Abstract
In this study we examined procedures that alter saccadic latencies and target selection to visual stimuli and to electrical stimulation of area V1 in the monkey. It has been shown that saccadic eye-movement latencies to singly presented visual targets form a bimodal distribution when the fixation spot is turned off a number of milliseconds prior to the appearance of the target (the gap period); the first mode has been termed express saccades and the second regular saccades. When the termination of the fixation spot is coincident with the appearance of the target (0 ms gap), express saccades are rarely generated. We show here that a bimodal distribution of saccadic latencies can also be obtained when an array of visual stimuli is presented prior to the appearance of the visual target, provided the elements of the array overlap spatially with the visual target. The overall latency of the saccadic eye movements elicited by electrical stimulation of area V1 is significantly shortened both when a gap is introduced between the termination of the fixation spot and the stimulation, and when an array is presented. However, under these conditions, the distribution of saccadic latencies is unimodal. When two visual targets are presented after the fixation spot, introducing a gap has no effect on which target is chosen. By contrast, when electrical stimulation is paired with a visual target, introducing a gap greatly increases the frequency with which the electrical stimulation site is chosen.
Keywords: Express saccades, rhesus macaque, electrical stimulation, area V1
Introduction
In exploring the visual scene we make about three saccadic eye movements per second, more than 160,000 saccades a day. With each shift in gaze two basic tasks must be accomplished: (1) to perform a high-resolution analysis of the visual scene that falls into the foveal area and (2) to decide where to shift the center of gaze next. The ability to carry out these tasks rapidly is important for survival in living organisms.
Conditions that define the latency with which saccadic eye movements can be initiated have been extensively studied (Fischer & Boch, 1983; Fischer & Ramsperger, 1984; Boch & Fischer, 1986; Fischer & Weber, 1993; Rohrer & Sparks, 1993; Sommer, 1994; Weber & Fischer, 1994; Cavegn & d'Ydewalle, 1996; Pare & Munoz, 1996; Weber et al., 1998; Schiller et al., 2004a, b; Schiller et al., 2004c; Schiller & Haushofer, 2005). An unexpected finding, first observed by Fischer and colleagues, is that saccadic latencies to singly appearing visual targets can yield a bimodal distribution in both humans and monkeys (Fischer & Boch, 1983; Fischer & Ramsperger, 1984; Rohrer & Sparks, 1993; Sommer, 1994; Pare & Munoz, 1996; Schiller et al., 2004a, b; Schiller et al., 2004c; Schiller & Haushofer, 2005). The first mode, which peaks at a latency of about 100 ms, has been termed express saccades; the second mode, which peaks around 160 ms, has been termed regular saccades. The frequency with which express saccades are generated has been shown to be greatly affected by when the fixation spot is terminated relative to the onset of the target stimulus; when the fixation spot remains on throughout or is terminated just when the target appears, relatively few express saccades are generated. However, when the fixation spot is terminated 50-250 ms prior to the onset of the target, numerous express saccades are made. It has been suggested that termination of the fixation spot disengages inhibitory processes that assure maintained fixation, thereby facilitating eye-movement generation in the express saccade range (Fischer & Boch, 1983; Kingstone & Klein, 1993). Most studies examining express saccade generation have reported that the frequency with which express saccades are made is also affected by the number of locations at which a target appears repeatedly; when a target appears at only one of two locations, the percentage of express saccades is much higher than when four or more target locations are used (Crawford & Muller, 1992; Kingstone & Klein, 1993; Tam & Stelmach, 1993; Carpenter & Williams, 1995; Pare & Munoz, 1996; Schiller et al., 2004a). Express saccades are rarely generated when several targets appear concurrently (McPeek & Schiller, 1994). This is true even when two identical targets appear concurrently (Schiller & Kendall, 2004). However, when these targets are temporally offset, even by just a few milliseconds (16-50 ms), monkeys choose the target that had appeared first and commonly make express saccades to them. One study did fail to demonstrate these differences (Kveraga et al., 2002).
When the pathway from area V1 to the brainstem oculomotor centers is disrupted by inactivation of the superior colliculus, express saccades are eliminated suggesting that this pathway is crucial for the production of these short-latency eye movements (Schiller et al., 1987). Inactivation of the frontal eye fields and the medial eye fields, which also play a central role in eye-movement generation, does not significantly alter express saccade generation (Schiller et al., 1987; Sommer et al., 1993; Schiller & Chou, 1998). By contrast, when the lateral geniculate nucleus is inactivated, monkeys no longer make any saccades to visual targets suggesting that the retino-geniculate pathway to cortex is essential for visually guided saccade generation both for regular and express saccades (Schiller et al., 1990).
Electrical stimulation of area V1 in humans has been shown to create a star-like image called a phosphene (Brindley & Lewin, 1968). In monkeys electrical stimulation also creates a phosphene, the spatial location of which is defined by the receptive field position of the stimulated neurons (Tehovnik et al., 2005). When regions of V1 are stimulated that represent two to four degrees of eccentricity in the visual field from the fovea, currents of 20 to 120 μA generate a percept of 6-12% contrast subtending 15-20 minutes of visual angle (Schiller et al., 2005). In numerous experiments, monkeys have been trained to make saccadic eye movements to the receptive field of the stimulated neurons (Schiller & Tehovnik, 2001; Bradley et al., 2005; Tehovnik et al., 2005). Prolonged stimulation of V1 creates a staircase of saccades just as it does when the superior colliculus and the frontal eye fields are electrically stimulated (Schiller, 1977).
The central aim of this study was to determine how saccadic latencies and target choice are different under conditions when visual targets are used and when electrical stimulation is applied to area V1. We asked to what extent electrical stimulation can generate a bimodal distribution of saccadic latencies, how the paired presentation of visual targets and electrical stimulation affect saccade generation, and whether saccadic latencies are modified for both visual and electrical stimulation when an array of stimuli precedes them.
Materials and methods
Subjects
Three rhesus monkeys (Monkeys C, H and J) were trained to make saccadic eye movements to visual targets; two of these animals (Monkeys C and H) were tested additionally with electrical stimulation of area V1. Each animal had a head post and a scleral search coil implanted to stabilize the head and to measure eye movements during the experimental sessions (Schiller et al., 1987). A stainless steel well was implanted in each animal through which glass coated platinum/iridium microelectrodes were lowered through the intact dura into area V1 using a microdrive (Schiller et al., 1976; Tehovnik et al., 2003). The electrodes were constructed in the laboratory. All surgical procedures were carried out in accordance with the NIH approved guidelines and have been approved by the Division of Comparative Medicine at MIT.
Testing procedures
The animals were seated in a monkey chair and faced a color monitor (Sony, Multiscan 20se) placed at a distance of 57.3 cm from the eye whereby a 1 cm extent on the screen corresponded to 1 degree of visual angle. After the animal was placed into the testing apparatus and its head was fixed, a tube for the delivery of apple juice was placed into its mouth. Each trial began with the appearance of a fixation spot in the center of the monitor. Each saccadic eye movement made to a visual target or to the receptive field site of the neurons electrically stimulated was rewarded with a drop of apple juice. The display monitor was calibrated to yield a gamma correction table for each phosphor.
Receptive field mapping
Each experimental session in which electrical stimulation was delivered commenced by lowering the electrode into area V1 and was followed by mapping the receptive field of the neurons at the electrode tip.
All the animals were trained to maintain fixation for 2-3 seconds on a red fixation spot until a red target appeared in one of four locations at an eccentricity of five degrees. A correct saccade made to this target was rewarded with a drop of apple juice. While the animal maintained fixation a bar of light was swept across the visual field in one of several orientations. The activity of the neurons was amplified and fed into a loud speaker to facilitate the mapping of receptive fields. The location of the bar was systematically varied as was its size, which was typically decreased in small steps until the receptive field of the neurons was accurately plotted. Most of the locations were in the lower contralateral visual field and were located between 2.8 to 4.5 degrees of visual angle away from the fixation spot. The size of the receptive fields was less than 0.5 degrees of visual angle.
Experimental procedures
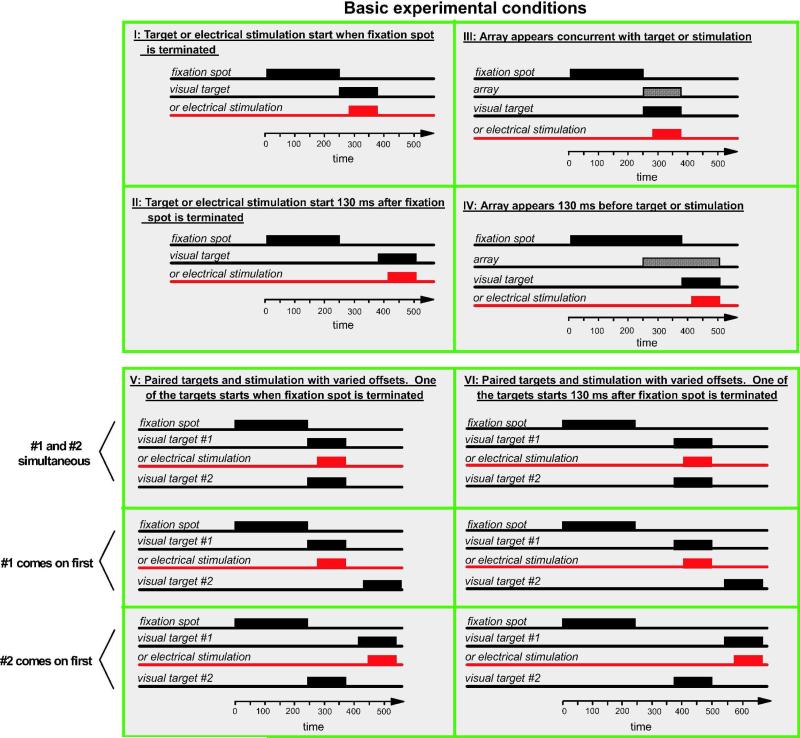
We used six basic experimental procedures which are outlined in Figure 1. In all cases each trial began with the appearance of a central fixation spot to which the animal had to direct his gaze for the trial to continue. For the first four procedures, shown in Figure 1, I- IV, subsequent to central fixation either a single stimulus appeared or area V1 was electrically stimulated. The size of the square fixation window was 50 minutes by 50 minutes of visual angle and the size of the target windows was 85 minutes by 85 minutes.
Figure 1.
The four basic conditions used in the experiments. A: After the monkey fixated the central fixation spot, either a target appeared at one of two or four locations or electrical stimulation was delivered to area V1. The termination of the fixation spot coincided with the onset of the target. B: The fixation spot was terminated 130 ms prior to the onset of the target or electrical stimulation. C: Upon termination of the fixation spot, the visual array and the target or electrical stimulation was turned on. D: The array appeared 130 ms prior to the target or electrical stimulation. The fixation spot was terminated at the same time the target or electrical stimulation was initiated.
Procedure I
The visual target or the electrical stimulation was initiated upon termination of the fixation spot (the 0 ms gap condition).
Procedure II
The visual target or the electrical stimulation was initiated 130 ms after the termination of the fixation spot (the 130 ms gap condition). This procedure is one commonly used in the classic express saccade experiments (Fischer & Boch, 1983).
Procedure III
An array of stimuli was presented on the monitor upon termination of the fixation spot. The array appeared concurrently with the target or the electrical stimulation. The spatial arrangement of the array was varied as described in the Results section and in Figures 2-8, including a condition in which the background illumination was changed homogeneously 130 ms before the target appeared as specified in Figure 2C.
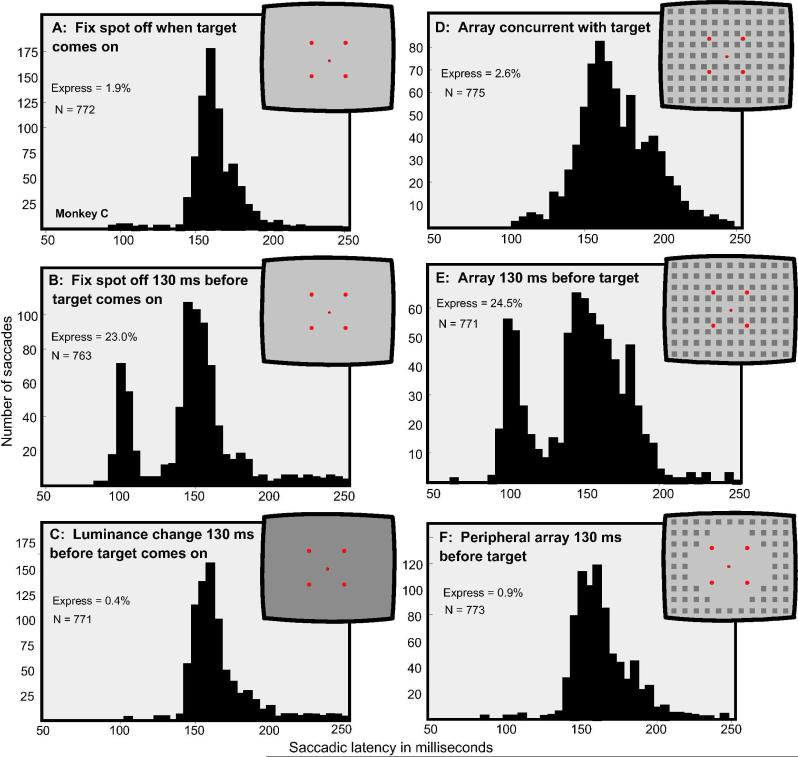
Figure 2.
The distribution of saccadic latencies to single visual targets appearing in one of four locations 3.3 degrees from central fixation under six conditions of presentation. The insets depict the experimental conditions. A: The target appeared upon termination of the fixation spot. B: The target appeared 130 ms after termination of the fixation spot. C: The overall illumination of the background was changed from 10.8 to 23 cd/m2 130 ms prior to the appearance of the target. D: The array, as shown in the inset, appeared concurrent with the target and the termination of the fixation spot. E: The array appeared 130 ms prior to the target. F: An array consisting only of elements peripheral to the target location, as shown in the inset, appeared 130 ms prior to the target. Conditions B and E produced numerous express saccades, the other conditions did not.
Figure 8.
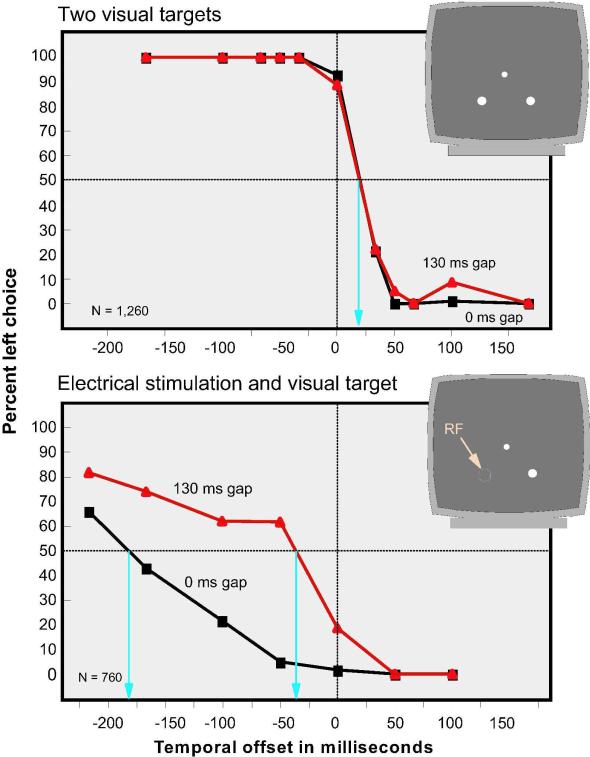
Percent left-target-site choice when two visual targets were presented and when a visual target was paired with electrical stimulation. For each of these conditions two gaps were used, 0 and 130 ms. The two visual targets and the visual target presented with electrical stimulation appeared with various temporal offsets, as indicated on the X axis. With two visual targets the two gap conditions yielded virtually identical results. With a gap of 130 ms, significant shifts occurred when electrical stimulation was paired with a visual target.
Procedure IV
This procedure used the same array presentation as Procedure III, but the array was presented 130 ms prior to the target or the electrical stimulation; the fixation spot was terminated at the same time as when the target appeared.
The monkey's response for these procedures was considered to be correct only when a single saccadic eye movement was made directly into the target window, which was centered on the target and measured 1.5 to 2.0 degrees in cross-section.
The central difference in the next two procedures from those presented so far in I-IV is that instead of a single target, two targets were presented with various temporal offsets or a visual target was paired with electrical stimulation:
Procedure V
Two identical targets appeared in the left and right visual hemifields at mirror image locations after the fixation spot was terminated or a visual target was paired with electrical stimulation. The two targets or the target paired with electrical stimulation were presented with various temporal offsets. The fixation spot was terminated concurrently with the appearance of the initial target or when the electrical stimulation was initiated first (0 ms gap).
Procedure VI
This procedure was similar to Procedure V but a 130 ms gap was introduced between the termination of the fixation spot and the appearance of the initial target or the electrical stimulation. Figure 1, V-VI show three basic sub-procedures: (1) when the stimuli were simultaneous, (2) when the target or electrical stimulation on the left appeared first and (3) when the target on the right appeared prior to the target or the electrical stimulation on the left. Several temporal offsets were used as shown in the figures.
Initially monkeys were trained on the paired visual targets and were rewarded only when they selected the one that appeared first. The location of the target that had appeared first was randomized. Monkeys have a strong natural tendency to pick the first target. After this training, during the experimental sessions when either two visual targets were presented or a visual stimulus was paired with electrical stimulation, monkeys were rewarded for every choice made.
The CIE coordinates for the maintained illumination of the monitor were 0.331 and 0.331. The background illumination was 10.8 cd/m2. The diameter of the circular fixation spot was 13.5 minutes of visual angle and the diameter of the circular targets was 20 minutes of visual angle. The contrast of these targets ranged between 7 and 11%. The stimulus array consisted of a grid of equally spaced squares with the spaces between the squares equal to their width. The illumination of the squares in the array was typically 4.63 cd/m2 (40% contrast) but we tested several different illumination levels (7-50% contrast) and spatial frequencies (1-10 cycles per degree).
Electrical stimulation
During experimental sessions when area V1 was electrically stimulated we first mapped the location of the receptive field as described above.
Subsequent to mapping the receptive field, electrical stimulation was delivered using a train of biphasic pulses delivered at 200 Hz for 100 ms with the width of each pulse being 0.2 ms using a Grass S88 stimulator attached to a pair of constant-current stimulus isolation units (Grass PSIU6B, Quincy, MA, USA). For each biphasic pulse, a cathodal pulse was followed immediately by an anodal pulse of the same amplitude and duration. Current was measured by the voltage drop across a 1000 Ω resistor in series with the return lead of the stimulator. The range of depths for the recordings was between 0.8 and 1.3 mm deep from the surface of the cortex. Current levels to evoke saccades ranged from 10 to 100 μA. During each experimental session electrical stimulation was randomly intermingled with the presentation of one to four visual stimuli that were placed at similar eccentricities in the contralateral and upper visual fields. Monkeys typically ran 1500-3000 trial per session. Electrical stimulation of area V1 was always initiated with a 30 ms delay relative to the presentation of visual targets because the signals from the visual stimuli that impinge on the retina take approximately 30 ms to reach area V1.
Results
The results are shown here in two sections. In section 1 the data obtained using Procedures I-IV are presented (Figures 2-7). In section 2 the data obtained using procedures V and VI are presented (Figure 8).
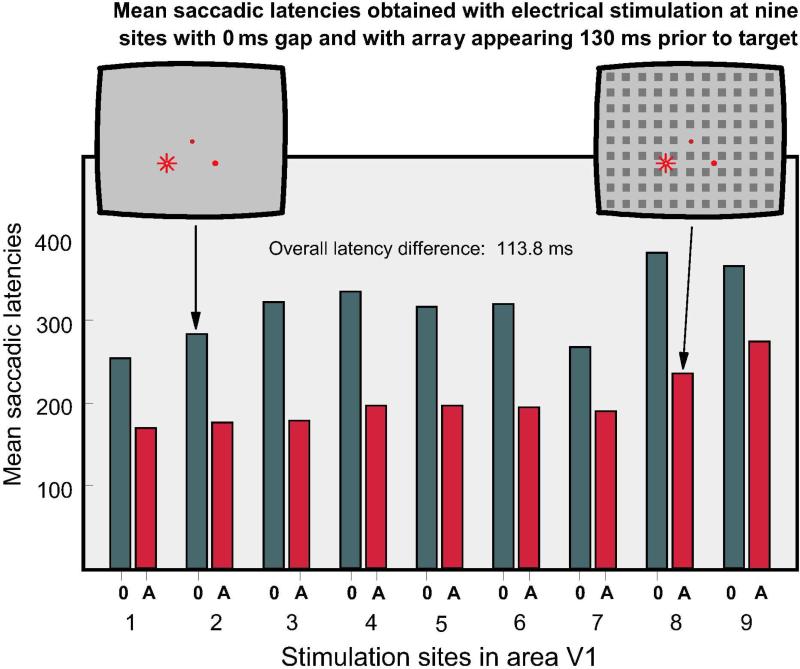
Figure 7.
Mean saccadic latencies obtained under two conditions at 9 stimulation sites: 0: electrical stimulation was applied upon termination of the fixation spot on a blank background. A: The whole field array was presented 130 ms prior to the target. At all sites the presentation of the array significantly shortened saccadic latencies.
Section 1, Procedures I-IV
In Figure 2 sections A, B, D and E show the temporal distribution of saccadic latencies obtained from Monkey C in response to singly appearing visual targets at one of four locations, as depicted in the figure insets. The data shown were acquired from using the four basic conditions delineated in Figure 1. When the target appeared on a homogeneous background concurrent with the termination of the fixation spot (Procedure I, Figure 1), few express saccades were generated (1.9% having latencies of 85-130 ms). As shown in Figure 2D, express saccades were also low in number when the array appeared concurrently with the target (Procedure III, Figure 1). When the target appeared 130 ms after the termination of the fixation spot and on a homogeneous background (Procedure II, Figure 1), express saccades were generated 23.0% of the time, as shown in Figure 2B. Express saccades were also generated, 24.5% of the time, when Procedure IV was used in which the stimulus array was presented 130 ms prior to the appearance of the target, as shown in Figure 2E.
The latency for generating regular saccades, which form the second mode of the bimodal distribution of saccadic latencies (illustrated in Figure 2 B and E), was largely unaffected by the various conditions shown in Figure 2. For Procedures I-III, respectively, the mean latencies in the regular saccade range (130 to 250 ms) were 164.6, 159.1 and 163.7 ms, showing that the latency of these regular saccades was largely unaffected by introducing a gap between fixation spot termination and target onset (Procedure II in Figure 1) or by introducing a luminance change (Procedure III). The presentation of the array, as shown in Figure 2D, E & F, also had little effect on the mean latency of regular saccades, which was, respectively, 172.2, 160.7 and 164.2 ms.
Data collected from Monkey J yielded similar results but with a higher overall percentage of express saccades. Based on a total of 3,704 trials, Procedure I produced 10.1% express saccades. Procedure II produced 55.6%, Procedure III 24%, and Procedure IV 79.4% express saccades.
To further identify the variables responsible for the generation of express saccades that occur when arrays are presented with or prior to the targets, we introduced two additional experimental conditions as shown in Figure 2C and F. In the first, when the overall luminance of the background was changed and no figures appeared, only 0.4% express saccades were generated, as shown in Figure 2C. Increasing the overall contrast, rather than decreasing it, yielded similar results (not shown). These findings show that a figural change is required rather than a luminance change to produce express saccades. In the second condition a stimulus array was presented in which the figural elements were confined to regions outside the spatial area within which the targets were presented, as shown in Figure 2F. In this case express saccades were generated only 0.9% on the time indicating that, in order for the figural elements in the array to be effective in generating express saccades, these elements must be within the spatial region of the targets.
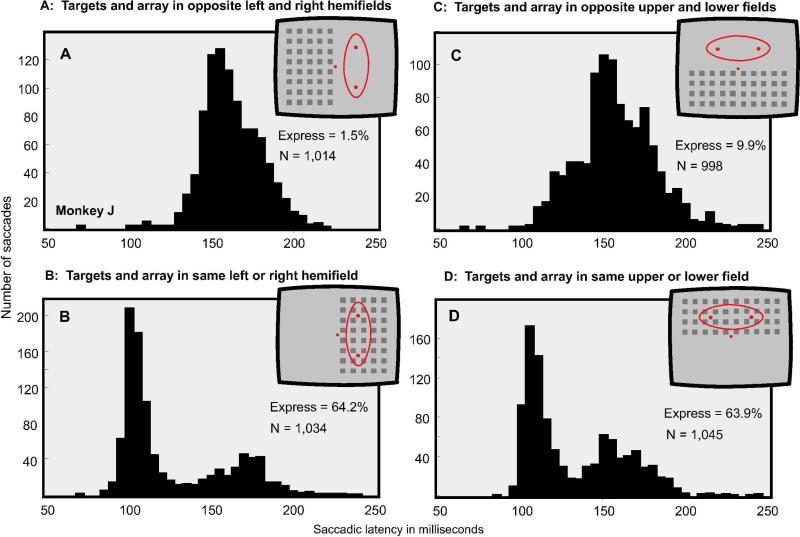
The connections in the visual system are such that images appearing in the left visual hemifield project to the right half of the brain and those in the right hemifield to the left half of the brain. On the other hand, the upper and lower portions of left and right hemifields project to the corresponding sides of the brain. Callosal connections provide some of the integration between the left and right visual hemifields whereas interconnections within each hemisphere provide integration between the upper and lower visual fields. These arrangements in connectivity raise the question as to whether there is a difference in express saccade generation when targets and the visual arrays are presented in opposite hemispheres or when they appear in the upper and lower portions of the same hemisphere. To test this we confined the arrays either to the left or right hemifields or to the upper or lower regions of the visual field. The targets were presented either on the same side as the array or contralateral to the array as depicted in the insets of Figure 3. The data shown in this figure were obtained from Monkey J. Express saccades occur profusely when the targets are in the same region as the array, but are rare when the targets appear in the homogeneous portion of the visual field. Similar results were obtained both for horizontal and vertical shifts suggesting that the principles of interconnectivity for express saccade generation are the same across hemispheres as within hemispheres. As noted for the data in Figure 2, the latencies of regular saccades were unaffected by the conditions of presentation. We have obtained similar data from Monkey C; based on a total for 1,887 trials, the percent of express saccades for the four conditions shown in Figure 3 were as follows: A: 1.7%, B: 36.9%, C = 4.7, D = 20.9%.
Figure 3.
Four conditions of array presentation. In all cases the array appeared 130 ms prior to the target which appeared in one of two spatial locations. The mode of presentation is indicated in the insets. A: The elements in the array appeared in the opposite hemifield from the targets. B: The elements in the array appeared in the same hemifield as the targets. C: The elements of the array appeared in the opposite upper or lower hemifield from the targets. D: The elements of the array appeared in the same upper or lower hemifield as the targets. The conditions of presentation were counterbalanced. Thus an equal number of trials were collected with targets in the same and opposite hemifields for left/right and upper/lower array and target locations. Express saccades were numerous only when the target and the array overlapped.
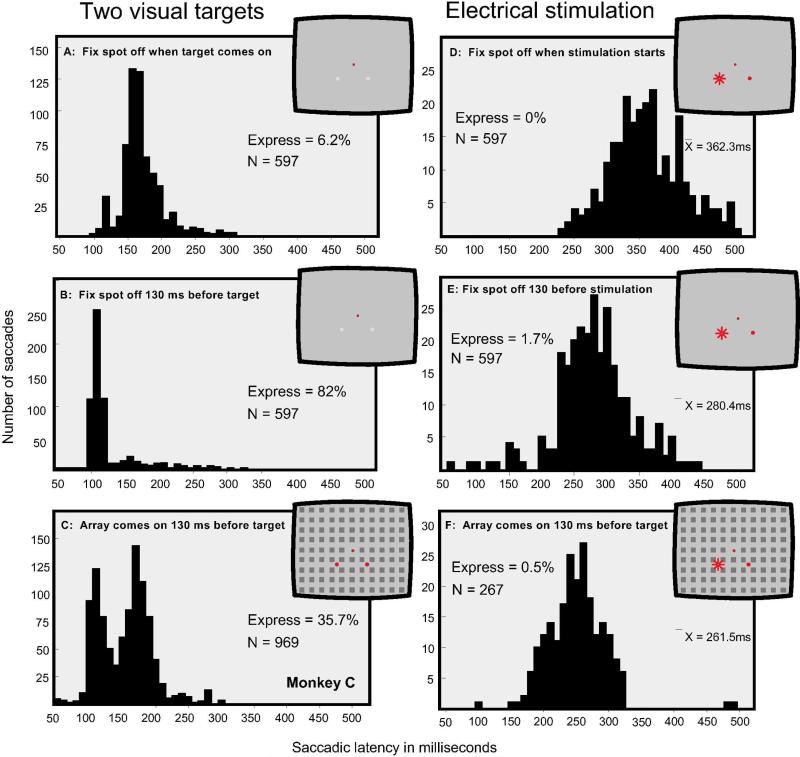
In Figure 4 data are shown comparing the distribution of saccadic latencies obtained to visual targets (panels A, B, C) and to electrical stimulation (panels D, E, F) under presentation Procedures I, II and IV (see Figure 1). Figure 4 A and D: The visual target or the electrical stimulation was presented when the fixation spot was turned off. Figure 4 B and E: The visual target or the electrical stimulation was initiated 130 ms after termination of the fixation spot. Figure 4 C and F: The visual target or the electrical stimulation was presented 130 ms after the appearance of the array, as depicted in the insets. For the conditions shown in panels A and B, the visual target had a contrast of 7%, which approximates the contrast of images created by electrical stimulation (Schiller et al., 2005). In all cases, the diameter of the circular visual targets was 20 minutes of visual angle. For the purposes of direct comparisons, the data plotted in Figure 4A, B and C are for the saccades made to the visual target that had appeared on the lower left; this target was positioned in the center of the receptive field of the stimulated neurons. The saccadic eye movement latencies produced by electrical stimulation using the same conditions appear in Figure 4D, E and F.
Figure 4.
Comparing the effects of electrical stimulation with visual target presentation. A-C shows data for visual targets and D-F for electrical stimulation. In all cases visual targets and electrical stimulation involved two spatial locations. A: The presentation of a single visual target upon termination of the fixation spot. B: The presentation of a single visual target 130 ms after termination of the fixation spot. C: The presentation of the array 130 ms prior to the visual target. D: Electrical stimulation initiated upon termination of the fixation spot. E: Electrical stimulation initiated 130 ms after termination of the fixation spot. F: Electrical stimulation initiated 130 ms after the onset of the array.
With the visual stimuli appearing at one of two locations, numerous express saccades were generated when the fixation spot was terminated 130 ms prior to the appearance of the target (82%, Figure 4B). When the array appeared 130 ms before the visual target, a clear bimodal distribution was evident (35.7% express saccades, Figure 4C). Few express saccades were generated with visual stimuli when target onset was concurrent with termination of the fixation spot (6.2%, Figure 4A). The overall temporal distribution to visual stimuli was quite narrow with very few saccades of latencies greater than 250 ms. In contrast with the distributions obtained with visual targets, electrical stimulation did not produce a bimodal distribution of saccadic latencies for any of the three conditions. The overall saccadic latencies to electrical stimulation were much longer than to visual stimuli, and the distributions were quite broad. However, saccadic latencies obtained with electrical stimulation using 130 ms gap (Figure 4E) were significantly faster than under the no gap condition (Figure 4D, t = 17.4, p < 0.001). The 130 ms gap condition with electrical stimulation yielded only 1.7 % saccades in the express range. The latency distribution to electrical stimulation using the arrays presented 130 ms prior to the target (Figure 4F) had a mean latency of 261.5 ms which was significantly faster than the no gap condition shown in Figure 4D ( t = 27, p < 0.01). The data obtained with visual targets and electrical stimulation were quite different.
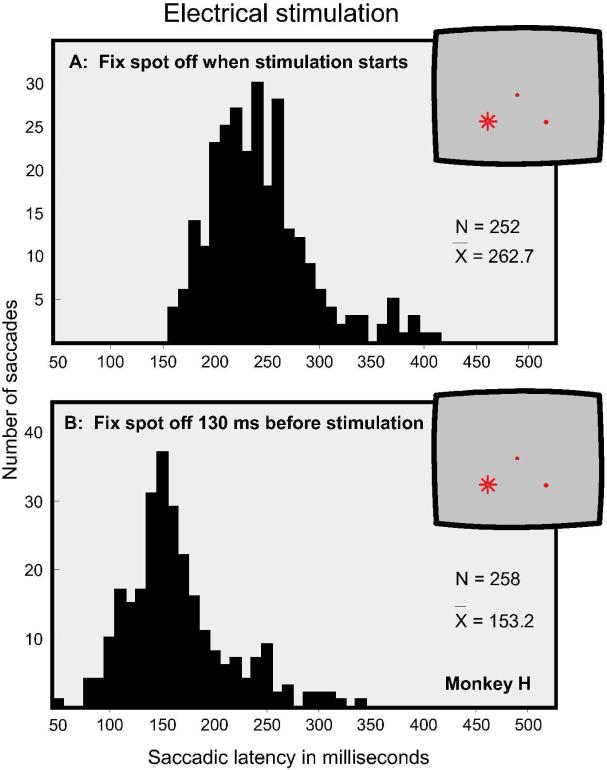
Figure 5D presents data from another monkey (Monkey C), which provides further verification of the overall shift of latencies to electrical stimulation. Figure 5A shows the distribution of saccadic latencies when electrical stimulation was initiated upon termination of the fixation spot, yielding a mean latency of 262.7 ms. Figure 5B shows the distribution of saccadic latencies when the fixation spot was terminated 130 ms prior to stimulation, yielding a mean latency of 153.2 ms. The latency difference between these two conditions is highly significant (t = 25.5, p < 0.001). In general, the saccadic latencies of this monkey were shorter than the latencies of the monkey shown in Figure 4.
Figure 5.
The effects of electrical stimulation in Monkey H under two conditions: A. Stimulation was initiated upon termination of the fixation spot. B. Stimulation was initiated 130 ms after the termination of the fixation spot.
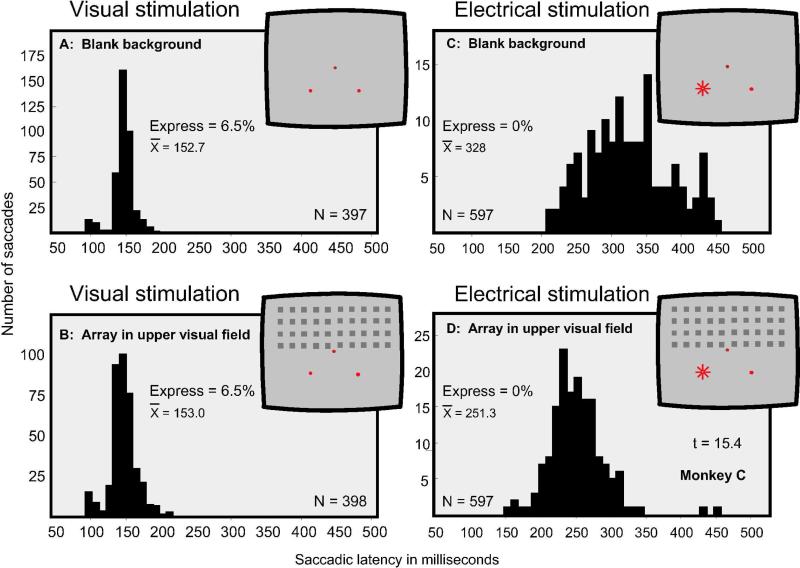
As has been shown in Figure 3, the presentation of an array of stimuli 130 ms prior to the visual targets was effective at generating express saccades only when the array was presented in the same region as the visual targets. Given the differences in the distribution of saccadic latencies we had observed between procedures involving visual targets and those involving electrical stimulation, we examined to what degree the spatial location of the array affects saccadic latencies to electrical stimulation. Figure 6 presents these findings. Here we directly compare saccadic latencies made to visual targets and to electrical stimulation when they are presented in the lower visual field and the array in the upper visual field. Figure 6A and C show saccadic latency distributions when the visual target or the electrical stimulation was presented upon termination of the fixation spot on a blank background. Figure 6B and D show the distribution of saccadic latencies when an array was presented 130 ms prior to the visual target and prior to electrical stimulation. For the visual target the two conditions yielded virtually identical results, with mean latencies of 152.7 and 153.0 ms. By contrast, the saccadic latencies to electrical stimulation were significantly shortened from 328.0 to 251.3 ms when the array was presented in the upper visual field (Figure 6D, t = 15.4, p < 0.001).
Figure 6.
Comparing the effects of visual stimulation and electrical stimulation under two conditions: A and C: Using a homogeneous background the target or electrical stimulation was delivered upon termination of the fixation spot. B and D: The array appeared in the upper visual field 130 ms prior to the target or electrical stimulation. The visual target and the receptive fields of the stimulated neurons were in the lower part of the visual field. With visual stimulation the two conditions produced similar results. With electrical stimulation the presentation of the array significantly shortened saccadic latencies.
Figure 7 shows mean latencies obtained with electrical stimulation at nine different sites in area V1 of Monkey C, comparing the effects of electrical stimulation when it was administered right after the fixation spot was terminated (dark bars) and when it was administered 130 ms after the appearance of the array (red bars). Each bar is based on 50 to 200 trials. All differences between the two conditions were statistically significant beyond the p < 0.01 level. The t-test values were as follows: 1 = 16.4 (df = 67), 2 = 6.16 (df = 70) 3 = 13.2 (df = 80), 4 = 20.5 (df = 51), 5 = 15.0 (df = 31), 6 = 10.9 (df = 34), 7 = 19.8 (df = 211), 8 = 20.8 (df = 373), 9 = 16.4 (df = 311). The mean latency difference for the nine sites was 113.8 ms.
Section 2, Procedures V and VI
Procedures V and VI differ from procedures I-IV in that following fixation either two visual targets appeared with various temporal offsets or a visual target was paired with electrical stimulation. Figure 8 shows data obtained with paired targets and a visual target presented with electrical stimulation using various temporal offsets. The first target was presented or electrical stimulation was delivered either concurrent with the termination of the fixation spot (0 ms gap) or with a delay (130 ms gap) as described in Figure 1, Procedures V and VI. The visual targets had a contrast of 11% and a size of 20 minutes. Electrical stimulation ranged between 20 and 100 μA. Data for the two gap conditions were collected in blocks, typically in ABBA or BAAB order (where A = 0 gap and B = 130 gap). In every set of four blocks the current levels were kept constant to assure that the only significant variable was the gap condition.
When the two visual targets were presented with various temporal offsets, performance was virtually identical for the 0 and 130 ms gaps, as shown in the upper section of Figure 8. The 50% cross-over point for both conditions is at plus 20 ms showing a slight bias in favor of the right target in this monkey for these locations. Such biases have been shown in other studies (Horstmann & Hoffmann, 2005; Scherberger et al., 2003).
When electrical stimulation was used instead of the left target, quite different effects were obtained. First, the overall frequency with which the electrical stimulation location was chosen was much lower than with the two visual targets. Second, the shift of choice with various temporal offsets was more gradual for the 130 ms gap condition. Third, the 130 ms gap significantly increased the frequency with which the electrical stimulation location was chosen compared with the 0 gap condition. The 50% cross-over point with the 130 ms gap shifted in favor of selecting the electrical stimulation location by 150 ms compared with the 0 ms gap condition. Thus with a gap of 0 ms the electrical stimulation was equivalent to the visual target when the electrical stimulation precedes the visual target by 182 ms whereas with a gap of 130 ms the electrical stimulation became equivalent to the visual stimulus when it was administered 36 ms prior to the appearance of the visual stimulus. A probit model was fitted to the percent correct data for the two gap conditions. The curve for the 130 ms gap was significantly shifted relative to the curve for the 0 ms gap (p < 0.05).
Discussion
In this paper we introduced a new paradigm which is capable of generating a bimodal distribution of saccadic latencies similar to that obtained when single visual targets are presented 50 to 200 ms after the termination of the fixation spot (Fischer & Boch, 1983; Fischer & Ramsperger, 1984; Rohrer & Sparks, 1993; Sommer, 1994; Pare & Munoz, 1996; Schiller et al., 2004a, b; Schiller et al., 2004c; Schiller & Haushofer, 2005). The presentation of an array of identical stimuli 130 ms prior to the appearance of the visual target (Figures 2-4) was effective in producing express saccades but only when its elements appeared in the same region of the visual field as did the visual targets.
The predominant theory about express saccade generation is that the termination of the fixation spot prior to the appearance of the visual target disengages the inhibitory mechanisms that assure the intent to maintain fixation (Fischer & Boch, 1983; Kingstone & Klein, 1993). By doing so, saccades can be generated with shorter latencies. The fact that express saccades can also be generated when an array of stimuli is presented prior to the target, even though the fixation spot stays on until the appearance of the visual target, suggests that additional factors are involved in the generation of express saccades. One possibility is that the array acts as a cue or primer for the appearance of the target thereby shortening saccadic latencies. However, the array was effective only when its elements appeared in the general proximity of the target. When four target locations were used the array was effective as long as the array elements were within all four of these locations (Figure 2E). Once the elements of the array were outside the location of the targets (Figure 2F) express saccades were not made. If the array serves as a cue or primer, it does so only when the elements are within the range of the target locations. An alternative possibility is that the array disengages the intent to fixate just as does the termination of the fixation spot. An additional line of evidence suggesting that more than pre-cuing is involved in the generation of express saccades is that when two visual targets are presented simulataneously or when an oddity task is used in which a target appears with several identical distracters, express saccades are not produced (Schiler et al., 2004a, 2004b, 2001).
In comparing the effects of saccade generation to visual stimuli and electrical stimulation of area V1 we have found six major differences: 1) Unlike to visual targets, electrical stimulation of V1 does not generate a bimodal distribution of saccadic latencies when the targets appear subsequent to the termination of the fixation spot and when the visual array is presented prior to visual targets or electrical stimulation. (2) The overall saccadic latencies are significantly shortened when electrical stimulation is applied 130 ms subsequent to the termination of the fixation spot and when the visual array is presented 130 ms prior to the termination of the fixation spot; with visual stimulation, these conditions generate express saccades without changing the latencies of regular saccades. (3) Presentation of a stimulus array prior to visual stimulation generates express saccades only when the elements of the array overlap with the targets in visual space; by contrast, presentation of the array significantly shortens saccadic latencies to electrical stimulation of V1 even when their receptive fields do not overlap with the elements of the array. (4) The saccades generated by electrical stimulation of V1 form a broad distribution of saccadic latencies for all conditions whereas saccades produced by visual stimuli form a narrow temporal distribution. (5) The overall saccadic latencies to electrical stimulation of V1 using current levels between 20 and 100 μA are significantly longer than those obtained with visual targets. The shortening of saccadic latencies when electrical stimulation is administered with a gap has also been shown to occur when the frontal and medial eye fields are stimulated (Tehovnik et al., 1999). We have yet to determine if a similar shortening occurs by way of pre-cueing using a visual array for these eye fields. (6) When two visual targets are presented with various temporal offsets, introducing a gap does not alter the relative frequency with which each target is chosen; by contrast, when a visual target is paired with electrical stimulation, introducing a gap significantly shifts the frequency in favor of the visual field location represented by the electrically stimulated neurons.
In earlier work we have proposed that the generation of saccadic eye movements involves two systems, the anterior and the posterior (Schiller & Tehovnik, 2001). The frontal and medial eye fields of the anterior system have direct access to the brain stem oculomotor centers. Area V1, extrastriate cortex and the lateral intraparietal area of the posterior system have access to the brainstem oculomotor centers via the superior colliculus. We believe that the generation of express saccades involves this posterior system. Two lines of evidence support this view: (1) lesions of the superior colliculus eliminate express saccades, an effect that persists even years after the lesion has been made (Schiller et al., 1987) and (2) individual or combined lesions of the frontal and medial eye fields do not affect express saccade generation but produce deficits in target choice when more than one visual stimulus is presented.
The retino-geniculate-striate system appears to play a central role in the generation of both express and regular saccades. Two systems that originate in the retina are the midget and the parasol. The midget system projects to the parvocellular layers of the lateral geniculate nucleus (LGN) and the parasol system to the magnocellular layers. The predominant projection from the LGN is to area V1 in monkeys and humans. Disruption of either the midget system, by blocking the parvocellular layers of the LGN, or the parasol system, by blocking the magnocellular layers of the LGN, does not significantly alter the generation of express saccades and normal saccades (Schiller & Lee, 1994). However, when the LGN in fully blocked, monkeys can no longer make saccadic eye movements of any sort to visual stimuli (Schiller et al., 1990). Along with the fact that SC lesions eliminate express saccades (Schiller et al., 1987), this indicates that the retino-geniculate-striate system is essential for processing visual stimuli for the generation of saccadic eye movements.
Why are there such dramatic differences in the generation of saccadic latencies to electrical stimulation of area V1 and to visual targets? Given the limited nature of our knowledge at this time we can only speculate. While electrical stimulation of V1 is believed to create a star-like image in monkeys (Schiller et al., 2005), it appears to also have several other consequences. The electrical stimulation activates several different types of cells indiscriminately, including excitatory and inhibitory neurons (Schiller & Tehovnik, 2003). Presumably, the generation of express saccades to visual stimuli involves the selective activation of complex circuitry that eventually drives cells in layer V of area V1 that project to the superior colliculus, the structure that is essential for express saccade generation (Schiller et al., 1987). The superior colliculus, furthermore, must receive input from several brain regions, including a number of cortical areas and the substantia nigra for the generation of a saccadic eye movement (Hikosaka & Wurtz, 1985; Schiller & Tehovnik, 2005). These inputs may be the outcome of decision processes that, under natural conditions, involve the selection of a specific target in the visual scene to which a saccade of a specific vector and amplitude is to be generated. Electrical stimulation of area V1 probably cannot tap into these multiple processes in a fashion similar to visual stimuli. Another line of evidence that highlights the difference in saccade generation produced by electrical stimulation and by visual targets, as seen at the level of the SC, is that simultaneous electrical stimulation at two sites elicits vector averaged saccades whereas when two simulaneously appearing visual targets are presented, the saccade is made to one or the other of the two targets (Robinson, 1972; Schiller, 1998; Schiller & Conway, 1979).
Regarding the shifts that occur for the two gap conditions (0 ms and 130 ms) in the selection of the electrically stimulated site that was demonstrated in Figure 8, we believe the effect ties in with the latency shifts described for the same two gap conditions to electrical stimulation shown in Figure 4 and 5. This shortening in latencies with the 130 ms gap may be the result of the brain being able to generate the visual percept and the decisions more quickly. Consequently, with the two-target task as shown in Figure 8, the point at which the visual target and the percept elicited by the electrical stimulation become simultaneous, occurs at smaller temporal differences between the two stimuli, thereby shifting the curve to the right when a 130 ms gap is used.
Acknowledgements
The research reported here was supported grant EY014884 from the National Eye Institute. The authors thank Christina E. Carvey for her assistance in the research and in putting together this manuscript.
References
- Boch R, Fischer B. Further observations on the occurrence of express-saccades in the monkey. Exp Brain Res. 1986;63:487–494. doi: 10.1007/BF00237472. [DOI] [PubMed] [Google Scholar]
- Bradley DC, Troyk PR, Berg JA, Bak M, Cogan S, Erickson R, Kufta C, Mascaro M, McCreery D, Schmidt EM, Towle VL, Xu H. Visuotopic mapping through a multichannel stimulating implant in primate V1. J Neurophysiol. 2005;93:1659–1670. doi: 10.1152/jn.01213.2003. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J Physiol. 1968;196:479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377:59–62. doi: 10.1038/377059a0. [DOI] [PubMed] [Google Scholar]
- Cavegn D, d'Ydewalle G. Presaccadic attention allocation and express saccades. Psychol Res. 1996;59:157–175. doi: 10.1007/BF00425831. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Muller HJ. Spatial and temporal effects of spatial attention on human saccadic eye movements. Vision Res. 1992;32:293–304. doi: 10.1016/0042-6989(92)90140-e. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res. 1983;260:21–26. doi: 10.1016/0006-8993(83)90760-6. [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res. 1984;57:191–195. doi: 10.1007/BF00231145. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behavioral and Brain Sciences. 1993;16:553–567. [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Horstmann A, Hoffmann KP. Target selection in eye-hand coordination: Do we reach to where we look or do we look to where we reach? Exp Brain Res. 2005;167:187–195. doi: 10.1007/s00221-005-0038-6. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Klein RM. Visual offsets facilitate saccadic latency: does predisengagement of visuospatial attention mediate this gap effect? J Exp Psychol Hum Percept Perform. 1993;19:1251–1265. doi: 10.1037//0096-1523.19.6.1251. [DOI] [PubMed] [Google Scholar]
- Kveraga K, Boucher L, Hughes HC. Saccades operate in violation of Hick's law. Exp Brain Res. 2002;146:307–314. doi: 10.1007/s00221-002-1168-8. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Schiller PH. The effects of visual scene composition on the latency of saccadic eye movements of the rhesus monkey. Vision Res. 1994;34:2293–2305. doi: 10.1016/0042-6989(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Pare M, Munoz DP. Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol. 1996;76:3666–3681. doi: 10.1152/jn.1996.76.6.3666. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Rohrer WH, Sparks DL. Express saccades: the effects of spatial and temporal uncertainty. Vision Res. 1993;33:2447–2460. doi: 10.1016/0042-6989(93)90125-g. [DOI] [PubMed] [Google Scholar]
- Scherberger H, Goodale MA, Andersen RA. Target selection for reaching and saccades share a similar behavioral reference frame in the macaque. J Neurophysiol. 2003;89:1456–1466. doi: 10.1152/jn.00883.2002. [DOI] [PubMed] [Google Scholar]
- Schiller PH. The effect of superior colliculus ablation on saccades elicted by cortical stimulation. Brain Res. 1977;122:154–156. doi: 10.1016/0006-8993(77)90672-2. [DOI] [PubMed] [Google Scholar]
- Schiller PH. The neural control of visually guided eye movements. In: Richards JE, editor. Cognitive neuroscience of attention : a developmental perspective. Erlbaum; Mahwah, N.J.: 1998. pp. 3–50. [Google Scholar]
- Schiller PH, Chou IH. The effects of frontal eye field and dorsomedial frontal cortex lesions on visually guided eye movements. Nat Neurosci. 1998;1:248–253. doi: 10.1038/693. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Finlay BL, Volman SF. Quantitative studies of single-cell properties in monkey striate cortex. I. Spatiotemporal organization of receptive fields. J Neurophysiol. 1976;39:1288–-1319. doi: 10.1152/jn.1976.39.6.1288. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Haushofer J. What is the coordinate frame utilized for the generation of express saccades in monkeys? Exp Brain Res. 2005;167:178–186. doi: 10.1007/s00221-005-0037-7. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Haushofer J, Kendall G. An examination of the variables that affect express saccade generation. Vis Neurosci. 2004a;21:119–127. doi: 10.1017/s0952523804042038. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Haushofer J, Kendall G. How do target predictability and precueing affect the production of express saccades in monkeys? Eur J Neurosci. 2004b;19:1963–1968. doi: 10.1111/j.1460-9568.2004.03299.x. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Kendall J. Temporal factors in target selection with saccadic eye movements. Exp Brain Res. 2004;154:154–159. doi: 10.1007/s00221-003-1653-8. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Lee K. The effects of lateral geniculate nucleus, area V4, and middle temporal (MT) lesions on visually guided eye movements. Vis Neurosci. 1994;11:229–241. doi: 10.1017/s0952523800001590. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Logothetis NK, Charles ER. Role of the color-opponent and broad-band channels in vision. Vis Neurosci. 1990;5:321–346. doi: 10.1017/s0952523800000420. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol. 1987;57:1033–1049. doi: 10.1152/jn.1987.57.4.1033. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Slocum WM, Carvey C, Tolias AS. Are express saccades generated under natural viewing conditions? Eur J Neurosci. 2004c;20:2467–2473. doi: 10.1111/j.1460-9568.2004.03663.x. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Look and see: how the brain moves your eyes about. Prog Brain Res. 2001;134:127–142. doi: 10.1016/s0079-6123(01)34010-4. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Cortical inhibitory circuits in eye-movement generation. Eur J Neurosci. 2003;18:3127–3133. doi: 10.1111/j.1460-9568.2003.03036.x. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Neural mechanisms underlying target selection with saccadic eye movements. Prog Brain Res. 2005;149:157–171. doi: 10.1016/S0079-6123(05)49012-3. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ, Weiner VS. Abstract Viewer/Itinerary Planner. Society for Neuroscience, 2005; Washington, DC: 2005. Preliminary studies examining the feasibility of a visual prosthetic device: 1. What does a monkey see when area V1 is stimulated electrically? Program No. 16.1. Online. [Google Scholar]
- Schiller PH, True SD, Conway JL. Paired stimulation of the frontal eye fields and the euperior colliculus of the rhesus monkey. Brain Res. 1979;179:162–164. doi: 10.1016/0006-8993(79)90500-6. [DOI] [PubMed] [Google Scholar]
- Sommer MA. Express saccades elicited during visual scan in the monkey. Vision Res. 1994;34:2023–2038. doi: 10.1016/0042-6989(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Schiller PH, McPeek RM. What neural pathways mediate express saccades? Behav Brain Sci. 1993;16:589–590. [Google Scholar]
- Tam WJ, Stelmach LB. Viewing behavior: ocular and attentional disengagement. Percept Psychophys. 1993;54:211–222. doi: 10.3758/bf03211758. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Carvey CE, Schiller PH. Phosphene induction and the generation of saccadic eye movements by striate cortex. J Neurophysiol. 2005;93:1–19. doi: 10.1152/jn.00736.2004. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Schiller PH. Behavioural conditions affecting saccadic eye movements elicited electrically from the frontal lobes of primates. Eur J Neurosci. 1999;11:2431–2443. doi: 10.1046/j.1460-9568.1999.00665.x. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Schiller PH. Saccadic eye movements evoked by microstimulation of striate cortex. Eur J Neurosci. 2003;17:870–878. doi: 10.1046/j.1460-9568.2003.02489.x. [DOI] [PubMed] [Google Scholar]
- Weber H, Durr N, Fischer B. Effects of pre-cues on voluntary and reflexive saccade generation. II. Pro-cues for anti-saccades. Exp Brain Res. 1998;120:417–431. doi: 10.1007/s002210050415. [DOI] [PubMed] [Google Scholar]
- Weber H, Fischer B. Differential effects of non-target stimuli on the occurrence of express saccades in man. Vision Res. 1994;34:1883–1891. doi: 10.1016/0042-6989(94)90312-3. [DOI] [PubMed] [Google Scholar]