Abstract
Background
Low levels of plasma adiponectin, an adipocytokine that possesses anti-inflammatory and anti-atherogenic properties, frequently observed among obese subjects correlate with higher prevalence of several cardiovascular diseases. This study investigated whether adiponectin modulates allograft rejection in MHC class II—mismatched cardiac transplants.
Methods
We heterotopically transplanted Bm12 allografts into adiponectin-deficient (APN−/−, C57BL/6 background) or wild-type (APN+/+) mice. Some APN−/− mice received adiponectin reconstitution by adenovirus. Histological analyses assessed allograft rejection, and real-time RT-PCR evaluated the genes for cytokines/chemokines associated with the immune and inflammatory responses. In addition, we tested the effect of adiponectin on proliferation and cytokine/chemokine production in mouse T lymphocytes stimulated in vitro with anti-CD3 antibodies.
Results
Allografts transplanted to APN−/− mice showed severe acute rejection relative to transplants in APN+/+ hosts accompanied by increased accumulation of CD4- and CD8-positive T lymphocytes and Mac3-positive macrophages. Adiponectin provision by adenovirus in APN−/− mice reversed these exacerbated responses to allografting. The rejected allografts in APN−/− mice contained significantly higher levels of TNFα, IFNγ, and RANTES. Moreover, adiponectin significantly suppressed proliferation and production of TNFα, IFNγ, RANTES, MCP-1, and IP-10 in mouse T lymphocytes stimulated in vitro with anti-CD3 antibodies.
Conclusions
These observations provide new mechanistic insight into immunoregulation in allograft recipients relative to obesity, an increasingly prevalent risk factor. Adiponectin may offer a new therapeutic target for allograft rejection after cardiac transplantation.
Keywords: Adiponectin, Cardiac transplantation, Rejection
Obesity, a condition associated with chronic systemic inflammation, constitutes a well-known risk factor for cardiovascular disease (1) (2). Grady et al. recently reported that postoperative obesity also increases the risk for morbidity and mortality after cardiac transplantation (3). However, the mechanism(s) that link obesity and rejection after heart transplantation remains unclear.
Adipose tissue secretes adipocytokines whose dysregulation associates closely with a variety of pathophysiologic abnormalities. In particular, obese individuals have low plasma levels of adiponectin, an anti-inflammatory and anti-atherogenic adipocytokine (4). Adiponectin appears to mitigate atherosclerosis, ischemic myocardial damage, and cardiac hypertrophy (5-7). No prior study has examined the effect of adiponectin in the setting of cardiac transplantation. This study therefore examined the effect of adiponectin on cardiac allograft rejection in vivo and in vitro.
Materials and Methods
Mouse Cardiac Transplantation
B6.C-H2<bm12>KhEg (bm12; H-2bm12, Jackson Laboratory, Bar Harbor, ME) donor hearts were transplanted heterotopically into B6 background adiponectin-deficient (APN−/−) (8) or littermate wild-type (APN+/+) mice (MHC class II mismatch) (9). For adiponectin reconstitution experiments, we injected adenovirus-expressing mouse adiponectin (Ad-APN) or β-galactosidase (Ad-βgal) into APN−/− mice through the tail veins 3 days before transplantation (2.0 ×108 plaque-forming unit / mouse). Plasma adiponectin levels were measured with mouse/Rat Adiponectin ELISA Kit (B-Bridge International, Mountain View, CA). Grafts were harvested 3 weeks (for gene analyses with quantitative RT-PCR) or 4 weeks (for histological analyses) after transplantation. Protocols for animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Harvard Medical School.
Histological Analyses
Hematoxylin-and-eosin and elastic fiber staining of paraffin-embedded tissue section were performed. Blinded observers scored parenchymal rejection (PR, 0 to 4, based on extent of inflammatory infiltrate and associated myocyte injury)(10). To quantify T lymphocytes and macrophages, cryosections were immunostained with antibodies against CD4, CD8, or Mac-3 (BD Biosciences, San Jose, CA) (11). Eight randomly chosen microscopic fields were examined to assess infiltrated CD4- or CD8-positive cells, as well as Mac-3 positive area (11).
Gene Expression Analysis by Quantitative RT-PCR
Total RNA isolated from allografts was reverse-transcribed, and real-time quantitative PCR with cDNA was performed (4). mRNA levels were quantified and expressed relative to GAPDH as an internal control. The Supplementary Table lists the sequence of primers used.
Splenocyte Proliferation and Proinflammatory Cytokine Production
For splenocyte proliferation assays, 2.0 × 105 naive splenocytes were treated with various concentrations of mouse recombinant adiponectin (BioVendor, Candler, NC) for 24 hours, washed, then stimulated in 96-well plates precoated with anti-CD3 mAb (1 μg/ml, BD Biosciences). Cells were cultured at 37°C in a 5% CO2 incubator for 4 days. After 4 days of stimulation (day 4), a portion of the culture supernatants was removed, and assayed for mouse inflammatory cytokine using a cytometric bead array kit (BD Biosciences) as well as ELISA kits for mouse RANTES and IP-10 (R&D, Minneapolis, MN). Lymphocyte proliferation was monitored by pulsing the wells for 6 h with 1 μCi/well of [3H]thymidine (New England Nuclear, Boston, MA). Proliferation was measured as incorporated radioactivity (cpm) (10).
Statistical Analyses
Data are shown as mean ± SEM. Groups were compared using the Student t-test. Between-group comparison of means was performed by ANOVA, followed by t-test. A value of p<0.05 was regarded as a significant difference.
Results and Discussion
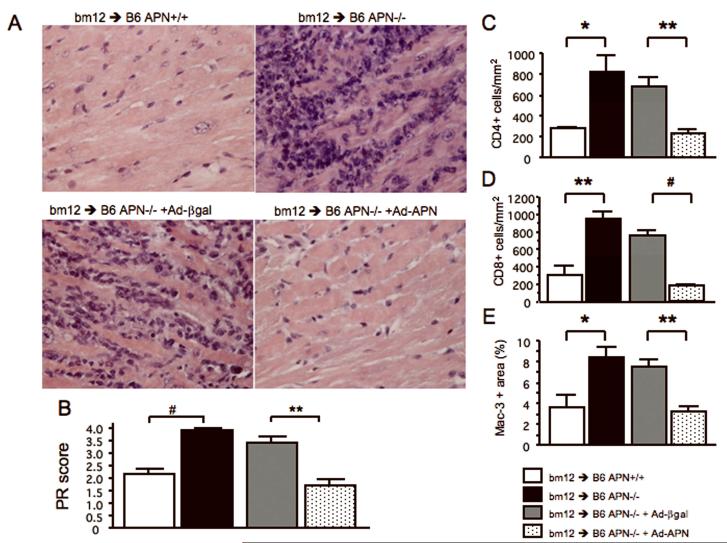
Four weeks after transplantation, palpation and inspection revealed markedly reduced contraction force in allografts transplanted to APN−/− mice. We consequently harvested all the allografts before or at that time point to perform a meaningful comparison of the extent and severity of rejection between control and experimental groups. Allograft histology, even within hours of cessation of contraction, is markedly confounded by a diffuse acute inflammatory response to necrobiotic tissue, and therefore does not provide an accurate assessment of rejection. These qualitative observations suggested marked functional impairment at earlier timepoints in allografts implanted into APN −/− hosts versus control wild-type animals. Even in the contracting APN−/− hosts, markedly increased numbers of leukocytes accumulated in allografts relative to wild-type APN+/+ mice; this was also accompanied by early tissue necrosis and hemorrhage, presumably related to rejection involving the allograft vasculature (Figure 1A and Supplementary Figure 1, upper panels). APN deficiency in recipients markedly exacerbated parenchymal rejection (PR) when compared at the same timepoints in viable beating hearts (APN+/+: 2.15±0.24, APN−/−: 3.92±0.08, p<0.0001, n=10 and 6 respectively, Figure 1B). We also injected adiponectin-expressing adenovirus into APN−/− mice (APN−/− +Ad-APN) to test the hypothesis that provision of adiponectin would reduce PR. When mice were killed, circulating adiponectin levels in APN−/− +Ad-APN were 19.9±2.4 μg/ml (within the physiological range) while those in APN+/+, APN−/− and APN−/− + Ad-β-gal were 16.7±0.5, <0.05, <0.05 μg/ml, respectively. Reconstitution of mouse adiponectin to APN−/− mice substantially ameliorated PR (Figure 1A and Supplementary Figure 1, lower panels) and significantly reduced PR scores (APN−/− + Ad-β-gal: 3.40±0.25 vs. APN−/− +Ad-APN: 1.70±0.26, p<0.01, n=5 each, Figure 1B). These findings demonstrate that adiponectin mitigates parenchymal rejection of allografts. The coronary arteries of allografts in APN−/− recipient mice showed marked lumenal accumulation of leukocytes yielding subtotal occlusion; adiponectin supplementation reversed this abnormality (Supplementary Figure 1B). We do not interpret the pathology as graft arterial disease (GAD) because GAD lesions contain a more fibrous component, consisting of vascular smooth muscle cells and extracellular matrix, and develop at later time points (usually 8-12 weeks after transplantation) in MHC class II—mismatched mice (12). However, this coronary occlusion due to leukostasis may cause myocardial ischemia leading to subsequent graft failure. Moreover, adiponectin may directly protect cardiomyocytes, as previously suggested (7).
Figure 1.
Representative sections of allografts stained with Hematoxylin-Eosin [4 weeks after transplantation with high magnification (A)]. PR (B) in allografts (transplanted to APN+/+, APN−/−, APN−/−+Ad-βgal, and APN−/−+Ad-APN mice; n=10, 6, 5, and 5 respectively) were assessed by blinded pathologic evaluation. Immunostaining for mouse CD4 (C), CD8 (D), and Mac-3 (E) in allografts (transplanted to APN+/+, APN−/−, APN−/−+Ad-βgal, and APN−/−+Ad-APN mice; n=4, 6, 5, and 5 respectively). The numbers of CD4- (C) and CD8- (D) positive cells and Mac-3-positive area are expressed as number of cells per mm2, and the percentage of positively stained areas for Mac-3 (E), respectively. Results are shown as mean ± SEM. *p<0.05, **p<0.01 and #p<0.0001.
Post-transplantation, parenchymal graft rejection involves both adaptive and innate immune responses. During parenchymal rejection, inflammatory cells, including T lymphocytes and macrophages, infiltrate the allografts and produce various cytokines and chemokines. Adiponectin deficiency in the recipient led to significantly greater accumulation of CD4 or CD8 positive T lymphocytes and Mac-3 positive macrophages in the transplanted hearts (CD4: 283±13.8 vs. 828±148.6 cells/mm2, p<0.05; CD8: 296±114.2 vs. 950±83.2 cells/mm2, p<0.01; Mac-3: 3.62±1.19 vs. 8.41±0.95 % p<0.05, n=4 and 6 respectively, Figure 1C-1E). Adiponectin reconstitution by adenovirus significantly reduced infiltration of these cells compared vs. control (CD4: 678±89.0 vs. 233±41.8 cells/mm2, p<0.01; CD8: 756±65.2 vs. 185±14.6 cells/mm2, p<0.0001; Mac-3: 7.52±0.73 vs. 3.21±0.53 %, p<0.01, n=5 each, Figure 1C-1E).
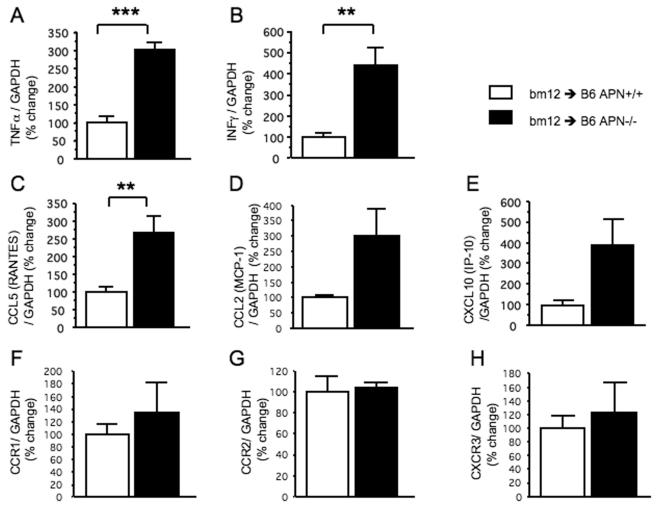
TNFα and IFNγ stimulate inflammation while RANTES and IP-10 recruit T lymphocytes and MCP-1 attracts mononuclear phagocytes. In addition, previous studies suggested the reciprocal association between adiponectin and RANTES or IP-10 and recruitment of T lymphocytes (5, 13). Allografts in APN−/− mice have significantly higher mRNA levels for TNFα(100.0±19.5 vs. 300.7±23.5%, n=5 each, p<0.001, Figure 2A) and IFNγ (100.0±20.3 vs. 441.3±85.6%, n=5 each, p<0.01, Figure 2B) compared with allografts in APN+/+ mice, as determined by real-time quantitative RT-PCR. Furthermore, APN−/− recipients had significantly higher levels of RANTES mRNA (CCL5, 100.0±15.7 vs. 268.5±46.0, n=5 each, p<0.01, Figure 2C), although APN−/− recipients tend to contain more mRNA for MCP-1 (CCL2) and IP-10 (CXCL10) (MCP-1; 100.0±7.6 vs. 301.3.±87.4, n=5 each, p=0.051, Figure 2D: IP-10; 100.0±22.7 vs. 385.9±129.7, n=5 each, p=0.062, Figure 2E). However, no difference emerged in expression of mRNAs that encode CCR1, CCR2, and CXCR3, cognate receptors for RANTES, MCP-1, and IP-10, between allografts in APN+/+ and APN−/− mice (Figure 2F-2H). For this analysis, we used allografts harvested at 3 weeks after transplantation. Allografts in APN−/− recipients harvested at 4 weeks contained diffuse regions of necrosis related to the loss of viability as reflected by loss of contractility (as shown in Figure 1A and Supplementary Figure 1, upper panels); such necrotic specimens did not allow the isolation of high-quality RNA. We therefore decided not to use 4-week samples for real-time quantitative RT-PCR analysis. Moreover, in the pathogenesis of allograft rejection, chemokine expression (e.g., RANTES, MCP-1, and IP-10) would logically precede leukocyte infiltration. Our data indicate that within 3 weeks of transplantation, the presence of recipient adiponectin correlated with suppression of chemokine expression within the allograft, with a consequent diminution of leukocyte infiltration at later timepoints (Figure 1C-1D, and Figure 2F-2H). We interpret these results to suggest that adiponectin can limit allograft rejection by suppressing the expression of local cytokine/chemokine ligands that mediate inflammation and immune cell recruitment.
Figure 2.
Real-time quantitative RT-PCR determined mRNA expression levels for inflammatory cytokines; TNFα (A), IFNγ (B), chemokines; CCL5 (RANTES, C), CCL2 (MCP-1,D), and CXCL10(IP-10, E), and chemokine receptors; CCR1 (F), CCR2 (G), and CXCR3 (H). The mRNA levels encoded by these genes were quantified and expressed after adjusting relative to those of GAPDH as an internal control (n=5 each group). Results are shown as mean ± SEM. **p<0.01, ***p<0.001.
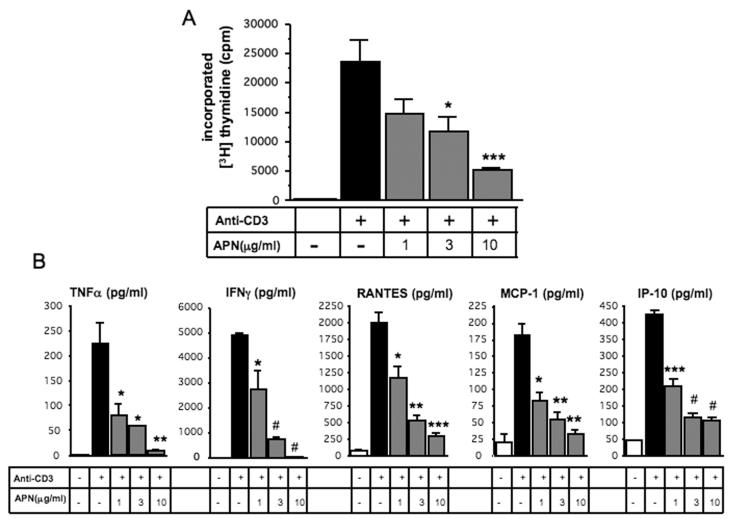
To test the hypothesis that adiponectin directly limits T lymphocyte proliferation and cytokine/chemokine production, we stimulated splenocytes from APN+/+ mice for four days with anti-CD3 antibody, a polyclonal activator of T cells. Adiponectin, within a physiological range, significantly reduced T lymphocyte proliferation in a concentration-dependent manner (by 78.2% at concentration of 10 μg/ml, p<0.001, n=6, Figure 3A). Moreover, based on assay of supernatants from these anti-CD3 stimulated splenocytes, adiponectin significantly suppressed production of cytokines/chemokines such as TNF-α, IFNγ, RANTES, MCP-1, and IP-10 in a concentration-dependent manner (by 96.0, 99.5, 85.2, 81.5, and 83.8%, at concentration of 10 μg/ml vs. anti-CD3 stimulation alone, p<0.01, <0.0001, <0.001, <0.01, and <0.0001, n=3, respectively, Figure 3B). Since reduced lymphocyte apoptosis can also increase accumulation of T lymphocytes in allograft, we assessed T cell apoptosis by TUNEL staining. Histological analysis by double staining of TUNEL and CD4 or CD8 did not demonstrate increased death of T cells (data not shown). These findings suggest that adiponectin inhibits the accumulation of T lymphocytes in MHC class II mismatched cardiac allograft by suppressing T lymphocyte proliferation as well. Since adiponectin circulates in the bloodstream and may modulate immunological reactions in the spleen, the spleen may play a part in the effect of adiponectin on cardiac allograft rejection observed in the present study.
Figure 3.
Splenocytes from C57BL/6 (APN+/+) mice were stimulated for 4 days with anti-CD3 antibody following treatment with the indicated concentrations of mouse adiponectin. Proliferation was evaluated by [3H]thymidine incorporation (A). TNFα, IFNγ, MCP-1, RANTES, and IP-10 were measured in the supernatant of these splenocytes as described in Materials and Methods (B). Results are shown as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 and #p<0.0001.
Taken together, higher plasma adiponectin levels may prove beneficial for outcome among recipients of cardiac transplant. Several drugs such as thiazolidinediones and statins increase plasma levels of adiponectin (14-16). Interestingly, such drugs may also protect against cardiac allograft rejection and transplant vasculopathy (9, 17, 18). Over all, the present study suggests that adiponectin reduces allograft rejection by blocking lymphocyte proliferation and recruitment. These observations provide new mechanistic insight into immunoregulation in allograft recipients in relation to obesity, a risk factor of increasing prevalence. Moreover, adiponectin may represent a new therapeutic target for allograft rejection after cardiac transplantation.
Supplementary Material
Supplementary Figure 1. Representative sections of allografts stained with Hematoxylin-Eosin (4 weeks after transplantation with low magnification).
Supplementary Figure 2. Representative sections of coronary arteries in allografts stained with elastin (4 weeks after transplantation with high magnification).
Acknowledgements
We thank Dr. Galina K. Sukhova, Ms. Eugenia Shvartz, and Ms. Lindsey MacFarlane for skillful technical assistance, and Ms. Joan Perry for editorial assistance.
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL-34636) and the Donald W. Reynolds Foundation to P.L., HL-43364, HL-67203, GM-67049, BW research excellence-bridge grant to R.N.M., from American Heart Association (Northeast Affiliate Postdoctoral Fellowship) and Eli Lilly (International Fellowship) to Y.O.
Abbreviations
- APN
adiponectin
- MHC
Major Histocompatibility Complex
- RT-PCR
Real-Time Polymerase Chain Reaction
- PR
Parenchymal Rejection
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- TNFα
Tumor Necrosis Factor α,
- IFNγ
Interferon γ,
- RANTES
Regulated upon Activation Normal T Expressed and presumably Secreted
- MCP-1
Monocyte Chemotactic Protein-1
- IP-10
Interferon γ inducible protein-10
- TUNEL
TdT-mediated dUTP-biotin Nick End Labeling
Footnotes
Authorship: Yoshihisa Okamoto1,2,3,4 Thomas Christen3,4, Koichi Shimizu3, Ken-ichi Asano3,4, Shinji Kihara1, Richard N. Mitchell1,2,4, and Peter Libby1,2,4
Participated in research design1, in the writing of the paper2, in the performance of the research3, and in data analysis4
The authors have no conflict of interest.
References
- 1.Vgontzas AN, Bixler EO, Papanicolaou DA, Chrousos GP. Chronic systemic inflammation in overweight and obese adults. Jama. 2000;283(17):2235. doi: 10.1001/jama.283.17.2235. author reply 2236. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 3.Grady KL, Naftel D, Pamboukian SV, et al. Post-operative obesity and cachexia are risk factors for morbidity and mortality after heart transplant: multi-institutional study of post-operative weight change. J Heart Lung Transplant. 2005;24(9):1424. doi: 10.1016/j.healun.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110(3):267. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto Y, Folco EJ, Minami M, et al. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102(2):218. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 6.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10(12):1384. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11(10):1096. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8(7):731. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu K, Aikawa M, Takayama K, Libby P, Mitchell RN. Direct anti-inflammatory mechanisms contribute to attenuation of experimental allograft arteriosclerosis by statins. Circulation. 2003;108(17):2113. doi: 10.1161/01.CIR.0000092949.67153.74. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu K, Schonbeck U, Mach F, Libby P, Mitchell RN. Host CD40 ligand deficiency induces long-term allograft survival and donor-specific tolerance in mouse cardiac transplantation but does not prevent graft arteriosclerosis. J Immunol. 2000;165(6):3506. doi: 10.4049/jimmunol.165.6.3506. [DOI] [PubMed] [Google Scholar]
- 11.Sukhova GK, Zhang Y, Pan JH, et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111(6):897. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest. 1997;100(3):550. doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115(8):1029. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 14.Maeda N, Takahashi M, Funahashi T, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50(9):2094. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 15.Hiuge A, Tenenbaum A, Maeda N, et al. Effects of peroxisome proliferator-activated receptor ligands, bezafibrate and fenofibrate, on adiponectin level. Arterioscler Thromb Vasc Biol. 2007;27(3):635. doi: 10.1161/01.ATV.0000256469.06782.d5. [DOI] [PubMed] [Google Scholar]
- 16.Takagi T, Matsuda M, Abe M, et al. Effect of pravastatin on the development of diabetes and adiponectin production. Atherosclerosis. 2008;196(1):114. doi: 10.1016/j.atherosclerosis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Kosuge H, Haraguchi G, Koga N, Maejima Y, Suzuki J, Isobe M. Pioglitazone prevents acute and chronic cardiac allograft rejection. Circulation. 2006;113(22):2613. doi: 10.1161/CIRCULATIONAHA.105.594101. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki J, Koga N, Kosuge H, et al. Pitavastatin suppresses acute and chronic rejection in murine cardiac allografts. Transplantation. 2007;83(8):1093. doi: 10.1097/01.tp.0000259650.67061.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Representative sections of allografts stained with Hematoxylin-Eosin (4 weeks after transplantation with low magnification).
Supplementary Figure 2. Representative sections of coronary arteries in allografts stained with elastin (4 weeks after transplantation with high magnification).