Abstract
CD137 (4-1BB) is a member of the TNFR-family with co-stimulatory function, triggering pro-survival signals in activated T-cells. Upregulation of CD137 upon stimulation allows identifying and isolating live, human antigen-specific CD8+ T-cells of all phenotypes and therefore provides a comprehensive detection method. Furthermore responses against antigen mixtures can be easily detected, enabling antigen discovery in a step-wise deconvoluting approach. In this article, we will discuss various aspects of this methodology, including potential pitfalls as well as a variety of applications, as illustrated by examples from our laboratory.
Introduction
CD137, a member or the TNFR-family,(1,2) functions as a costimulatory molecule, promoting proliferation and survival of activated T-cells, as demonstrated in vivo and in vitro.(3–6) Expression of CD137 is highly restricted to recent activation by a signal through the T-cell-receptor (TCR) and can therefore be exploited to identify and select antigen-specific CD8+ T-cells.(7) Such a live-cell assay has advantages over those immune-monitoring assays that require fixation or permeabilization such as intracellular cytokine staining in that antigen-specific T-cells can be isolated and expanded for further characterization or use in adoptive immunotherapy.(8) Furthermore the method detects specifically activated T-cells even if initially stimulated by a complex mixture of antigens hence facilitating antigen-discovery by employing a step-wise reductionist approach. As CD137 is expressed on the cell surface, analysis can be performed by straightforward direct staining. Upregulation of CD137 occurs on CD8+ T-cells of all phenotypes (e.g. naïve T-cells, early and late memory effector T-cells), and therefore provides a more comprehensive detection method than analysis for the production of a single cytokine. Consequently, selection of CD137+ cells after antigen stimulation results in enrichment of the specific T-cells regardless of individual functional characteristics, making it possible to study the complete reactive T-cell repertoire.
Methodology
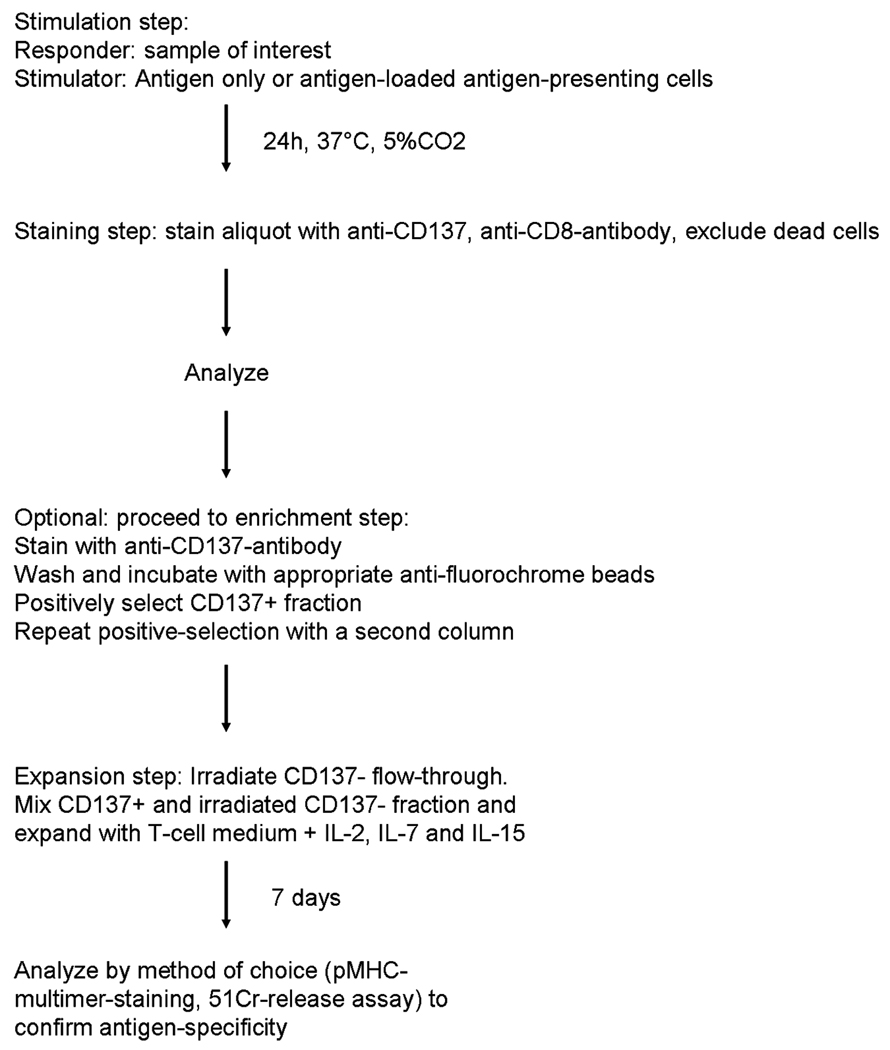
Similar to other functional assays measuring cytokine production(9,10) or degranulation,(11) the assay consists of a first activation step, followed by a brief incubation period (optimally 20–24h), and subsequent staining for CD137. If CD137+ cells are to be selected for further expansion, cells can be sorted or more rapidly processed by using paramagnetic beads (Fig.1). Note that additives like brefeldin A of monensin are not required for this assay.
Figure 1.
Flow-chart for the detection and isolation of CD137+ T-cells.
Choosing an efficient method for presentation of a particular antigen is crucial for this assay similar to all assays that detect responding T cells, as this influences the extent of antigen-specific upregulation of CD137 as well as the possible non-specific activation of T-cells. Different antigen-presenting cells can be used in order to achieve activation: for example, freshly isolated PBMC can be stimulated directly ex vivo by addition of a defined peptide of interest or a pool of peptides (Fig. 2a). When testing purified T-cells (either directly ex vivo or as cultured T-cell lines or clones), peptide pulsed antigen-presenting cells should be used (Fig. 2b). In our hands, autologous T-cell-depleted PBMC, dendritic cells, B-cells and monocytes are all suitable, although differences in the level of activation may exist, requiring adjustments of the experimental system to the scientific question; e.g. expansion of antigen-specific T-cells, if present at a low precursor frequency, requires repeated stimulation; therefore PBMC rather than dendritic cells should be used as the latter may induce activation induced cell death.(12,13)
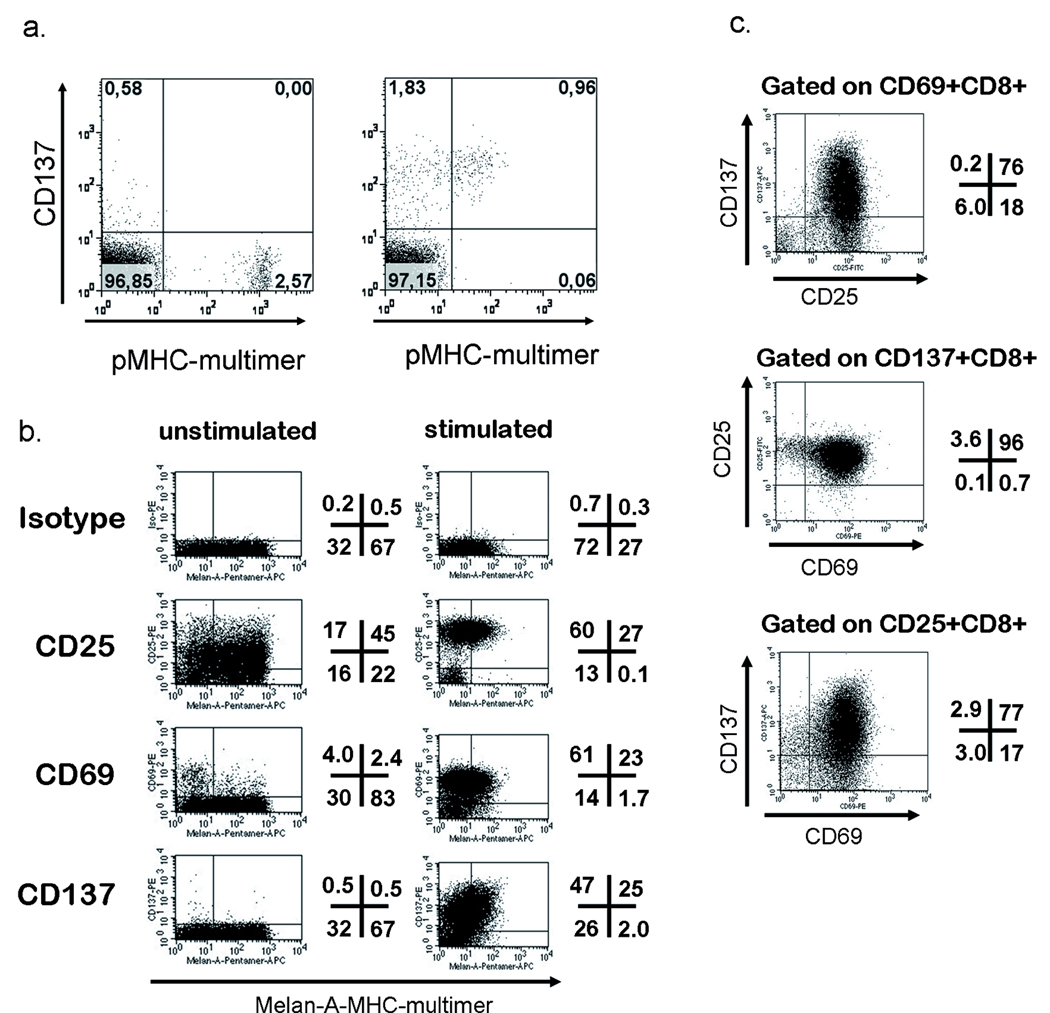
Figure 2.
Upregulation of CD137 upon peptide-specific activation. a. PBMC from an HLA-B7+, CMV+ donor were thawed, rested overnight and then incubated either without (left) or with pp65(417–426) peptide (5µg/ml) for 24h followed by staining with CD8-FITC, CD137-APC, 7-AAD (all from Pharmingen) and B7-pp65-Tetramer-PE (in-house-production). Plots were obtained by excluding dead cells by 7-AAD, gating on CD8+ lymphocytes. b. A polyclonal Melan-A(26–35L)-specific T-cell line was generated by 2 in vitro stimulations and assessed for baseline expression and specific upregulation of the indicated markers in response to peptide-pulsed T2-cells (numbers indicate the percentage of viable CD8+ T-cells in each quadrant). C. Triple staining for the activation markers (CD25-FITC, CD69-PE and CD137-APC, co-stained with CD8-PerCP (all antibodies from Pharmingen) of the same polyclonal, Melan-A-specific T-cell line as in B. For analysis in Figure C all 4 available channels were used for specific markers, therefore dead cells were excluded by light scatter properties only, otherwise 7-AAD was used.
Following activation, the cells are incubated for 24h, and then analyzed using antibodies to CD137. As for all questions related to the detection of rare events, fluorochrome formulations with a low signal-to-noise ratio are required. In our hands APC-labelled, directly conjugated CD137 antibody (clone 4B4-1, Pharmingen) was superior to PE-labelled antibody, but other dyes or use of quantum dots may also be suitable.(14) Controls should include the unstimulated sample without the addition of antigen-presenting cells, as well as a sample of cells incubated with antigen-presenting cells not expressing the antigen or pulsed with an irrelevant antigen.
CD137+ T-cells can be selected either by flow-sorting or by the use of paramagnetic beads, with the latter allowing a higher throughput if larger cell numbers need to be processed resulting in better survival of the cells. T-cells labelled with CD137-APC are incubated with anti-APC beads (Miltenyi) and then selected using the appropriate columns (MS or LS depending on the processed cell number). Note that if the very few antigen-specific cells are being isolated (<1%), culture medium can be added to the column for the last rinse and positive cells can then be flushed out using medium rather than buffer avoiding the need for further washing steps that would require an additional centrifugation step. Also, if the experimental goal is to expand the CD137+ T-cells, a culture system that must contain feeder cells is required. The most straightforward approach is to collect the flow-through of CD137 negative cells, irradiate these cells and mix them again with the CD137+ T-cells.
Problems
As described above, the use of CD137 as a surface marker is a straightforward approach. In PBMC from healthy donors, background expression of CD137 in the unstimulated sample is generally very low. An overnight resting period after thawing the PBMC prior to stimulation with the antigen further improves upregulation of CD137 on reactive cells versus non-reactive cells. If in vitro cultured T-cells or clones are to be tested, antigen-presenting cells need to be added as stimulators. Background due to these antigen-presenting cells is variable: in our hands mature dendritic cells commonly induced a stronger non-specific activation than monocytes, B-cells or even immature DC. For analysis, it is crucial to clearly identify single T-cells, as CD137 expression may be detected on a number of other cells such as dendritic cells, monocytes and B-cells.(15) Conjugates of T-cells with such antigen-presenting cells can therefore also stain positive for CD137 and T-cell-markers. Furthermore, especially when PBMC are tested, further improvements may be achieved by using a ‘dump’ channel that allows exclusion of monocytes, B-cells and dead cells to further increases signal-to-background discrimination. One difficulty, especially in terms of quantification of rare events, is establishing a stringent gating system: the highest variation is usually found in setting the FSC/SSC-gate, as activated T-cells increase in size and granularity and may shift out of the gate if the lymphocyte gate is set too narrow. As discussed at the MASIR 2008 meeting, a standardized gating strategy is a challenge yet to be resolved for all assays measuring specifically activated T-cells (16,17).
Some of the questions arising during selection of CD137+ cells from cultures with a very low frequency prior to selection have already been addressed above. The purity of antigen-specific T-cells after selection with paramagnetic beads and after an additional expansion phase (usually one week), is correlated with the frequency of antigen-specific T-cells prior to enrichment. For example, good enrichment (10–20 fold) of antigen-specific T-cells is reproducibly achieved, when starting with a frequency in the range of 1–5% of antigen-specific T-cells prior to enrichment. Although processing of polyclonal responding T-cell populations with an even lower precursor frequency (< 1%) also results in good relative enrichment (up to 100 fold in terms of increase), the absolute purity tends to be lower due to a higher percentage of contaminating CD137− cells. A simila r correlation of purity and precursor frequency has also been reported for other enrichment methods such as the cytokine secretion assay (M. Assenmacher, oral presentation, MASIR 2008). Purity of the selection, however at the cost of reduced total recovery, can be improved by including a repeated purification step with a new column. Use of a biotinylated antibody and streptavidin microbeads has also been reported to be superior to anti-fluorochrome beads for depletion of alloreactive CD137+ T-cells.(18)
Potential applications
We have shown previously that CD137 expression is a favourable marker for identifying specifically activated CD8+ T-cells as it is not expressed on resting, cultured T-cells or peripheral blood T-cells and is strongly upregulated 24h after antigen stimulation.(7) Figure 2a gives an example of monitoring a response against the HLA-B7-restricted CMVpp65-derived epitope(417–426). The number of pMHC-multimer+ T-cells prior to stimulation (2.57%) and the number of CD137+ T-cells after stimulation (2.79%) correlated well. In this example, downregulation of the TCR was not complete (which may depend on stimulating conditions, peptides, etc), thus allowing simultanous staining of CD137 with pMHC-multimer. However it has to be noted, that pMHC-staining 16–24h after activation is usually not a reliable method to identify antigen-specific T-cells A small percentage of pMHC multimer+ T-cells did not upregulate CD137 upon stimulation (Fig.2a and b). We interpret the lack of CD137 expression as a sign of insufficiently stimulated T-cells, however the existence of rare T-cell populations which become fully activated but do not upregulate CD137 cannot be formally excluded. In comparison to the well-characterized activation markers CD69 and CD25, CD137 permits better discrimination of positive cells than CD69, due to a higher and more robust expression level, and is more specific than CD25 which is often already detectable at an intermediate expression level on cultured T-cells without recent stimulation through the TCR. Multi-color-analysis has also demonstrated that the number of CD137+ cells correlates closely, and better than CD69+ or CD25+ cells, with the pMHC multimer+ cells in the un-stimulated control- largely due to the absence of false positives with CD137 analysis. This is in part evidenced by the fact that most CD137+ cells also express CD69 and CD25 (triple positive), whereas a significant number of CD69+ and CD25+ cells are not triple positive (Fig. 2c).
As a method useful for the enrichment of live T-cells, selection on the basis of CD137 expression upon stimulation is suitable for selecting T-cells responding to antigen mixtures, without the need to know the exact epitope, which subsequently – by deconvoluting the antigen mixture – may lead to epitope discovery. In our recent work (7), a T-cell line was generated against a peptide library spanning the Wilms Tumor antigen 1 protein, a transcription factor important for leukaemic progress.(19) As the precursor frequency in healthy individuals against this self antigen is below the detection limit of conventional methods, we used the CD137-enrichment method to expand antigen-specific T-cells in order to obtain numbers above the threshold level for identification by cytokine flow cytometry. Using this approach, a number of immunogenic peptides were identified, including WT1(286–293), which was described in more detail in our recent work. The peptide library consisted of 110 15mers, overlapping by 11, and the complete peptide-pool was pulsed onto dendritic cells for generating the responding T-cell line, making it possible to analyze the reponse to the entire protein without having to resort to pre-selection of candidate petides by in silico analysis of potential HLA binding based on motifs. Implementing the CD137-enrichment step is not imperative when strong immune responses, e.g. a memory responses against viral proteins, are to be resolved and mapped.(20,21) However, with antigens that are over-expressed normal proteins such as most candidate tumor antigens, immune responses are often weak, and most high affinity T-cells have been deleted from the repertoire due to thymic or peripheral based on self-reactivity. The method can also be used for the generation of T-cells reactive against CMV-derived epitopes from CMV seronegative individuals (Jedema et al., American Society of Hematology, 2007, Meeting abstract no. 1053), opening therapeutic options to reduce the risk of acute CMV infection for CMV seropositive patients receiving an allogeneic transplant from CMV seronegative donors.
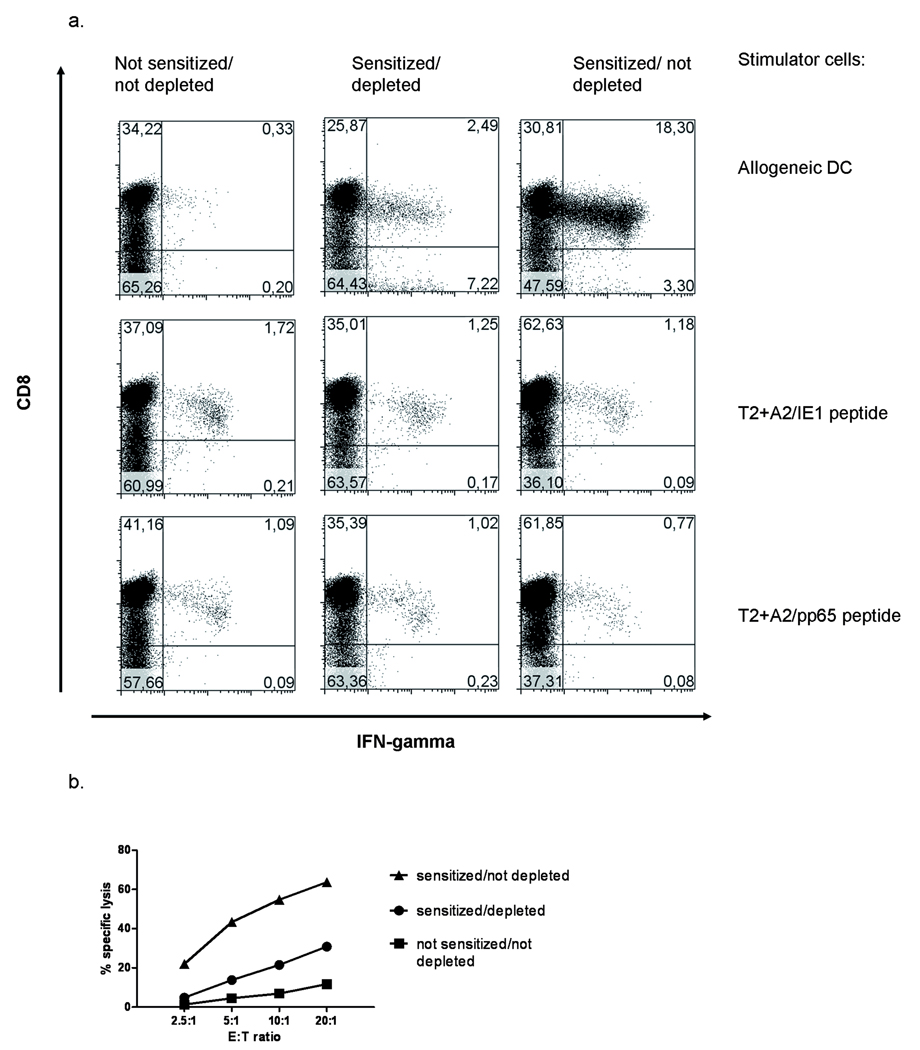
Depletion of antigen-reactive T-cells is relevant in the clinical setting of allogeneic stem cell transplantation. Graft-versus-Host-Disease (GVHD) is often mediated by donor-derived CD4+ and CD8+ T-cells and several methods to purge the graft from alloreactive T-cells have been evaluated.(22) One approach is to sensitize donor T-cells with patient-derived antigen-presenting cells and deplete reactive T-cells. Markers previously used for detection of these pre-sensitized, alloreactive T-cells have been CD25, CD95 and CD69. (23–26) Wehler et al. demonstrated that depletion of cells expressing CD137 following stimulation with allogeneic antigen-presenting cells can significantly reduce alloreactivity.(18) This was demonstrated for both, CD4 and CD8+ T-cells suggesting analogous upregulation of this protein on CD4+ T-cells. In our own series of experiments, we confirmed the utility of this approach, with T-cells reactive with fully mismatched antigen-presenting cells reduced by about one log. Most importantly, the depletion was specific, with virus-specific memory responses retained as demonstrated by the detection of CMV-specific T-cells (Fig. 3). Although promising, in a clinical setting the use of any method that includes in vitro activation prior to depletion bears the risk of enhancing GVHD due to the few remaining, sensitized alloreactive T-cells. Thus more work must be done to improve the completeness of the depletion and assess the safety of this approach.
Figure 3.
Depletion of CD137+ T-cells results in decreased alloreactivity and maintaine d T-cell memory. PBMC were stimulated (sensitized) with mature allogeneic dendritic cells on day 0. To ensure survival of the T-cells in the unstimulated control, cells were kept in culture with IL2, 50IU/ml throughout the whole culture period. For stimulated cultures, cytokines were added only after depletion on day 2. To ensure best depletion results, CD137+ T-cells were depleted twice, first at the peak of CD137-expression after 24h and then after 48h to remove T-cells that were activated at a later time point by the remaining dendritic cells. Removal was performed using anti-CD137-APC and binding to anti-APC-beads (Pharmingen/Miltenyi). A control group (sensitized/not depleted) was stained with CD137-PE followed by the same depletion procedure using anti-APC-beads and LD-columns. All groups were then resuspended in fresh medium containing IL2, IL7 and IL15. a. On day 8 of culture, the cells were restimulated with mature, allogeneic DC from the original donor, and intracellular IFNγ production was assessed after 6h of incubation. Responses to the HLA-A0201 restricted CMV-derived peptides IE1(316–324) and pp65(495–504) were assessed using peptide pulsed T2-cells.
b. The same groups as in a were assessed for their lytic capacity by incubation for 4h with of 1×104 51Chromium-labelled allogeneic, mature dendritic cells as targets. The assay was performed in triplicate. SEM of any the data points was in the range of 0.17–2.98). Statistical analysis using one-way ANOVA analysis showed a stastically significant difference between all groups (p=0.0005).
Sensitivity, background and comparison to other technologies
Comparison of immune-monitoring based on CD137 expression with results based on IFNγ production and TNFα production showed an excellent correlation for quantitating memory CD8+ T cell responses, as reflected by analysis of CMV-specific immune responses directly ex vivo or following generation of T-cell lines in vitro (p<0.002, r2>0.9).(7) Comparison with induced expression of CD107, which reflects degranulation, also revealed a strong correlation (p=0.007), but with a slightly lower coefficient of correlation r2 (0.87), which may reflect the different functional requirements for degranulation versus full activation. As for many assays involving T-cell stimulation, background staining is variable and depends on multiple factors (donor dependency, experimental stimulation conditions, etc.). The published data on quantitation of antigen-specific T-cells against a single epitope will have to be extended using peptide pools or proteins. Besides assay optimization, further work is necessary using samples from larger cohorts, to accurately determine the sensitivity and the quanification limit and address the issue of assay-validation as has been shown for intracellular cytokine staining (16,17)
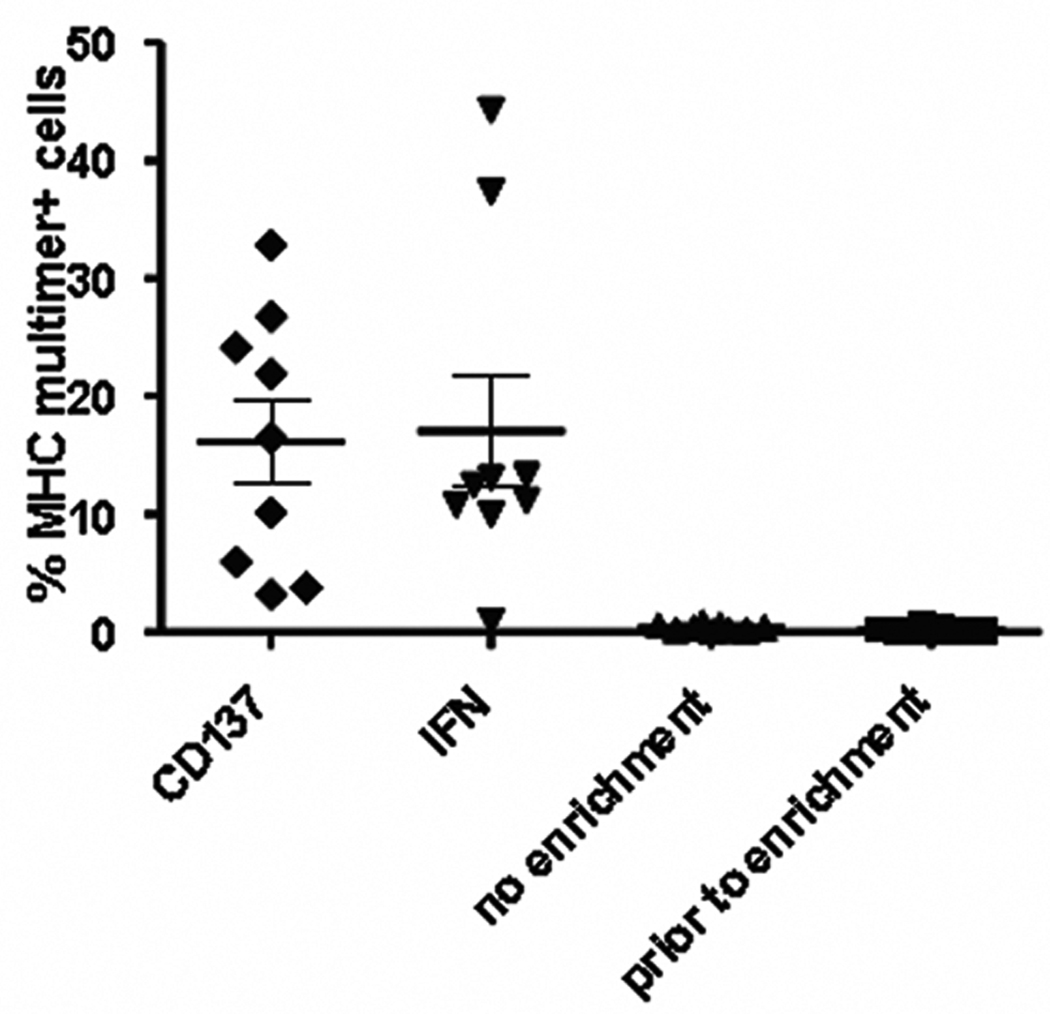
For enrichment of antigen-specific T-cells, we compared CD137-selection to the IFNγ -capture method (Miltenyi), which is a commercially available assay for the selection of IFNγ secreting cells (27). Each assay was set up in parallel and performed for multiple T-cell lines containing low numbers of antigen-specific T-cells (0.07–0.61%). For IFNγ –capture a 5h stimulation period was followed by a 45min secretion period, which reflects the peak of the IFNγ reponse, when peptide antigens are being used. As CD137 only peaks after prolonged incubation with antigen for 18–24h, this assay was performed after an overnight culture. Under these conditions, performance of both tests was similar as demonstrated in Fig. 4. Wehler et al. have reported that in their system CD137 selection outperformed the IFNγ capture method (American Society of Hematology, 2007, Meeting abstract no. 1972). Due to TCR downregulation after antigen stimulation, the enriched T-cell fraction cannot be tested for specificity directly after enrichment (e.g. with pMHC multimers) and a further culture period is required to allow re-expression of the TCR. Therefore CD137 negative cells carried over from the original T-cell population might also expand as cytokine-driven bystander cells. Further technical advances like the use of the biotinylated antibodies are currently being evaluated to address these issues.
Figure 4.
Comparison of CD137-enrichment to IFNγ secretion assay for enrichment of antigen-specific T-cells from a low precursor frequency. T-cells lines were induced by stimulating T-cells enriched for naïve T-cells by depletion of RO+ memory cells, with autologous dendritic cells, pulsed with the HCV NS3(1406–1415) peptide. After one week of culture in medium containing IL7 and IL15, each well was split and cells were processed either using the IFNγ secretion assay (Miltenyi) or the CD137-enrichment procedure. Analysis of each well by MHC-multimer-staining (Proimmune) was performed 7 days post enrichment. The irradiated negative flow-through was used as feeder cells. Each data point represents an enrichment procedure from an individual well. Results are pooled from 2 independent experiments.
The practical advantages of the CD137-enrichment method in comparison to the IFNγ capture method are evident: the ability to use a more prolonged incubation time (24h) as opposed to the usual 5–6h for the IFNγ secretion assay leads to a less time-sensitive, more robust activation, especially when weak antigens are to be tested or the kinetics of antigen-presentation is unclear (e.g. when using whole protein antigens such as a cell lysate). Furthermore cross-staining of bystander cells by secreted IFNγ is a well-known caveat for the secretion assay (27), but it is irrelevant in the CD137-assay. Moreover no additives (like the cytokine catch reagent) or shakers are necessary.
Future directions
It is presently unclear if, in patients with acute or chronic diseases, activated CD137+ T-cells circulate through the blood and may be detected ex vivo without additional in vitro restimulation. We have recently begun examining patients suffering from GVHD after allogeneic stem cell transplantation to determine if CD137+ T-cells could be detected directly ex vivo, as the interaction of CD137 and its ligand has been shown to be important for mediating GVHD in mouse models. (28,29) Preliminary results from a cohort of patients with acute or chronic GVHD do not indicate a correlation with disease activity, although the study is still ongoing (Wölfl, Eyrich, Schlegel, unpublished results). This may be explained by activated T-cells being more likely to be found in lymph nodes and affected organs, such as skin and liver. Thus, it might prove more informative to study CD137 expression in inflamed tissue, such as during chronic GVHD and chronic viral infections like HCV, as well the lymphocytes infiltrating tumors. Isolation and characterization of CD137+ cells at these sites may provide a means to identify the specific antigens being recognized by the responding T cells.
Besides conventional CD8+ αβT-cells, γδT-cells also upregulate CD137. γδT-cells can recognize a broad variety of malignant cells through less limited mechanisms than αβT-cells, and without recognition of allo-antigens that could result in GVHD.(30) As CD137 is also upregulated on γδT-cells upon stimulation with tumor cells (J.Kuball, personnel observation), isolation of tumor-reactive γδT-cells based on CD137 expression upon stimulation might provide a means to more efficiently isolate tumor-reactive γδT-cells.
Adoptive immunotherapy has proven to be effective under certain conditions targeting cancer cells or preventing viral diseases in severely immunocompromised hosts.(8,31,32) However the generation and expansion of these antigen-specific T-cells has been very time- and labour-intensive, especially when T-cells from the naïve repertoire are to be recruited.(12) An enrichment method would greatly accelerate the procedure and may therefore be key to facilitate clinical trials exploring adoptive immunotherapy. Implementation of such a method, however, will require further efforts from both, academia and industry, to meet the criteria of good manufacturing practice.
The strategy described here was developed mostly using synthetic peptides pulsed onto antigen-presenting cells as stimulators. It is also possible to generate, select for and expand antigen-specific T-cells using antigen-presenting cells which endogenously process the antigen (M.Wölfl, unpublished observation). Although the TCR stimulus from such antigen-presenting cells may be weaker and the resulting T-cell response smaller, it should still be possible to use this strategy to identify novel antigens capable of inducing a T-cell response from the available repertoire. Besides antigen identification such an approach holds promise to increase the efficacy to generate T-cell responses against malignant cells or cell lysates, which, e.g. in the setting of allogeneic stem cell transplantation, may be used to improve the graft-versus-leukaemia-effect by patient-tailored, donor-derived T-cell preparations. Especially when in vitro priming against a mixture of antigens such as peptide libraries, whole tumor cells or tumor lysates is pursued, CD137 expression can be exploited in two ways: first by enriching for antigen-specific T-cells and secondly by easily screening large numbers of sub-cloned T-cells for antigen-reactivity. For example, T-cell clones are expanded from single cells, which generally requires testing of hundreds of wells. In this setting CD137 can be used as a surrogate marker for antigen-specificity after stimulation of an aliquot of the T-cell clone with antigen-presenting cells. As the method is easily adapted to a 96-well-plate format, detection by CD137 expression is suitable for a high-throughput approach using a flow cytometer with a plate-reader function. In contrast, current procedures for testing the reactivity of T-cell clones are generally done either by Chromium-release assays or by staining with pMHC-multimers, both of which have disadvantages in terms of cost, logistics (radioactivity) and availability (pMHC multimers).
Footnotes
Research support: MW and JK were fellows of the Deutsche Krebshilfe e.V., Germany. MW currently receives a stipend from the Child Philipp Foundation for Cancer Research (T237/16586/2007), Germany.
This work is based on a presentation given at the MASIR meeting 2008 in La Plagne, France.
References
- 1.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A. 1989;86(6):1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 3.Wen T, Bukczynski J, Watts TH. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J Immunol. 2002;168(10):4897–4906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- 4.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169(9):4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 5.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002;3(6):536–541. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 6.Dawicki W, Watts TH. Expression and function of 4-1BB during CD4 versus CD8 T cell responses in vivo. Eur J Immunol. 2004;34(3):743–751. doi: 10.1002/eji.200324278. [DOI] [PubMed] [Google Scholar]
- 7.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, Greenberg PD. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110(1):201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3(5):431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 9.Maino VC, Picker LJ. Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry. 1998;34(5):207–215. doi: 10.1002/(sici)1097-0320(19981015)34:5<207::aid-cyto1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Maino VC, Maecker HT. Cytokine flow cytometry: a multiparametric approach for assessing cellular immune responses to viral antigens. Clin Immunol. 2004;110(3):222–231. doi: 10.1016/j.clim.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1–2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 12.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods. 2006;310(1–2):40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Mehrotra S, Stevens R, Zengou R, Chakraborty NG, Butterfield LH, Economou JS, Dorsky DI, Mukherji B. Regulation of melanoma epitope-specific cytolytic T lymphocyte response by immature and activated dendritic cells, in vitro. Cancer Res. 2003;63(17):5607–5614. [PubMed] [Google Scholar]
- 14.Chattopadhyay PK, Price DA, Harper TF, Betts MR, Yu J, Gostick E, Perfetto SP, Goepfert P, Koup RA, De Rosa SC, et al. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat Med. 2006;12(8):972–977. doi: 10.1038/nm1371. [DOI] [PubMed] [Google Scholar]
- 15.Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim KK, Kim YJ, Wang S, Gentz R, Yu GL, et al. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272(22):14272–14276. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- 16.Hural J, Horton H, Huang Y, McElrath MJ, De Rosa SC. Validation of Intracellular Cytokine Staining Assays for Evaluation of T Cell Responses in Vaccine Trials. Cytometry. 2008;(THIS ISSUE) [Google Scholar]
- 17.Nomura L, Maino VC, Maecker HT. Standardization and Optimization of Multiparameter Flow Cytometric Studies. Cytometry. 2008;(THIS ISSUE) doi: 10.1002/cyto.a.20602. [DOI] [PubMed] [Google Scholar]
- 18.Wehler TC, Nonn M, Brandt B, Britten CM, Grone M, Todorova M, Link I, Khan SA, Meyer RG, Huber C, et al. Targeting the activation-induced antigen CD137 can selectively deplete alloreactive T cells from antileukemic and antitumor donor T-cell lines. Blood. 2007;109(1):365–373. doi: 10.1182/blood-2006-04-014100. [DOI] [PubMed] [Google Scholar]
- 19.Ariyaratana S, Loeb DM. The role of the Wilms tumour gene (WT1) in normal and malignant haematopoiesis. Expert Rev Mol Med. 2007;9(14):1–17. doi: 10.1017/S1462399407000336. [DOI] [PubMed] [Google Scholar]
- 20.Kern F, Surel IP, Brock C, Freistedt B, Radtke H, Scheffold A, Blasczyk R, Reinke P, et al. Schneider-Mergener J, Radbruch A and others. T-cell epitope mapping by flow cytometry. Nat Med. 1998;4(8):975–978. doi: 10.1038/nm0898-975. [DOI] [PubMed] [Google Scholar]
- 21.Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, Proesch S, Reinke P, Volk HD, Lehmkuhl H, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201(7):1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mielke S, Solomon SR, Barrett AJ. Selective depletion strategies in allogeneic stem cell transplantation. Cytotherapy. 2005;7(2):109–115. doi: 10.1080/14653240510018172. [DOI] [PubMed] [Google Scholar]
- 23.Amrolia PJ, Muccioli-Casadei G, Yvon E, Huls H, Sili U, Wieder ED, Bollard C, Michalek J, Ghetie V, Heslop HE, et al. Selective depletion of donor alloreactive T cells without loss of antiviral or antileukemic responses. Blood. 2003;102(6):2292–2299. doi: 10.1182/blood-2002-11-3516. [DOI] [PubMed] [Google Scholar]
- 24.Amrolia PJ, Muccioli-Casadei G, Huls H, Adams S, Durett A, Gee A, Yvon E, Weiss H, Cobbold M, Gaspar HB, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108(6):1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartwig UF, Nonn M, Khan S, Meyer RG, Huber C, Herr W. Depletion of alloreactive T cells via CD69: implications on antiviral, antileukemic and immunoregulatory T lymphocytes. Bone Marrow Transplant. 2006;37(3):297–305. doi: 10.1038/sj.bmt.1705238. [DOI] [PubMed] [Google Scholar]
- 26.Hartwig UF, Nonn M, Khan S, Link I, Huber C, Herr W. Depletion of alloreactive donor T lymphocytes by CD95-mediated activation-induced cell death retains antileukemic, antiviral, and immunoregulatory T cell immunity. Biol Blood Marrow Transplant. 2008;14(1):99–109. doi: 10.1016/j.bbmt.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Assenmacher M. Cytokine capture system (CCS) for enrichment of T cells for adoptive transfer. Cytometry. 2008;(THIS ISSUE) [Google Scholar]
- 28.Blazar BR, Kwon BS, Panoskaltsis-Mortari A, Kwak KB, Peschon JJ, Taylor PA. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J Immunol. 2001;166(5):3174–3183. doi: 10.4049/jimmunol.166.5.3174. [DOI] [PubMed] [Google Scholar]
- 29.Blazar BR, Lees CJ, Martin PJ, Noelle RJ, Kwon B, Murphy W, Taylor PA. Host T cells resist graft-versus-host disease mediated by donor leukocyte infusions. J Immunol. 2000;165(9):4901–4909. doi: 10.4049/jimmunol.165.9.4901. [DOI] [PubMed] [Google Scholar]
- 30.Beetz S, Marischen L, Kabelitz D, Wesch D. Human gamma delta T cells: candidates for the development of immunotherapeutic strategies. Immunol Res. 2007;37(2):97–111. doi: 10.1007/BF02685893. [DOI] [PubMed] [Google Scholar]
- 31.O'Reilly RJ, Doubrovina E, Trivedi D, Hasan A, Kollen W, Koehne G. Adoptive transfer of antigen-specific T-cells of donor type for immunotherapy of viral infections following allogeneic hematopoietic cell transplants. Immunol Res. 2007;38(1–3):237–250. doi: 10.1007/s12026-007-0059-2. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]