Summary
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that functions as a key regulator of cell growth, protein synthesis, and cell-cycle progression through interactions with a number of signaling pathways, including PI3K/AKT, ras, TCL1, and BCR/ABL. Many haematologic malignancies have aberrant activation of the mTOR and related signaling pathways. Accordingly, mTOR inhibitors, a class of signal transduction inhibitors that were originally developed as immunosuppressive agents, are being investigated in preclinical models and clinical trials for a number of haematologic malignancies. Sirolimus and second generation mTOR inhibitors such as temsirolimus and everolimus, are safe and relatively well-tolerated, making them potentially attractive as single agents or in combination with conventional cytotoxics and other targeted therapies. Promising early clinical data suggests activity of mTOR inhibitors in a number of haematologic diseases, including acute lymphoblastic leukemia, chronic myelogenous leukemia, mantle cell lymphoma, anaplastic large cell lymphoma, and lymphoproliferative disorders. This review describes the rationale for using mTOR inhibitors in a variety of haematologic diseases with a focus on their use in leukemia.
Keywords: mTOR, Leukemia, Signal Transduction, Lymphoma, Sirolimus
Targeting mTOR Signaling
mTOR Inhibitors (MTIs) are a class of signal transduction inhibitors developed as immunosuppressive agents. Because mTOR signaling is aberrantly activated in a number of malignancies, mTOR inhibitors are being investigated in a number of tumor types in both pre-clinical models and clinical trials. Sirolimus (rapamycin), a macrocyclic lactone produced by Streptomyces hydroscopicus, was the first MTI to be used in a clinical setting.(Schmelzle and Hall 2000) Sirolimus is FDA-approved as an immunosuppressive agent in solid organ transplantation, but the drug has clear anti-neoplastic activity and is in phase II-III trials against a variety of cancers.(Baldo, et al 2008) Sirolimus has poor aqueous solubility and variable bioavailability, requiring therapeutic drug monitoring. A number of second-generation MTIs, including temsirolimus (CCI-779), everolimus (RAD001), and deferolimus (AP23573) have been developed to circumvent those problems. These agents are also being investigated in a number of malignancies. Temsirolimus was the first mTOR inhibitor to gain FDA approval for any malignancy, having been approved for the treatment of advanced renal cell carcinoma.(Baldo, et al 2008)
mTOR inhibitors when used as monotherapy are relatively well-tolerated. Unlike the commonly used immunophilins, cyclosporine and tacrolimus, mTOR inhibitors cause little nephrotoxicity and neurotoxicity. MTIs may cause hyperlipidemia, mild myelosuppression, hypertension, and mucositis.(Baldo, et al 2008) The toxicities of combining mTOR inhibitors with conventional cytotoxic agents have not been fully explored in both preclinical and clinical studies.
Because mTOR signaling has been demonstrated to be important in cell growth and survival in a number of haematologic malignancies, MTIs are being investigated in a myriad of diseases. Preclinical studies have demonstrated MTIs have activity either when used as single agents and/or when used in combination with cytotoxic chemotherapeutics and other targeted agents in acute lymphoblastic leukemia, acute myelogenous leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia, multiple myeloma, non-hodgkins lymphoma, myelodysplastic syndrome, and non-malignant hematologic disorders, including lymphoproliferative disorders. Numerous clinical trials are underway for these diseases and early clinical data has shown potential activity in a number of these conditions. This review will focus on the use of mTOR inhibitors in leukemia, but will also briefly summarize on-going work in other malignant and non-malignant haematologic disorders.
The mTOR Signaling Network
mTOR is a 210 kD protein that has C-terminal homology to PI-3 kinase and is therefore a member of the PI-3 kinase-related kinase family.(Wullschleger, et al 2006) mTOR is a serine/threonine kinase that acts as a central regulator of cell growth, survival, metabolism, and proliferation and functions as a sensor to ensure that the cell is in an appropriate nutritional and bioenergenic state to support these processes prior to committing to growth and division.(Schmelzle and Hall 2000) When mTOR is activated, a number of cellular processes occur, including an increase in ribosomal biogenesis, cap-dependent translation (initiation of translation involving 5'-end of mRNA), TOP-protein translation (translation of specific class of mRNAs containing oligopyrimine tracts in 5'untranslated region), expression of metabolism-related genes, cell growth, nutrient and amino acid uptake, and an increase in cell cycle transit time.(Wullschleger, et al 2006) Conversely, the activation of mTOR leads to an inhibition of apoptosis and autophagy.(Asnaghi, et al 2004, Zeng and Kinsella 2008) The import of nutrients and amino acids is critical for the generation of ATP and cell metabolism. mTOR regulates these processes in part by up-regulating the protein translation machinery which results in the synthesis of nutrient and amino acid transporters (e.g. Glut 1), as well as key molecules that promote cell growth and survival, such as Hif-1a, Cyclin D1, and myc.(Gera, et al 2004, Majumder, et al 2004)
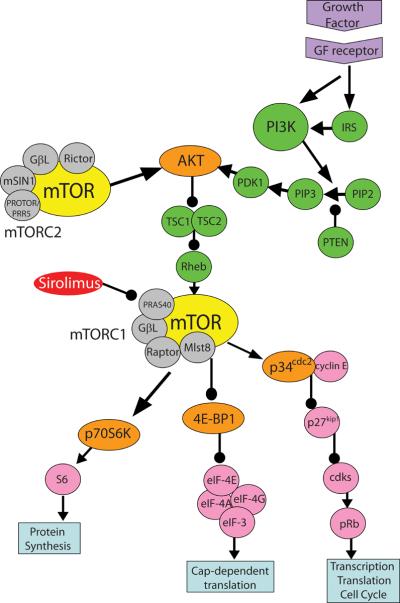
mTOR can form two distinct complexes, mTORC1 and mTORC2 (Figure 1).(Bhaskar and Hay 2007) mTORC1 is sensitive to mTOR inhibitors (MTIs), including sirolimus, and is thought to regulate cell growth, proliferation, autophagy and translation in response to nutrients and energy availability. Data suggest that mTORC2 is insensitive to MTIs in some cell types whereas mTORC2 remains sensitive to MTIs in other cancer cell types.(Rosner and Hengstschlager 2008) mTORC2's function, regulation and response to MTIs remains unclear, and seems to vary by cell type. mTOR is activated by a number of upstream signaling pathways, including PI3K/AKT, RAS/MAPK/RSK, cytokine signaling (IKK), TCL1, BCR-ABL, and nutrient (amino acid) sensing via the Rag GTP-binding proteins. (Kharas, et al 2008, Shaw and Cantley 2006) The main downstream targets of activated mTORC1 are S6K1 and the inhibitor of cap-dependent translation, 4E-BP-1.(Wullschleger, et al 2006) Figure 1 summarizes the mTOR signaling pathway.
Figure 1.
mTOR Signaling Cascade. mTOR regulates a number of key cellular processes in mammalian cells, including protein translation. mTOR can bind to GβL, Mlst8, PRAS40, and RAPTOR, forming the MTI sensitive complex, mTORC1.(Bhaskar and Hay 2007) In comparison, the components of mTORC2 include mTOR, GβL, mSIN1, RICTOR, and PROTOR/PRR5.(Bhaskar and Hay 2007) mTORC2, in concert with PDK1, activates AKT by phosphorylation. Activation of growth factor receptors, including IL-7R, IGF-1R, c-kit, and flt-3, via insulin, hormones, and growth factors, leads to activation of IRS-1. The activation of IRS-1 in turn leads to PI3K up-regulation. PI3K can also be activated by directly associating with the growth factor receptor at the cell membrane. Activated PI3K generates PIP3 which can recruit AKT to the cell membrane so that PDK1 and TORC2 can activate it.(Wullschleger, et al 2006) The tumor suppressor PTEN negatively regulates PI3K by dephosphorylating its second messengers, i.e. PIP3.(Mills, et al 2001) Inactivating mutations of PTEN, which are found in many tumor types, leads to excess activation of AKT, mTOR, p70S6 kinase 1 (S6K1) and can increase sensitivity to mTOR inhibition.(Neshat, et al 2001) Activated AKT then can phosphorylate TSC2, resulting in the inactivation of the TSC1:TSC2 complex, allowing for RHEB to activate mTORC1.(Wullschleger, et al 2006) The main downstream targets of activated mTORC1 are S6K1 and the inhibitor of cap-dependent translation, 4E-BP-1.(Wullschleger, et al 2006) The mTORC1 kinase phosphorylates S6K1. Phosphorylated- S6K1 induces TOP-translation and ribosomal biosynthesis as well as blocks apoptosis by phosphorylating the pro-apoptotic molecule BAD. In addition, P-S6K1 acts as a negative feedback mechanism for the mTOR pathway by down-regulating IRS-1.(Harrington, et al 2005) mTORC1 regulates cap-dependent protein translation via phosphorylation of 4E-BP-1.(Huang, et al 2003) When hypophosphorylated, 4E-BP1 binds tightly to eIF-4E, blocking the association of eIF-4E with eIF-4G. This blocks the formation of the eIF-4F translation initiation complex which is necessary for cap-dependent translation. When 4E-BP1 is phosphorylated by mTORC1, it is released from eIF4E, thereby facilitating translational initiation of mRNAs for a number of key intracellular proteins, including c-MYC, cyclin D1, and ornithine decarboxylase.(Faivre, et al 2006). Cyclin D1 forms a complex with CDK4 (cyclin dependent kinase 4) which is required for activation via phosphorylation of Rb (retinoblastoma protein).(Ewen, et al 1993) mTOR also facilitates the elimination of the cyclin dependent kinase inhibitor p27kip1 through interactions with p34cdc2, allowing cell cycle progression under the regulation of cyclin-dependent kinases, including cyclin-A.(Faivre, et al 2006) Arrows represent activation; Lines with circles represent inhibition. mTOR = mammalian target of rapamycin; PI3K = phosphoinositide 3-kinase; IRS = insulin receptor substrates; PTEN = phosphatase and tensin homologue deleted on chromosome ten; TSC = tuberous sclerosis; Rheb = ras homologue enriched in brain; p34cdc2 = cyclin-dependent controlling kinase p34; p27kip1 = cyclin-dependent kinase inhibitor kip1; cdks = cyclin-dependent kinases; pRb = retinoblastoma protein; S6 = ribosomal protein S6; 4E-BP1: eIF-4E binding protein; eIF = eukaryotic initiation factors; GβL = G protein beta subunit-like; mTORC = mTOR complex; PIP2 = phosphatidylinositol bisphosphate; PIP3 = phosphatidylinositol triphosphate; PDK1 = pyruvate dehydrogenase kinase, isozyme 1; Mlst8 = mammalian lethal with sec-13; PRAS40 = proline-Rich Akt substrate of 40kDa; mSIN1 = mammalian stress-activated protein kinase-interaction protein 1; and, PROTOR/PRR5 = Protein observed with Rictor-1/Proline-rich protein 5; GF = growth factor. Colour schematic: Yellow = mTOR; grey = Other proteins that bind to mTOR to form mTORC1/2; orange = targets phosphorylated by mTOR; pink = other down-stream-effectors; green = up-stream signaling molecules; purple = growth factor and receptor; red = drug.
A number of mechanisms that lead to mTOR deregulation have been identified. These include overexpression of growth factors (such as IGF), overexpression or mutations of growth factor receptors (e.g. IGFR, HER/EGFR), point mutations in the PIK3CA (p110alpha PI3K) gene, loss of tumor suppressor genes (e.g. PTEN or TSC1:TSC2 complex), and gain-of-function mutations in mTOR or mTOR-linked pathways (e.g. formation of the aberrant protein BCR-ABL in Ph+ leukemia cells or stimulation of PI3K by aberrant ras/raf/MAPK pathway intermediates).(Inoki, et al 2005) Of these mechanisms, the most common ones are related to the overexpression or constitutive activation of PI3K or AKT and/or the loss of PTEN.(Inoki, et al 2005) Through mTOR-mediated deregulated signaling or pathway cross-talk, increased mTOR activity supports cancer cells by stimulating the synthesis of proteins necessary for cell growth, proliferation, survival, angiogenesis, nutrient uptake and metabolism.
Leukemia
Acute Lymphoblastic Leukemia (ALL)
ALL is a malignancy of lymphoid origin, arising from transforming events that occur in early B cell progenitors. It is the most common cancer in children, accounting for 35% of new pediatric cancer diagnoses.(Plasschaert, et al 2004) Unfortunately, 20–25% of children and 80% of adults with ALL relapse and the majority of these patients succumb to their cancer despite aggressive therapy.(Plasschaert, et al 2004) Current ALL treatment protocols use combinations of multiple cytotoxic chemotherapeutics with overlapping toxicity and and the potential for long term sequelae, especially in the most intensively treated patients. New agents with activity against ALL are needed, and targeted biologic agents have the potential to add efficacy without additional toxicity in patients with refractory ALL.
As mTOR inhibitors have activity against lymphocytes and abnormal signaling can lead to neoplastic transformation, our group hypothesized that ALL cells may be dependent on mTOR signaling and studied the effects of MTIs on ALL blasts.(Brown, et al 2003) We demonstrated that sirolimus inhibited proliferation and induced apoptosis in ALL cell lines and improved survival in a Eu-RET transgenic mouse model of leukemia/lymphoma.(Brown, et al 2003) Since that initial report, the mTOR signaling pathway has been extensively studied by our group and others in preclinical models of ALL.(Avellino, et al 2005, Brown, et al 2003, Brown, et al 2007, Hirase, et al 2008, Houghton, et al 2008, Teachey, et al 2006b, Teachey, et al 2008) MTIs (sirolimus, temsirolimus, and everolimus) have been shown to be effective not only against cell lines and transgenic mouse models, but also against primary human ALL cells using in vitro (bone marrow stromal cell culture) and in vivo (NOD/SCID xenografts) models.(Avellino, et al 2005, Crazzolara, et al 2007, Teachey, et al 2006b)
The use of primary human ALL cells xenografted into immunodeficient mouse strains such as NOD/SCID mice is a powerful tool to study ALL biology and response to therapy. ALL cell lines can be useful tools, especially for signal transduction experiments, but they are difficult to establish from primary blasts, do not represent the heterogeneity of primary disease, and are thus suboptimal models for many preclinical studies. NOD/SCID xenografted ALL maintains its phenotypic characteristics even after serial passage and response of leukemic blasts to chemotherapeutic agents in the NOD/SCID xenograft model has been shown to correlate directly with human response to therapy.(Liem, et al 2004) Since treatment response in the mice correlates with human disease, these models can be used to compare chemotherapeutic responders to non-responders to delineate mechanisms of resistance. One problem with laboratory investigation of primary leukemia cells has been the limited quantity of blasts for analysis. The NOD/SCID xenograft model allows for significant expansion of ALL in the mouse in order to generate sufficient quantities of cells for study.
Our group has studied the activity of mTOR inhibitors using xenografts generated from 13 different ALL patients and found MTIs were effective in 62% of samples.(Brown, et al 2008) mTOR inhibitors appear to be active against both pre-B and pre-T ALL; however, MTIs may be most active in pre-T cell disease.(Houghton, et al 2008) While MTIs have been clearly shown to kill ALL cells, debate exists in the literature as to whether it is through apoptosis or autophagy.(Avellino, et al 2005, Crazzolara, et al 2007) As more data is accumulating that suggests ALL cells are dependent on the PI3K/AKT/mTOR signaling pathway, the activity of PI3K inhibitors, AKT inhibitors, and multi-kinase inhibitors (mTOR plus PI3K) is being investigated in ALL. (Brown, et al 2008, Kharas, et al 2008, Levy, et al 2008) Also, as cancer cells may become resistant to mTOR inhibitors through up-regulation of other intermediaries in the PI3K/AKT/mTOR signaling pathway, combinations of mTOR inhibitors with PI3K inhibitors and AKT inhibitors are being actively explored to overcome mTOR resistance with promising results. (Breslin, et al 2005, Brown, et al 2008, Kharas, et al 2008)
Because mTOR inhibitors are less likely to be effective in a clinical setting when used as single agents against leukemia, combination treatment is the next logical step in the therapeutic development of MTIs in ALL. It is important to choose rationally-designed combinations, building on an understanding of the mechanism(s) of action of MTIs in ALL blasts. MTIs have been shown to be effective and potentially synergistic in combination with a number of chemotherapeutics in vitro, including methotrexate, dexamethasone, etoposide, asparaginase, and doxorubicin.(Saydam, et al 2005, Teachey, et al 2008) MTIs have also been studied in combination with methotrexate and vincristine in vivo using NOD/SCID models with a marked response to both combination regimens.(Crazzolara, et al 2007, Teachey, et al 2008) The combination of temsirolimus and methotrexate resulted in cure in some xenografted animals. ALL cells treated with temsirolimus had marked reduction of cyclin D1 and dihydrofolate reductase, potentially increasing the sensitivity of ALL cells to methotrexate and explaining the combined effect.(Teachey, et al 2008) While this combination appears extremely promising in preclinical studies, both methotrexate and MTIs can cause mucositis and clinical trials are needed to determine tolerability. Nevertheless, everolimus and methotrexate have been successfully used in combination in patients with rheumatoid arthritis without significant toxicity, including mucositis.(Bruyn, et al 2008)
Another promising combination studied by our group and others in ALL is corticosteroids with MTIs.(Brown, et al 2008, Teachey, et al 2008, Wei, et al 2006) Wei et. al. screened a database of drug-associated gene expression profiles in ALL cells to evaluate gene expression signatures of glucocorticoid sensitivity as compared to resistance.(Wei, et al 2006) They found the profile generated by sirolimus matched the signature of glucocorticoid sensitivity and demonstrated that sirolimus could restore steroid sensitivity to steroid-resistant ALL. Similar work performed by Gu et. al. suggests that MTIs may reverse glucocorticoid resistance in ALL cells, an important finding especially since ALL patients frequently develop steroid resistance at relapse.(Gu, et al 2008, Haarman, et al 2008)
Patients with ALL that express the Philadelphia chromosome (Ph+ALL) have a particularly poor prognosis; however, the development of tyrosine kinase inhibitors against Bcr-Abl appears promising and will hopefully improve outcome. Resistance to these tyrosine kinase inhibitors is a real clinical concern. Because BCR-ABL is upstream of the PI3K/AKT/mTOR signaling pathway, MTIs maybe effective in Ph+ALL, including Bcr-Abl TKI resistant disease.(Kharas, et al 2004) Promising data suggests that Ph+ALL cells may be especially sensitive to mTOR inhibition (see section on CML below).(Hirase, et al 2008, Kharas, et al 2008)
Based on the preclinical work investigating MTIs in ALL, a number of clinical trials evaluating MTIs in patients with ALL as single agents and in combination with other agents are on-going (Table 1). Two Phase I/II trials of MTIs in patients with relapsed or refractory malignances, including patients with ALL, have been completed.(Rizzieri, et al 2008, Yee, et al 2006) Both of these trials had one patient each with ALL and both patients tolerated therapy, but neither had an objective response. Rheingold, et. al. recently reported interim results of an on-going phase 1 trial of sirolimus in children with relapsed/refractory ALL.(Rheingold, et al 2007) All patients tolerated sirolimus and 3 of 7 patients had stable disease.
Table 1.
Ongoing Clinical Trials
| Disease | Phase | Location(s) | Clinical Trials.gov number | Additional Information |
|---|---|---|---|---|
| ALL | II | COG Transplant Centers | NCT00795886 | Randomized trial comparing sirolimus plus standard GVHD ppx vs standard GVHD ppx alone after stem cell transplant for ALL* |
| ALL/NHL | I | Philadelphia, PA | NCT00068302 | Sirolimus for relapsed/refractory ALL or NHL |
| ALL/NHL CML** | I/II | Philadelphia, PA | NCT00776373 | Sirolimus plus etoposide and cytarabine for relapsed/refractory lymphoid malignancies |
| AML | I/II | Philadelphia, PA | NCT00780104 | Sirolimus plus MEC chemotherapy for high risk AML |
| AML | I | Melbourne, Australia | NCT00636922 | Everolimus plus cytarabine in elderly with AML |
| AML | I | Paris, France | NCT00544999 | Everolimus plus cytarabine and daunorubicin in relapsed AML |
| AML | II | Rome, Italy | NCT00775593 | Temsirolimus and clofarabine for relapsed or refractory AML |
| AML | I/II | Bavaria, Germany | NCT00762632 | Everolimus plus nilotinib for c-kit+ CML |
| CLL/B-NHL | II | Houston, TX | NCT00290472 | Temsirolimus for relapsed/refractory CLL or B cell NHL |
| CML** | I | Multiple centers in U.S, China, and Singapore | NCT00101088 | Temsirolimus and imatinib for CML accelerated phase |
| NHL | I | Ontario, Canada | NCT00659568 | Temsirolimus for advanced lymphoma |
| NHL | I | Cleveland, OH | NCT00671112 | Everolimus plus bortezomib for relapsed refractory MCL and other NHL |
| NHL | II | Multiple centers U.S. | NCT00436618 | Everolimus for refractory or advanced NHL |
| NHL | I | Tokyo, Japan | NCT00622258 | Everolimus for refractory or relapsed NHL |
| NHL/HD | I/II | Multiple centers U.S. | NCT00704054 | Deforolimus for relapsed/refractory NHL HD |
| MCL | II | Munich, Germany | NCT00727207 | Everolimus for relapsed/refractory MCL |
| NHL/HD MM | I/II | Multiple centers U.S. | NCT00474929 | Everolimus and Sorafenib for relapsed or refractory NHL, HD, or MM |
| MM | I | New York, NY | NCT00317798 | Sirolimus and ATG for relapsed MM |
| MM | I | Multiple centers U.S. | NCT00729638 | Everolimus and lenalidomide for relapsed MM |
| Advanced malignancies | I | Houston, TX | NCT00678233 | Temsirolimus plus IMC-A12 (anti-IGF-1R ab) for locally advanced or metastatic malignancy, including hematologic |
| Advanced malignancies | I | San Antonio, TX | NCT00060645 | Deforolimis for relapsed/refractory malignancies, including NHL, HD, and MM |
A number of clinical trials are on-going using sirolimus post-stem cell transplant as part of GVHD prophylaxis in patients with hematologic maligancies. ASCT0431 is the only one that randomizes patients to sirolimus versus no sirolimus with the hypothesis that sirolimus will improve survival via a direct action of sirolimus on ALL blasts.
CML in late accelerated phase or blast crisis
AML = acute myelogenous leukemia; ALL = acute lymphoblastic leukemia; CLL = chronic lymphocytic leukemia; CML = chronic myelogenous leukemia; NHL = Non-hodgkins lymphoma; HD = hodgkins disease; MM = multiple myeloma; GVHD = graft vs host disease; ppx = prophylaxis; MEC = mitoxantrone, cytarabine, etoposide
Given the potential activity of MTIs against ALL, and considering that haematopoietic stem cell transplantation (HSCT) is used as a major salvage strategy for patients with relapsed or refractory ALL and sirolimus has been used as graft-versus-host disease prophylaxis in a number of transplant trials, the use of sirolimus in the the post-HSCT setting has been proposed. This was tested in a phase II study, with promising results.(Pulsipher, et al 2008) As a result, the Children's Oncology Group has initiated a nationwide phase III randomized trial, ASCT0431, evaluating the addition of the mTOR inhibitor sirolimus to graft-versus-host disease (GVHD) prophylaxis during HSCT for relapsed ALL. The primary hypothesis of this trial is that the addition of sirolimus to GVHD prophylaxis will increase leukemia free survival compared to a regimen of standard agents, through the novel benefit of using a drug which has the potential to both control GVHD and directly suppress leukemic blasts. A strategy such as this would be a major advance in antileukemia therapy and transplantation.
Acute Myelogenous Leukemia (AML) and Myelodysplastic Syndromes (MDS)
AML is a clonal disorder of myeloid haematopoietic stem/progenitor cells. While the prognosis for patients with AML has been poor in general the outcome has improved with aggressive chemotherapeutic regimens and HSCT, at the cost of increased toxicities and long-term sequelae. A number of newer and more targeted agents with promise are in use and under development, including gemtuzumab ozogamicin, FLT-3 inhibitors, and farnesyl transferase inhibitors. Nevertheless, the only targeted therapies that have made significant improvements to date in the prognosis of AML have been used in patients with acute promyelocytic leukemia with the additions of arsenic and all-trans retinoic acid.(Sanz, et al 2008)
Recent interest has focused on targeting the PI3K/AKT/mTOR pathway in AML, as a majority of patient's blasts have constitutive activation of AKT with subsequent phosphorylation of down-stream targets of mTOR, including 4E-BP1 and S6K1.(Xu, et al 2003) As direct inhibitors of AKT and PI3K inhibitors remain in early development, the primary focus thus far has been evaluating MTIs in AML. Promising results have been demonstrated using monotherapy with MTIs in preclinical models of AML; however, these have not translated into substantial clinical benefit in early phase trials.(Perl and Carroll 2007, Recher, et al 2005, Rizzieri, et al 2008, Yee, et al 2006) This discrepancy may be due to the fact that early phase clinical trials are performed in patients with more aggressive disease (relapsed and/or refractory disease), and/or that the majority of preclinical work testing MTIs in AML has occurred in vitro. Until very recently, there were no murine models of AML that would allow testing of agents in vivo after the development of measurable disease. Prior preclinical work involved exposing AML cells to drugs in vitro and then testing the ability of cells to engraft in xenografted animals. This is in contrast to xenograft models of other hematologic diseases, including ALL, where agents can be tested in vivo after the development of measurable disease. Despite these findings, interest remains as targeting the mTOR pathway may enhance the cytotoxicity of existing chemotherapeutic agents and other targeted agents. Xu et. al. demonstrated that sirolimus enhanced the sensitivity of AML blasts to etoposide in vitro and the combination could prevent engraftment of AML cells in NOD/SCID mice better than either single agent alone if cells were treated in vitro prior to injection.(Xu, et al 2005a) Other groups have demonstrated that blocking mTOR increases the sensitivity of AML cells to HDAC (histone deacetylase) inhibitors and inhibitors of glycolysis.(Nishioka, et al 2008, Xu, et al 2005b) As other inhibitors of the PI3K/AKT/mTOR pathway are developed and tested in clinical trials, these agents may prove superior to mTOR inhibitors, since targeting PI3K with LY294002, AKT with perifosine, and both mTOR and PI3K with the dual inhibitor PI103 have shown promise in preclinical studies.(Papa, et al 2008, Park, et al 2008, Xu, et al 2003) Because combination therapy with mTOR inhibitors and either cytotoxics or biologics may be beneficial in patients with AML, clinical trials testing MTIs in AML are actively enrolling patients (Table 1). At the University of Pennsylvania, a phase I/II trial of sirolimus plus etoposide, mitoxantrone, and cytarabine has compelling preliminary data and is being broadened into a multi-center randomized trial.(Luger, et al 2006) In addition, trials using mTOR inhibitors in elderly patients with AML who cannot tolerate more aggressive cytotoxic therapy and in combination with cytotoxics or biologic agents in relapsed or refractory AML are ongoing (Table 1).
MDS are a heterogenous group of disorders characterized by cytopenias from defects in haematopoietic stem cell differentiation which frequently transform into acute myelogenous leukemia. Patients are classified by the IPSS (International Prognostic Scoring System) into low, intermediate (groups 1 and 2), and high prognostic risk groups based on patient characteristics, pathology, and tumor biology.(Greenberg, et al 1997) As PI3K/AKT/mTOR signaling has been shown to be important in cellular proliferation and malignant transformation, Follo et. al. hypothesized that aberrant activation of these survival signals may lead to transformation of MDS into AML.(Follo, et al 2007) This group found that mTOR and its downstream intermediates, S6K1 and 4E-BP1, were activated in high risk MDS patients (IPSS: intermediate risk group-2 and high risk) and were not activated in low risk patients. (intermediate risk group-1 and low risk). They also found that sirolimus was effective in vitro against the CD34+ cells from high risk patients but not low risk or normal controls. MTIs were noted to be active in some patients with MDS, resulting in either stable disease or improvement in cytopenias in early phase clinic trials and additional trials are under development.(Rizzieri, et al 2008, Yee, et al 2006)
Chronic Lymphocytic Leukemia (CLL)
CLL is the most common form of leukemia and arises from transforming events in CD5+ B cells. With aggressive chemotherapy, stem cell transplant, and novel therapeutics, including monoclonal antibodies, the prognosis for CLL has improved; however, it remains largely an incurable disease.(Lin 2008) Two cell populations are thought to exist in CLL: a very large non-proliferating population of peripheral blood B lymphocytes and a smaller pool of cycling malignant B cells found in pseudofollicles in lymph nodes, spleen, and bone marrow.(Dighiero and Hamblin 2008) The crux of targeted therapy is currently being directed at the smaller cycling compartment.
As MTIs have been shown to inhibit proliferation of malignant and non-malignant B lymphocytes and because PI3K was found to be constitutively active in CLL cells from patients, it has been hypothesized that targeting the PI3K/AKT/mTOR signaling pathway may be effective in patients with CLL.(Ringshausen, et al 2005, Ringshausen, et al 2002) Preclinical studies have shown mTOR inhibitors do not induce apoptosis in CLL cells but can cause cell cycle arrest through targeting the expression of cyclins D3, E, A and survivin.(Decker, et al 2003, Ringshausen, et al 2005) In addition, sirolimus was shown to improve survival in a CLL transgenic mouse generated by overexpressing TCL1 under the control of the μ immunoglobulin gene enhancer.(Zanesi, et al 2006) These preclinical studies led to a phase I clinical trial of everolimus in patients with CLL.(Decker, et al 2008) Three patients on this trial had stable disease and one patient had a partial response. Unfortunately, this trial was stopped after only seven patients were enrolled because 4 patients developed opportunistic infections. Patients on this trial did not receive infectious prophylaxis, and all had received aggressive and immunosuppressive regimens prior to everolimus. Interestingly, a high infectious risk has not been seen in other trials using everolimus even when used in combination with other immunosuppressives, including corticosteroids and cyclosporine.(Decker, et al 2008, Dunn and Croom 2006) Smith et. al. recently presented similar preliminary results from a phase II non-randomized single institution trial at M.D. Anderson, demonstrating stable disease and partial responses in a number of patients with CLL who were treated with temsirolimus.(Smith, et al 2008) Infections were noted but not to a degree that required the trial to be stopped. Other clinical trials using mTOR inhibitors in CLL are ongoing (Table 1).
Chronic Myelogenous Leukemia (CML)
CML is a myeloproliferative disorder characterized by malignant cells with a Bcr-Abl (9;22) translocation. The bcr-abl oncogene in CML encodes a 210kDa oncoprotein, whereas in Ph+ALL the translocation produces a 190kDa oncoprotein. Both fusion proteins have aberrant tyrosine kinase activity.(Piccaluga, et al 2007) Prior to the development of tyrosine kinase inhibitors (TKIs) with activity against Bcr-Abl, CML was only curable with HSCT. Over the past few years, complete responses have been documented with a number of targeted agents, including imatinib, nilotinib, and dasatinib, and front-line use of a TKI is now the standard of care for the disease.(Gora-Tybor and Robak 2008) Imatinib was the first TKI to be used in CML; unfortunately, approximately 25% of patients will either have innate resistance or more commonly acquire resistance to imatinib, because of Bcr-Abl mutations.(Gora-Tybor and Robak 2008) The majority of these patients will respond to second line TKIs, but a subset have a particular mutation (T3151) that is not treatable with current Bcr-Abl targeting TKIs.(Gora-Tybor and Robak 2008) Three reasons to develop alternative agents for patients with CML are: (1) to treat those with resistant disease; (2) to identify relatively non-toxic agents that target pathways down-stream of Bcr-Abl and could be used in combination with Bcr-Abl targeting TKIs front-line in certain high-risk patients to potentially prevent the development of resistant clones; (3) to treat patients in accelerated phase or blast crisis as they have a particularly poor prognosis and are less likely to respond to Bcr-Abl targeting TKIs as monotherapy.
Multiple groups have demonstrated that mTOR-dependent pathways are activated in Bcr-Abl transformed cells both in CML and in Ph+ALL.(Kim, et al 2005, Ly, et al 2003, Mayerhofer, et al 2005) Bcr-Abl has been shown to regulate translation of critical targets in CML, including S6 and 4EBP-1 via mTOR.(Ly, et al 2003) In preclinical studies, sirolimus has been shown to not only be effective when used as a single agent against Bcr-Abl transformed cells, but also to be potentially synergistic when combined with imatinib.(Mayerhofer, et al 2005, Mohi, et al 2004) Sirolimus has also been shown to be effective in vitro against resistant CML, including cells with T3151 mutations.(Sillaber, et al 2008) Similar results have been described in Ph+ALL.(Hirase, et al 2008, Kharas, et al 2008) Accordingly, clinical trials are underway using mTOR inhibitors in patients with relapsed/refractory CML and Ph+ALL. Sillaber et. al. treated 6 patients with imatinib-resistant CML with sirolimus and two patients had a major response and two others had a minor response.(Sillaber, et al 2008) Weltzer and colleagues recently completed a phase I-II study of everolimus in combination with imatinib in patients with imatinib-resistant CML (clinicaltrials.gov). Results of this trial are not currently available.
Other Haematologic Malignancies
Targeting mTOR signaling has also been investigated in Hodgkin's and non-Hodgkin's lymphoma (NHL), post-transplant lymphoproliferative disorder, and multiple myeloma. Arguably the subtype of lymphoma that has been the most studied and has the most potential for clinical benefit from targeting mTOR is mantle cell lymphoma.(Younes 2008) Mantle cell lymphoma is an extremely aggressive and incurable form of B-cell lymphoma with a median overall survival of 3 to 5 years.(Schmidt and Dreyling 2008) Mantle cell lymphoma is characterized by a t(11;14) translocation juxtaposing cyclin D1 with the immunoglobulin heavy chain, resulting in increased production of cyclin D1.(Hartmann, et al 2008) Cyclin D1 is a down-stream target of mTOR and MTIs can inhibit the cap-dependent translation of this protein in many cell types.(Hipp, et al 2005) Accordingly, Hipp et. al. hypothesized that targeting mTOR would be an effective treatment in mantle cell lymphoma by down-regulating cyclin D1 expression.(Hipp, et al 2005) They found that sirolimus was effective against mantle cell lymphoma cell lines in vitro; however, while other cyclins (D3, E, and A) were reduced, cyclin D1 expression did not change.(Hipp, et al 2005)
Subsequent work by Rudelius et. al., demonstrated that AKT, mTOR, and a number of mTOR down-stream signaling intermediates are constitutively activated in mantle cell lymphoma, giving an alternative explanation for the potential effectiveness of targeting AKT/PI3K/mTOR signaling, results confirmed by Peponi et. al.(Peponi, et al 2006, Rudelius, et al 2006) Other preclinical work has demonstrated that mTOR inhibitors synergize in vitro with a number of agents used to treat mantle cell, including rituximab, vincristine, doxorubicin, and bortezomib.(Haritunians, et al 2007) These findings led to a phase II trial of temsirolimus in patients with relapsed lymphoma. Results from this study are impressive, with a 41% overall response rate and a median time to progression of 6 months.(Ansell, et al 2008) Based on these results, additional trials are underway (Table 1).
AKT and the down-stream intermediates, 4E-BP1 and S6K1, were shown to be activated in Hodgkin's lymphoma cell lines; however, targeting either PI3K with LY294002 or mTOR with sirolimus only showed a modest effect in vitro. Nevertheless, a combination of doxorubicin and sirolimus was found to be synergistic and profoundly inhibited the same cell lines.(Dutton, et al 2005) A more pronounced single agent effect was demonstrated in a NOD/SCID xenograft model of Hodgkin's treated with everolimus.(Jundt, et al 2005) mTOR has also been shown to be activated in follicular lymphoma through a Syk-dependent mechanism, and sirolimus has some activity in follicular lymphoma cell lines.(Calastretti, et al 2001, Leseux, et al 2008) Similarly, mTOR and its intermediates have been shown to be activated in ALK-positive anaplastic large cell lymphoma and mTOR inhibition is effective in preclinical models of the disease.(Jundt, et al 2005, Vega, et al 2006) Chumsri and colleagues report that treating a patient with refractory cutaneous anaplastic large cell lymphoma with sirolimus resulted in a durable complete response.(Chumsri, et al 2008) Targeting mTOR has also been shown to be effective in preclinical models of diffuse large B cell lymphoma.(Wanner, et al 2006) All of these studies have led to a number of phase I-III clinical trials of mTOR inhibitors as single agents or in combination in aggressive and/or refractory lymphomas (Table 1).
Post-transplant lymphoproliferative disorder (PTLD) is a rare but serious complication of transplant (solid organ or HSCT), resulting from a defective cytotoxic T-cell response to viral infection, primarily EBV, in the setting of chronic immunosuppression.(Lewin 1997) The goals of treatment are to reduce and/or alter immunosuppression to allow partial T cell recovery and/or to target the EBV-infected B-cells with rituximab or anti-viral agents.(Lewin 1997) Some patients with PTLD have very aggressive disease with transformation to lymphoma and need definitive chemotherapy. Recently, El-Salem et. al. demonstrated that there is constitutive activation of mTOR signaling in patients with PTLD, regardless of the EBV genome expression status, and preclinical studies have demonstrated efficacy of MTIs in PTLD.(El-Salem, et al 2007, Majewski, et al 2003) Accordingly, a number of investigators have changed immunosuppression to MTIs in PTLD patients (sirolimus and everolimus) with improvement in PTLD and, in some cases, documented complete responses.(Boratynska and Smolska 2008, Pascual 2007)
Multiple myeloma is a plasma cell malignancy characterized by monoclonal proliferation of B cells producing a single immunoglobulin and affecting bone marrow and osseous bone.(Kyle and Rajkumar 2008) Treatment has improved over the past decade with the introduction of thalidomide (and thalidomide analogs) and bortezomib; however, even with these agents, aggressive chemotherapy, and stem cell transplant, median overall survival is 2 years in patients with high-risk disease.(Kyle and Rajkumar 2008) The PI3K/AKT/mTOR pathway is altered frequently in multiple myeloma with constitutive activation of AKT and loss of PTEN function.(Pene, et al 2002, Shi, et al 2002) AKT and PI3K inhibitors have profound effects against multiple myeloma in preclinical models; however, mTOR inhibitors were found to have lesser effects, demonstrating efficacy in cells with loss of PTEN function but showing significantly less activity in cells with normal PTEN function.(Hideshima, et al 2006, Pene, et al 2002, Shi, et al 2002) In a xenograft model of multiple myeloma, temsirolimus demonstrated efficiacy. In other studies, sirolimus was found to sensitize multiple myeloma cells to dexamethasone, and mTOR inhibitors were found to synergize with other targeted agents, including sunitinib, a HSP90 inhibitor, and lenalidomide.(Francis, et al 2006, Frost, et al 2004, Ikezoe, et al 2006, Raje, et al 2004, Yan, et al 2006) Clinical trials in multiple myeloma are ongoing (Table 1).
Non-malignant haematologic disorders
In addition to the recent interest of MTIs in haematologic malignancies, targeting mTOR signaling has been studied in non-malignant haematologic disorders, particularly autoimmune disorders, for two reasons: (1) MTIs can cause apoptosis in abnormal lymphocytes, whereas many immunosuppressive agents only inhibit growth; and (2) mTOR inhibitors increase peripheral blood regulatory T cells (Tregs). Tregs are a T cell population that suppresses the immune system. Tregs are frequently decreased in autoimmune diseases and increasing Tregs may improve autoimmune disorders.(Brusko, et al 2008) We have studied the activity of sirolimus in a rare pediatric haematologic disorder, Autoimmune Lymphoproliferative Syndrome (ALPS). ALPS is a disorder of disrupted lymphocyte homeostasis caused by defective fas-mediated apoptosis, leading to chronic lymphoproliferation, systemic autoimmunity, and a propensity to develop secondary cancers.(Bleesing, et al 2000) The most common autoimmune manifestation is autoimmune cytopenias and many patients have severe disease. We hypothesized targeting mTOR would be effective in ALPS through inducing apoptosis in the abnormal lymphocytes found in the disease and/or by increasing Tregs. We found marked improvement in lymphoproliferation and autoimmunity when we treated murine models of ALPS.(Teachey, et al 2006a) Based on those results, we have treated 6 refractory ALPS patients with sirolimus and found profound improvement in all patients with complete responses in the majority of patients.(Teachey, et al 2009) We have an open clinical trial for ALPS patients and plan to expand the trial to include patients with chronic severe and/or refractory autoimmune cytopenias either as an idiopathic condition (immune thrombocytopenic purpura, autoimmune hemolytic anemia, autoimmune neutropenia, or Evans syndrome) or as a consequence of a syndrome (systemic lupus erythematosis, rheumatoid arthritis, or inflammatory bowel disease).
Sirolimus has also been used as a immunosuppressive salvage regimen in patients who develop transplant associated microangiopathy or autoimmune cytopenias (Jubelirer, et al. in preparation, (Yango, et al 2002) Finally, mTOR inhibitors are under investigation in preclinical models and/or clinical trials in aplastic anemia and for haematologic manifestations of systemic lupus erythematosis.(Ramos-Barron, et al 2007, Tisdale, et al 2000)
Conclusion
Interest in mTOR inhibitors has grown considerably over the recent years as the important role of mTOR signal transduction in cell growth and proliferation has become better elucidated. As a class, mTOR inhibitors are safe and well-tolerated. Because aberrant activation of the AKT/PI3K/mTOR signaling network is a common finding in many haematologic and non-malignant diseases, mTOR inhibitors have the potential to be efficacious in a variety of disorders. To date, mTOR inhibitors have been demonstrated substantial activity against abnormal haematopoietic cells of multiple lineages. Nevertheless, as MTIs are unlikely to be curative as single agents in many malignancies, elucidating the most effective way of combining MTIs with conventional cytotoxic agents and new targeted therapies is imperative in order to improve cure rates in these difficult-to-treat diseases.
Acknowledgments
Support: Supported by a Larry and Helen Hoag Foundation Clinical Translational Research Career Development Award, ASCO Young Investigator and Career Development Awards, and the Leukemia and Lymphoma Society (DTT); NIH 1 K08 CA104882-01A1, grant # IRG-78-002-30 from the American Cancer Society, the Children's Cancer Fund, the Florence R.C. Murray Program at the Children's Hospital of Philadelphia and WW Smith Charitable Trust (VIB); and NIH CA102646, CA1116660, ACS RSG0507101, and the Weinberg Fund of the Children's Hospital of Philadelphia (SAG).
References
- Ansell SM, Inwards DJ, Rowland KM, Jr., Flynn PJ, Morton RF, Moore DF, Jr., Kaufmann SH, Ghobrial I, Kurtin PJ, Maurer M, Allmer C, Witzig TE. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnaghi L, Calastretti A, Bevilacqua A, D'Agnano I, Gatti G, Canti G, Delia D, Capaccioli S, Nicolin A. Bcl-2 phosphorylation and apoptosis activated by damaged microtubules require mTOR and are regulated by Akt. Oncogene. 2004 doi: 10.1038/sj.onc.1207698. [DOI] [PubMed] [Google Scholar]
- Avellino R, Romano S, Parasole R, Bisogni R, Lamberti A, Poggi V, Venuta S, Romano MF. Rapamycin stimulates apoptosis of childhood acute lymphoblastic leukemia cells. Blood. 2005;106:1400–1406. doi: 10.1182/blood-2005-03-0929. [DOI] [PubMed] [Google Scholar]
- Baldo P, Cecco S, Giacomin E, Lazzarini R, Ros B, Marastoni S. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr Cancer Drug Targets. 2008;8:647–665. doi: 10.2174/156800908786733513. [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Bleesing JJ, Straus SE, Fleisher TA. Autoimmune lymphoproliferative syndrome. A human disorder of abnormal lymphocyte survival. Pediatr Clin North Am. 2000;47:1291–1310. doi: 10.1016/s0031-3955(05)70272-8. [DOI] [PubMed] [Google Scholar]
- Boratynska M, Smolska D. Inhibition of mTOR by sirolimus induces remission of post-transplant lymphoproliferative disorders. Transpl Int. 2008;21:605–608. doi: 10.1111/j.1432-2277.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Breslin EM, White PC, Shore AM, Clement M, Brennan P. LY294002 and rapamycin co-operate to inhibit T-cell proliferation. Br J Pharmacol. 2005;144:791–800. doi: 10.1038/sj.bjp.0706061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VI, Fang J, Alcorn K, Barr R, Kim JM, Wasserman R, Grupp SA. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc Natl Acad Sci U S A. 2003;100:15113–15118. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VI, Hulitt J, Fish J, Sheen C, Bruno M, Xu Q, Carroll M, Fang J, Teachey D, Grupp SA. Thymic stromal-derived lymphopoietin induces proliferation of pre-B leukemia and antagonizes mTOR inhibitors, suggesting a role for interleukin-7Ralpha signaling. Cancer Res. 2007;67:9963–9970. doi: 10.1158/0008-5472.CAN-06-4704. [DOI] [PubMed] [Google Scholar]
- Brown VI, Seif AE, Reid GS, Teachey DT, Grupp SA. Novel molecular and cellular therapeutic targets in acute lymphoblastic leukemia and lymphoproliferative disease. Immunol Res. 2008;42:84–105. doi: 10.1007/s12026-008-8038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- Bruyn GA, Tate G, Caeiro F, Maldonado-Cocco J, Westhovens R, Tannenbaum H, Bell M, Forre O, Bjorneboe O, Tak PP, Abeywickrama KH, Bernhardt P, van Riel PL. Everolimus in patients with rheumatoid arthritis receiving concomitant methotrexate: a 3-month, double-blind, randomised, placebo-controlled, parallel-group, proof-of-concept study. Ann Rheum Dis. 2008;67:1090–1095. doi: 10.1136/ard.2007.078808. [DOI] [PubMed] [Google Scholar]
- Calastretti A, Rancati F, Ceriani MC, Asnaghi L, Canti G, Nicolin A. Rapamycin increases the cellular concentration of the BCL-2 protein and exerts an anti-apoptotic effect. Eur J Cancer. 2001;37:2121–2128. doi: 10.1016/s0959-8049(01)00256-8. [DOI] [PubMed] [Google Scholar]
- Chumsri S, Zhao M, Garofalo M, Burger A, Hamburger A, Zhao F, Rapoport A. Inhibition of the mammalian target of rapamycin (mTOR) in a case of refractory primary cutaneous anaplastic large cell lymphoma. Leuk Lymphoma. 2008;49:359–361. doi: 10.1080/10428190701809214. [DOI] [PubMed] [Google Scholar]
- Crazzolara R, Cisterne A, Thien M, Hewson J, Bradstock K, Bendall L. The mTOR Inhibitor RAD001 (Everolimus) Improves Survival in Preclinical Models of Primary Human ALL. Blood (ASH Annual Meeting Abstracts) 2007;110 [Google Scholar]
- Decker T, Hipp S, Ringshausen I, Bogner C, Oelsner M, Schneller F, Peschel C. Rapamycin-induced G1 arrest in cycling B-CLL cells is associated with reduced expression of cyclin D3, cyclin E, cyclin A, and survivin. Blood. 2003;101:278–285. doi: 10.1182/blood-2002-01-0189. [DOI] [PubMed] [Google Scholar]
- Decker T, Sandherr M, Goetze K, Oelsner M, Ringshausen I, Peschel C. A pilot trial of the mTOR (mammalian target of rapamycin) inhibitor RAD001 in patients with advanced B-CLL. Ann Hematol. 2008 doi: 10.1007/s00277-008-0582-9. [DOI] [PubMed] [Google Scholar]
- Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371:1017–1029. doi: 10.1016/S0140-6736(08)60456-0. [DOI] [PubMed] [Google Scholar]
- Dunn C, Croom KF. Everolimus: a review of its use in renal and cardiac transplantation. Drugs. 2006;66:547–570. doi: 10.2165/00003495-200666040-00009. [DOI] [PubMed] [Google Scholar]
- Dutton A, Reynolds GM, Dawson CW, Young LS, Murray PG. Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin's lymphoma cells through a mechanism involving Akt kinase and mTOR. J Pathol. 2005;205:498–506. doi: 10.1002/path.1725. [DOI] [PubMed] [Google Scholar]
- El-Salem M, Raghunath PN, Marzec M, Wlodarski P, Tsai D, Hsi E, Wasik MA. Constitutive activation of mTOR signaling pathway in post-transplant lymphoproliferative disorders. Lab Invest. 2007;87:29–39. doi: 10.1038/labinvest.3700494. [DOI] [PubMed] [Google Scholar]
- Ewen ME, Sluss HK, Sherr CJ, Matsushime H, Kato J, Livingston DM. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- Follo MY, Mongiorgi S, Bosi C, Cappellini A, Finelli C, Chiarini F, Papa V, Libra M, Martinelli G, Cocco L, Martelli AM. The Akt/mammalian target of rapamycin signal transduction pathway is activated in high-risk myelodysplastic syndromes and influences cell survival and proliferation. Cancer Res. 2007;67:4287–4294. doi: 10.1158/0008-5472.CAN-06-4409. [DOI] [PubMed] [Google Scholar]
- Francis LK, Alsayed Y, Leleu X, Jia X, Singha UK, Anderson J, Timm M, Ngo H, Lu G, Huston A, Ehrlich LA, Dimmock E, Lentzsch S, Hideshima T, Roodman GD, Anderson KC, Ghobrial IM. Combination mammalian target of rapamycin inhibitor rapamycin and HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin has synergistic activity in multiple myeloma. Clin Cancer Res. 2006;12:6826–6835. doi: 10.1158/1078-0432.CCR-06-1331. [DOI] [PubMed] [Google Scholar]
- Frost P, Moatamed F, Hoang B, Shi Y, Gera J, Yan H, Gibbons J, Lichtenstein A. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood. 2004;104:4181–4187. doi: 10.1182/blood-2004-03-1153. [DOI] [PubMed] [Google Scholar]
- Gera JF, Mellinghoff IK, Shi Y, Rettig MB, Tran C, Hsu JH, Sawyers CL, Lichtenstein AK. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279:2737–2746. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- Gora-Tybor J, Robak T. Targeted drugs in chronic myeloid leukemia. Curr Med Chem. 2008;15:3036–3051. doi: 10.2174/092986708786848578. [DOI] [PubMed] [Google Scholar]
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- Gu L, Gao J, Li Q, Zhu YP, Jia CS, Fu RY, Chen Y, Liao QK, Ma Z. Rapamycin reverses NPM-ALK-induced glucocorticoid resistance in lymphoid tumor cells by inhibiting mTOR signaling pathway, enhancing G1 cell cycle arrest and apoptosis. Leukemia. 2008;22:2091–2096. doi: 10.1038/leu.2008.204. [DOI] [PubMed] [Google Scholar]
- Haarman EG, Kaspers GJ, Pieters R, Rottier MM, Veerman AJ. Circumvention of glucocorticoid resistance in childhood leukemia. Leuk Res. 2008;32:1417–1423. doi: 10.1016/j.leukres.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Haritunians T, Mori A, O'Kelly J, Luong QT, Giles FJ, Koeffler HP. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2007;21:333–339. doi: 10.1038/sj.leu.2404471. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hartmann EM, Ott G, Rosenwald A. Molecular biology and genetics of lymphomas. Hematol Oncol Clin North Am. 2008;22:807–823. doi: 10.1016/j.hoc.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson PG, Anderson KC. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp S, Ringshausen I, Oelsner M, Bogner C, Peschel C, Decker T. Inhibition of the mammalian target of rapamycin and the induction of cell cycle arrest in mantle cell lymphoma cells. Haematologica. 2005;90:1433–1434. [PubMed] [Google Scholar]
- Hirase C, Maeda Y, Takai S, Kanamaru A. Hypersensitivity of Ph-positive lymphoid cell lines to rapamycin: Possible clinical application of mTOR inhibitor. Leuk Res. 2008 doi: 10.1016/j.leukres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Morton CL, Kolb EA, Gorlick R, Lock R, Carol H, Reynolds CP, Maris JM, Keir ST, Billups CA, Smith MA. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- Ikezoe T, Nishioka C, Tasaka T, Yang Y, Komatsu N, Togitani K, Koeffler HP, Taguchi H. The antitumor effects of sunitinib (formerly SU11248) against a variety of human hematologic malignancies: enhancement of growth inhibition via inhibition of mammalian target of rapamycin signaling. Mol Cancer Ther. 2006;5:2522–2530. doi: 10.1158/1535-7163.MCT-06-0071. [DOI] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Jundt F, Raetzel N, Muller C, Calkhoven CF, Kley K, Mathas S, Lietz A, Leutz A, Dorken B. A rapamycin derivative (everolimus) controls proliferation through down-regulation of truncated CCAAT enhancer binding protein {beta} and NF-{kappa}B activity in Hodgkin and anaplastic large cell lymphomas. Blood. 2005;106:1801–1807. doi: 10.1182/blood-2004-11-4513. [DOI] [PubMed] [Google Scholar]
- Kharas MG, Deane JA, Wong S, O'Bosky KR, Rosenberg N, Witte ON, Fruman DA. Phosphoinositide 3-kinase signaling is essential for ABL oncogene-mediated transformation of B-lineage cells. Blood. 2004;103:4268–4275. doi: 10.1182/blood-2003-07-2193. [DOI] [PubMed] [Google Scholar]
- Kharas MG, Janes MR, Scarfone VM, Lilly MB, Knight ZA, Shokat KM, Fruman DA. Ablation of PI3K blocks BCR-ABL leukemogenesis in mice, and a dual PI3K/mTOR inhibitor prevents expansion of human BCR-ABL+ leukemia cells. J Clin Invest. 2008;118:3038–3050. doi: 10.1172/JCI33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, Salgia R, Podar K, Griffin JD, Sattler M. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leseux L, Laurent G, Laurent C, Rigo M, Blanc A, Olive D, Bezombes C. PKC zeta mTOR pathway: a new target for rituximab therapy in follicular lymphoma. Blood. 2008;111:285–291. doi: 10.1182/blood-2007-04-085092. [DOI] [PubMed] [Google Scholar]
- Levy DS, Kahana JA, Kumar R. AKT inhibitor, GSK690693, induces growth inhibition and apoptosis in acute lymphoblastic leukemia cell lines. Blood. 2008 doi: 10.1182/blood-2008-02-137737. [DOI] [PubMed] [Google Scholar]
- Lewin KJ. Post-transplant lymphoproliferative disorders. Pathol Oncol Res. 1997;3:177–182. doi: 10.1007/BF02899918. [DOI] [PubMed] [Google Scholar]
- Liem NL, Papa RA, Milross CG, Schmid MA, Tajbakhsh M, Choi S, Ramirez CD, Rice AM, Haber M, Norris MD, MacKenzie KL, Lock RB. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103:3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- Lin TS. Novel agents in chronic lymphocytic leukemia: efficacy and tolerability of new therapies. Clin Lymphoma Myeloma. 2008;8(Suppl 4):S137–143. doi: 10.3816/CLM.2008.s.009. [DOI] [PubMed] [Google Scholar]
- Luger S, Perl AE, Kemner A, Stadtmauer E, Porter D, Schuster SJ, Goldstein S, Tsai D, Loren N, Dierov J, Vogl D, Andreadis C, Emerson SG, Carroll M. A phase 1 dose escalation study of the mTOR inhibitor sirolimus and MEC chemotherapy targeting signal transduction in leukemic stem cells for acute myeloid leukemia. Blood (ASH Annual Meeting Abstracts) 2006;108 [Google Scholar]
- Ly C, Arechiga AF, Melo JV, Walsh CM, Ong ST. Bcr-Abl kinase modulates the translation regulators ribosomal protein S6 and 4E-BP1 in chronic myelogenous leukemia cells via the mammalian target of rapamycin. Cancer Res. 2003;63:5716–5722. [PubMed] [Google Scholar]
- Majewski M, Korecka M, Joergensen J, Fields L, Kossev P, Schuler W, Shaw L, Wasik MA. Immunosuppressive TOR kinase inhibitor everolimus (RAD) suppresses growth of cells derived from posttransplant lymphoproliferative disorder at allograft-protecting doses. Transplantation. 2003;75:1710–1717. doi: 10.1097/01.TP.0000063934.89714.19. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Mayerhofer M, Aichberger KJ, Florian S, Krauth MT, Hauswirth AW, Derdak S, Sperr WR, Esterbauer H, Wagner O, Marosi C, Pickl WF, Deininger M, Weisberg E, Druker BJ, Griffin JD, Sillaber C, Valent P. Identification of mTOR as a novel bifunctional target in chronic myeloid leukemia: dissection of growth-inhibitory and VEGF-suppressive effects of rapamycin in leukemic cells. FASEB J. 2005;19:960–962. doi: 10.1096/fj.04-1973fje. [DOI] [PubMed] [Google Scholar]
- Mills GB, Lu Y, Kohn EC. Linking molecular therapeutics to molecular diagnostics: inhibition of the FRAP/RAFT/TOR component of the PI3K pathway preferentially blocks PTEN mutant cells in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:10031–10033. doi: 10.1073/pnas.191379498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohi MG, Boulton C, Gu TL, Sternberg DW, Neuberg D, Griffin JD, Gilliland DG, Neel BG. Combination of rapamycin and protein tyrosine kinase (PTK) inhibitors for the treatment of leukemias caused by oncogenic PTKs. Proc Natl Acad Sci U S A. 2004;101:3130–3135. doi: 10.1073/pnas.0400063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H, Sawyers CL. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka C, Ikezoe T, Yang J, Koeffler HP, Yokoyama A. Blockade of mTOR signaling potentiates the ability of histone deacetylase inhibitor to induce growth arrest and differentiation of acute myelogenous leukemia cells. Leukemia. 2008;22:2159–2168. doi: 10.1038/leu.2008.243. [DOI] [PubMed] [Google Scholar]
- Papa V, Tazzari PL, Chiarini F, Cappellini A, Ricci F, Billi AM, Evangelisti C, Ottaviani E, Martinelli G, Testoni N, McCubrey JA, Martelli AM. Proapoptotic activity and chemosensitizing effect of the novel Akt inhibitor perifosine in acute myelogenous leukemia cells. Leukemia. 2008;22:147–160. doi: 10.1038/sj.leu.2404980. [DOI] [PubMed] [Google Scholar]
- Park S, Chapuis N, Bardet V, Tamburini J, Gallay N, Willems L, Knight ZA, Shokat KM, Azar N, Viguie F, Ifrah N, Dreyfus F, Mayeux P, Lacombe C, Bouscary D. PI-103, a dual inhibitor of Class IA phosphatidylinositide 3-kinase and mTOR, has antileukemic activity in AML. Leukemia. 2008;22:1698–1706. doi: 10.1038/leu.2008.144. [DOI] [PubMed] [Google Scholar]
- Pascual J. Post-transplant lymphoproliferative disorder--the potential of proliferation signal inhibitors. Nephrol Dial Transplant. 2007;22(Suppl 1):i27–35. doi: 10.1093/ndt/gfm088. [DOI] [PubMed] [Google Scholar]
- Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F, Lacombe C, Bouscary D. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–6597. doi: 10.1038/sj.onc.1205923. [DOI] [PubMed] [Google Scholar]
- Peponi E, Drakos E, Reyes G, Leventaki V, Rassidakis GZ, Medeiros LJ. Activation of mammalian target of rapamycin signaling promotes cell cycle progression and protects cells from apoptosis in mantle cell lymphoma. Am J Pathol. 2006;169:2171–2180. doi: 10.2353/ajpath.2006.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl AE, Carroll M. Exploiting signal transduction pathways in acute myelogenous leukemia. Curr Treat Options Oncol. 2007;8:265–276. doi: 10.1007/s11864-007-0043-z. [DOI] [PubMed] [Google Scholar]
- Piccaluga PP, Paolini S, Martinelli G. Tyrosine kinase inhibitors for the treatment of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Cancer. 2007;110:1178–1186. doi: 10.1002/cncr.22881. [DOI] [PubMed] [Google Scholar]
- Plasschaert SL, Kamps WA, Vellenga E, de Vries EG, de Bont ES. Prognosis in childhood and adult acute lymphoblastic leukaemia: a question of maturation? Cancer Treat Rev. 2004;30:37–51. doi: 10.1016/S0305-7372(03)00140-3. [DOI] [PubMed] [Google Scholar]
- Pulsipher M, Wall D, Goyal R, Grupp SA, Bunin N. Sirolimus (SRL)-Based GVHD Prophylaxis after TBI/TT/Cy Allogeneic HSCT in Pediatric Patients with HR ALL: Results of a Multi-Insitutional Pilot Study. Biol. Blood Marrow Transplant 2008 ASBMT Annual Meeting Abstracts. 2008;14:28. [Google Scholar]
- Raje N, Kumar S, Hideshima T, Ishitsuka K, Chauhan D, Mitsiades C, Podar K, Le Gouill S, Richardson P, Munshi NC, Stirling DI, Antin JH, Anderson KC. Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood. 2004;104:4188–4193. doi: 10.1182/blood-2004-06-2281. [DOI] [PubMed] [Google Scholar]
- Ramos-Barron A, Pinera-Haces C, Gomez-Alamillo C, Santiuste-Torcida I, Ruiz JC, Buelta-Carrillo L, Merino R, de Francisco AL, Arias M. Prevention of murine lupus disease in (NZBxNZW)F1 mice by sirolimus treatment. Lupus. 2007;16:775–781. doi: 10.1177/0961203307081401. [DOI] [PubMed] [Google Scholar]
- Recher C, Dos Santos C, Demur C, Payrastre B. mTOR, a new therapeutic target in acute myeloid leukemia. Cell Cycle. 2005;4:1540–1549. doi: 10.4161/cc.4.11.2159. [DOI] [PubMed] [Google Scholar]
- Rheingold S, Sacks N, Chang YJ, Brown VI, Teachey DT, Lange B, Grupp SA. A phase I trial of sirolimus (rapamycin) in pediatric patients with relapsed/refractory leukemia. Blood (ASH Annual Meeting Abstracts) 2007;110 [Google Scholar]
- Ringshausen I, Peschel C, Decker T. Mammalian target of rapamycin (mTOR) inhibition in chronic lymphocytic B-cell leukemia: a new therapeutic option. Leuk Lymphoma. 2005;46:11–19. doi: 10.1080/10428190400005353. [DOI] [PubMed] [Google Scholar]
- Ringshausen I, Schneller F, Bogner C, Hipp S, Duyster J, Peschel C, Decker T. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100:3741–3748. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- Rizzieri DA, Feldman E, Dipersio JF, Gabrail N, Stock W, Strair R, Rivera VM, Albitar M, Bedrosian CL, Giles FJ. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2008;14:2756–2762. doi: 10.1158/1078-0432.CCR-07-1372. [DOI] [PubMed] [Google Scholar]
- Rosner M, Hengstschlager M. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet. 2008;17:2934–2948. doi: 10.1093/hmg/ddn192. [DOI] [PubMed] [Google Scholar]
- Rudelius M, Pittaluga S, Nishizuka S, Pham TH, Fend F, Jaffe ES, Quintanilla-Martinez L, Raffeld M. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006;108:1668–1676. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Buchner T, Dohner H, Burnett AK, Lo-Coco F. Guidelines on the management of acute promyelocytic leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2008 doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- Saydam G, H C, P C, Bertino JR, Erickan-Abali E. mTOR inhibition leads to increased sensitivity to methotrexate. AACR 98th Annual Meeting; 2005. Abstract No. 3303. [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Dreyling M. Therapy of mantle cell lymphoma: current standards and future strategies. Hematol Oncol Clin North Am. 2008;22:953–963. doi: 10.1016/j.hoc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Shi Y, Gera J, Hu L, Hsu JH, Bookstein R, Li W, Lichtenstein A. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027–5034. [PubMed] [Google Scholar]
- Sillaber C, Mayerhofer M, Bohm A, Vales A, Gruze A, Aichberger KJ, Esterbauer H, Pfeilstocker M, Sperr WR, Pickl WF, Haas OA, Valent P. Evaluation of antileukaemic effects of rapamycin in patients with imatinib-resistant chronic myeloid leukaemia. Eur J Clin Invest. 2008;38:43–52. doi: 10.1111/j.1365-2362.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- Smith MS, Pro B, Cisneros A, Smith S, Stiff P, Lester E, Modi S, Dancey JE, Vokes EE, van Besian K. Activity of single agent temsirolimus (CCI-779) in non-mantle cell non-Hodgkin lymphoma subtypes. J Clin Oncol (ASCO Annual Meeting) 2008;26(suppl 8514) [Google Scholar]
- Teachey DT, Greiner R, Seif AE, Attiyeh EF, Bleesing JJ, Choi JK, Manno CS, Rappaport E, Schwabe D, Sheen C, Sullivan KE, Zhaung H, Wechsler DS, Grupp SA. Treatment with sirolimus results in complete responses in patients with Autoimmune Lymphoproliferative Syndrome. Br J Haematol. 2009 doi: 10.1111/j.1365-2141.2009.07595.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Obzut DA, Axsom K, Choi JK, Goldsmith KC, Hall J, Hulitt J, Manno CS, Maris JM, Rhodin N, Sullivan KE, Brown VI, Grupp SA. Rapamycin improves lymphoproliferative disease in murine autoimmune lymphoproliferative syndrome (ALPS) Blood. 2006a;108:1965–1971. doi: 10.1182/blood-2006-01-010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Obzut DA, Cooperman J, Fang J, Carroll M, Choi JK, Houghton PJ, Brown VI, Grupp SA. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood. 2006b;107:1149–1155. doi: 10.1182/blood-2005-05-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Sheen C, Hall J, Ryan T, Brown VI, Fish J, Reid GS, Seif AE, Norris R, Chang YJ, Carroll M, Grupp SA. mTOR inhibitors are synergistic with methotrexate: an effective combination to treat acute lymphoblastic leukemia. Blood. 2008;112:2020–2023. doi: 10.1182/blood-2008-02-137141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale JF, Dunn DE, Maciejewski J. Cyclophosphamide and other new agents for the treatment of severe aplastic anemia. Semin Hematol. 2000;37:102–109. doi: 10.1016/s0037-1963(00)90034-9. [DOI] [PubMed] [Google Scholar]
- Vega F, Medeiros LJ, Leventaki V, Atwell C, Cho-Vega JH, Tian L, Claret FX, Rassidakis GZ. Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res. 2006;66:6589–6597. doi: 10.1158/0008-5472.CAN-05-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner K, Hipp S, Oelsner M, Ringshausen I, Bogner C, Peschel C, Decker T. Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br J Haematol. 2006;134:475–484. doi: 10.1111/j.1365-2141.2006.06210.x. [DOI] [PubMed] [Google Scholar]
- Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, Opferman JT, Sallan SE, den Boer ML, Pieters R, Golub TR, Armstrong SA. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–342. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005a;106:4261–4268. doi: 10.1182/blood-2004-11-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RH, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P. Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia. 2005b;19:2153–2158. doi: 10.1038/sj.leu.2403968. [DOI] [PubMed] [Google Scholar]
- Yan H, Frost P, Shi Y, Hoang B, Sharma S, Fisher M, Gera J, Lichtenstein A. Mechanism by which mammalian target of rapamycin inhibitors sensitize multiple myeloma cells to dexamethasone-induced apoptosis. Cancer Res. 2006;66:2305–2313. doi: 10.1158/0008-5472.CAN-05-2447. [DOI] [PubMed] [Google Scholar]
- Yango A, Morrissey P, Monaco A, Butera J, Gohh RY. Successful treatment of tacrolimus-associated thrombotic microangiopathy with sirolimus conversion and plasma exchange. Clin Nephrol. 2002;58:77–78. doi: 10.5414/cnp58077. [DOI] [PubMed] [Google Scholar]
- Yee KW, Zeng Z, Konopleva M, Verstovsek S, Ravandi F, Ferrajoli A, Thomas D, Wierda W, Apostolidou E, Albitar M, O'Brien S, Andreeff M, Giles FJ. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- Younes A. Therapeutic activity of mTOR inhibitors in mantle cell lymphoma: clues but no clear answers. Autophagy. 2008;4:707–709. doi: 10.4161/auto.6232. [DOI] [PubMed] [Google Scholar]
- Zanesi N, Aqeilan R, Drusco A, Kaou M, Sevignani C, Costinean S, Bortesi L, La Rocca G, Koldovsky P, Volinia S, Mancini R, Calin G, Scott CP, Pekarsky Y, Croce CM. Effect of rapamycin on mouse chronic lymphocytic leukemia and the development of nonhematopoietic malignancies in Emu-TCL1 transgenic mice. Cancer Res. 2006;66:915–920. doi: 10.1158/0008-5472.CAN-05-3426. [DOI] [PubMed] [Google Scholar]
- Zeng X, Kinsella TJ. Mammalian target of rapamycin and S6 kinase 1 positively regulate 6-thioguanine-induced autophagy. Cancer Res. 2008;68:2384–2390. doi: 10.1158/0008-5472.CAN-07-6163. [DOI] [PubMed] [Google Scholar]