Abstract
Wallis, Craig (Baylor University College of Medicine, Houston, Tex.) and Joseph L. Melnick. Thermosensitivity of poliovirus. J. Bacteriol. 86:499–504. 1963.—Polioviruses are thermosensitive agents, although thermoresistant strains have been obtained and reported in the literature. Such resistant strains can be developed by exposure of the virus to cystine during multiple-cycle yields. Thermoresistant strains can be converted to the thermosensitive state by passing the virus in cells maintained in a cystine-free medium, or by reducing the virus with glutathione. The thermoresistant variants seem to result from the conditions under which virus is grown and harvested. Consequently, many such thermostable polioviruses actually represent phenotypic rather than genotypic variation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
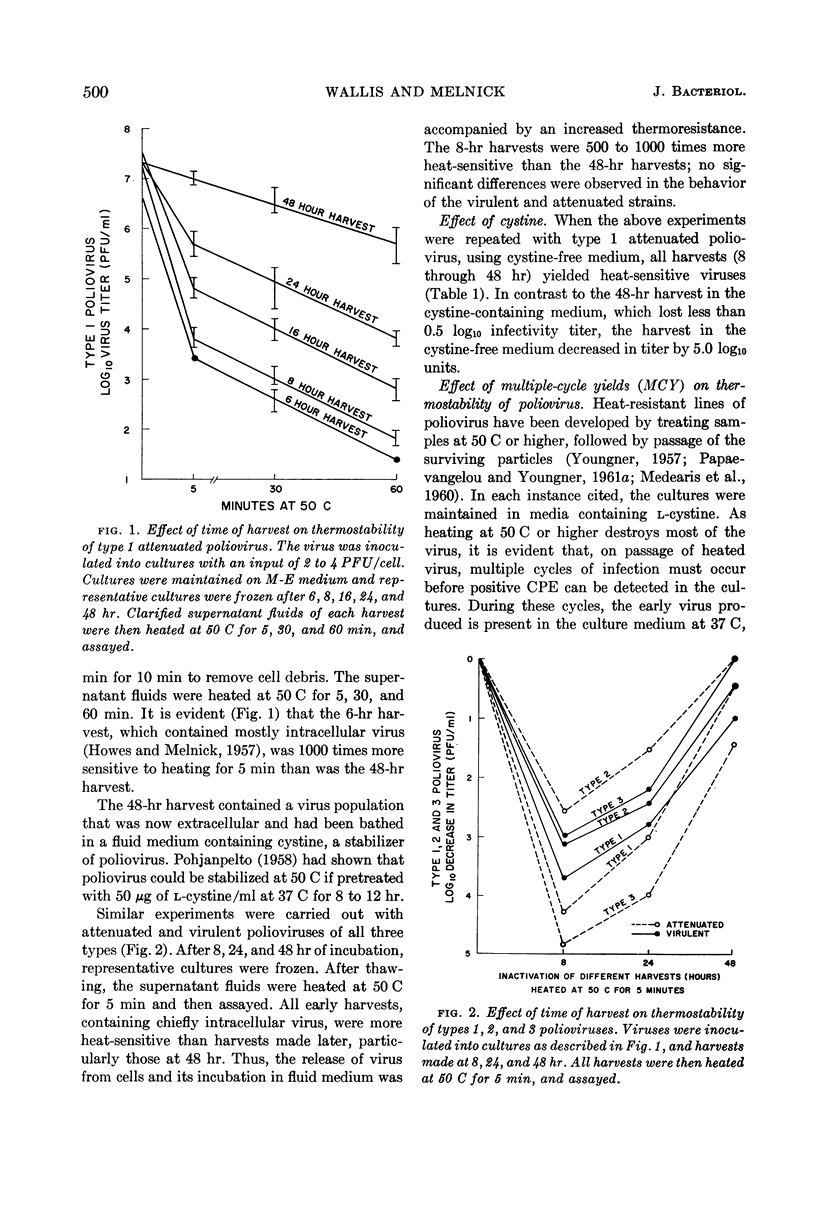
- HOWES D. W., MELNICK J. L. The growth cycle of poliovirus in monkey kidney cells. I. Maturation and release of virus in monolayer cultures. Virology. 1957 Aug;4(1):97–108. doi: 10.1016/0042-6822(57)90045-4. [DOI] [PubMed] [Google Scholar]
- KAPLAN A. S., MELNICK J. L. Effect of milk and cream on the thermal inactivation of human poliomyelitis virus. Am J Public Health Nations Health. 1952 May;42(5 Pt 1):525–534. doi: 10.2105/ajph.42.5_pt_1.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
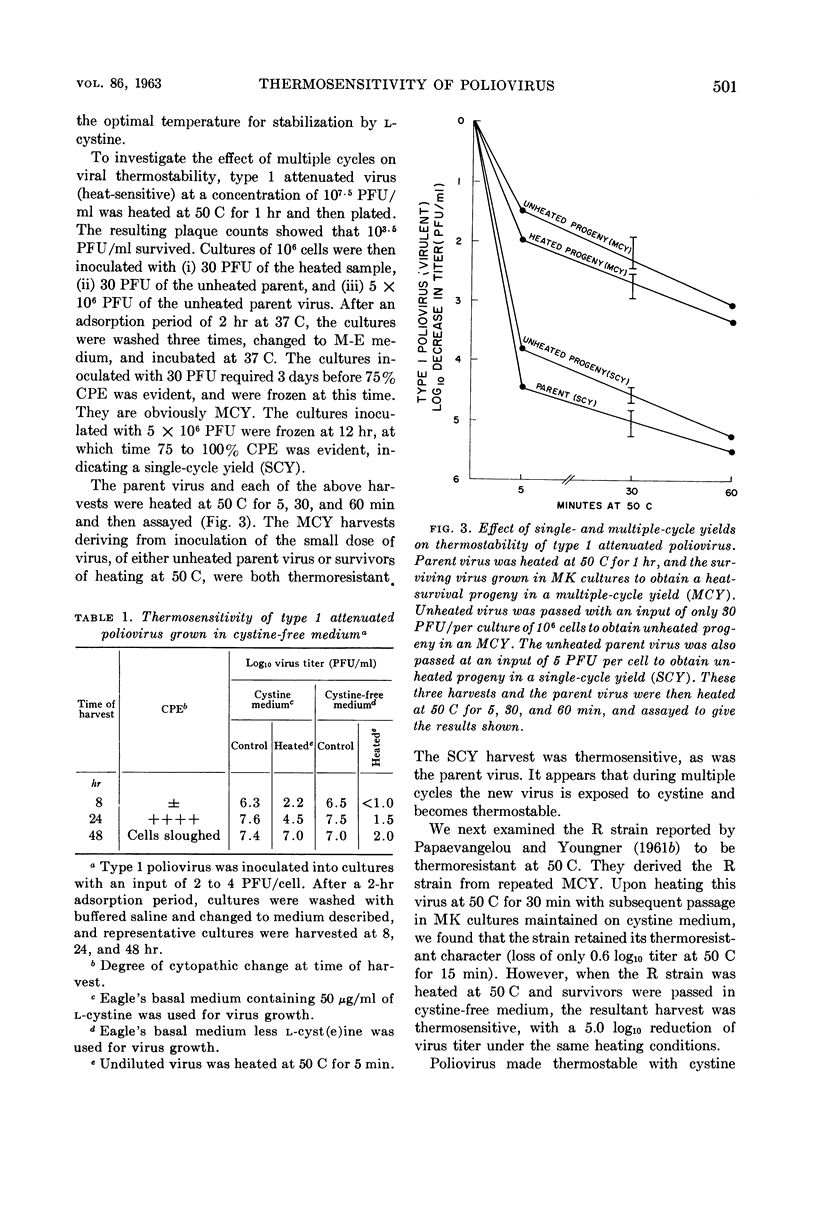
- PAPAEVANGELOU G. J., YOUNGNER J. S. Correlation between heat-resistance of polioviruses and other genetic markers. Proc Soc Exp Biol Med. 1961 Nov;108:505–507. doi: 10.3181/00379727-108-26979. [DOI] [PubMed] [Google Scholar]
- PAPAEVANGELOU G. J., YOUNGNER J. S. Thermal stability of ribonucleic acid from poliovirus mutants. Virology. 1961 Dec;15:509–510. doi: 10.1016/0042-6822(61)90120-9. [DOI] [PubMed] [Google Scholar]
- POHJANPELTO P. Response of enteroviruses to cystine. Virology. 1961 Nov;15:225–230. doi: 10.1016/0042-6822(61)90352-x. [DOI] [PubMed] [Google Scholar]
- POHJANPELTO P. Stabilization of poliovirus by cystine. Virology. 1958 Oct;6(2):472–487. doi: 10.1016/0042-6822(58)90095-3. [DOI] [PubMed] [Google Scholar]
- POHJANPELTO P. Two different thermostable variants of poliovirus. Virology. 1961 Nov;15:231–236. doi: 10.1016/0042-6822(61)90353-1. [DOI] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L. Magnesium chloride enhancement of cell susceptibility to poliovirus. Virology. 1962 Feb;16:122–132. doi: 10.1016/0042-6822(62)90287-8. [DOI] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L. Thermal inactivation of poliovirus under anaerobic conditions. J Bacteriol. 1962 Sep;84:389–392. doi: 10.1128/jb.84.3.389-392.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESE C. Thermal inactivation of animal viruses. Ann N Y Acad Sci. 1960 Jan 13;83:741–751. doi: 10.1111/j.1749-6632.1960.tb40943.x. [DOI] [PubMed] [Google Scholar]
- YOUNGNER J. S. Thermal inactivation studies with different strains of poliovirus. J Immunol. 1957 Apr;78(4):282–290. [PubMed] [Google Scholar]



