Abstract
Crowell, Richard L. (Hahnemann Medical College, Philadelphia, Pa.). Specific viral interference in HeLa cell cultures chronically infected with Coxsackie B5 virus. J. Bacteriol. 86:517–526. 1963.—The presence of large amounts of Coxsackie B5 virus in culture fluids of a HeLa subline, serially propagated over a 3-year period, provided evidence for attainment of a viral carrier state. Human serum in the growth medium of carrier cultures appeared prerequisite for maintenance of a stable virus-cell equilibrium. Virus was eliminated from HeLa cells by addition of B5 antiserum to the growth medium, whereas subcultivation in calf serum medium resulted in cellular degeneration by virus. HeLa cells, chronically infected by B5 virus, retained normal morphology in monolayer cultures and were found preservable by freezing. Persistently infected HeLa cells formed colonies with high efficiency in a medium containing B5 antiserum, to provide evidence that the majority of cells in carrier populations were not fatally infected. The significance of occurrence of small and large plaque variants of B5 virus in the carrier system remains to be determined. Coxsackie B5-carrier cultures were found specifically resistant to superinfection by all members of Coxsackie group B. This resistance, due to viral interference, was not extended to three immunological types of Coxsackie group A, poliovirus types 1 to 3, adenovirus T1, or vaccinia virus. Viral interference was found to be a consequence of altered surfaces of carrier cells, as reflected by decreased adsorption kinetics and cell penetration by Coxsackie group B viruses. The data suggested that Coxsackie group B viruses share a unique requirement, distinct from that of polioviruses, for reception and eclipse by HeLa cells. Interference between polioviruses and members of Coxsackie group B is discussed.
Full text
PDF


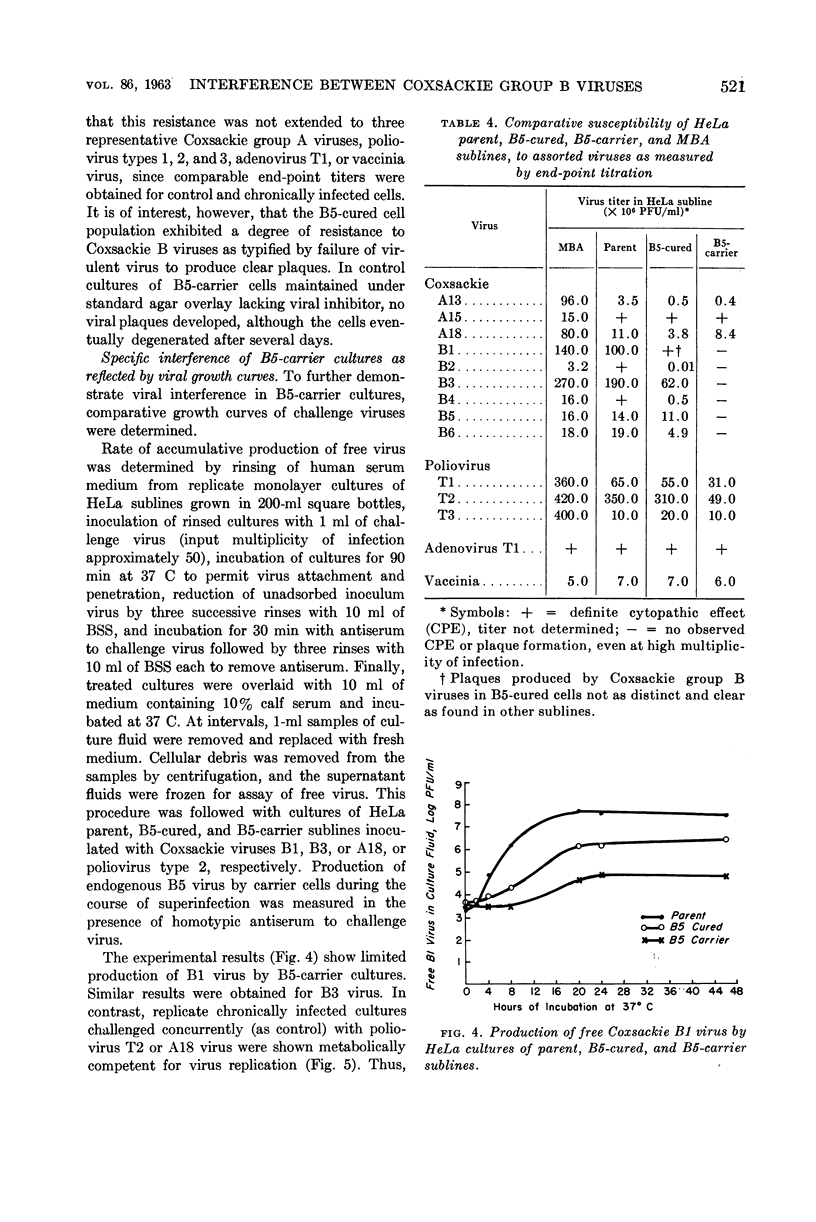
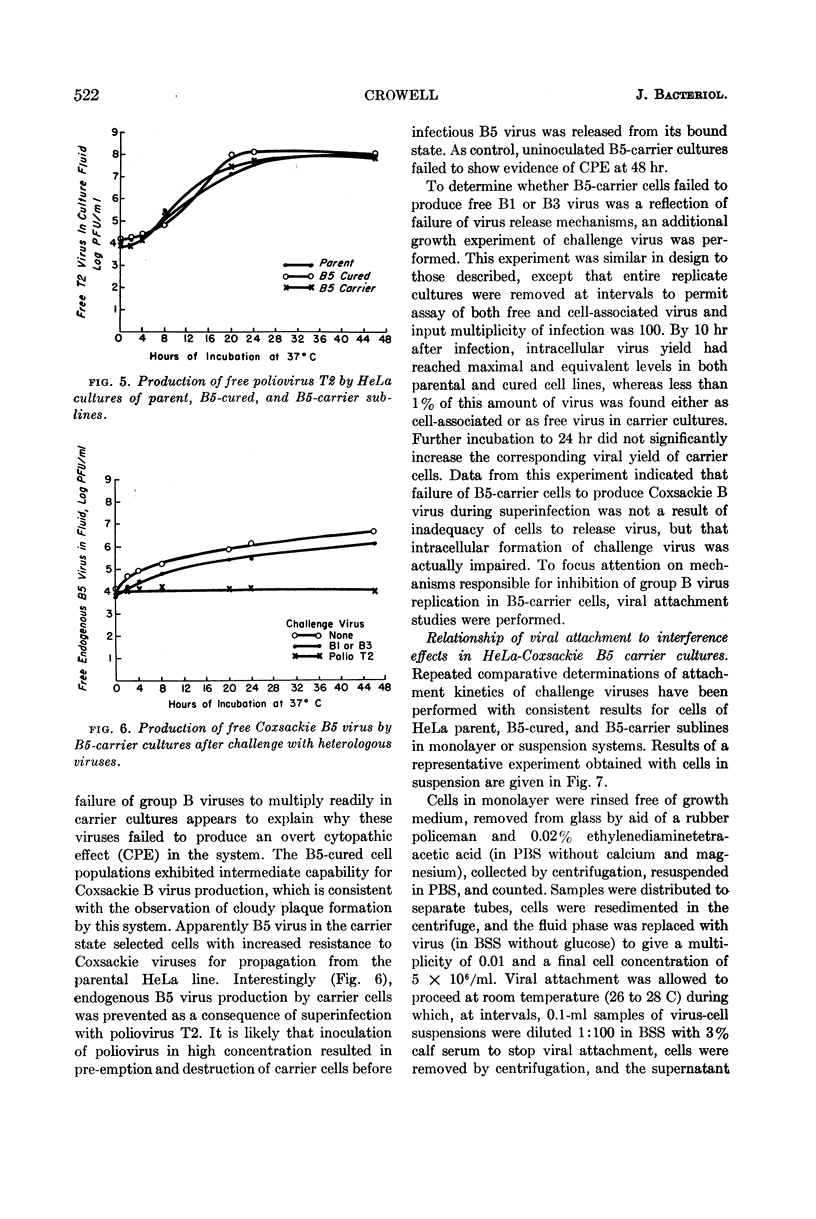
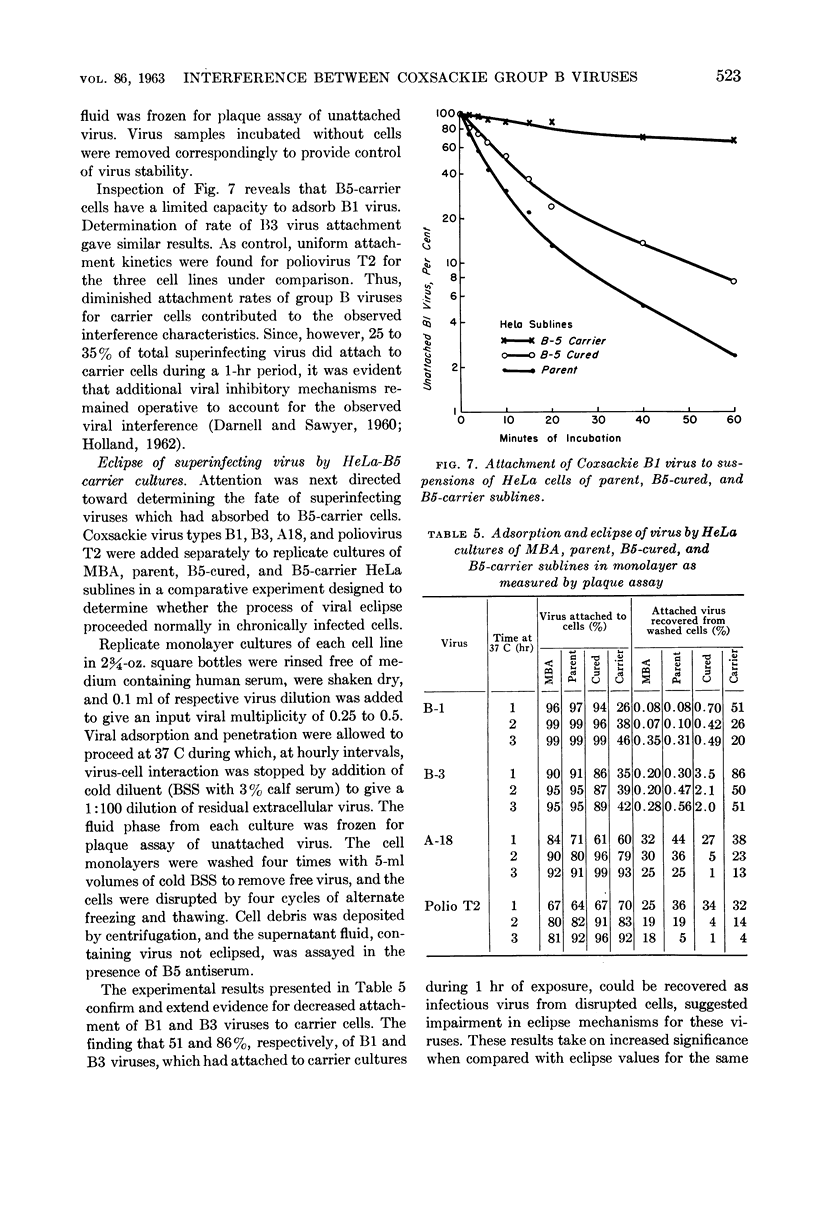



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALUDA M. A. Loss of viral receptors in homologous interference by ultraviolet-irradiated Newcastle disease virus. Virology. 1959 Mar;7(3):315–327. doi: 10.1016/0042-6822(59)90201-6. [DOI] [PubMed] [Google Scholar]
- CORIELL L. L. Detection and elimination of contaminating organisms. Natl Cancer Inst Monogr. 1962 Apr;7:33–53. [PubMed] [Google Scholar]
- CROWELL R. L., SYVERTON J. T. The mammalian cell-virus relationship. VI. Sustained infection of HeLa cells by Coxsackie B3 virus and effect on superinfection. J Exp Med. 1961 Feb 1;113:419–435. doi: 10.1084/jem.113.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, SAWYER T. K. The basis for variation in susceptibility to poliovirus in HeLa cells. Virology. 1960 Aug;11:665–675. doi: 10.1016/0042-6822(60)90113-6. [DOI] [PubMed] [Google Scholar]
- HABEL K., HORNIBROOK J. W., GREGG N. C., SILVERBERG R. J., TAKEMOTO K. K. The effect of anticellular sera on virus multiplication in tissue culture. Virology. 1958 Feb;5(1):7–29. doi: 10.1016/0042-6822(58)90003-5. [DOI] [PubMed] [Google Scholar]
- HALPERN S., SULKIN S. E. Mixed viral infections: interactions between Coxsackie virus and poliovirus in mice and tissue cultures. Tex Rep Biol Med. 1961;19:585–612. [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The location and nature of enterovirus receptors in susceptible cells. J Exp Med. 1961 Aug 1;114:161–171. doi: 10.1084/jem.114.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO M., ENDERS J. F. Further studies on an inhibitor of viral activity appearing in infected cell cultures and its role in chronic viral infections. Virology. 1959 Nov;9:446–477. doi: 10.1016/0042-6822(59)90135-7. [DOI] [PubMed] [Google Scholar]
- HSIUNG G. D. Multiplication of poliovirus in tissue cultures previously infected with other enteroviruses. Arch Gesamte Virusforsch. 1961;11:343–354. doi: 10.1007/BF01249590. [DOI] [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. Alteration plaque morphology of EMC virus with polycations. Virology. 1961 Aug;14:502–504. doi: 10.1016/0042-6822(61)90349-x. [DOI] [PubMed] [Google Scholar]
- MANDEL B. Reversibility of the reaction between polio-virus and neutralizing antibody of rabbit origin. Virology. 1961 Jul;14:316–328. doi: 10.1016/0042-6822(61)90317-8. [DOI] [PubMed] [Google Scholar]
- PACSA S. Poliovirus-carrying lines of HeLa cells: their establishment and sensitivity to viruses. Acta Microbiol Acad Sci Hung. 1961;8:329–332. [PubMed] [Google Scholar]
- QUERSIN-THIRY L. Action of anticellular sera on virus infections. I. Influence on homologous tissue cultures infected with various viruses. J Immunol. 1958 Sep;81(3):253–260. [PubMed] [Google Scholar]
- SABIN A. B. Status of field trials with an orally administered, live attenuated poliovirus vaccine. J Am Med Assoc. 1959 Oct 17;171:863–868. doi: 10.1001/jama.1959.03010250001001. [DOI] [PubMed] [Google Scholar]
- SOLOVYOV V. D., GULEVICH N. E. Studies on antiviral immunity using tissue culture methods. II. Obtaining cells resistant to poliomyelitis virus. Acta Virol. 1960 Jul;4:220–226. [PubMed] [Google Scholar]
- TAKEMORI N., NOMURA S., NAKANO M., MORIOKA Y., HENMI M., KITAOKA M. Mutation of polioviruses to resistance to neutralizing substances in normal bovine sera. Virology. 1958 Feb;5(1):30–55. doi: 10.1016/0042-6822(58)90004-7. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., HABEL K. Virus-cell relationship in a carrier culture of HeLa cells and Coxsackie A9 virus. Virology. 1959 Jan;7(1):28–44. doi: 10.1016/0042-6822(59)90175-8. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Properties of a HeLa cell culture with increased resistance to poliomyelitis virus. Virology. 1958 Jun;5(3):425–434. doi: 10.1016/0042-6822(58)90037-0. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R. Cellular resistance to viral infection, with particular reference to endogenous interferon. Bacteriol Rev. 1963 Mar;27:72–86. doi: 10.1128/br.27.1.72-86.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]



