General anesthetics are highly lipid soluble and can dissolve in every membrane, penetrate into organelles and interact with numerous cellular constituents. Their actions have long been considered rapid and fully reversible, with the pharmacodynamic time course of anesthesia dictated solely by the pharmacokinetic profiles of anesthetic uptake and elimination. But recent laboratory data call for a cautious reassessment of this assumption. In the last decade, it has become apparent that anesthetics can affect gene expression, protein synthesis and processing, and cellular function in poorly understood ways that provide plausible biochemical substrates for durable long-term effects in a number of tissues. While in most patients physiological homeostasis is restored soon following general anesthesia, anesthetics have potentially profound and long-lasting effects that, in animal models, appear particularly consequential in specific developmental periods and pathophysiological contexts.
Could a class of drugs employed for many decades without evidence of long-term damage have insidious and heretofore unrecognized neurotoxic effects? Here we critically evaluate available scientific evidence for general anesthetic ‘neurotoxicity’ and the potential clinical implications. Since the scope of this clinical commentary is limited, numerous deserving laboratory investigations could not be mentioned in this focused overview. Our goal as physician-scientists not directly involved in this area of research is to summarize the diverse directions of research in this field, while highlighting the limitations of existing data with respect to clinical practice in order to provide the reader with a rational platform for discussions with colleagues and patients. The barriers that must be overcome to permit clinical translation of the available laboratory research are emphasized, along with the need for further research, as recently highlighted in a consensus statement by a group including authoritative researchers in this field.1 As with any emerging field of research, new evidence continues to accrue such that conclusions are by necessity preliminary and will require frequent reappraisal.
Why suspect long-term neurological effects of anesthetics?
The degree of reversible changes induced by transient exposure to general anesthetic drugs is immense: deep coma and suppression of nocifensive reflexes compatible with a diagnosis of brain death is followed by a rapid return to normal consciousness with discontinuation of the anesthetic. Given such profound, though reversible, alterations of central nervous system [CNS]) function, some degree of long-lasting effects might well be anticipated. Anesthetic preconditioning, which minimizes ischemic damage to the heart or brain, provides a precedent for anesthetic-induced changes that outlast the physical presence of the agents. The possibility that subtle long-lasting changes might be more widespread and not necessarily beneficial in nature is therefore quite real. But caution is necessary in assessing claims of irreversible anesthetic effects. Although observations of prolonged and even irreversible cognitive changes after anesthesia have appeared over the years, it is difficult to attribute these often dramatic effects to the anesthetic drugs per se. Only studies performed using tightly controlled intraoperative and postoperative conditions can really contribute to understanding the scope and nature of the potential problem given the multiple confounding variables now recognized.
The International Study of Post-Operative Cognitive Dysfunction convincingly established the existence and prevalence of postoperative cognitive dysfunction (POCD) as a defined clinical entity in elderly patients.2 The major risk factor identified for POCD was advanced age, but mechanisms remain unknown, and could involve effects of accumulating chronic disease, declining organ function and/or altered drug responses.3 Exposure to surgery and anesthesia has also been suspected to accelerate preexisting but heretofore latent neurodegenerative disorders such as Alzheimer's disease (AD)4 that might manifest in the postoperative period as POCD or mild cognitive impairment, a possible prelude to AD. Although not substantiated to date, the idea that anesthetic drugs might affect the progression of neurodegenerative disorders or injure the brain via common pathways has had significant impact on research in mechanisms of anesthetic toxicity.
At the opposite extreme of age, the observation that early exposure to ethanol causes neurodevelopmental abnormalities such as the fetal alcohol syndrome prompted researchers to investigate general anesthetics (alcohols are model anesthetics) for their neurodegenerative potential in neonatal animal models.5 The immature rodent CNS is clearly susceptible to damage induced by commonly used anesthetic drugs (ketamine or a cocktail of midazolam, nitrous oxide and isoflurane).6 Although concerning, the relevance of these findings to human infants has been questioned on theoretical grounds. Recent experiments in primates provide a reassuring but preliminary new perspective7 on the rodent data. Two recent retrospective studies failed to conclusively resolve the issue.8,9 For instance, the incidence of learning disabilities in children with multiple anesthetic exposures before the age of four years was found to be associated with a doubled incidence of learning disability diagnoses in later life.8 Despite the striking nature of this significant finding, the authors rightly point out that the methodological constraints of the study preclude conclusions as to the role played by anesthesia versus surgery and other associated conditions for this long-term outcome. In the meantime further laboratory and clinical studies are underway to clarify the clinical implications.10 Since it might take years to accurately define these risks, it is appropriate to comment on the existing laboratory data from the clinical perspective.
Extensive clinical trials have failed to prove or exclude a direct causal link between general anesthetics and long-term cognitive impairment.2,11,12 Given the logistical difficulties and time requirements inherent in clinical outcome studies, it is now clear that animal-based research is essential to advance our understanding and help target clinical research.1 Some of the most pressing currently unresolved questions are:
Do anesthetic drugs per se have measurable and reproducible long-term cognitive effects in the healthy human brain, particularly at the extremes of age, and/or do cognitive deficits result from other perioperative factors such as surgical trauma, concomitant disease, sleep disturbance, etc.?
Are long-term anesthetic effects drug-specific (e.g., inhaled vs. intravenous), or the result of the (un)physiological state of pharmacological coma they produce?
What are the molecular/cellular mechanisms of neuronal toxicity, and what are the predisposing factors to injury (e.g., age/developmental stage, presymptomatic neurodegenerative disease, dementia)?
If clinically relevant anesthetic neurotoxicity occurs in humans (no data support this assumption thus far), is prophylaxis or treatment possible, or can new, nontoxic agents or drug combinations be developed?
The mature and aging brain: a clinical problem in search of experimental evidence?
Neurobehavioral models of learning and memory have been used to probe for long-term deficits following general anesthetic exposure of adult and aged rodents to simulate behavioral aspects of POCD. Seemingly paradoxical improvements have been observed in memory consolidation in adult rats.13 With refinement in the models, impaired learning was reported consistently within two to three weeks after exposure to anesthetic combinations in aged rats.14 Conversely, anesthetic exposure caused a transient improvement in cellular and behavioral measures of learning and memory on the day after exposure in adult mice15 as well as lasting improvements in memory performance of adult rats exposed to anesthetics at two months of age (i.e., as young adults).16 In summary, when effects on learning and memory were observed, be it improvement in adult or deterioration in aged rodents, they appeared to last for weeks, which is considerably longer than predicted by the pharmacokinetic properties of potent inhalational agents. Moreover, a 4-h exposure of aged rats to nitrous oxide alone has been reported to cause learning impairment for up to 2 weeks,17 possibly providing a behavioral correlate to the earlier finding of neuronal damage induced by nitrous oxide exposure.18 When tested in the same behavioral model, propofol did not produce delayed cognitive impairment in aged rats.19 These studies suggest that, in the absence of surgical stress, some general anesthetics have sustained effects on spatial learning and memory that outlast their physical presence in the body, while others do not. Confirmation of these observations (for equivalent anesthetic depths and under surgical stress) would indicate that lasting cognitive impairment is likely to be due to a drug-specific effect on the aged brain, rather than result from the anesthetic-induced comatose state per se.
The events leading to prolonged alterations of memory and their relation to in vitro biochemical changes or clinical observations are unclear, however. Current hypotheses attribute the delayed neurocognitive deficits produced by anesthetics to direct neurotoxic effects (e.g., via alteration of intracellular calcium homeostasis in the case of isoflurane20), to enhancement of endogenous neurodegenerative mechanisms, to neuroinflammatory mechanisms triggered by surgically induced systemic inflammation,21 or to age-sensitive suppression of neuronal stem cell proliferation or differentiation.16,22 Any combination of these causes or a heretofore unidentified one may also be plausible.
Anesthetic toxicity and neurodegeneration
Although the mechanism of neurodegeneration in AD is still unclear, the leading theory implicates toxic effects mediated by the accumulation and aggregation of amyloid-peptides into a variety of soluble oligomers.23-26 Amyloid β peptides of various lengths are constitutively released after proteolytic cleavage of the amyloid precursor protein (APP). Depending on various conditions, these peptides can self-assemble into toxic oligomers which correlate with the severity of cognitive impairment in AD. Insoluble deposits of Aβ that appear amorphous under light microscopy are found in abnormally high amounts in the brains of AD patients and to a lesser extent in normal age-matched humans. While it is not entirely clear how these products of oligomerization cause neurodegeneration, considerable evidence supports the ‘Aβ hypothesis of AD’. Specifically, mutations of APP that increase cleavage of APP to the toxic form of Aβ (Aβ42) are associated with AD, synthetic Aβ peptides are toxic to hippocampal and cortical neurons in vitro, and injection of Aβ into the cerebral ventricles reversibly prevents induction of long-term potentiation, a cellular form of learning and memory.27 Within the framework of the Aβ hypothesis, the neurotoxic element is the intermediate-sized Aβ oligomer rather than the mature fibril. The possibility that volatile anesthetics might favor oligomerization of Aβ42 under physiologically relevant conditions is therefore intriguing.
A current hypothesis attributes a pivotal role to neurotoxic effects of Aβ42. A 2-hour isoflurane anesthetic in mice activated biomarkers compatible with a transient neurotoxic effect: an increase in caspase 3 (a marker of apoptosis) 6 h after anesthesia was followed by elevated Aβ 24 h after exposure.28 Whether these biochemical alterations persist beyond 24 h and/or correlate with neurobehavioral deficits was not reported. On the other hand, hyperphosphorylation of the protein τ, an important step in the genesis of neurofibrillary tangles, has been reported in vivo in mice immediately after anesthesia-induced hypothermia with various anesthetic drugs. It is noteworthy that τ-hyperphosphorylation was observed in the absence of demonstrable changes in Aβ levels (at least after chloral hydrate - Aβ levels were not reported for other anesthetics).29 The preventability of these ‘prodegenerative’ changes (by normothermia) does not directly support a simple toxicity-promoting role of general anesthetics, and demonstrates again the importance of careful control of physiological variables, both clinically and in studies of neurotoxicity.
A causal link between anesthetic-induced changes in biochemical markers of neurotoxicity and the development long-term cognitive dysfunction assessed with a variety of behavioral tests is required to solidify the proposed link between inhaled anesthetics (the largest body of incriminating evidence implicates isoflurane) and neurodegenerative changes. If such a relationship is indeed demonstrated, it will likely be dose- and context-specific. For example, Aβ is physiologically released in the healthy brain and picomolar (soluble, not oligomeric) concentrations of Aβ actually improve, while higher concentrations degrade, long-term potentiation and memory in rodents.30,31
The degeneration-prone brain
Are anesthetics particularly toxic to a brain in the early (presymptomatic) stages of a neurodegenerative disorder? This clinically important question has been addressed in an animal model of AD (Tg2576 transgenic mice) that harbors the human APP-Swedish mutation of APP.32 The affected hemizygous mice produce increasing amounts of Aβ and show age-dependent memory deficits by 9-10 months of age. Daily exposure of female mice to either halothane or isoflurane for 2 h over 5 consecutive days was bracketed by behavioral tests and followed by terminal immunohistochemistry two weeks after the anesthetic exposures. Increased cognitive impairment in the wake of anesthetic exposure was observed only in isoflurane-exposed wild-type mice, not in the baseline impaired AD mutants, while an increase in amyloid plaque load without worsened cognitive impairment was present in halothane-exposed AD-prone mutant mice. These results are difficult to reconcile with the hypothesis that anesthetic-induced increases in Aβ lead to cognitive impairment, but could reflect a delay in the cognitive functional effects of anesthetic-induced changes in amyloid formation. Thus the link between anesthetics and neurodegenerative disorders is far from conclusive, and alternative hypotheses should continue to be explored in this experimentally challenging area.
The role of neuroinflammation
The context of the anesthetic exposure might be critical to long-term neurocognitive effects. Clinical studies have not identified an effect of general versus regional anesthesia which suggests that the surgical insult itself might play a critical role in POCD.33 Recent experiments provide evidence for a role of inflammation in early cognitive abnormalities, at least in adult animals. Neurolept anesthesia with fentanyl and droperidol together with a surgical insult (splenectomy) in rats resulted in signs of CNS inflammation and cognitive impairment on the first and third postoperative days, while control and anesthesia only groups showed neither inflammatory changes nor behavioral effects.21 Immune-mediated sickness behavior is among the multiple physiological and pharmacological derangements occurring in the perioperative period that may impact postoperative cognitive function and must therefore be considered.
Insights from experiments in vitro
Experiments performed in cell-free systems and in cell or neuronal cultures cannot demonstrate neurocognitive effects, but provide important mechanistic insights for correlation with laboratory studies. A major effort has focused on linking anesthetic exposure to the generation of amyloid or direct neurotoxicity. Halothane and isoflurane at supraclinical concentrations accelerate oligomerization of Aβ42 and increase the amount of insoluble oligomers.34 In cultured pheochromacytoma cells, isoflurane and halothane were not toxic alone but augmented Aβ42-induced toxicity. These effects were specific for volatile anesthetics since propofol and ethanol at clinically relevant concentrations were ineffective. If similar processes occurred in vivo under clinically relevant conditions, volatile anesthetics could increase oligomerized Aβ42, a neurotoxic form of amyloid, in susceptible brains.34
Cultured human neuroglioma (H4) cells transfected to express human APP served as a model to probe isoflurane effects on AD-related pathologic processes. A 6-h exposure to isoflurane increased apoptosis and Aβ secretion, but these effects did not appear to be linked.35 The primary effect of isoflurane was proposed to be increased secretion of Aβ42 leading to induction of apoptosis. Aggregation of secreted Aβ peptides, in turn, was facilitated by isoflurane and the oligomerized Aβ was proposed to promote toxicity in a cell culture model.36 The primary toxic effect of isoflurane could be related to intracellular Ca2+ homeostasis since isoflurane-induced apoptosis was traced to excessive release of Ca2+ from the endoplasmic reticulum via activation of IP3 receptors in cultured pheochromocytoma cells and chicken lymphocytes.20 The survival of cultured neurons was reduced by exposure to isoflurane for 24 h, but not to sevoflurane, via a mechanism linked to release of Ca2+ from intracellular stores.37 The relevance of these findings, especially those obtained from genetically modified and nonneuronal cells, for the brain in vivo are unknown, but warrant further studies.1
The immature brain: Experimental evidence in search of clinical relevance
Anesthesia, apoptosis and long-term consequences
Receptors for the most abundant excitatory (l-glutamate) and/or inhibitory (γ-aminobutyric acid - GABA) neurotransmitters are affected by all known anesthetic drugs at concentrations achieved during clinical anesthesia, but the contributions of these and other known drug-receptor interactions to their desirable or undesirable effects are far less clear. It is important to note that the signaling systems activated by these two transmitters undergo dramatic changes during the maturation of the CNS. In contrast to the mature brain, transient pharmacologic blockade of N-methyl-d-aspartate (NMDA) receptors in the developing rodent brain causes excessive neuronal apoptosis,38 while neuronal degeneration caused by ethanol is even more widespread, perhaps due to its ability to enhance GABAA receptors in addition to blocking NMDA receptors.39
Even though ethanol and other alkanols are used as model anesthetics in mechanistic research, and the neurotoxic potential of ketamine is established,38,40 it was the demonstration that drug combinations used commonly in clinical practice produce long-lasting neurological deficits in neonatal rats that shocked the medical community. Rat pups anesthetized on P7 with combinations of midazolam, isoflurane and nitrous oxide (triple cocktail) sufficient to maintain a surgical plane of anesthesia for 6 h showed excessive neuronal apoptosis in the short term, impaired electrophysiological and behavioral measures of learning and memory as juveniles, and impaired cognitive function as adults.6 This publication has since achieved landmark status due to its persuasive combination of histopathology, electrophysiology and behavioral experiments and the observations on subjects exposed to the triple cocktail into adulthood. Only isoflurane, when given alone, increased apoptosis in the neonate but each additional drug (double and triple cocktails) further increased the number of apoptotic neurons (up to 15-fold). Exposure to the triple cocktail exactly at P7 caused impaired hippocampal long-term potentiation and mild deficits in spatial reference memory as adolescents (P28-32). Deficits in hippocampus-dependent learning and memory persisted into adulthood.
A number of experimental studies have confirmed the immediate neurotoxic effect of anesthetics in immature rodents and have largely excluded the possibility of confounding alterations of homeostasis (e.g., hypoglycemia, hypothermia, hypoxia, etc.). However, these studies generally did not address the important question of long-tem cognitive consequences. Recent reports confirmed the early cellular-level toxicity of both isoflurane41 and sevoflurane42 when administered at P7 to P10, but differed significantly with respect to long-term cognitive outcomes. Behavioral deficits persisting into adulthood were found in the experiments using sevoflurane but not isoflurane. An interesting difference between these studies that both exposed the animals for 6 h to comparable concentrations of the respective inhalational agent lies in the different strains of mice used to conduct the experiments (hybrid vs. inbred). The genetically more variable crossbred mice showed no long-term impairment, indicating that the strain of mice used must be considered when interpreting experiments. In rats, by contrast, isoflurane caused both acute toxicity and delayed deficits in spatial memory, but the behavioral effect was only present after a 4-h isoflurane anesthetic and not with shorter exposures.43 Recent studies of injectable drugs suggest that anesthetic agents somewhat specific for NMDA or GABAA receptors (ketamine, thiopental, propofol, either alone or in combination) when administered to P10 mice can also cause behavioral deficits or alterations in adulthood.44,45 but it should be noted that the specificity of, for example, ketamine for NMDA receptors may be less than previously thought and hence attributing any outcomes to specific drug-receptor interactions could be premature. Overall, this evidence, suggesting that anesthetics with different molecular mechanisms of action can potentially increase apoptosis in the rodent brain and lead to long-lasting neurobehavioral consequences, is compatible with the notion that prolonged pharmacologic coma might be detrimental irrespective of the causative condition.
Mechanisms of neurotoxicity
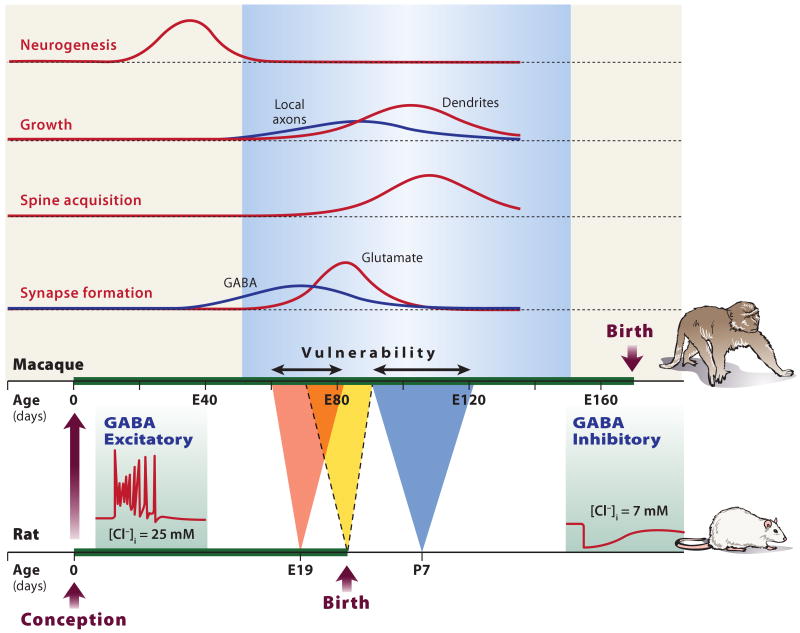
A consistent requirement for anesthetic neurotoxicity is that exposure to anesthetic drugs must be substantial both in terms of duration and dose as well as precisely timed to coincide with the brain growth spurt, a period of intense neurogenesis and synaptogenesis.6,43 Many more neurons and synapses are formed throughout development than survive into adulthood to form the ∼1011 neurons and ∼1014 synapses in the adult human brain. Programmed cell death (apoptosis) and synaptic pruning are critical to plasticity and stabilization of circuits in the developing nervous system. These are active processes that are tightly controlled by neurotrophin signaling mechanisms to ensure normal development, facilitate synaptic plasticity, and prevent neoplasia. It is commonly assumed that apoptosis is induced by an unphysiologic ‘black out’ during this critical period which, if long enough, causes synaptic connections to degenerate and thus ‘disconnected’ neurons to undergo apoptosis. The mechanistic details have yet to be established. An excitotoxic effect of GABAergic anesthetic drugs acting to potentiate excitatory GABAA receptors during early development is a prime candidate (fig. 1).46 Blockade of NMDA receptors has been suggested to produce excitotoxicity due to compensatory increases in NMDA receptor expression following sustained blockade. After withdrawal of the blocker, increased Ca2+ influx could lead to both apoptotic and necrotic cell death.7 Evidence also suggests that isoflurane can affect the balance between the forms of the neurotrophin BDNF that promote neuron survival or death and neuronal spine stability or retraction.47,48 What other pathways for toxicity exist and whether there are overlaps with neurotoxicity in the aged brain remain to be clarified.
Fig. 1. Developmental vulnerability to neurotoxicity due to paradoxical GABAergic excitation.
Animal models have provided convincing evidence for drug-induced apoptotic neurodegeneration. The peaks of vulnerability to early (E19) and late (P7) apoptotic neurodegeneration induced by ethanol in rat brain47 are indicated. The development of rat brain compared to that of the macaque is shown in the lower axes with approximate corresponding developmental milestones. Neurogenesis occurs before E80 in primate, which corresponds to birth in rat. A single day in rat neurodevelopment (P7) translates to 32 days in the primate. Note that axons develop before dendrites and spines, and GABA synapses before glutamate synapses. Excitotoxicty resulting from GABAA-enhancing anesthetics is considered a plausible model for neurodegeneration. During development, GABA changes from excitatory (depolarizing) to inhibitory (hyperpolarizing) because the intracellular chloride concentration decreases due to changes in the expression of the two major chloride cotransporters, KCC2 and NKCC1 that are differentially expressed during development. Paradoxical excitation of immature neurons by activation of GABAA receptors generates an efflux of chloride and excitation in immature neurons (left inset), in contrast to the influx of chloride with inhibition in mature neurons (right inset.
Stem cells/apoptosis?
Anesthetic-induced apoptosis of neural stem cells is a possible mediator of neurotoxicity that could explain effects at both extremes of age. Recent evidence indicates that functionally important neurogenesis continues at a low level in specific brain regions throughout life, particularly in the dentate gyrus of the hippocampus, where its selective blockade causes delayed memory deficits in adult animals.49 Neural precursor cells obtained from postnatal day 2 (P2) rat pups and grown in culture did not undergo apoptosis or necrosis after exposure to 4 h of 3.7% isoflurane but were slowed in their proliferation and showed accelerated neuronal differentiation.22 Exposure to 4 h of 1 minimum alveolar concentration isoflurane also decreased progenitor proliferation in P7 but not young adult rats.16 Whether and how these changes relate to long-term cognitive deficits remains to be determined but it appears that these changes may be more relevant for the immature than the mature brain.
Clinical relevance
The relevance of these findings to clinical anesthesia in pediatric patients has naturally generated a vigorous and ongoing debate resulting from a conflict between concerning preclinical laboratory findings and established clinical practices. While available retrospective clinical trials are not incompatible with the existence of associations between early anesthetic exposure and long-term neurocognitive function, the limitations mentioned above indicate that time-consuming randomized prospective trials are required.11 Two principal arguments have been raised by skeptics of the clinical relevance of anesthetic neurotoxicity. The first is that neuronal damage and its sequelae (in rodent models without surgical intervention) are due to causes other than the anesthetic drugs, an argument that has been largely refuted. The second argument is based on the profound differences between mammalian species in neurodevelopmental time course and hence timing and relative duration of exposure in potentially anesthetic-sensitive critical periods. Related to timing is also the extrapolation of the duration of exposure necessary to inflict damage.
The neonatal rodent is the flagship animal model for studies of neonatal anesthetic toxicity. Rats and mice, however, are altricial while other species, e.g., guinea pigs, primates and ungulates, are precocial. The brain growth spurt occurs later in the former, predominantly prenatally or both pre- and postnatally in the latter. Accordingly, histopathologic evidence of toxicity in guinea pigs was documented following exposure to anesthetics in utero, but its reproducibility postnatally as well as behavioral relevance are unknown. The important question is therefore when the developing primate brain is vulnerable and what constitutes a critical threshold (dose × time) of anesthetic exposure. Only a partial answer is available and only for ketamine: Exposure of rhesus monkey fetuses and newborns to ketamine for 24 h caused neurodegeneration at day 122 of gestation (75-80% of normal term, roughly comparable to P7 in rat and nominally to 20-28 weeks of gestation in humans, but see the milestone-based calculation below) and on P5, but not on P35. Conversely, a 3-h exposure at P5 had no neurodegenerative effect.7 While ketamine has the most consistently reproducible, dose-dependent effects in rodent models50 and these data might seem reassuring, the animal numbers examined were small and behavioral consequences were not assessed. Studies to detect subtle changes and whether other anesthetics follow a similar profile are necessary. Despite these caveats, the data obtained from rhesus monkeys are consistent with contemporary comparative neurobiological data using a developmental milestones-based approach by which the brain of a P7 rat pup corresponds to the brain of a human embryo of 16-22 weeks gestation*, and are also compatible with previously published estimates for the peak vulnerability of human fetuses to excitotoxic damage.51 An additional, purely quantitative, factor that could complicate interspecies extrapolation is the varying fraction of neurons that normally undergo apoptosis during gestation. In the human cerebral cortex, the number of neurons peaks at 28-32 weeks of gestation and is reduced by 70% by the early postnatal period. If this fraction is substantially different from rodents, the long-term impact of additional, drug-induced apoptosis on cognitive function might also differ. Differences between rodent and mammalian brain with respect to developmental flexibility, compensatory mechanisms and susceptibility to exogenous modulators could also be involved.
Conclusions
In light of the existing evidence reviewed above, the question is not so much whether long-lasting anesthetic-induced changes in the CNS can occur, but whether they have any identifiable or preventable deleterious impact in the long-term when used clinically. Convincing evidence to that effect is lacking but is probably unattainable due to methodological constraints of studies in human subjects. Nevertheless, both retrospective52 and prospective10 outcome studies are underway in an effort to define the impact of general anesthetics on long-term cognitive function. In the meantime, more evidence is required from animal models that more closely mimic the clinical reality. Considering that there are no alternatives to the available anesthetic drugs in most cases (to the best of our knowledge, long-term consequences of silencing the developing spinal cord have not been studied and acute local anesthetic neurotoxicity is beyond the scope of this review), it becomes incumbent on anesthesiologists and intensivists to use existing drugs judiciously while developing ways to minimize potential for harm. Given the major gaps in our understanding of the neurocognitive consequences of anesthetic exposure with and without surgical stress, and the paucity of convincing clinical evidence implicating or acquitting anesthetic drugs per se, it is appropriate to remember that no drugs are free of side-effects but also to reassure our patients about their general safety. Cause for concern? Yes. Cause for continued research efforts? Definitely. Cause to adjust sound clinical practice? No. Stay tuned….
Acknowledgments
Support for Dr. Perouansky was provided solely from institutional and/or departmental sources. Support for Dr. Hemmings was provided by departmental funds and by a grant from the National Institutes of Health gant GM 58055 (Bethsda Maryland).
Footnotes
Summary statement: Accumulating evidence indicates that general anesthetics can have persistent adverse neurological effects. Identification of risk factors and understanding their mechanisms is important in order to identify and minimize the risks.
www.translatingtime.net, last accessed August 5, 2009.
References
- 1.Baranov D, Bickler PE, Crosby GJ, Culley DJ, Eckenhoff MF, Eckenhoff RG, Hogan KJ, Jevtovic-Todorovic V, Palotas A, Perouansky M, Planel E, Silverstein JH, Wei H, Whittington RA, Xie Z, Zuo Z. Consensus statement: First International Workshop on Anesthetics and Alzheimer's disease. Anesth Analg. 2009;108:1627–30. doi: 10.1213/ane.0b013e318199dc72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 3.Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176–81. doi: 10.1097/ALN.0b013e3181a10207. [DOI] [PubMed] [Google Scholar]
- 4.Bohnen NI, Warner MA, Kokmen E, Beard CM, Kurland LT. Alzheimer's disease and cumulative exposure to anesthesia: A case-control study. J Am Geriatr Soc. 1994;42:198–201. doi: 10.1111/j.1532-5415.1994.tb04952.x. [DOI] [PubMed] [Google Scholar]
- 5.Olney JW, Young C, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C. Do pediatric drugs cause developing neurons to commit suicide? Trends Pharmacol Sci. 2004;25:135–9. doi: 10.1016/j.tips.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–58. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 8.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805–12. doi: 10.1097/ALN.0b013e31819c7124. [DOI] [PubMed] [Google Scholar]
- 10.Davidson AJ, McCann ME, Morton NS, Myles PS. Anesthesia and outcome after neonatal surgery: The role for randomized trials. Anesthesiology. 2008;109:941–4. doi: 10.1097/ALN.0b013e31818e3f79. [DOI] [PubMed] [Google Scholar]
- 11.Bryson GL, Wyand A. Evidence-based clinical update: General anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can J Anaesth. 2006;53:669–77. doi: 10.1007/BF03021625. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–6. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 13.Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96:1004–9. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- 14.Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg. 2004;99:1393–7. doi: 10.1213/01.ANE.0000135408.14319.CC. [DOI] [PubMed] [Google Scholar]
- 15.Rammes G, Starker LK, Haseneder R, Berkmann J, Plack A, Zieglgansberger W, Ohl F, Kochs EF, Blobner M. Isoflurane anaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology. 2009;56:626–36. doi: 10.1016/j.neuropharm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 17.Culley DJ, Raghavan SV, Waly M, Baxter MG, Yukhananov R, Deth RC, Crosby G. Nitrous oxide decreases cortical methionine synthase transiently but produces lasting memory impairment in aged rats. Anesth Analg. 2007;105:83–8. doi: 10.1213/01.ane.0000266491.53318.20. [DOI] [PubMed] [Google Scholar]
- 18.Jevtovic-Todorovic V, Beals J, Benshoff N, Olney JW. Prolonged exposure to inhalational anesthetic nitrous oxide kills neurons in adult rat brain. Neuroscience. 2003;122:609–16. doi: 10.1016/j.neuroscience.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Lee IH, Culley DJ, Baxter MG, Xie Z, Tanzi RE, Crosby G. Spatial memory is intact in aged rats after propofol anesthesia. Anesth Analg. 2008;107:1211–5. doi: 10.1213/ane.0b013e31817ee879. [DOI] [PubMed] [Google Scholar]
- 20.Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–60. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 21.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: A possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–43. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Sall JW, Stratmann G, Leong J, McKleroy W, Mason D, Shenoy S, Pleasure SJ, Bickler PE. Isoflurane inhibits growth but does not cause cell death in hippocampal neural precursor cells grown in culture. Anesthesiology. 2009;110:826–33. doi: 10.1097/ALN.0b013e31819b62e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings BJ, Cotman CW. Image analysis of beta-amyloid load in Alzheimer's disease and relation to dementia severity. Lancet. 1995;346:1524–8. doi: 10.1016/s0140-6736(95)92053-6. [DOI] [PubMed] [Google Scholar]
- 24.Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PT, Jones BW, Fernandez SJ, Lacor PN, Horowitz P, Finch CE, Krafft GA, Klein WL. Self-assembly of Abeta(1-42) into globular neurotoxins. Biochemistry. 2003;42:12749–60. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- 25.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 26.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 27.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 28.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–27. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, Krishnamurthy P, Herman M, Wang L, Schachter JB, Nelson RB, Lau LF, Duff KE. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci. 2007;27:3090–7. doi: 10.1523/JNEUROSCI.4854-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–45. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Osta A, Alberini CM. Amyloid beta mediates memory formation. Learn Mem. 2009;16:267–72. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–10. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: A systematic review. Anesthesiology. 2007;106:572–90. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-β oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–9. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid β protein levels. Anesthesiology. 2006;104:988–94. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–54. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–47. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 39.Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–60. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 40.Scallet AC, Schmued LC, Slikker W, Jr, Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F, Hanig JP. Developmental neurotoxicity of ketamine: Morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81:364–70. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- 41.Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, Danzer SC. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 42.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 43.Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–61. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 44.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and γ-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–36. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 45.Viberg H, Ponten E, Eriksson P, Gordh T, Fredriksson A. Neonatal ketamine exposure results in changes in biochemical substrates of neuronal growth and synaptogenesis, and alters adult behavior irreversibly. Toxicology. 2008;249:153–9. doi: 10.1016/j.tox.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Ari Y. Excitatory actions of gaba during development: The nature of the nurture. Nat Rev Neurosci. 2002;3:728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 47.Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006;11:1603–15. doi: 10.1007/s10495-006-8762-3. [DOI] [PubMed] [Google Scholar]
- 48.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–25. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W, Wang C. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108:149–58. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev. 1991;26:61–7. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- 52.Hansen TG, Flick R. Anesthetic effects on the developing brain: Insights from epidemiology. Anesthesiology. 2009;110:1–3. doi: 10.1097/ALN.0b013e3181915926. [DOI] [PubMed] [Google Scholar]