Abstract
The Infant Development Environment and Lifestyle (IDEAL) study is investigating the effects of prenatal methamphetamine (MAMP) exposure on infant and child development; potential concurrent exposure to cannabis and tobacco also are evaluated. Maternal self-reported drug use and/or meconium toxicology results defined drug exposure status. It is unclear how the frequency, duration and magnitude of maternal MAMP exposure affect qualitative and quantitative meconium results.
Materials and Methods
Interviews regarding maternal drug use were collected shortly after birth; meconium specimens were screened for amphetamines, cannabis and cotinine by immunoassay and confirmed by gas chromatography mass spectrometry (GCMS).
Results
The majority of MAMP- and cannabis-exposed infants were identified by maternal interview alone. Meconium tests were more likely to be positive if the mother reported MAMP and cannabis use, particularly in the third trimester. Less than half of immunoassay-positive amphetamines (31.0%) and cannabis (17.9%) meconium results were confirmed by GCMS. Tobacco exposure was equally detected by immunoassay cotinine screen and maternal report. Meconium concentrations did not correlate with maternal self-report status or trimester of use, frequency or route of MAMP use.
Discussion
Maternal self-report was more sensitive than meconium testing for identifying MAMP and cannabis-exposed neonates; however, the timing of drug exposure may influence meconium toxicology results. Most women ceased MAMP and cannabis use before the third trimester. In the first trimester, meconium has not yet formed, and based on our recent results for opiates and cocaine, drug use in the second trimester appears to be poorly reflected in meconium.
Conclusion
Low confirmation rates in meconium reinforce the need for confirmatory testing following positive screening results and additional research to identify alternative biomarkers.
Keywords: methamphetamine, pregnancy, meconium, in utero, cannabis, tobacco, prenatal drug exposure, amphetamines
Despite widespread methamphetamine (MAMP) and amphetamine (AMP) abuse, little is known about the effect of amphetamines on the developing fetus. The Infant Development, Environment and Lifestyle (IDEAL) study is investigating prenatal MAMP exposure effects on neonatal outcomes and child development in the largest cohort of amphetamines-exposed infants evaluated to date. Lower birth weights, higher incidences of small-for-gestational age, and increased physiological stress were observed among these MAMP-exposed infants.1, 2
Maternal self-report and/or toxicological testing are often employed to identify drug-exposed infants. Drug use histories provided by pregnant women often are thought to be unreliable because of guilt, fear of prosecution or genuine inability to accurately remember drug use details. Meconium, the first neonatal feces, objectively identifies a variety of prenatal drug exposures and offers a wider window of drug detection and easier collection than neonatal urine.3
Yet, it is unclear if maternal self-report or meconium testing is the most sensitive method for specifically detecting prenatal MAMP use. Other large scale investigations identified few MAMP-exposed neonates by self-report or meconium testing4, 5; however, these studies targeted areas with little MAMP abuse. To be most inclusive, maternal admission of amphetamines use and/or positive meconium toxicology results defined exposure status in the IDEAL cohort.
The primary aim of this report was to describe maternal self-reported drug use and meconium toxicology results from the IDEAL study, focusing primarily on amphetamines. We determined MAMP confirmation rates, and compared timing, frequency, and route of MAMP abuse with meconium toxicology results in a large cohort. Cannabis and tobacco exposure detection by meconium analysis and maternal self-report also is described. Evaluating the ability of meconium to identify amphetamines-exposed neonates provides objective guidance for clinicians in interpreting meconium amphetamines results and suggests needed prenatal MAMP research.
Materials and Methods
Four US communities with high prevalence of MAMP abuse participated in the IDEAL study: Los Angeles, California; Des Moines, Iowa; Tulsa, Oklahoma; and Honolulu, Hawaii. The study was approved by Institutional Review Boards at each hospital, the University of Maryland, Warren Alpert Medical School of Brown University, and National Institute on Drug Abuse (NIDA); a NIDA Certificate of Confidentiality was obtained to facilitate honest recall of drug use history. Procedures and protocols were standardized across locations.6 Maternal and infant exclusion criteria were fully described by Arria et al.6 Among the most frequent reasons for ineligibility were the inability to speak English, maternal age < 18 years, multiple births and opiate use during pregnancy.
After providing informed consent, mothers completed a Substance Use Inventory to recall the amount and frequency of alcohol, tobacco, and drug (cannabis, hashish, MAMP, ecstasy, AMP, benzodiazepine/tranquilizers, barbiturates/sedatives, and cocaine/crack) consumption during each trimester and the three months prior to becoming pregnant. Quantity descriptions varied by drug, and frequency was classified by the number of days per week drugs were consumed: every day, almost every day, 3–4 times per week, 1–2 times per week, 2–3 times per month, once a month, or 1–2 times in 3 months. Route of administration, insufflation, ingestion, smoking and/or intravenous use, were recorded.
Meconium was collected from diapers until the appearance of milk stool. Specimens remained refrigerated until transported overnight (2-day for Hawaii) to the United States Drug Testing Laboratories (Des Plaines, IL) for analysis. Meconium (0.5 g) was homogenized in methanol and centrifuged. Supernatants were buffered and extracted using mixed mode solid phase extraction columns (ZSDAU020, United Chemical Technologies, Bristol, PA). Specimens were screened within 24 hours of receipt with Syva enzyme multiplied immunoassay technique (EMIT) II Plus (Dade Behring, Cupertino, CA) for cannabinoids (cutoff: 40 ng/g), cocaine metabolite (75 ng/g), opiates (150 ng/g) and amphetamines (500 ng/g) on an Olympus AU640 analyzer (Center Valley, PA). According to the manufacturer, the following compounds substantially cross-reacted (>5%) with the amphetamines immunoassay: d,l-MAMP (71%), benzphetamine (71%), phenmetrazine (70%), phentermine (55%), d,l-AMP (48%), l-MAMP (38%), mephentermine (33%), 3,4-methylenedioxyamphetamine (MDA) (29%), l-AMP (13%), fenfluramine (12.5%), 4-chloramphetamine (11%), tranylcypromine (8%), 3,4-methylenedioxyethylamphetamine (7%), norpseudoephedrine (7%), and MDMA (5%).7 If positive, separate aliquots were extracted and confirmed by GCMS within 48 h at the following cutoffs: 2 ng/g 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH); 5 ng/g cocaine, cocaethylene, benzoylecgonine, and/or m-hydroxybenzoylecgonine; 5 ng/g codeine and morphine; and 5 ng/g AMP, MAMP, MDMA, and MDA.8 The tobacco biomarker cotinine was screened by enzyme-linked immunosorbent assay (ELISA, International Diagnostics, St. Joseph, MI) with a 10 ng/g cutoff. A more specific chromatographic method for cotinine in meconium was not available for confirmation. Maternal opiate use was exclusionary and cocaine use without concurrent MAMP use also was exclusionary; thus, meconium results for these drug classes are not presented, as data are not representative.
SPSS version 16.0 for Windows (Chicago, IL) and Microsoft Excel were employed for data analysis and statistical evaluation. Mann-Whitney tests evaluated analyte concentration and metabolite ratio differences between groups; p-values <0.05 were considered statistically significant.
Results
California contributed the most mother/infant dyads (N=1292), followed by Iowa (N=947), Hawaii (N=755), and Oklahoma (N=711) for a total of 3705 participants. Approximately 5.5% reported consuming amphetamines (MAMP, AMP and/or ecstasy) during pregnancy. Most only consumed MAMP (N=190), while others reported MAMP and AMP (N=4), MAMP and ecstasy (N=2), MAMP, AMP, and ecstasy (N=1), AMP only (N=3) and ecstasy only (N=2). The overall percentages positive for amphetamines by EMIT screening and GCMS confirmation were 5.3 and 1.3%, respectively, with variable percentages across locations (Table 1). By GCMS, 39.0% of immunoassay-positive meconium specimens contained both MAMP and AMP, except for one specimen with MAMP only; none were MDMA- or MDA-positive. MAMP concentrations ranged from 26–19,376 ng/g (median 1,971 ng/g), with AMP ranging from 11–2,765 ng/g (median 389 ng/g). MAMP concentrations exceeded AMP in all cases except one; AMP/MAMP ratios were 0.01–1.14 (median 0.23).
Table 1.
Number (%) of total positive maternal self-reports and meconium enzyme multiplied immunoassay technique (EMIT) or enzyme-linked immunosorbent assay (ELISA) screening and gas chromatography mass spectrometry (GCMS) confirmation results by location.
| Iowa | Oklahoma | Hawaii | California | Total | |
|---|---|---|---|---|---|
|
Amphetamines* | |||||
| N | 947 | 711 | 755 | 1292 | 3705 |
| Self-report | 19 (2.0) | 27 (3.8) | 77 (10.2) | 79 (6.1) | 202 (5.5) |
| EMIT | 26 (2.7) | 35 (4.9) | 55 (7.3) | 81 (6.3) | 197 (5.3) |
| GCMS | 3 (0.3) | 5 (0.7) | 31 (4.1) | 22 (1.7) | 61 (1.6) |
|
Cannabinoids | |||||
| N | 947 | 711 | 754 | 1291 | 3703 |
| Self-report | 22 (2.3) | 81 (11.3) | 33 (4.4) | 89 (6.9) | 225 (6.1) |
| EMIT | 69 (7.3) | 126 (17.7) | 81 (10.7) | 165 (12.8) | 441 (11.9) |
| GCMS | 7 (0.7) | 39 (5.4) | 7 (0.9) | 27 (2.1) | 80 (2.2) |
|
Cotinine | |||||
| N | 843 | 605 | 670 | 953 | 3071 |
| Self-report | 150 (17.8) | 229 (37.9) | 186 (27.8) | 173 (18.2) | 738 (24.0) |
| ELISA | 148 (17.5) | 225 (37.2) | 197 (29.4) | 176 (18.5) | 746 (20.2) |
includes methamphetamine, amphetamine and ecstasy
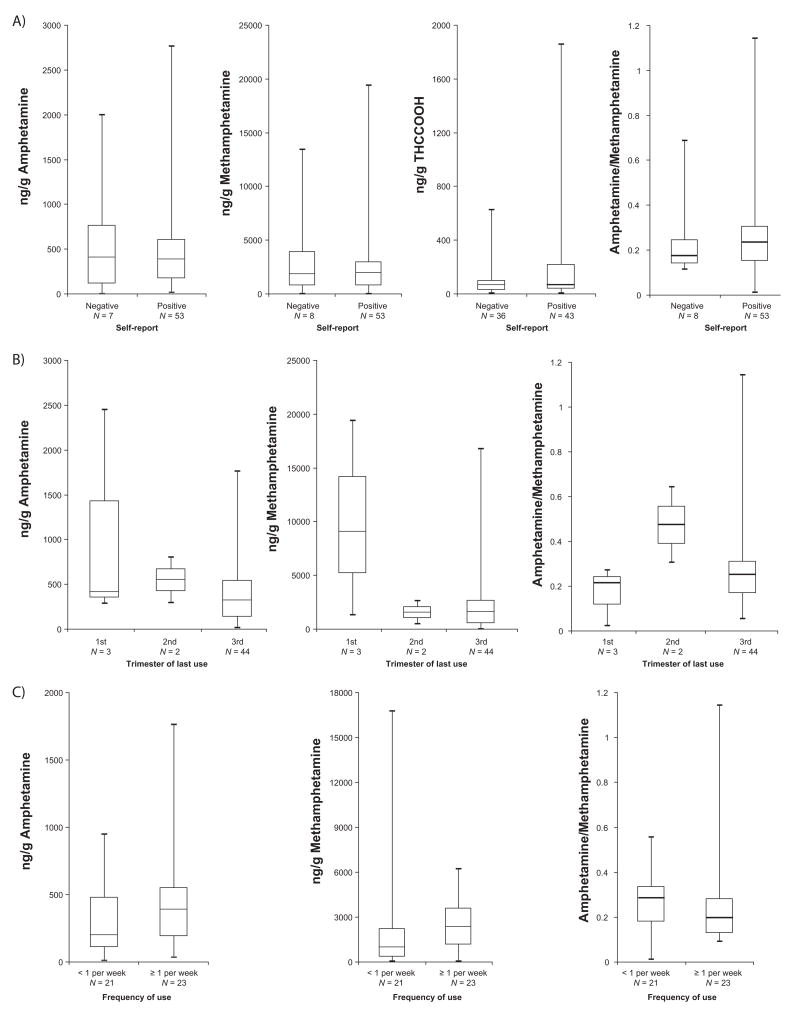
Maternal admission of use was associated with an increased percentage of positive meconium screening and confirmation tests (Table 2), yet only 25% of amphetamines-positive self-reports had positive meconium screens (N=56) or confirmations (N=53). Interestingly, one meconium specimen contained a high MAMP concentration despite maternal admission of only AMP consumption, highlighting the uncertain composition and purity of illicit drug preparations. Two women reporting only ecstasy exposure had negative meconium results. Among those denying amphetamines consumption, 141 meconium specimens screened positive, and only eight (0.2% of negative self-reports) confirmed by GCMS. No MAMP or AMP concentration or AMP/MAMP ratio differences were observed between those with positive and negative maternal self-reports (Figure 1a). Overall, 210 (5.7%) neonates were identified as amphetamines-exposed; 71.0% were identified by maternal self-report only, 25.2% by self-report and meconium results, and 3.8% by meconium analysis only. Thus, with current analytical procedures, maternal self-report was more sensitive than meconium analysis for detecting prenatal amphetamines exposure.
Table 2.
Number (% of reported) positive meconium enzyme multiplied immunoassay technique (EMIT) or enzyme-linked immunosorbent assay (ELISA) screening and gas chromatography mass spectrometry (GCMS) confirmation results by maternal self-report, and if positive, trimester of last use
| N | EMIT/ELISA (% of N) | GCMS (% of N) | |
|---|---|---|---|
|
Amphetamines* | |||
| Negative self-report | 3503 | 141 (4.0) | 8 (0.2) |
| Positive self-report1 | 202 | 56 (27.7) | 53 (26.2) |
| First trimester only | 68 | 5 (7.4) | 3 (4.4) |
| Second trimester | 46 | 3 (6.5) | 2 (4.3) |
| Third trimester | 81 | 44 (54.3) | 44 (54.3) |
|
Cannabis | |||
| Negative self-report | 3477 | 357 (10.3) | 36 (1.0) |
| Positive self-report1 | 225 | 82 (36.4) | 43 (19.1) |
| First trimester only | 28 | 7 (25.0) | 1 (3.6) |
| Second trimester | 20 | 5 (25.0) | 0 (0.0) |
| Third trimester | 25 | 16 (64.0) | 12 (48.0) |
|
Cotinine | |||
| Negative self-report | 2333 | 270 (11.6) | |
| Positive self-report | 738 | 476 (64.5) | |
includes methamphetamine, amphetamine and ecstasy
Trimester data not available for 7 participants using amphetamines and 152 for cannabis.
Figure 1.
Methamphetamine, amphetamine, 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH) concentrations and methamphetamine/amphetamine concentration ratios in positive meconium specimens by A) maternal self-report, B) trimester of last use and C) frequency of use during the third trimester.
Most women decreased MAMP exposure as their pregnancy progressed.2 To compare the timing of drug exposure and meconium results, trimester of drug use was classified as: first trimester only, first and/or second trimester, and first, second and/or third trimester (Table 2); trimester use data were not available for 7 women reporting amphetamines use and 152 cannabis smokers. If concurrent MAMP, AMP and/or MDMA use was declared, classification was assigned according to last trimester used, irrespective of the specific drug. As expected, first trimester exposure was associated with low percentages of EMIT- and confirmed-positive cases. Second trimester exposure results were similar to the first trimester, suggesting that meconium does not reliably reflect use prior to the third trimester. If mothers reported third trimester drug consumption, a higher percentage of meconium specimens were positive, yet, 45.7% still had negative meconium results for amphetamines. Too few positive results for first and second trimester exposure precluded statistical comparisons of MAMP and AMP concentrations and AMP/MAMP concentration ratios by trimester of last use (Figure 1b).
Meconium results were divided by frequency of amphetamines consumption (Table 3). More frequent use during the first and second trimesters was not associated with more positive meconium results, while in the third trimester, slightly more positive meconium results were observed when use was greater than once per week. Frequency of use during the last reported trimester, MAMP and AMP concentrations, and AMP/MAMP concentration ratios were not statistically correlated (Figure 1c).
Table 3.
Number (%) positive meconium enzyme multiplied immunoassay technique (EMIT) screening and gas chromatography mass spectrometry (GCMS) confirmation results by frequency and trimester of self-reported methamphetamine consumption
| First trimester | Second trimester | Third trimester | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | EMIT (%) | GCMS (%) | N | EMIT (%) | GCMS (%) | N | EMIT (%) | GCMS (%) | |
| 1–2 times in 3 mo | 22 | 0 (0.0) | - | 15 | 1 (6.7) | 0 | 34 | 14 (41.2) | 14 (41.2) |
| 1 time per mo | 1 | 0 (0.0) | - | 1 | 0 (0.0) | - | 8 | 4 (50.0) | 4 (50.0) |
| 2–3 times per mo | 6 | 1 (16.6) | 1 (16.6) | 6 | 1 (16.7) | 1 (16.7) | 7 | 3 (42.9) | 3 (42.9) |
| 1–2 times per wk | 11 | 0 (0.0) | - | 8 | 0 (0.0) | - | 13 | 10 (76.9) | 10 (76.9) |
| 3–4 times per wk | 18 | 2 (11.1) | 1 (5.6) | 9 | 1 (11.1) | 1 (11.7) | 9 | 6 (66.7) | 6 (66.7) |
| Almost everyday | 1 | 0 (0.0) | - | 2 | 0 (0.0) | - | 5 | 4 (80.0) | 4 (80.0) |
| Daily | 5 | 1 (20.0) | 1 (20.0) | 5 | 0 (0.0) | - | 5 | 3 (60.0) | 3 (60.0) |
Women were asked their preferred route(s) of MAMP administration (smoking, insufflation, ingestion, injection, or multiple routes). Not surprisingly, smoking was the most common method while injection and ingestion were less common. Meconium results from neonates exposed during the third trimester were assessed by maternal administration route to determine MAMP biomarker disposition, but no significant differences were observed between any route of administration, positive screen and confirmation rates (Table 4), MAMP and AMP concentrations or AMP/MAMP concentration ratios.
Table 4.
Number (%) meconium enzyme multiplied immunoassay technique (EMIT) screening and gas chromatography mass spectrometry (GCMS) confirmation results by route of third trimester methamphetamine administration
| Route of Administration | N | EMIT (%) | GCMS (%) |
|---|---|---|---|
| Smoke only | 51 | 31 (60.8) | 31 (60.8) |
| Sniff only | 13 | 8 (61.5) | 8 (61.5) |
| Ingest only | 2 | 1 (50.0) | 1 (50.0) |
| Multiple routes | 14 | 4 (28.6) | 4 (28.6) |
In addition to amphetamines, cannabis and tobacco use were investigated by maternal self-report and meconium to identify potential co-exposures. For two participants, cannabis testing was not performed because insufficient meconium remained. Only individuals with maternal self-report and meconium tests were included in this report. Oklahoma had the highest percentage of women reporting cannabis use, as well as the highest percentage of cannabinoid-positive meconium (Table 1). Over 6% admitted smoking cannabis (N=225), but meconium specimens screened and confirmed positive in 36.4% and 19.1%, respectively (Table 2). Independent of maternal self-report, 441 meconium specimens screened positive, and only 82 (18.6%) contained THCCOOH, with concentrations ranging from 3–1,856 ng/g (median 66 ng/g). No significant concentration differences were observed between positive and negative self-report groups (Figure 1a). Similar to amphetamines, few meconium specimens screened or confirmed positive when the mother declared cannabis use in the first and/or second trimester (Table 2). Meconium specimens were more likely to be positive if exposure continued into the third trimester; however, also like amphetamines, 52% of neonates exposed to cannabis late in pregnancy had negative meconium results. Overall, 261 (7.0%) neonates were cannabis-exposed. Maternal self-report alone identified 69.7%; 16.5% had positive self-report and meconium results, and 13.8% were detected by meconium analysis alone.
For tobacco exposure, 634 participants were not evaluated for cotinine because of lack of adequate specimen Again, Oklahoma had the highest percentages of tobacco positive self-report and meconium screens (Table 1). Most women reporting tobacco use (64.5%) had presumptive positive cotinine screens, while 11.6% of those denying use screened positive. Among neonates with meconium analysis and maternal self-report, 1008 (32.8%) were tobacco-exposed. Maternal admission alone identified 26.0%, whereas positive maternal self-report and meconium detected 47.2%. Meconium testing alone identified the remaining 26.8% of nicotine-exposed neonates. These results indicate that maternal self-report and meconium testing have equal sensitivity for detection of tobacco exposure.
Discussion
The IDEAL study is the first prospective, longitudinal investigation of prenatal amphetamines exposure. Over 3,700 meconium specimens and maternal interviews were obtained in four geographically and ethnically diverse locations. We investigated the relative ability of maternal self-report and meconium analysis to identify amphetamines and other prenatal drug exposures, and compared maternal drug use patterns (timing, frequency, and magnitude) to meconium outcomes. Based on the combination of maternal self-report and meconium analysis, 210 (5.7%) of neonates were amphetamines-exposed. Of these, 204 are participating in ongoing evaluations to determine the impact of prenatal amphetamines exposure on infant and child development.
Pregnant women often deny or misrepresent drug consumption because of societal perceptions surrounding gestational drug use or fear of losing parental custody or prosecution.9 Thus, our reliance on maternal self-reported drug use histories is a limitation; however, several techniques were employed to improve recall accuracy and minimize bias in this investigation. First, a NIDA Certificate of Confidentiality was obtained, protecting mothers from mandatory reporting requirements, except in instances of child abuse or endangerment. Secondly, staff administered structured, scripted interviews and were centrally trained to standardize questioning procedures. Third, in addition to the drug class, common street or slang terms and brand names were provided. Lastly, participants were given a calendar including holidays and personal events to serve as a memory aid. However, in spite of these efforts, completely accurate histories cannot be guaranteed. Other objective measures could have been applied to corroborate maternal claims, but these also have limitations. Participants were enrolled at delivery; thus, a biological matrix with a long drug detection window would be required to asses drug use throughout pregnancy. Maternal hair testing has a sufficiently long window, but may reflect environmental drug exposure rather than actual ingestion and cannot provide drug consumption details like frequency, timing and magnitude.10 Additionally, hair would not reflect the most recent maternal drug use.
Surprisingly, maternal self-report was more sensitive for detecting prenatal amphetamines and cannabis exposure than meconium analysis. This result is in stark contrast to other prenatal drug exposure studies where meconium was more sensitive than self-report, and most positive self-reports were corroborated by positive meconium tests.5,11 In the “Meconium Project,” a large scale European investigation of prenatal drug exposure prevalence, liquid chromatography mass spectrometric analysis of meconium specimens for opiates and cocaine identified ~2.5 – 7 times more affected neonates than maternal self-report, and all self-reported instances of opiate and/or cocaine use were corroborated by positive meconium results.5 In the same cohort, cannabis exposure was evaluated by maternal self-report and meconium analysis for Δ9-tetrahydrocannabinol, 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC) and THCCOOH by GCMS analysis. In total, 52 meconium specimens were cannabinoid-positive, with only 11 corresponding with positive maternal self-report.11 In our study, mothers were assured confidentiality of self-reported information and also offered educational and medical resources for the child and family. These measures may have improved the accuracy of self-reported drug use history, as compared to the “Meconium Project.” There also was a major difference in the primary drug classes evaluated. Opiate and cocaine use were the focus of the “Meconium Project”, but were exclusionary in the IDEAL population. Thus, it is not possible to directly compare results between studies. For cannabis, detailed maternal consumption characteristics were not reported in the “Meconium Project,” so the frequency or magnitude of cannabis exposure could differ between studies, and as a result, meconium results may be dissimilar. Of the 17 women reporting cannabis use in the “Meconium Project”, six declared consumption only in the first trimester and, as in our study, all corresponding meconium specimens were negative. The analytical differences between studies may also contribute to the disparate results. Foregoing immunoassay cannabis screening and including additional cannabis biomarkers in GCMS analysis, notably 11-OH-THC, potentially increased positivity rates in the “Meconium Project.” In their population, 13.5% of positive specimens were identified by 11-OH-THC alone.
Other factors may contribute to the low number of positive meconium results observed in our study. First, meconium begins forming in the twelfth gestational week, theoretically collecting endogenous and exogenous wastes from the second trimester. Hence, if drug use ceased in the first trimester, as was the case for many women, meconium results would be negative. Not surprising, 95.6% of meconium specimens were negative when amphetamines and cannabis consumption was limited to the first trimester.
Additionally, data suggest that second trimester drug exposure is poorly reflected in meconium, contrary to the currently accepted detection window. Casanova et al observed negative meconium results when cocaine exposure occurred more than one month before delivery, whereas all meconium specimens were positive if mothers used cocaine within that time frame.12 Similarly, Kacinko et al. reported that neonates exposed to opiates or cocaine within 10 days of delivery all had positive meconium results, while neonates exposed more than 20 days prior to delivery frequently had negative meconium; infants with positive meconium also were drug-exposed more frequently in the third trimester, as documented by thrice weekly maternal urinalysis.13 The only animal study to our knowledge investigating MAMP exposure timing and meconium disposition corroborates the human data. MAMP was detectable in guinea pig meconium following maternal administration on gestational day 63 of a 68 day gestation, but not from earlier exposures.14 In our study, amphetamines exposure during the second trimester was associated with far fewer positive meconium results than when exposure continued into the third trimester, supporting a shorter detection window than currently thought for meconium. The described animal study also observed increased AMP with advancing gestational age, suggesting increased fetal metabolic function.14 In our specimens, there were no MAMP or AMP concentration or MAMP/AMP ratio differences by exposure timing or frequency. Meconium MAMP and AMP concentrations may better reflect the quantity of drug consumed by the mother, rather than frequency. Mothers were asked how much drug was consumed, but the responses are of limited use because of differences in reporting. Some women reported the quantity by monetary value, weight, or non-standardized street terminology. The unknown purity of illicit drug preparations further complicates dose-concentration relationships.
While early exposure may explain a large proportion of negative tests, nearly half of neonates exposed during the third trimester still were amphetamines negative. It is possible that “negative” meconium may contain MAMP and/or AMP biomarkers but at concentrations below the 500 ng/g immunoassay cutoff, a concentration in keeping with available meconium testing procedures.15, 16 By lowering the screening cutoff concentration, more true positive specimens might have been identified; however, more false positive tests also would be expected, along with increased cost and turnaround time. Further, by maintaining the commercial laboratory’s normal amphetamines screening cutoff, our meconium results are easily extrapolated to routine clinical specimens and may help guide result interpretation.
Confirming presumptive immunoassay results with a more specific analytical technique, such as GCMS, is standard procedure for most analytical laboratories; however, some hospital laboratories and research investigators forego confirmatory analyses and base exposure status solely on immunoassay results. As meconium results frequently inform child protective service investigations and differentiate drug-exposed from control groups in research settings, relying on immunoassay data alone is inappropriate because of the high false-positive rates observed in meconium, especially for amphetamines.17 Nearly 70% of IDEAL meconium specimens screening positive for amphetamines were not confirmed for MAMP and/or AMP by GCMS. EMIT assays targeting MAMP/AMP cross-react with other sympathomimetic amines, including structurally-related compounds from over-the-counter cold medications and with minor MAMP metabolites. Recently, our laboratory discovered three novel meconium MAMP/AMP biomarkers, p-hydroxymethamphetamine, p-hydroxyamphetamine and norephedrine,18 but additional research is needed to determine if more amphetamines-exposed neonates can be identified with these new biomarkers.
Cannabis and tobacco use also were monitored to identify co-exposures and to exclude other potentially confounding drug exposures. Results for cannabis exposure were similar to the amphetamines in that maternal self-report was more sensitive than meconium analysis for identifying cannabis exposure, more meconium specimens screened and confirmed positive for cannabinoids when use continued into the third trimester and the confirmation rate of positive screening specimens was low. As with the amphetamines, most cannabis use was confined to early gestation and could account for the poor detection rate by meconium analysis.
Not surprisingly, tobacco smoking was far more common than exposure to illicit drugs. Maternal self-report and meconium cotinine screening were equally sensitive for detecting prenatal nicotine exposure. It is interesting that many tobacco-smoking women chose not to report use of this licit drug. Guilt for using tobacco during pregnancy and disapproval of tobacco-smoking by medical professionals could explain this phenomenon. Meconium cotinine screening without chromatographic confirmation was employed to objectively assess maternal self-reported smoking status. However, caution should be used when interpreting the cotinine data, since presumptive positives were not confirmed and 634 neonates were not evaluated by meconium testing, as too little specimen remained. Furthermore, smoking data may be underestimated based solely on cotinine immunoassay results. Recently, our laboratory identified 25% more tobacco-exposed neonates with a liquid chromatography tandem mass spectrometry assay for nicotine and trans-3′-hydroxycotinine, as well as cotinine.19
The low prevalence of MAMP and cannabis biomarkers in meconium questions the utility of meconium analyses to document prenatal drug exposure. Despite these research findings, we believe that meconium analysis for assessing prenatal drug exposure is still beneficial. First, as discussed above, the confidentiality certificate obtained for this study protected participants’ self-reported drug use history from disclosure, which is not the case for most pregnant women. Second, specific resources were made available for the child and family that offered considerable value for MAMP-exposed infants. Third, our focus was limited to amphetamines and cannabis, and these data cannot be extrapolated to other drugs of abuse, particularly opiates and cocaine. In previous research4,5, meconium analysis was shown to be more sensitive for detecting cocaine and opiate exposure than maternal self-report. Fourth, our research and others are identifying new biomarkers in meconium for prenatal drug exposure that should increase the accuracy and efficacy of this testing.18 And finally, our laboratory demonstrated that meconium concentrations may predict adverse neonatal outcomes; specifically, buprenorphine and metabolite concentrations in meconium are related to the severity and duration of neonatal abstinence syndrome.20 At this time, it is not clear if concentrations of other drugs in meconium, including amphetamines and cannabis, may be predictive of poor neonatal and/or child development.
Conclusion
Maternal self-report identified more MAMP and cannabis-exposed neonates than meconium analysis; however, our data suggest that meconium analysis poorly reflects first and second trimester consumption, when the majority of women ceased use. Most positive amphetamines and cannabis meconium screens were not confirmed, highlighting the need for confirmatory testing following positive screening results.
Acknowledgments
This research was funded by IDEAL grant NIDA R01DA014948 and the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health (TRG and MAH).
This research was funded by the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health and IDEAL grant NIDA R01DA014948.
References
- 1.Smith LM, LaGasse LL, Derauf C, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–56. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 2.Smith LM, Lagasse LL, Derauf C, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30:20–8. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray T, Huestis M. Bioanalytical procedures for monitoring in utero drug exposure. Anal Bioanal Chem. 2007;388:1455–1465. doi: 10.1007/s00216-007-1228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lester BM, ElSohly M, Wright LL, et al. The maternal lifestyle study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107(2):309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 5.Pichini S, Puig C, Zuccaro P, et al. Assessment of exposure to opiates and cocaine during pregnancy in a Mediterranean city: preliminary results of the “Meconium Project”. Forensic Sci Int. 2005;153:59–65. doi: 10.1016/j.forsciint.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Arria AM, Derauf C, Lagasse LL, et al. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) study. Matern Child Health J. 2006;10:293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- 7.Syva Company. EMIT II Plus Monoclonal Amphetamine/Methamphetamine Assay. 1999 [Google Scholar]
- 8.Lewis DL. Forensically-acceptable determinations of gestational fetal exposure to drugs and other chemical agents. 5,532,131. United States Patent and Trademark Office. 1996
- 9.Jacobson SW, Jacobson JL, Sokol RJ, et al. Maternal recall of alcohol, cocaine, and marijuana use during pregnancy. Neurotoxicol Teratol. 1991;13:535–540. doi: 10.1016/0892-0362(91)90062-2. [DOI] [PubMed] [Google Scholar]
- 10.Boumba V, Ziavrou K, Vougiouklakis T. Hair as a biological indicator of drug use, drug abuse or chronic exposure to environmental toxicants. Int J Toxicol. 2006;25:143–63. doi: 10.1080/10915810600683028. [DOI] [PubMed] [Google Scholar]
- 11.Lozano J, Garcia-Algar O, Marchei E, et al. Prevalence of gestational exposure to cannabis in a Mediterranean city by meconium analysis. Acta Paediatr. 2007;96:1734–7. doi: 10.1111/j.1651-2227.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 12.Casanova OQ, Lombardero N, Behnke M, et al. Detection of cocaine exposure in the neonate. Analyses of urine, meconium, and amniotic fluid from mothers and infants exposed to cocaine. Arch Pathol Lab Med. 1994;118:988–993. [PubMed] [Google Scholar]
- 13.Kacinko SL, Jones HE, Johnson RE, et al. Correlations of maternal buprenorphine dose, buprenorphine, and metabolite concentrations in meconium with neonatal outcomes. Clin Pharmacol Ther. 2008;84:604–12. doi: 10.1038/clpt.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura KT, Ayau EL, Uyehara CF, et al. Methamphetamine detection from meconium and amniotic fluid in guinea pigs depends on gestational age and metabolism. Dev Pharmacol Ther. 1992;19:183–190. doi: 10.1159/000457483. [DOI] [PubMed] [Google Scholar]
- 15.ElSohly MA, Stanford DF, Murphy TP, et al. Immunoassay and GC-MS procedures for the analysis of drugs of abuse in meconium. J Anal Toxicol. 1999;23:436–445. doi: 10.1093/jat/23.6.436. [DOI] [PubMed] [Google Scholar]
- 16.Franssen RME, Stolk LML, van den Brand W, et al. Analysis of morphine and amphetamine in meconium with immunoassay and HPLC-diode-array detection. J Anal Toxicol. 1994;18:294–295. doi: 10.1093/jat/18.5.294. [DOI] [PubMed] [Google Scholar]
- 17.Moore C, Lewis D, Leikin J. False-positive and false-negative rates in meconium drug testing. Clin Chem. 1995;41(11):1614–1616. [PubMed] [Google Scholar]
- 18.Gray TR, Kelly T, LaGasse LL, et al. Novel biomarkers of prenatal methamphetamine exposure in human meconium. Ther Drug Monit. 2008;31:70–5. doi: 10.1097/FTD.0b013e318195d7cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray TR, Magri R, Shakleya DM, et al. Meconium Nicotine and Metabolites by Liquid Chromatography-Tandem Mass Spectrometry: Differentiation of Passive and Nonexposure and Correlation with Neonatal Outcome Measures. Clin Chem. 2008;54:2018–2027. doi: 10.1373/clinchem.2008.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kacinko S, Jones H, Johnson R, et al. Correlations of Maternal Buprenorphine Dose, Buprenorphine, and Metabolite Concentrations in Meconium with Neonatal Outcomes. Clin Pharmacol Ther. 2008 doi: 10.1038/clpt.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]