observed in patients that have undergone callosotomy (Devinsky and Laff, 2003). Such language deficits are associated with problems in social interaction and are symptoms of the autism spectrum disorders (Paul et al., 2007). In addition to language deficits, CC structural abnormalities have shown a correlation with impairments in motor tasks involving bimanual finger co-ordination. These motor impairments are observed in patients with agenesis of the CC, in patients that have undergone callosotomy, in children in whom the CC is not fully myelinated, and also in MS patients (Kennerley et al., 2002; Bonzano et al., 2008; Muetzel et al., 2008).
The CC is a widely studied CNS tract for rodent models of MS based on the experimental advantages of being the largest myelinated tract in the brain, with demyelination readily induced by injection of toxins or antibodies, or by ingestion of cuprizone (Gensert and Goldman, 1997; Matsushima and Morell, 2001; Armstrong et al., 2002; Decker et al., 2002). Additionally, the CC is located adjacent to the subventricular zone, a potential source for neural stem cells, making the CC ideally located to monitor neuroregenerative responses for repair of demyelination. A behavioural assessment that correlates with demyelination in the mouse CC would provide insights into the role of the CC in rodents and would facilitate non-invasive evaluation of CC demyelination for longitudinal studies throughout demyelination and remyelination. Importantly, a behavioural assay correlating with CC pathology is necessary to quantify deficits during acute demyelination and to subsequently test strategies to promote remyelination.
In the present study, we use cuprizone ingestion in mice to induce a reproducible pattern of CC demyelination followed by remyelination. In the present study, two different behavioural assays are modified and evaluated to identify functional deficits related to the cuprizone-induced demyelination. Our results indicate that both a complex wheel test of bilateral limb sensorimotor coordination and a resident–intruder test of social interaction correlate with CC demyelination status in the cuprizone model. Importantly, these two assays evaluate distinct functional modalities and can be used in conjunction. Furthermore, the complex wheel activity can be used in longitudinal analysis, whereas the resident–intruder assay is useful for analysis at a specific treatment time point. These assays detected differences which correlated with CC demyelination during both acute and chronic cuprizone treatment paradigms. Both assays were highly robust in that significant differences were distinguished among small cohorts of mice which will be important for analyses within a cohort size that is feasible for studies of cellular and molecular mechanisms involved in remyelination. Our analysis also demonstrates that immunohistochemical evidence of extensive remyelination may not indicate full recovery to normal action potential transmission along previously demyelinated axons.
MATERIALS AND METHODS
Animals
Young adult male C57Bl/6 mice were provided from Jackson Laboratories at 6 weeks of age and housed singly on-site in the animal facility according to the guidelines of the National Institutes of Health and the Institutional Animal Care and Use Committee of the Uniformed Services University of the Health Sciences. All mice were kept on a 12 h light/dark cycle (06:00–18:00 light; 18:00–06:00 dark) in cages that measured 23.62 cm×35.3 cm×19.56 cm.
Cuprizone model
Male C57Bl/6 mice (8–9 weeks of age; Jackson Laboratories) were fed ad libitum a diet of 0.2% (w/w) cuprizone [oxalic bis-(cyclohexylidenehydrazide); Sigma–Aldrich] mixed into milled chow pellets (Certified LM-485 code 7012CM; Harland Teklad), which can be administered for specific periods to induce reproducible patterns of demyelination and remyelination of the CC (Matsushima and Morell, 2001; Mason et al., 2001; Armstrong et al., 2002, 2006). To induce an initial phase of demyelination (designated as the ‘acute' condition), mice were fed cuprizone continuously for 6 weeks. After 6 weeks of cuprizone feeding, some of these mice were fed normal chow for a subsequent 6 week period to allow progression of spontaneous remyelination (designated as the ‘recovery' condition). To induce prolonged demyelination (designated as the ‘chronic' condition), mice were fed cuprizone continuously for 12 weeks. Controls were age-matched male C57Bl/6 mice fed normal chow. Cohorts were followed longitudinally to compare behavioural performance within-subjects in addition to the comparison between-subjects. No vert neurological deficits were apparent in treated or non-treated mice. Mice were weighed at the beginning of each week of analysis (0, 6 and 12 weeks of treatment or matched no-treatment controls). For the respective 0, 6 and 12 week cuprizone time points, the weights (means±S.D. in g) for non-treated mice were 24.4±1.2, 26.9±1.9 and 28.3±1.6, whereas the respective weights for cuprizone-treated mice were 22.4±1.5, 23.3±1.2 and 24.1±0.9 (0 week, P>0.05; 6 week, P<0.001; 12 week, P<0.001; measured using two-way ANOVA).
Bilateral sensorimotor limb coordination: complex wheel-running assay
A running assay using wheels with irregularly spaced rungs has been shown to detect functional differences in mice with surgical transection of the CC (Schalomon and Wahlsten, 2002) and with cuprizone-induced acute demyelination (Liebetanz and Merkler, 2006). These methods were modified to test the application of this assessment for use in longitudinal studies, especially with small cohorts as is feasible for remyelination studies, for detection of continued effects during chronic demyelination, and for use in conjunction with an assessment of non-motor modalities (see the section on the ‘Social interaction: resident–intruder assay' below). Cohorts of mice were housed in cages equipped with an optical sensor to detect the number of wheel revolutions per time interval (Lafayette Instruments). A training wheel with all 38 rungs intact was present in the cages for 2 weeks to normalize running behaviour. On the third week, mice were introduced to the ‘complex' wheel with 22 rungs missing at intervals according to the pattern shown (Figure 1A). After the third week, the running wheel was removed from the cage. This cycle of 2 weeks on the training wheel and 1 week on the complex wheel has been referred to as a MOSS (motor skills sequence) (Liebetanz and Merkler, 2006). This 3 week sequence was carried out prior to the start of cuprizone and then repeated for the acute, chronic and recovery time points (Figure 1B). Age-, weight- and sex-matched control mice were treated identically, but did not have cuprizone in the chow pellets. Using the Activity Wheel Monitoring Software, wheel revolutions were recorded throughout 10 min intervals during the light phase, when mice were relatively inactive, and throughout 1 min intervals during the more active dark phase. Results from each 24 h period were exported to a Microsoft Excel file in which total distance run, maximum velocity among daily runs, number of runs and maximum run interval (i.e. duration of run) were calculated. A total of 29 mice were tested of which nine were age-matched controls. Of these 20 cuprizone-treated mice, each was tested prior to the beginning of cuprizone feeding in order to serve as within-subject controls. All mice showed spontaneous running activity so that none were excluded on this basis, in contrast with the study by Liebetanz and Merkler, (2006).
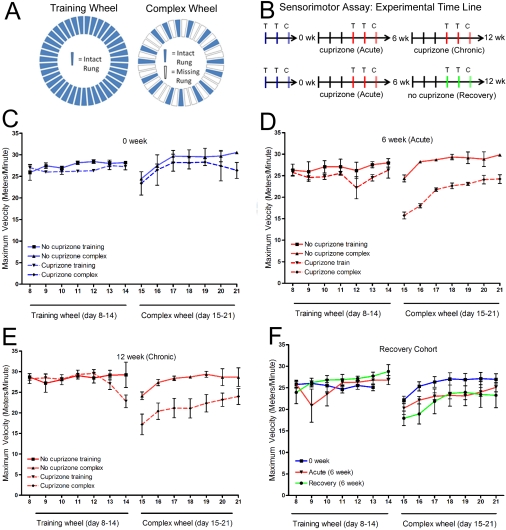
Figure 1. Mice with cuprizone-induced demyelination can be distinguished using complex wheel activity that challenges bilateral sensorimotor coordination.
(A) Home cages were equipped with either a training wheel (all rungs in place) or a complex wheel (one or two rungs removed in an alternating pattern). (B) Timeline showing availability of training wheel (T) or complex wheel (C) in the cages. Each set of wheel availability consisted of 1 week on the training wheel to stabilize the behaviour, a second week on the training wheel to record values, and a subsequent week on the complex wheel. Wheel sets were scheduled so that the complex wheel period ended at the start of cuprizone feeding (0 week, blue), at the end of the acute demyelination phase (6 week, red), at the end of the chronic demyelination phase (12 week, red), or at the end of the 6 week period for recovery following acute demyelination (recovery, green). (C–F) The maximum velocity (Vmax) recorded is shown for each 24 h period. Each graph shows the second week of activity on the training wheel (days 8–14) and the subsequent week on the complex wheel (days 15–21). (C–E) An example from a matched set of six mice separated into two cohorts of three mice for cuprizone treatment and three non-treated mice. On the training wheel, Vmax values are not significantly different (P>0.05) between non-treated mice and those fed cuprizone for any conditions tested. On the complex wheel, Vmax values are not significantly different prior to the start of cuprizone treatment (C; P>0.05), but are significantly decreased during week 6 of cuprizone feeding (D; P<0.0001) and continue to be significantly lower during week 12 of continuous cuprizone feeding (E; P<0.0001). When assessed in parallel with the same mice followed longitudinally, the complex wheel Vmax distinguishes treated from non-treated mice with this small cohort size. (F) An example of a cohort of six mice followed longitudinally to test the effect of a recovery period that should correlate with significant remyelination. No significant differences in Vmax were observed on the training wheel within the cohort across disease stages (P>0.05). On the complex wheels, compared with the values prior to the start of cuprizone (blue, week 0), the mice treated with cuprizone for 6 weeks had significantly reduced Vmax values both during week 6 of cuprizone feeding (red compared with blue; P<0.01) and during week 6 of the recovery period following cuprizone treatment (green compared with blue; P<0.01). Therefore, during remyelination, the complex wheel Vmax still detected a significant difference among mice that had experienced an episode of demyelination.
Social interaction: resident–intruder assay
As a broad screen of activities, the resident–intruder assay (Bolivar et al., 2007) was modified to use video recording to quantify all distinct behaviours of cuprizone-treated and non-treated resident mice in the presence and absence of an intruder mouse in combination with analysis of movement to assess overall activity level and overt neurological differences. Individual cuprizone treated or non-treated C57Bl/6 male mice (with the observer blinded to treatment category) were placed in a clean plastic housing cage identical with the home cage (23.62 cm×35.3 cm×19.56 cm) for 15 min to establish each as the ‘resident' mouse. An age-matched, weight-matched non-treated male mouse was introduced as the ‘intruder' for a second 15 min period. Both 15 min sessions were video recorded to facilitate scoring of all identifiable distinct behaviours. The number of distinct behaviours was scored for the resident mouse (15 min alone; 15 min with intruder) and for the intruder mouse (15 min with resident).
The independent behaviours included sniffing the environment, rearing alongside the cage, rearing independently, digging, circling clockwise, circling counter-clockwise, allogrooming, freezing and scratching. Interactive behaviours included sniffing the other mouse, following, grooming, rearing at the other mouse, sitting or laying next to the other mouse, backing or running away from the other mouse, biting, boxing or wrestling, mounting, pinning and tail-rattling. Video files were also analysed using an ObjectTracker program on ImageJ software. This analysis yielded Microsoft Excel data files with object coordinates within the cage for every 1 s of video recording. From these data sets the distance travelled for each 15 min video recording session was extrapolated. Unpaired Student's t tests were used to assess differences between age-matched cohorts for both independent and interactive behaviours. A total of 46 resident mice (25 cuprizone-treated and 21 non-treated matched controls) were examined using the resident–intruder assay.
Analysis of myelination status
Following behavioural analysis, mice were perfused with 4% (w/v) paraformaldehyde. Coronal brain sections (15 μm) were cut for immunohistochemistry. Analysis within the body of the CC was performed using cryostat sections taken between approx. Bregma levels 0 to −1 mm, whereas the hippocampal analysis was performed on more caudal sections, approx. Bregma levels −1 to −2 mm. To examine the cerebellar cortex, sagittal sections (40 μm) were cut on a vibratome using brains from a separate set of mice that were not in the behavioural cohorts. MOG (myelin oligodendrocyte glycoprotein) was detected with the monoclonal antibody 8-18C5 [from Dr Minettia Gardinier (Department of Pharmacology, University of Iowa, Iowa City, IA, U.S.A.)] followed by incubation with donkey anti-mouse IgG F(ab′)2 conjugated with Cy3 (Jackson Immunoresearch) (Armstrong et al., 2006). MOG-immunostained sections viewed on an Olympus IX-70 microscope were photographed with a Spot 2 CCD (charge-coupled device) digital camera using Spot Advanced image acquisition software (Diagnostic Instruments). Panels of images were prepared using Adobe Photoshop 7.0.
Quantification of CC myelination was estimated from MOG immunofluorescence using Metamorph software (Armstrong et al., 2006). Pixel intensity values were normalized between sections by thresholding to exclude values below the level in the dorsal fornix, which is an adjacent white matter tract that is not demyelinated by cuprizone. The percentage area of the CC (midline unilaterally to under the cingulum apex) with MOG pixel intensities above the threshold level was then used as an estimate of the myelinated area. Similarly, quantification of cerebral cortex myelination was estimated from MOG immunofluorescence in the same tissue sections used for CC analysis. Pixel intensity values were normalized between sections by thresholding to distinguish myelinated cortical radiation fibres in the cortex. The percentage area of the cortex (over the CC from the midline unilaterally to over the apex of the cingulum) with MOG immunoreactivity above the threshold level was then used as an estimate of the myelinated area.
Statistical analysis
For each experimental approach, each category analysed included three or more mice per condition. Analysis of tissue sections included at least three sections per mouse. Prism 5.0 (GraphPad Software) was used for statistical analyses. Unpaired Student's t tests were used to assess differences between age-matched cohorts for a single time point. One-way ANOVA with post-hoc Tukey's multiple comparison was used to determine significant differences across time points. Two-way ANOVA with post-hoc Tukey's multiple comparisons was used for analysis across time points and treatments. Statistical significance was determined as P<0.05.
RESULTS
Complex wheel assay of bilateral sensorimotor coordination
Although the CC is a common site for analysis of demyelination and remyelination for many reasons, including those noted above, these analyses are constrained by the lack of a measure of CC function that can be applied in longitudinal studies to monitor the effect of demyelination, as well as to functionally evaluate manipulations to promote remyelination. Cuprizone ingestion results in widespread, reproducible demyelination of the CC in mice (Mason et al., 2001; Matsushima and Morell, 2001; Armstrong et al., 2002, 2006) making the cuprizone model ideal for testing functional behavioural correlates of CC myelination status. A complex wheel assessment which incorporates irregularly spaced wheel rungs was selected for longitudinal analysis of CC function because of the required integration of sensory and motor information bilaterally in a non-uniform pattern, which would reduce the influence of pattern-generating spinal cord locomotion expected to correspond with uniform or rhythmic movements (Figures 1A and 1B). Differences detected with the complex wheel assay have been previously correlated with surgical transection of the CC (Schlamomon and Wahlsten, 2002) and with acute (6 week) cuprizone treatment (Liebetanz and Merkler, 2006).
The parameters recorded daily from the wheel running behaviour included the total number of runs, total distance run, maximum run interval and the maximal running velocity (Vmax). The Vmax appeared to be a relevant parameter for the complex wheel differentiation of bilateral sensorimotor co-ordination as shown for a representative cohort of mice in Figures 1(C) and 1(D). Baseline measures of Vmax were recorded on the training wheel and the complex wheel for all mice prior to the start of cuprizone treatment. No differences in baseline values were observed between the cohorts of mice tested (0 weeks; Figure 1C). Compared with the matched non-treated cohort, the treated mice had slower Vmax values on the complex running wheel after both acute (6 weeks; P<0.0001) and chronic (12 weeks; P<0.0001) cuprizone ingestion (Figures 1D and 1E). During the week on the complex wheel, the sum of the Vmax values was reduced by 24.7% for mice fed cuprizone for 6 weeks and 23.7% for mice fed cuprizone for 12 weeks, with each compared with the respective matched cohort. These results indicate that the Vmax values exhibit significant differences during both acute and chronic stages that are not abrogated from the repeated exposure to the complex wheel in these longitudinal studies. Values for the other wheel-running parameters had greater variability than Vmax and during chronic demyelination did not distinguish treated from non-treated mice on the complex wheel (number of runs, P = 0.8369; maximum run interval, P>0.05), with the exception of the total distance run (P<0.01), which had velocity as a contributing factor. Also, on the training wheels, Vmax values were not significantly different between the cuprizone-treated and non-treated mice throughout the time course (Figures 1C–1E). Therefore the complex wheel can detect motor co-ordination deficits that are not apparent with the uniformly spaced rungs on the training wheel. These lack of differences on the training wheel also indicates that the cuprizone treatment did not cause an overall decrease of interest in the wheel or impair the general ability to run rapidly.
Mice were also tested longitudinally at time points of before cuprizone (0 week), after acute demyelination (6 weeks of cuprizone) and again after a period of recovery (6 weeks on normal chow) (Figure 1F). Again, Vmax values on the training wheel were not significantly different among mice tested at different disease time points. In contrast, the mice had significantly slower Vmax values on the complex wheel at the acute (reduced by 11.5%) and recovery (reduced by 15.8%) time points than at the pre-cuprizone time point (Figure 1F; P<0.01 for each time point). The values did not change significantly between the acute and recovery time points on the complex wheel (Figure 1F). These findings indicate that Vmax on the complex wheel is sensitive to demyelination status both between- and within-subjects. Furthermore, the complex wheel Vmax values indicated continued effects on this behaviour even after a 6-week period for recovery, which is typically associated with extensive remyelination, indicating continued neurophysiological abnormalities.
Resident–intruder paradigm for assessment of social interaction
A social interaction paradigm was tested as a distinct behavioural assessment to complement the sensorimotor assessment from the complex wheel assay. The resident–intruder test of social interaction (Bolivar et al., 2007) was modified to evaluate overt neurological function and to quantify overall activity, as well as all distinct behaviours identified for each mouse (Figure 2A). The resident mouse was either cuprizone-treated or a non-treated control, whereas the intruder mouse was always a non-treated control. No overt neurological symptoms were observed in any of the mice examined during acute or chronic cuprizone treatment. Overall distance travelled during the recording period was calculated to determine whether a difference in overall movement of the mice may contribute to the increased interactive behaviours observed (Figure 2B). This analysis of movement showed no difference in total distance travelled between cuprizone-treated animals and age-matched controls. Across the battery of activities recorded, a grouping became apparent in that interactive behaviours were consistently increased in frequency among mice with acute demyelination (6 weeks cuprizone) compared with the control cohort. Conversely, independent behaviours were consistently less frequent among the mice with acute demyelination.
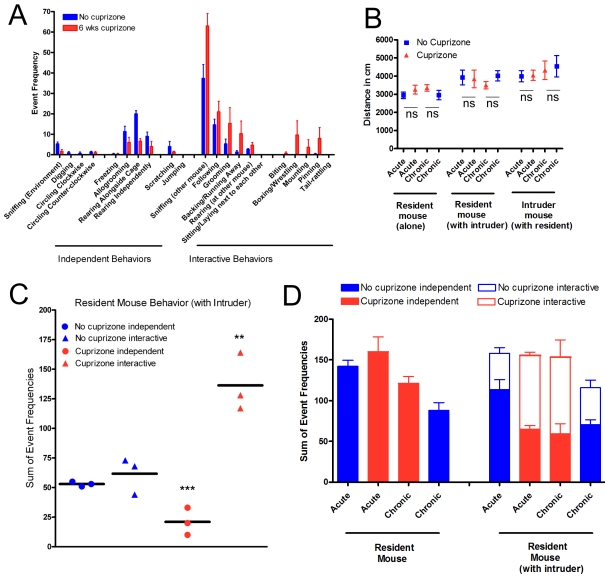
Figure 2. Mice with cuprizone-induced demyelination can be distinguished using the resident–intruder social interaction paradigm.
(A) An example of the battery of distinct behaviours quantified among mice filmed during the period with both a resident mouse (with or without cuprizone feeding) and an intruder mouse (always without cuprizone) in the cage. Grouping of behaviours according to treatment condition reveals that, compared with non-treated mice (blue), mice treated with cuprizone for 6 weeks (red) have a lower frequency of behaviours that can be categorized as ‘independent' and a higher frequency of behaviours involving the intruder mouse that can be categorized as ‘interactive' behaviours (n = 3 for each cohort of mice). (B) Total distance travelled during each filming was measured as an indicator of overall activity. In each condition, no significant (ns) differences were observed in acute (6 week) or chronic (12 week) cuprizone-treated mice relative to the age-matched control mice (n = 3 for each cohort of mice; all comparisons, P>0.1). (C) Resident mouse behaviour frequencies were combined to sum those that indicated independent or interactive events, as noted in (A). The cuprizone-treated mice showed significantly decreased independent behaviours and more dramatically elevated interactive events (n = 3 mice per condition; P = 0.0091 and P = 0.0112 respectively). Values for individual resident cuprizone-treated mice separated clearly from control mice. (D) Comparison of cohorts of acute and chronic cuprizone-treated mice with age-matched controls. In the absence of an intruder mouse, an age-dependent decrease of independent activities is observed across this prolonged disease time course (P = 0.0007). There is a marginally significant treatment effect among the resident mice while observed alone (P = 0.0436). In the presence of an intruder mouse, resident mice treated with cuprizone had a significantly decreased frequency of independent behaviours (P = 0.0045) and increased frequency of interactive behaviours (P = 0.0028) compared with the age-matched non-treated mice. In the presence of an intruder mouse, there was again an age-dependent effect among the independent behaviours (P = 0.018). Statistical comparisons are from two-way ANOVA with six mice per group.
The social interaction data were further analysed to determine whether significant differences could be demonstrated for individual mice within each cohort (Figure 2C). To take advantage of the distribution of behaviours observed (Figure 2A) the set of activities quantified was summed for independent compared with interactive behaviours. Individual mouse values clearly separate based on treatment condition. Indeed, the interactive behaviours for each acute cuprizone-treated mouse are clearly distinct from the values for the non-treated mice.
This distinction of interactive compared with independent behaviours was also tested for applicability across acute through chronic demyelination stages (Figure 2D). Compared with the matched controls, the resident mice with acute demyelination showed a significant shift of behaviours, with a 51.0% increase in interactive behaviours and 42.9% decrease in independent behaviours. Importantly, the mice with chronic demyelination continued to show an increase in interactive behaviours (49.2%). These differences in treated compared with non-treated mice across both time points were significant based on a two-way ANOVA (interactive behaviours, P = 0.0028; independent behaviours, P = 0.0045). Also, a decrease in independent activity frequency was notable between ages among the control mice, indicating an age-dependent effect among the resident mice (P = 0.0007 without intruder; P = 0.018 with intruder). Therefore the frequency of interactive behaviours appears to be a better parameter than independent behaviours for comparisons across this prolonged disease course.
Resident mice with acute demyelination followed by a 6 week recovery period still showed a high frequency of interactive behaviours (111.7±18.9) and low frequency of independent behaviours (61.3±18.6) in the presence of the intruder mouse. Although only a set of three mice was tested for this recovery cohort, the values are very close to those of the acute and chronic cohorts, indicating a lack of functional recovery during the remyelination period that is also consistent with the lack of full recovery on the complex wheel (Figure 1F). Ideally, direct comparison of values for the same mice evaluated at both the acute and recovery time points would be optimal to evaluate changes longitudinally, which was not done with this recovery cohort. In general, mice should be socially naïve before testing in a resident–intruder paradigm (Bolivar et al., 2007). Therefore this social interaction paradigm is not expected to be appropriate for repeated testing of the same resident mouse for longitudinal studies, presumably because repetition may dampen interest of the resident mouse to interact with the novel intruder mouse. Surprisingly, we performed a preliminary test of a matched cohort of treated (n = 3) and non-treated (n = 3) mice evaluated at both 6 and 12 weeks of cuprizone and found significant differences across both acute and chronic stages (P = 0.0427 for independent behaviours; P = 0.0186 for interactive behaviours; as measured by two-way ANOVA). This finding may reflect that the cuprizone effect is unusually robust for this behavioural assay and so maintained a significant difference upon repetition. However, the major advantage of the resident–intruder social interaction paradigm is for analysis on a specific day during the course of demyelination in that the video recording can be accomplished in 1 h and requires no specific training of the mice.
Myelination status of mice used in behavioural studies
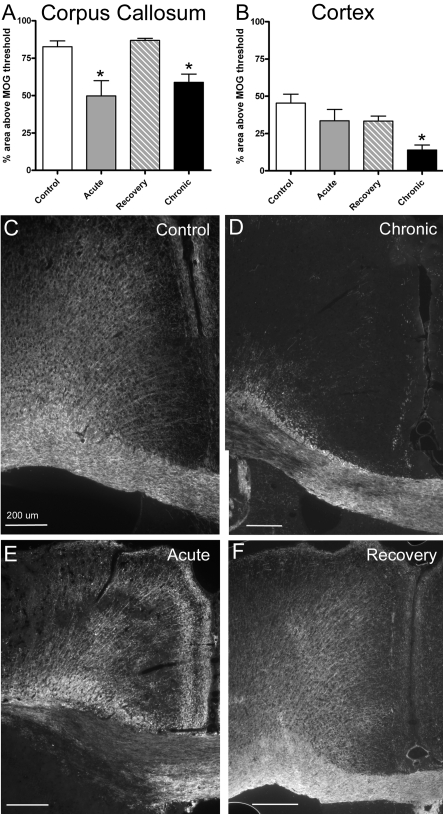
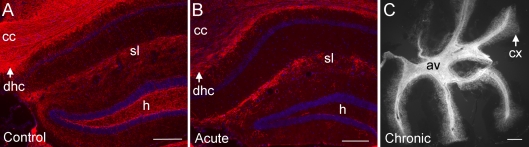
Upon completion of behavioural assessments, coronal sections through the body of the CC were immunostained for MOG to estimate the myelination status at specific stages of cuprizone treatment and recovery (Figure 3). The expected demyelination of the CC after 6 and 12 weeks of continuous cuprizone ingestion is evident when compared with normally myelinated non-treated control mice (Figures 3A and 3C–3E; P = 0.0027 for both 6 and 12 weeks of cuprizone compared with control values). In addition, after acute demyelination, extensive spontaneous remyelination of the CC is evident following the subsequent 6 weeks of recovery on normal chow (Figures 3A and 3F). Myelination of the adjacent cerebral cortex was also examined for a potential contribution to the observed behavioural effects. The cerebral cortex showed significantly reduced myelination after chronic cuprizone treatment (P = 0.0207), but not following acute cuprizone treatment (Figures 3B–3E). To evaluate myelination status in additional neuroanatomical areas for which cuprizone demyelination has been reported, we also performed MOG immunohistochemistry on coronal brain sections at more caudal levels to include hippocampal areas and on sagittal sections through the cerebellum (Figure 4). Demyelination of hippocampal fibres was evident after 6 weeks of cuprizone in the dorsal hippocampal commissure and within the hippocampus, which was especially marked in the hilus area in comparison with non-treated mice (Figures 4A and 4B). In contrast, the cerebellar tissue did not show evidence of demyelination in the arbor vitae or the cerebellar cortex, even when examined after 12 weeks of cuprizone treatment (Figure 4C). Therefore the behavioural changes observed correlate with demyelination of the CC with co-incident demyelination in hippocampal fibres.
Figure 3. Quantitative analysis of myelination following behavioural assessments.
CC (A) and cerebral cortical (B) myelination was estimated in coronal brain sections based on the percentage area immunolabelled for MOG. The region analysed for the CC extended from the midline to under the apex of the cingulum. The region analysed in the adjacent cerebral cortex was outlined from the midline along the pial surface with the lateral border parallel to the midline down to the apex of the cingulum and continuing with the inferior boundary as the border with the cingulum and CC. (A) Compared with non-treated control mice, during cuprizone treatment myelination of the CC was significantly reduced at acute (6 weeks; P = 0.0027) and chronic (12 weeks; P = 0.0027) time points, whereas the percentage area myelinated returned to control levels in the mice allowed a recovery period (6 weeks of cuprizone feeding followed by 6 weeks on normal chow). (B) Compared with non-treated mice, only the chronic (12 week) cuprizone condition showed a significant reduction in the percentage area myelinated in the adjacent cortical region (P = 0.0207). (C and D) Representative images of immunofluorescence for MOG to illustrate myelination patterns in the CC and adjacent cortex in coronal sections. Comparison with a non-treated mouse (C, Control) shows demyelinated areas in both the CC and adjacent cortex after 12 weeks of cuprizone treatment (D, Chronic). After cuprizone treatment for 6 weeks (E, Acute) demyelination is much more apparent in the CC than in the adjacent cortex and the CC is no longer markedly demyelinated after a subsequent 6 week period on normal chow (F, Recovery). Scale bar = 200 μm.
Figure 4. Myelination of hippocampal and cerebellar regions.
(A and B) MOG immunohistochemistry in coronal brain sections shows myelinated hippocampal fibres in non-treated mice (A) that are markedly reduced in mice with acute demyelination following 6 weeks of cuprizone treatment (B). cc, corpus callosum; dhc, dorsal hippocampal commissure; h, hilus; sl, stratum lacunosum of CA1. (C) MOG immunohistochemistry in a sagittal section through the cerebellum shows heavy myelination of the arbor vitae (av) with myelinated fibres extending throughout the cerebellar cortex (cx), which illustrates a lack of cerebellar demyelination in mice treated with cuprizone for 12 weeks. Scale bar = 200 μm.
DISCUSSION
In the present study, we employed both bilateral sensorimotor coordination and social interaction assessments for complementary tests of CC function for studies of cuprizone-induced demyelination in mice. Cuprizone ingestion induces a reproducible pattern of extensive CC demyelination in mice (Mason et al., 2001; Matsushima and Morell, 2001; Armstrong et al., 2002, 2006). The results of the present study indicate that both a complex wheel test of sensorimotor co-ordination and a resident–intruder test of social interaction correlated with CC demyelination status to test distinct functional modalities independently or in conjunction with one another. The complex wheel assay maintained sensitivity when performed at repeated intervals for longitudinal analyses. Both assays distinguished treated from control mice during both acute and chronic demyelination. In addition, both assays were extremely sensitive and detected significant differences between cohorts as small as three mice. Interestingly, the data distinguish treated from non-treated mice following acute demyelination and a subsequent recovery period of spontaneous remyelination that was immunohistochemically confirmed in the same mice, indicating that normal action potential transmission has not yet fully recovered along previously demyelinated axons. These behavioural measurements of CC function in mice will be important to non-invasively monitor demyelination as well as to functionally evaluate manipulations to promote remyelination.
In humans, marked structural changes in the CC are associated with impairments in complex interhemispheric functions such as those related to language skills (Friederici et al., 2007), dichotic listening (Gadea and Espert, 2009), bimanual finger opposition movements (Bonzano et al., 2008) and social interaction and social communication (Badaruddin et al., 2007). Abnormal CC development has also shown correlations in autism spectrum disorders (Cody et al., 2002; Nicolson and Szatmari, 2003; Alexander et al., 2007; Anstey et al., 2007; Paul et al., 2007). Although analogous tasks for some of these skills can be difficult to assess in experimental animals, consistent findings have been observed for social interactions and bilateral coordination. Sociability measurements have been correlated with CC size among mouse strains (Fairless et al., 2008). Bilateral limb co-ordination, as assessed by wheel running on a complex wheel, exhibited deficits as a result of CC agenesis or callosotomy (Schalomon and Wahlsten, 2002) and acute cuprizone demyelination (Liebetanz and Merkler, 2006). The direct effect of cuprizone acute demyelination on CC function has been demonstrated with electrophysiological approaches. Recordings from ex vivo slice preparations showed impaired compound action potentials corresponding with acute CC demyelination, and after 6 weeks of cuprizone ingestion subsequent recovery is incomplete after 6 weeks on normal chow (Tiwari-Woodruff et al., 2009). Similarly, cuprizone treatment increased the response latency between left and right cortical neurons and partial recovery was observed after removal of cuprizone from the diet (Bando et al., 2008).
Consistent with predicted coordination deficits with CC demyelination, several reports indicate motor activity deficits with cuprizone treatment in C57Bl/6 mice, but do not demonstrate applicability for longitudinal studies. After 5 weeks of cuprizone ingestion, deficits are evident as an increase in the frequency of falls on the rotarod, although the sensitivity of the rotarod to measure CC myelination status is unclear since a significant difference was not found at 6 weeks of cuprizone but was present after 6 weeks of cuprizone and 6 weeks of recovery (Franco-Pons et al., 2007). Wheel running with a sequence of training and complex rung patterns showed significant differences in maximal velocity on the complex wheel at 6 weeks of cuprizone treatment (Liebetanz and Merkler, 2006), as was confirmed in the present studies (Figure 1). Both the rotarod and complex wheel assays indicate that significant differences continue even after a 6 week recovery period for remyelination (Liebetanz and Merkler, 2006; Franco-Pons et al., 2007). Each of these previous studies tested activity only at one time point prior to sacrifice so that comparison could only be made between separate cohorts sacrificed at each disease stage. Importantly, the present study was designed to determine whether the complex wheel assessment would be sufficiently sensitive when repeated on a given mouse, as needed for longitudinal studies, or when used to analyse small cohorts of mice, as would be practical in typical remyelination studies. Factors such as training and novelty effects can influence the use of behavioural assessments for repeated measures in longitudinal studies. As our results demonstrate, the complex wheel assay can be used for a within-subjects comparison of pre-treatment, demyelination and remyelination time points. In addition, significant differences were observed with cohorts of only three mice in the present study design as compared with typically larger cohort sizes in behavioural studies, such as the design in the acute cuprizone study with a complex wheel assessment that used 12 mice per cohort (Liebetanz and Merkler, 2006).
Behavioural assessments of anxiety, memory and social interaction have also been reported with cuprizone treatment in C57Bl/6 mice (Franco-Pons, et al. 2007; Xu et al. 2009). In contrast with our findings of increased interactive behaviours among the cuprizone-treated mice in the resident–intruder assay (Figure 2), Xu et al. (2009) reported a significantly decreased social interaction among mice treated with cuprizone for 4–6 weeks. However, the analysis of social interaction used by Xu et al. (2009) was automated and based on the two mice being closer than 5 cm for 0.2 s without being validated by manual observation of an interactive behaviour involving both mice, as was quantified in the present study. Furthermore, open-field assessments in cuprizone-treated mice showed a tendency towards the field centre (Franco-Pons et al., 2007; Xu et al., 2009), indicating decreased anxiety which may be consistent with the increased interactive behaviours observed in our resident–intruder assays. Spatial working memory deficits were similar at weeks 2, 3, 4, 5 and 6 of cuprizone treatment (Xu et al., 2009), indicating a poor correlation of these assessments with the dramatic changes in myelination status of the CC over this time course. In our assessment, using the resident–intruder assay, the increase in interactive behaviours was evident at both acute (6 week) and chronic (12 week) cuprizone-treatment stages, which correlates well with significant CC demyelination at each time point. We also note an age-dependent effect on the frequency of independent behaviours in the resident–intruder assay over this prolonged disease time course. This age effect is not as evident among cuprizone-treated resident mice when an intruder is present, but may be masked in the treated mice since the level of independent behaviours is already reduced significantly by the treatment effect in the younger mice. To minimize the potential involvement of this age effect among independent behaviours, the frequency of interactive behaviours is likely to give a more direct differentiation of treated compared with non-treated mice.
At the completion of our behavioural analysis, the progression of demyelination and remyelination was quantified using immunohistochemistry for MOG, which correlates well with ultrastructural measurements of remyelination (Lindner et al., 2008). Our findings of deficits in the complex wheel running and of increased interactive behaviours were consistently observed during both acute and chronic demyelination (Figures 1 and 2). Both time points correlate with the presence of extensive demyelination in the CC (Figure 3). The proportion of CC demyelination at 12 weeks of cuprizone treatment in our mice exposed to the running wheels is similar to that observed at this time point in a previous analysis (Armstrong et al., 2006). Following acute demyelination, spontaneous remyelination of the CC was observed which was also consistent with our previous studies (Armstrong et al., 2006). Therefore the wheel access, which is a form of behavioural enrichment, is unlikely to have altered the extent of CC demyelination or remyelination obtained relative to our standard cuprizone protocol. Interestingly, evidence of remyelination during the recovery period after acute demyelination was not accompanied by a similar return of normal activity, indicating that the complex wheel assay is sensitive to persisting abnormalities, possibly in the nodal or other axon–myelin structures.
After cuprizone ingestion, the cerebral cortex can also show signs of demyelination with a temporal pattern that is delayed relative to the CC (Skripuletz et al., 2008; Gudi et al., 2009). The complex wheel activity and social interaction changes were observed during acute demyelination at a time when cortical demyelination was not yet evident (Figure 3). Therefore CC demyelination may be sufficient for the observed differences in complex wheel activity and social interaction after 6 weeks of cuprizone treatment. The interpretation that impaired CC function is sufficient to cause decreased velocity of running on the complex wheel assay is supported by studies showing similar results with surgical transection of the CC (Schalomon and Wahlsten, 2002).
The extent to which changes in myelination are evident at specific stages following cuprizone treatment may also vary with the detection methodology. After 6 weeks of cuprizone treatment, significant cortical demyelination was not detected with LFB (Luxol Fast Blue) staining (Skripuletz et al., 2008), but was readily detected by immunoreactivity for PLP (proteolipid protein) and MBP (myelin basic protein) (Skripuletz et al., 2008; Gudi et al., 2009). Hippocampal and cerebellar cortical demyelination has also been reported in the murine cuprizone model (Koutsoudaki et al., 2009; Norkute et al., 2009; Skripuletz et al., 2009). As with the cerebral cortex, hippocampal demyelination was noted with PLP immunostaining, but was not detected with LFB staining 6 weeks after cuprizone treatment (Koutsoudaki et al., 2009). However, it is important to note that LFB staining and MOG immunoreactivity correlated more closely than immunoreactivity for PLP or MBP when compared with electron microscopic quantification of myelination in the CC (Lindner et al., 2008). Our experience with LFB myelin staining (Armstrong et al., 2002; Song et al., 2005) correlates well with our MOG immunoreactivity (Armstrong et al., 2006; and the present study) and with the findings reported by other laboratories using cuprizone (e.g. Matsushima and Morell, 2001). Indeed, at 6 weeks after cuprizone treatment, with both LFB staining and with MOG immunohistochemistry, our studies indicate demyelination of hippocampal fibres, including the dorsal hippocampal commissure, but cerebral cortical demyelination is not marked (Armstrong et al., 2002; Song et al., 2005) (Figures 3 and 4). In addition, relative to cerebellar cortical demyelination, Groebe et al. (2009) did not detect cerebellar cortical demyelination using PLP immunohistochemistry or electron microscopy, which is consistent with our preliminary studies with MOG immunohistochemistry after 12 weeks of cuprizone (Figure 4C). Our interpretation is that hippocampal demyelination is co-incident with CC demyelination, whereas cerebral cortical demyelination is slightly delayed and cerebellar cortical demyelination was not observed in the cuprizone treatment conditions used in the present studies. Relative to our observed behavioural effects, hippocampal demyelination would be likely to have an impact on functions associated with learning and memory, in contrast with the sensorimotor co-ordination and social assessments used in the present study targeting CC analysis, or contribute to the epileptic seizures as has also been reported with cuprizone treatment (Hoffman et al., 2008).
Differences in mouse weight may indicate an underlying variability in the extent of white matter pathology among studies even when a 0.2% (w/w) cuprizone diet is reported. For example, a 30% reduction in weight between treated compared with non-treated mice was reported in the study that indicated development of epileptic seizures among the cuprizone-treated mice (Hoffman et al., 2008). In the present study, the mice did not lose weight during cuprizone treatment, but simply did not gain weight at the same rate as non-treated mice (see the Materials and methods section). Cuprizone has a dose-dependent toxicity effect on mouse weight (Hiremath et al., 1998). Therefore variations in weight loss among studies may reflect variations in the cuprizone toxicity experienced. These differences may carry over to differences in the extent of white matter pathology and in the general health and behaviour with cuprizone treatment as well. For example, during acute cuprizone treatment Liebetanz and Merkler, (2006) reported differences in running parameters on the training wheels that were speculated as due to impairment of general health, whereas differences on the training wheels were not observed in the present study. Importantly, the Liebetanz and Merkler, (2006) study used female C57Bl/6 mice with an average starting weight of 16.5±1.2 g so that the effect of the 0.2% cuprizone dose may have been more severe than in the present study using male C57Bl/6 mice with an average starting weight of 22.4±1.5 g (see the Materials and methods section for other time points).
In conclusion, the resident–intruder social interaction paradigm and the complex wheel-running assay can behaviourally phenotype mice according to CC myelination status in the context of the cuprizone model. These non-invasive functional assays can identify affected mice during longitudinal studies. These assays will be especially useful for characterizing acute and chronic disease severity to identify manipulations to promote remyelination. These findings should also facilitate studies to exploit mouse genetics to examine mechanisms of pathogenesis and repair in demyelinating diseases and take advantage of mouse models to determine involvement of the CC in other CNS injuries and disorders.
Footnotes
This work was supported by the National Institutes of Health [grant number NS39293]; the National Multiple Sclerosis Foundation [grant number RG3515]; and the Department of Defense [grant number G170TP].
REFERENCES
- Adamo AM, Paez PM, Escobar Cabrera OE, Wolfson M, Franco PG, Pasquini JM, Soto EF. Remyelination after cuprizone-induced demyelination in the rat is stimulated by apotransferrin. Exp Neurol. 2006;198:519–529. doi: 10.1016/j.expneurol.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, Bigler ED, Lainhart JE. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Mack HA, Christensen H, Li SC, Reglade-Meslin C, Maller J, Kumar R, Dear K, Easteal S, Sachdev P. Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia. 2007;45:1911–1920. doi: 10.1016/j.neuropsychologia.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Le TQ, Frost EE, Borke RC, Vana AC. Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. J Neurosci. 2002;22:8574–8585. doi: 10.1523/JNEUROSCI.22-19-08574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Le TQ, Flint NC, Vana AC, Zhou YX. Endogenous cell repair of chronic demyelination. J Neuropathol Exp Neurol. 2006;65:245–256. doi: 10.1097/01.jnen.0000205142.08716.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaruddin DH, Andrews GL, Bolte S, Schilmoeller KJ, Schilmoeller G, Paul LK, Brown WS. Social and behavioral problems of children with agenesis of the corpus callosum. Child Psychiatry Hum Dev. 2007;38:287–302. doi: 10.1007/s10578-007-0065-6. [DOI] [PubMed] [Google Scholar]
- Bando Y, Takakusaki K, Ito S, Terayama R, Kashiwayanagi M, Yoshida S. Differential changes in axonal conduction following CNS demyelination in two mouse models. Eur J Neurosci. 2008;28:1731–1742. doi: 10.1111/j.1460-9568.2008.06474.x. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M. Callosal contributions to simultaneous bimanual finger movements. J Neurosci. 2008;28:3227–3233. doi: 10.1523/JNEUROSCI.4076-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody H, Pelphrey K, Piven J. Structural and functional magnetic resonance imaging of autism. Int J Dev Neurosci. 2002;20:421–438. doi: 10.1016/s0736-5748(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Decker L, Picard-Riera N, Lachapelle F, Baron-Van Evercooren A. Growth factor treatment promotes mobilization of young but not aged adult subventricular zone precursors in response to demyelination. J Neurosci Res. 2002;69:763–771. doi: 10.1002/jnr.10411. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Laff R. Callosal lesions and behavior: history and modern concepts. Epilepsy Behav. 2003;4:607–617. doi: 10.1016/j.yebeh.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, Talbot K, Arnold SE, Abel T, Brodkin ES. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain Res. 2008;1230:211–217. doi: 10.1016/j.brainres.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Pons N, Torrente M, Colomina MT, Vilella E. Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol Lett. 2007;169:205–213. doi: 10.1016/j.toxlet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Friederici AD, von Cramon DY, Kotz SA. Role of the corpus callosum in speech comprehension: interfacing syntax and prosody. Neuron. 2007;53:135–145. doi: 10.1016/j.neuron.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Gadea M, Espert R. A comparison of the effects for sustained versus shifted attention on dichotic listening performance. Laterality. 2009;14:315–328. doi: 10.1080/13576500802440707. [DOI] [PubMed] [Google Scholar]
- Gean-Marton AD, Vezina LG, Marton KI, Stimac GK, Peyster RG, Taveras JM, Davis KR. Abnormal corpus callosum: a sensitive and specific indicator of multiple sclerosis. Radiology. 1991;180:215–221. doi: 10.1148/radiology.180.1.2052698. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Groebe A, Clarner T, Baumgartner W, Dang J, Beyer C, Kipp M. Cuprizone treatment induces distinct demyelination, astrocytosis, and microglia cell invasion or proliferation in the mouse cerebellum. Cerebellum. 2009 doi: 10.1007/s12311-009-0099-3. [DOI] [PubMed] [Google Scholar]
- Gudi V, Moharregh-Khiabani D, Skripuletz T, Koutsoudaki PN, Kotsian A, Skuljec J, Trebst C, Stangel M. Regional differences between grey and white matter in cuprizone induced demyelination. Brain Res. 2009;1283:127–138. doi: 10.1016/j.brainres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Hiremath MM, Saito Y, Knapp GW, Ting JP, Suzuki K, Matsushima GK. Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 1998;92:38–49. doi: 10.1016/s0165-5728(98)00168-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Lindner M, Groticke I, Stangel M, Loscher W. Epileptic seizures and hippocampal damage after cuprizone-induced demyelination in C57BL/6 mice. Exp Neurol. 2008;210:308–321. doi: 10.1016/j.expneurol.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Jean I, Lavialle C, Barthelaix-Pouplard A, Fressinaud C. Neurotrophin-3 specifically increases mature oligodendrocyte population and enhances remyelination after chemical demyelination of adult rat CNS. Brain Res. 2003;972:110–118. doi: 10.1016/s0006-8993(03)02510-1. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Diedrichsen J, Hazeltine E, Semjen A, Ivry RB. Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nat Neurosci. 2002;5:376–381. doi: 10.1038/nn822. [DOI] [PubMed] [Google Scholar]
- Koutsoudaki PN, Skripuletz T, Gudi V, Moharregh-Khiabani D, Hildebrandt H, Trebst C, Stangel M. Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci Lett. 2009;451:83–88. doi: 10.1016/j.neulet.2008.11.058. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Merkler D. Effects of commissural de- and remyelination on motor skill behaviour in the cuprizone mouse model of multiple sclerosis. Exp Neurol. 2006;202:217–224. doi: 10.1016/j.expneurol.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Lindner M, Heine S, Haastert K, Garde N, Fokuhl J, Linsmeier F, Grothe C, Baumgartner W, Stangel M. Sequential myelin protein expression during remyelination reveals fast and efficient repair after central nervous system demyelination. Neuropathol Appl Neurobiol. 2008;34:105–114. doi: 10.1111/j.1365-2990.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- Martola J, Stawiarz L, Fredrikson S, Hillert J, Bergstrom J, Flodmark O, Kristoffersen Wiberg M. Progression of non-age-related callosal brain atrophy in multiple sclerosis: a 9-year longitudinal MRI study representing four decades of disease development. J Neurol Neurosurg Psychiatry. 2007;78:375–380. doi: 10.1136/jnnp.2006.106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Langaman C, Morell P, Suzuki K, Matsushima GK. Episodic demyelination and subsequent remyelination within the murine central nervous system: changes in axonal calibre. Neuropathol Appl Neurobiol. 2001;27:50–58. doi: 10.1046/j.0305-1846.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, Schissel MA, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage. 2008;39:1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson R, Szatmari P. Genetic and neurodevelopmental influences in autistic disorder. Can J Psychiatry. 2003;48:526–537. doi: 10.1177/070674370304800804. [DOI] [PubMed] [Google Scholar]
- Norkute A, Hieble A, Braun A, Johann S, Clarner T, Baumgartner W, Beyer C, Kipp M. Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J Neurosci Res. 2009;87:1343–1355. doi: 10.1002/jnr.21946. [DOI] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Schalomon PM, Wahlsten D. Wheel running behavior is impaired by both surgical section and genetic absence of the mouse corpus callosum. Brain Res Bull. 2002;57:27–33. doi: 10.1016/s0361-9230(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Lindner M, Kotsiari A, Garde N, Fokuhl J, Linsmeier F, Trebst C, Stangel M. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am J Pathol. 2008;172:1053–1061. doi: 10.2353/ajpath.2008.070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T, Bussmann JH, Gudi V, Koutsoudaki PN, Pul R, Moharregh-Khiabani D, Lindner M, Stangel M. Cerebellar cortical demyelination in the murine cuprizone model. Brain Pathol. 2009 doi: 10.1111/j.1750-3639.2009.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Tiwari-Woodruff SK, Crawford DK, Song B, Sofroniew MV, Mangiardi M. A critical window: functional recovery of callosal axons following demyelination. J Neurochem. 2009;108(Suppl 1):65. doi: 10.1016/j.neuroscience.2009.09.069. [DOI] [PubMed] [Google Scholar]
- Xu H, Yang HJ, Zhang Y, Clough R, Browning R, Li XM. Behavioral and neurobiological changes in C57BL/6 mice exposed to cuprizone. Behav Neurosci. 2009;123:418–429. doi: 10.1037/a0014477. [DOI] [PubMed] [Google Scholar]