Abstract
In children with acute leukemia, measurements of minimal residual disease (MRD) provide unique information on treatment response and have become a crucial component of contemporary treatment protocols. In acute lymphoblastic leukemia (ALL), the most useful MRD assays are based on polymerase chain reaction (PCR) amplification of antigen-receptor genes, and on flow cytometric detection of abnormal immunophenotypes. The latter is the only MRD assay available for most patients with acute myeloid leukemia (AML). PCR amplification of chromosomal breakpoints and fusion transcripts can also be used to track MRD in a minority of patients with ALL or AML. Because of the strong correlation between MRD levels and risk of relapse, several ongoing regimens include treatment intensification for children with higher MRD. Treatment deintensification for patients with early MRD clearance is also being tested. In addition to their direct clinical application, MRD measurements can be used to better understand the molecular and cellular mechanisms of drug resistance in vivo. The identification of new markers of leukemia and the use of increasingly sophisticated technologies for detection of rare cells should further facilitate routine monitoring of MRD and elucidate the features of drug-resistant leukemic cells.
Keywords: acute lymphoblastic leukemia, acute myeloid leukemia, flow cytometry, polymerase chain reaction, treatment response
Rationale for minimal residual disease testing
Monitoring response to treatment by periodic examination of bone marrow aspirates is an integral part of the clinical management of patients with acute leukemia. The presence of residual leukemia and the overall status on normal hematopoiesis, as determined by the cellular appearance of bone marrow smears, provide an indication of the sensitivity of leukemic cells to chemotherapy and of the degree of hematopoietic regeneration occurring during treatment intervals. Because the morphology of leukemic cells generally resembles that of normal lymphohematopoietic progenitors, it is difficult to identify leukemic cells with confidence. In fact, identification of individual leukemic cells scattered among normal bone marrow cells might not be possible even for an experienced hemopathologist, unless leukemic cells have striking morphologic traits such as Auer rods. Therefore, a patient can have nearly 5% leukemic cells that morphologically appear as leukemic blasts and yet be considered in remission. Only a minority of patients have leukemic cells above this threshold at the end of remission induction therapy but those with a lower proportion of blasts may still harbor a considerable leukemic burden (Campana & Pui, 1995). Hence, a significant proportion of patients are likely to receive post-remission therapy that is less intensive than required. The morphologic similarities between leukemic cells and normal hematopoietic cells may also lead to the opposite error, i.e. an overestimation of the leukemia burden due to the mistaken identification of normal cells as leukemic cells. This error could trigger unnecessary treatment intensification and toxicities.
Over the last 2–3 decades there has been an intense effort to develop methods that could determine the degree of residual leukemic present in patients considered to be in morphologic remission, that is to measure minimal residual disease (MRD). These efforts have resulted in assays whose sensitivity is much higher (100 times or more) than that of morphology (Szczepanski et al, 2001;Campana, 2003). MRD assays are also more objective because they rely on specific leukemia markers rather than on the subjective recognition of morphologic patterns. Studies reported during the last decade, discussed below, have unequivocally demonstrated the prognostic importance of MRD in childhood leukemia. Therefore, the more stringent definition of remission provided by MRD assays is now preferentially used at many cancer centers.
Methodologies to detect MRD
Targets and methods
The common principle underlying all MRD assays is that the leukemogenic process has resulted in molecular and cellular changes that distinguish leukemic cells from their normal counterparts (Szczepanski et al, 2001;Campana, 2003). These leukemia-associated features are identified at diagnosis (or at relapse) and then used to monitor MRD. Table 1 summarizes the applicability and sensitivity of the most widely used assays.
Table 1.
Methods for monitoring MRD in childhood leukemia
| Method | ALL | AML | ||
|---|---|---|---|---|
| % of cases with marker | Sensitivity | % of cases with marker | Sensitivity | |
| Flow cytometric detection of abnormal phenotypes | 98% | 10−4 | 93% | 10−3−10−4 |
| PCR amplification of IG or TCR genes | 90% (10−5) | 10−4−10−5 | <10% | |
| RT-PCR amplification fusion transcripts | <50% | 10−3−10−5 | <20% | 10−3−10−5 |
One of the distinguishing features of leukemic cells is the expression of cell markers in abnormal patterns. These abnormal cell profiles are best detected with multiparameter flow cytometry (Campana, 2003). The sensitivity of this approach depends on two main factors: the degree of dissimilarity between the immunophenotypes of leukemic cells and those of normal cells, and the number of cells available for study. Leukemic lymphoblasts in nearly all patients with acute lymphoblastic leukemia (ALL) express immunophenotypes that are sufficiently distinct to allow the detection of 1 leukemic cells among 10,000 normal cell (Coustan-Smith et al, 2002a;Campana & Coustan-Smith, 1999). Distinctive markers can also be identified in most patients with acute myeloid leukemia (AML), although in approximately 40% of patients the routine sensitivity that can achieved is not higher than 1 in 1,000, owing to a partial overlap between the phenotype of leukemic cells and those of normal hematopoietic cells (Coustan-Smith et al, 2003).
Flow cytometry-based assays are rapid and provide an accurate quantitation of MRD while gaining information on the status of normal hematopoietic cells at the same time. The number of antibody combinations used to identify leukemic cells and the stability of the markers targeted are important factors for the reliability of this approach. In general, it is advisable to use multiple sets of antibodies to compensate for immunophenotypic switches (Van Wering et al, 1995;Coustan-Smith et al, 1998;Baer et al, 2001;Gaipa et al, 2005). In addition to the skills necessary for reliable leukemia immunophenotyping, productive MRD studies by flow cytometry require great care to avoid sample contamination at all stages of processing as well as a solid knowledge of the immunophenotypic patters found in normal and regenerating bone marrow cells, particularly of immature myeloid and lymphoid cells (Campana & Coustan-Smith, 1999).
A second distinguishing feature of leukemic cells is the clonal rearrangement of immunoglobulin (IG) and T-cell receptor (TCR) genes. This leukemia-specific molecular signature can be found in the majority of cases of ALL (Pongers-Willemse et al, 1999), but in less than 10% of AML cases (Boeckx et al, 2002). “Real-time” PCR is the preferred method to detect cells with such rearrangements because it allows a precise quantitation of the PCR product (van der Velden et al, 2003), hence of MRD (each cell has one copy of the rearranged gene and the PCR product is directly proportional to the leukemic cell number). PCR analysis of IG and TCR genes allows the routine detection of 1 leukemic cells in 10,000–100,000 normal cells.
Monitoring the persistence of clonal antigen-receptor genes during treatment provides a sensitive and objective assessment of MRD. The reliability of the method can be affected by the presence of multiple rearrangements in the same leukemic cell population. Thus, a minor clone at diagnosis may become predominant during the course of the disease and remain undetected because only a major clone present at diagnosis is being monitored (Szczepanski et al, 2002;van der Velden et al, 2004). To prevent this potential problem, it is advisable to use sets of probes matching two or more different rearrangements (Pongers-Willemse et al, 1999;Flohr et al, 2008). Flohr et al. reported that among 3341 patients studied, two or more targets that allow PCR analysis with a 1 in 10,000 sensitivity or better were found in 2365 (71%); 671 (20%) additional patients had only one such target (Flohr et al, 2008). Alternately, the use of two independent MRD methods (e.g., PCR and flow cytometry) should greatly reduce the risk of false-negative results. Extensive standardization of the methods for PCR amplification of antigen-receptor genes has been performed by the BIOMED collaborative group, which has published specific guidelines for optimal performance in a clinical setting (van der Velden et al, 2007).
A third leukemia-associated feature that can be used to distinguish leukemic from normal cells is represented by chromosomal abnormalities and resulting gene fusions (van Dongen et al, 1999;Gabert et al, 2003). Fusion transcripts, such as BCR-ABL, MLL-AF4, E2A-PBX1, and TEL-AML1 in ALL, and AML1-ETO, CBFβ-MYH11 and PML-RARA in AML can be used as target for amplification; real-time PCR provides the most accurate way to measure their level (van Dongen et al, 1999;Gabert et al, 2003). Overall, less than one-third of patients with ALL or AML have leukemic cells with genetic abnormalities that can be studied with the typical assays performed in molecular pathology laboratories, allowing the detection of 1 leukemic cell in 1000 to 100,000 normal bone marrow cells.
An advantage of monitoring MRD by targeting fusion transcripts is the strong association between the molecular abnormality and the leukemic clone, irrespective of the presence of intraclonal differentiation and cellular changes caused by therapy. Although earlier studies had reported the detection of leukemia gene fusions in apparently healthy individuals (Bose et al, 1998), this does not seem to be a major problem, particularly at the detection levels used to monitor MRD clinically. The main disadvantage of targeting fusion transcripts is that the number of transcripts per leukemic cell may vary from patient to patient with the same genetic leukemia subtype and among different cells within the leukemic clone, and might be affected by therapy (Gabert et al, 2003). Therefore, precise quantitation of MRD with this technique can be difficult.
It has been suggested that overexpression of WT-1 could be used as a leukemia marker for MRD studies (Ogawa et al, 2002;Cilloni et al, 2003). Because WT1 is also expressed in normal CD34+ bone marrow cells (Maurer et al, 1997), the use of this approach in a clinical setting may not be straightforward. The ever-increasing understanding of the molecular lesions that participate to leukemogenesis might reveal new genomic alterations that can be used as targets for MRD assays (Mullighan et al, 2007).
Consideration for clinical use of MRD assays
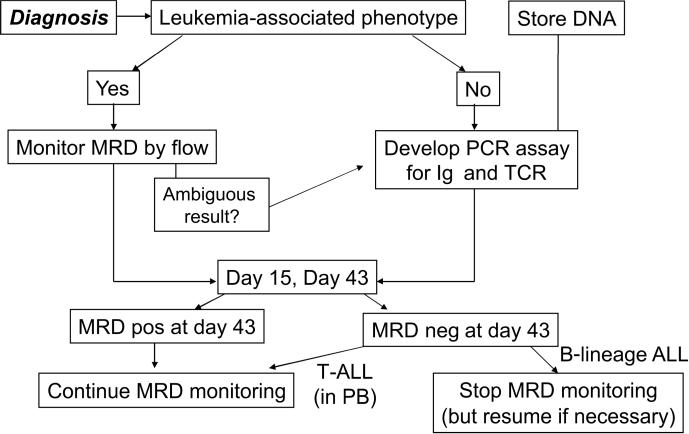
If the signal detected by an MRD assay corresponds closely to the number of leukemic cells present in the sample tested, it is expected that different MRD assays would yield concordant MRD estimates. Indeed, we and others found that flow cytometry and PCR amplification of IGH and TCR genes estimated similar levels of MRD in most remission samples obtained from children with ALL (Neale et al, 2004;Kerst et al, 2005). Given that the two technique yield similar results when MRD is present at levels of 0.01% or above, which is the best method for routine monitoring of MRD? Flow cytometry is more likely to be readily available (flow cytometers and methods for leukemia immunophenotyping are used at virtually every cancer center) but MRD monitoring requires expertise beyond that needed for leukemia immunophenotyping. Without such specific expertise, the likelihood of errors in MRD estimates is very high; laboratories that are unprepared to perform the assay correctly should resist the pressure to delivery MRD results until the methodology has been validated. Flow cytometry is generally quicker than PCR but both methods can produce MRD estimates within 24 hours of sample collection. For studies at early time points during therapy, e.g. day 15, flow cytometry has an advantage, as the development of a patient-tailored PCR assay currently requires more than two weeks. Conversely, PCR may be preferable for studies at the end of therapy, when the higher sensitivity of PCR might reveal MRD undetectable by flow cytometry. Although flow cytometry is often regarded to be less expensive, many variables must be factored in, such as the type of antibody panels used, the number of molecular targets studied, and the cost of sequencing and probe preparation for PCR. Based on our experience, we estimate the cost of the two methods to be similar overall. In sum, it is difficult to pick a clear winner on the basis of speed, accuracy, complexity and cost for MRD studies in ALL. Because either assay must be performed in specialized laboratories with proven expertise, the type of expert laboratory available to a cancer center or a cooperative group may ultimately be the decisive factor in selecting the method to be used. The strategy currently used at our institution, where both methods are available, is outlined in Fig. 1. In the case of AML there is no contest as flow cytometry is the only method that can study MRD in most patients.
Figure 1.
MRD monitoring strategy used in the current Total XVI study at St. Jude Children's Research Hospital.
While flow cytometry and PCR amplification of antigen-receptor genes typically yield similar MRD estimates in patients with ALL, the relation between these estimates and those obtained by PCR amplification of fusion transcripts is not entirely clear because systematic comparisons including the latter technique have not yet been performed. Such comparisons might provide unique insights in leukemia biology. For example, clinically silent preleukemic clones in patients with TEL-AML1 ALL (Hong et al, 2008) (and possibly other leukemia subtypes) might be detectable by PCR targeting of the fusion transcript but may lack the abnormal phenotypes and clonal antigen-receptor gene rearrangements observed in the leukemic cells at diagnosis. Persistent stem cell populations bearing BCR-ABL might become undetectable during treatment with methods that do not directly target the gene fusion.
MRD assays are complex, expensive and time-consuming in relation to other routine diagnostic assays for leukemia, which might preclude the wider application of MRD-directed therapy. The observation that normal lymphoid progenitors in the bone marrow, identified by the expression of CD19, CD10 and/or CD34, are exquisitely sensitive to corticosteroids and other antileukemic drugs (Coustan-Smith et al, 2006), suggested to us that this immunophenotype could be useful to monitor early response to therapy in patients with B-lineage ALL. Since lymphoblasts in most cases of this leukemia subtype express CD19, CD10 and/or CD34, the detection of such cells during remission induction therapy should reflect persistent disease. We therefore developed a 3-antibody assay and studied MRD in bone marrow samples collected on day 19 of remission induction therapy from 380 children with B-lineage ALL (Coustan-Smith et al, 2006). The results of the simplified assay correlated well with those of the standard flow cytometric assay and those of PCR amplification of antigen-receptor genes. We expect that this assay will facilitate the implementation of MRD measurements in centers that have limited resources to invest in MRD testing.
Must MRD studies be performed in bone marrow or can peripheral blood be used instead? In patients with B-lineage ALL (Brisco et al, 1997;Coustan-Smith et al, 2002b;van der Velden et al, 2002), and in those with AML (E. Coustan-Smith, D. Campana, et al., unpublished observations) MRD is usually detected at higher levels in bone marrow. Therefore, studies of blood might be less informative about the patient remission status than those in marrow. It is possible, however, that detection of MRD in peripheral blood may indicate a higher risk of relapse, as suggested by preliminary observations in patients with B-lineage ALL (Coustan-Smith et al, 2002b). The pattern of MRD distribution is different in patients with T-lineage ALL, where MRD levels in peripheral blood are similar to those in bone marrow (Coustan-Smith et al, 2002b;van der Velden et al, 2002). In these patients, sequential MRD testing can be performed in blood, a practice that we currently follow in the current St Jude Total XVI study (Fig. 1).
Prognostic significance of MRD in ALL
Many studies have demonstrated the prognostic importance of MRD as detected by flow cytometry in children with ALL (Coustan-Smith et al, 1998;Coustan-Smith et al, 2000;Coustan-Smith et al, 2002a;Dworzak et al, 2002;Borowitz et al, 2008). We found that patients who had MRD of 0.01% or higher in bone marrow at any of the time point during treatment had a significantly higher risk of relapse (Coustan-Smith et al, 1998;Coustan-Smith et al, 2000;Coustan-Smith et al, 2002a). Patients with MRD 1% or higher at the end of remission induction therapy and those with MRD 0.1% or higher during continuation therapy had an extremely high relapse hazard. MRD testing also identified a group of patients with a particularly favorable prognosis. Thus, among 112 patients studied on day 19 of remission induction therapy, the 53 who were MRD negative had a the 3-year cumulative incidence of relapse of less than 5% (Coustan-Smith et al, 2002a). Investigators of the Children's Oncology Group (COG) monitored MRD in peripheral blood specimens collected on day 8 and in bone marrow specimens collected on day 29 (end of remission induction therapy) in over 2000 children with B-lineage ALL (Borowitz et al, 2008). The presence of MRD (0.01% or higher) at either interval predicted a poorer outcome. The MRD results obtained in the day 29 bone marrow were the strongest prognostic indicator, superior to other commonly used prognostic parameters in childhood ALL. Of note, MRD predicted both early and late relapses.
Studies of MRD by PCR also showed clearly the prognostic importance of MRD (Brisco et al, 1994;Cave et al, 1998;van Dongen et al, 1998;Zhou et al, 2007;Flohr et al, 2008). Investigators of the International Berlin-Frankfurt-Munster (I-BFM) Study Group found that by combining the MRD information from day 33 and day 78 they could identify three groups of patients with a significantly different outcome: 43% of patients had MRD negative results at both time points and a 3-year relapse rate of only 2%; 15% of patients had MRD levels of 0.1% or higher at both time points and a relapse rate of 75%; the remaining patients (43%) had a relapse rate of 23%. These data were recently updated by Flohr et al.(Flohr et al, 2008) who reported 10-year event-free survivals of 93% for the low MRD risk group, 16% for the high MRD risk group and 74% for the intermediate risk group. Investigators of the Dana-Farber Cancer Institute ALL Consortium studied MRD in 284 children with B-lineage ALL. The 5-year risk of relapse was 5% in 176 children with no detectable MRD at end of remission induction and 44% in the 108 children with detectable MRD (P < 0.001) (Zhou et al, 2007). An MRD cut-off level of 0.1% was found to be the one that best predicted 5-year relapse hazard: 72% for patients with higher levels of MRD and 12% for those with lower levels.
MRD was also an independent predictor of subsequent relapse in patients with ALL who had a relapse and then achieved a second remission, irrespective of whether MRD was measured by PCR amplification of antigen-receptor genes (Eckert et al, 2001), or by flow cytometry (Coustan-Smith et al, 2004). We studied 35 patients with first relapsed ALL in second remission and detected MRD 0.01% or higher in 19 (54%). The 2-year cumulative incidence of second leukemia relapse was 70% for the MRD-positive patients and 28% for MRD-negative patients (P <0.01). Among patients with a first relapse off therapy, 2-year second relapse rates were 49% in the 12 MRD-positive and 0% in the 11 MRD-negative patients (P = 0.014); among those who received only chemotherapy after first relapse, the 2-year second relapse rates were 82%(n = 12) and 25% (n = 13), respectively (P <0.01). Time of first relapse and MRD were the only two significant predictors of outcome in a multivariate analysis.
MRD monitoring using PCR amplification of antigen-receptor gene rearrangements predicts outcome in patients undergoing hematopoietic stem cell transplantation (HSCT) (Knechtli et al, 1998;van der Velden et al, 2001;Bader et al, 2002;Uzunel et al, 2001;Krejci et al, 2003;Goulden et al, 2003). In patients receiving T-cell-depleted grafts, high levels of MRD-PCR positivity (0.1% to 1%) before HSCT were consistently associated with relapse post-transplant, and patients with lower levels of MRD had a 35% to 50% 2-year event-free survival as compared to 70% for MRD-negative patients (Knechtli et al, 1998;Bader et al, 2002;Uzunel et al, 2001;Krejci et al, 2003).
Prognostic significance of MRD in AML
Initial MRD studies in AML were performed in adult patients by using either RT-PCR amplification of fusion transcripts (LoCoco & Ammatuna, 2007;Tobal et al, 2000;Marcucci et al, 2001), or flow cytometry (Campana et al, 1990;San Miguel et al, 2001;Venditti et al, 2003;Buccisano et al, 2006). These studies demonstrated the potential clinical usefulness of monitoring MRD in AML.
Investigators of the COG detected MRD in the bone marrow of 41 of 252 children with AML, all of whom had achieved remission (Sievers et al, 2003). These patients had a 4.8 higher relapse hazard in a multivariate model, with MRD being the strongest prognostic factor. We studied MRD by flow cytometry in 46 children with de novo AML enrolled in the St. Jude Children's Research Hospital AML97 study and observed that the mean 2-year survival estimates for patients with MRD positivity (0.1% or higher) after induction therapy was 33% as compared to 72% for those with lower levels or no detectable MRD. MRD was the strongest predictor of outcome also in this cohort. Among patients tested after the first cycle of remission induction therapy, those in morphologic remission but with detectable MRD were 3.8 times more likely to die than those who were MRD negative. Similar observations were made for tests post-induction 2: MRD-positive patients were 6.2 times more likely to die than those with undetectable disease. Langebrake et al. (Langebrake et al, 2006) studied residual disease by flow cytometry in 150 children enrolled in the AML-BFM 98 study. Detection of residual disease was significantly associated with a lower event-free survival, with positive patients at the earlier time points having a more than 2-fold risk of relapse. When considered in combination with other prognostic factors, however, residual disease findings loss statistical significance in this series.
Clinical applications of MRD assays in childhood leukemia
In childhood ALL, slow clearance of leukemic cells during remission induction therapy as assessed by morphologic examination of peripheral blood or bone marrow predicts an inferior treatment outcome (Gajjar et al, 1995;Steinherz et al, 1996;Schrappe et al, 2000;Gaynon et al, 2000;Sandlund et al, 2002). The application of MRD assays to measure early response to therapy is considerably more powerful because a substantial proportion of poor responders by MRD criteria would not have been identified by morphologic analysis. In the recently completed Total XV study for newly diagnosed children with ALL at our institution, remission induction therapy was intensified for patients who had MRD 1% or higher on day 19 of remission induction therapy; post-remission therapy was intensified for standard risk patients who had MRD 0.01% or higher on day 46 (Pui et al, 2001). Moreover, any patient with MRD 1% or higher on day 46, or 0.1% or higher during continuation therapy was considered as candidate for allogeneic HSCT. The BFM group uses MRD levels on days 33 and 78 as a guide for treatment intensification (Flohr et al, 2008), and other groups worldwide are planning to introduce MRD in their risk-assignment schema.
Early clearance of MRD indicates a high chemosensitivity of the leukemic clone, and was associated with an excellent overall outcome in correlative studies (Panzer-Grumayer et al, 2000;Coustan-Smith et al, 2002a). Following this observation and considering that in past trials nearly half of children with ALL could be cured with therapy less intensive than that of today (Rivera et al, 1993), we hypothesize that patients who achieve MRD negativity after 2–3 weeks of remission induction chemotherapy can be cured with less intensive therapy. The need for treatment deintensification is particularly pressing in developing countries, where contemporary therapies for childhood ALL may have unacceptably high toxicities (Eden, 2002;Howard et al, 2004;Ribeiro & Pui, 2005). To this end, a protocol that incorporates reduction in treatment intensity for patients with negative MRD in bone marrow on day 19 as determined by a simplified flow cytometric assay (Coustan-Smith et al, 2006) has been implemented in Recife (Brazil). These studies are ongoing and the validity of this approach awaits evaluation.
In addition to measuring early response to chemotherapy, MRD assays have several other applications in the clinical management of children with ALL. For example, they can uncover impending relapse, thus giving a head start in the planning of salvage therapy and/or HSCT. Since the risk of relapse after HSCT is strongly related to levels of MRD before transplant (Krejci et al, 2003;Goulden et al, 2003), MRD measurements can also be used to determine the timing of HSCT. MRD measurements post-HSCT can be used to guide the administration of donor lymphocyte infusions or other agents. Finally, in children who relapse and achieve a second remission, MRD assays can be used to help selecting the optimal post-remission treatment, i.e. chemotherapy versus HSCT.
In patients with AML, MRD assays can also be applied to guide treatment decisions (Goulden et al, 2006). In our recently completed AML02 study, patients with MRD 1% or higher at the end of remission induction therapy were classified as high-risk and offered HSCT. In addition, patients with MRD 0.1% or higher received gentuzumab ozogamicin; if MRD persisted, they became candidates for HSCT. In addition, as described for childhood ALL, MRD can be used to optimized timing of HSCT and selection of post-HSCT therapy. It should be clear, however, that the overall clinical benefits of changes in therapy based on MRD findings remain to be proven.
Use of MRD for correlative studies with cellular and molecular features of leukemic cells
MRD measurements provide an indication of the drug sensitivity of leukemic cells. Therefore, they can be used to identify genes that are associated with multiagent chemoresistance in vivo.
Cario et al. (Cario et al, 2005) compared gene expression profiles of lymphoblasts in 21 B-lineage ALL patients with high MRD and 30 with low MRD enrolled in the BFM ALL-2000 protocol; leukemic cells in all patients lacked known genetic abnormalities predictive of outcome. Several genes whose expression was strongly associated with MRD were found; those with low expression in high-MRD cases were predominantly associated with cell-cycle progression and apoptosis.
We analyzed gene expression of diagnostic lymphoblasts from 189 children with ALL and compared the findings with MRD on days 46 of remission induction treatment (Flotho et al, 2006). Caspase 8 associated protein 2 (CASP8AP2) gene was of particular interest because of its strong association with MRD (patients with lower CASP8AP2 had higher MRD) and its reported role in apoptosis and glucocorticoid signaling. Low levels of CASP8AP2 expression were associated with a lower propensity of leukemic lymphoblasts to undergo apoptosis and predicted a lower event-free survival and a higher rate of leukemia relapse. We also compared gene expression in the same cohort with MRD results obtained on day 19 of remission induction treatment (Flotho et al, 2007). We identified 674 probe sets that were associated with MRD on day 19; 40 of the identified genes predicted relapse in the independent cohort of 99 patients. Among these, 14 showed independent prognostic significance. More than half of the 40 genes and nearly all of the 14 genes were functionally related, as indicated by their roles in the regulation of cell proliferation. Underexpression of genes promoting cell proliferation was associated with resistance to chemotherapy.
Conclusions
Currently available MRD assays ensure the objective and sensitive assessment of treatment response in most patients with acute leukemia. For example, in our recently closed Total XV study, 481 of 482 (99.8%) children with ALL could be monitored by flow cytometry and/or PCR amplification of IG and TCR genes; the only patient whose cells lacked suitable markers for these techniques had MLL-AF4 and response to therapy could be monitored by PCR amplification of the corresponding fusion transcript. It is now important to further simplify the assays so they can be implemented widely. To this end, new markers emerging from genome-wide expression studies might help reducing the number of antibodies required for flow cytometric analysis of MRD (Chen et al, 2001).
MRD studies can be used to quickly assess the effectiveness of novel antileukemic agents, and support innovative designs for Phase II studies. In this regard, an exciting opportunity is the possibility of determining the status of cell signaling pathways in the leukemic cell population (Irish et al, 2006). Taking advantage of new flow cytometers capable of detecting 9 or more parameters, it should be possible to assess whether the signaling pathways targeted by tyrosine kinase inhibitors are affected in the MRD cell population.
Acknowledgments
This work was supported by grants CA60419, CA115422 and CA21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
References
- Bader P, Hancock J, Kreyenberg H, Goulden NJ, Niethammer D, Oakhill A, Steward CG, Handgretinger R, Beck JF, Klingebiel T. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16:1668–1672. doi: 10.1038/sj.leu.2402552. [DOI] [PubMed] [Google Scholar]
- Baer MR, Stewart CC, Dodge RK, Leget G, Sule N, Mrozek K, Schiffer CA, Powell BL, Kolitz JE, Moore JO, Stone RM, Davey FR, Carroll AJ, Larson RA, Bloomfield CD. High frequency of immunophenotype changes in acute myeloid leukemia at relapse: implications for residual disease detection (Cancer and Leukemia Group B Study 8361) Blood. 2001;97:3574–3580. doi: 10.1182/blood.v97.11.3574. [DOI] [PubMed] [Google Scholar]
- Boeckx N, Willemse MJ, Szczepanski T, van der Velden V, Langerak AW, Vandekerckhove P, van Dongen JJ. Fusion gene transcripts and Ig/TCR gene rearrangements are complementary but infrequent targets for PCR-based detection of minimal residual disease in acute myeloid leukemia. Leukemia. 2002;16:368–375. doi: 10.1038/sj.leu.2402387. [DOI] [PubMed] [Google Scholar]
- Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, Linda S, Martin PL, Pullen DJ, Viswanatha DS, Willman CL, Winick N, Camitta BM. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors. a Children's Oncology Group study. Blood. 2008 doi: 10.1182/blood-2008-01-132837. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Deininger M, Gora-Tybor J, Goldman JM, Melo JV. The presence of typical and atypical BCR-ABL fusion genes in leukocytes of normal individuals: biologic significance and implications for the assessment of minimal residual disease. Blood. 1998;92:3362–3367. [PubMed] [Google Scholar]
- Brisco MJ, Condon J, Hughes E, Neoh SH, Sykes PJ, Seshadri R, Toogood I, Waters K, Tauro G, Ekert H. Outcome prediction in childhood acute lymphoblastic leukaemia by molecular quantification of residual disease at the end of induction. Lancet. 1994;343:196–200. doi: 10.1016/s0140-6736(94)90988-1. [DOI] [PubMed] [Google Scholar]
- Brisco MJ, Sykes PJ, Hughes E, Dolman G, Neoh SH, Peng LM, Toogood I, Morley AA. Monitoring minimal residual disease in peripheral blood in B-lineage acute lymphoblastic leukaemia. Br J Haematol. 1997;99:314–319. doi: 10.1046/j.1365-2141.1997.3723186.x. [DOI] [PubMed] [Google Scholar]
- Buccisano F, Maurillo L, Gattei V, Del PG, Del Principe MI, Cox MC, Panetta P, Consalvo MI, Mazzone C, Neri B, Ottaviani L, Fraboni D, Tamburini A, Lo-Coco F, Amadori S, Venditti A. The kinetics of reduction of minimal residual disease impacts on duration of response and survival of patients with acute myeloid leukemia. Leukemia. 2006;20:1783–1789. doi: 10.1038/sj.leu.2404313. [DOI] [PubMed] [Google Scholar]
- Campana D. Determination of minimal residual disease in leukemia patients. Br J Haematol. 2003;121:823–838. doi: 10.1046/j.1365-2141.2003.04393.x. [DOI] [PubMed] [Google Scholar]
- Campana D, Coustan-Smith E. Detection of minimal residual disease in acute leukemia by flow cytometry. Cytometry. 1999;38:139–152. doi: 10.1002/(sici)1097-0320(19990815)38:4<139::aid-cyto1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Campana D, Coustan-Smith E, Janossy G. The immunologic detection of minimal residual disease in acute leukemia. Blood. 1990;76:163–171. [PubMed] [Google Scholar]
- Campana D, Pui CH. Detection of minimal residual disease in acute leukemia: methodologic advances and clinical significance. Blood. 1995;85:1416–1434. [PubMed] [Google Scholar]
- Cario G, Stanulla M, Fine BM, Teuffel O, Neuhoff NV, Schrauder A, Flohr T, Schafer BW, Bartram CR, Welte K, Schlegelberger B, Schrappe M. Distinct gene expression profiles determine molecular treatment response in childhood acute lymphoblastic leukemia. Blood. 2005;105:821–826. doi: 10.1182/blood-2004-04-1552. [DOI] [PubMed] [Google Scholar]
- Cave H, van der Werff ten Bosch, Suciu S, Guidal C, Waterkeyn C, Otten J, Bakkus M, Thielemans K, Grandchamp B, Vilmer E. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer--Childhood Leukemia Cooperative Group. N Engl J Med. 1998;339:591–598. doi: 10.1056/NEJM199808273390904. [DOI] [PubMed] [Google Scholar]
- Chen JS, Coustan-Smith E, Suzuki T, Neale GA, Mihara K, Pui CH, Campana D. Identification of novel markers for monitoring minimal residual disease in acute lymphoblastic leukemia. Blood. 2001;97:2115–2120. doi: 10.1182/blood.v97.7.2115. [DOI] [PubMed] [Google Scholar]
- Cilloni D, Gottardi E, Fava M, Messa F, Carturan S, Busca A, Guerrasio A, Saglio G. Usefulness of quantitative assessment of the WT1 gene transcript as a marker for minimal residual disease detection. Blood. 2003;102:773–774. doi: 10.1182/blood-2003-03-0980. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Behm FG, Sanchez J, Boyett JM, Hancock ML, Raimondi SC, Rubnitz JE, Rivera GK, Sandlund JT, Pui CH, Campana D. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet. 1998;351:550–554. doi: 10.1016/S0140-6736(97)10295-1. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Gajjar A, Hijiha N, Razzouk BI, Ribeiro RC, Rivera GK, Rubnitz JE, Sandlund JT, Andreansky M, Hancock ML, Pui CH, Campana D. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia after first relapse. Leukemia. 2004;18:499–504. doi: 10.1038/sj.leu.2403283. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Ribeiro RC, Rubnitz JE, Razzouk BI, Pui CH, Pounds S, Andreansky M, Behm FG, Raimondi SC, Shurtleff SA, Downing JR, Campana D. Clinical significance of residual disease during treatment in childhood acute myeloid leukemia. Br J Haematol. 2003;123:243–252. doi: 10.1046/j.1365-2141.2003.04610.x. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Ribeiro RC, Stow P, Zhou Y, Pui CH, Rivera GK, Pedrosa F, Campana D. A simplified flow cytometric assay identifies children with acute lymphoblastic leukemia who have a superior clinical outcome. Blood. 2006;108:97–102. doi: 10.1182/blood-2006-01-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustan-Smith E, Sancho J, Behm FG, Hancock ML, Razzouk BI, Ribeiro RC, Rivera GK, Rubnitz JE, Sandlund JT, Pui CH, Campana D. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood. 2002a;100:52–58. doi: 10.1182/blood-2002-01-0006. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Sancho J, Hancock ML, Boyett JM, Behm FG, Raimondi SC, Sandlund JT, Rivera GK, Rubnitz JE, Ribeiro RC, Pui CH, Campana D. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000;96:2691–2696. [PubMed] [Google Scholar]
- Coustan-Smith E, Sancho J, Hancock ML, Razzouk BI, Ribeiro RC, Rivera GK, Rubnitz JE, Sandlund JT, Pui CH, Campana D. Use of peripheral blood instead of bone marrow to monitor residual disease in children with acute lymphoblastic leukemia. Blood. 2002b;100:2399–2402. doi: 10.1182/blood-2002-04-1130. [DOI] [PubMed] [Google Scholar]
- Dworzak MN, Froschl G, Printz D, Mann G, Potschger U, Muhlegger N, Fritsch G, Gadner H. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002;99:1952–1958. doi: 10.1182/blood.v99.6.1952. [DOI] [PubMed] [Google Scholar]
- Eckert C, Biondi A, Seeger K, Cazzaniga G, Hartmann R, Beyermann B, Pogodda M, Proba J, Henze G. Prognostic value of minimal residual disease in relapsed childhood acute lymphoblastic leukaemia. Lancet. 2001;358:1239–1241. doi: 10.1016/S0140-6736(01)06355-3. [DOI] [PubMed] [Google Scholar]
- Eden T. Translation of cure for acute lymphoblastic leukaemia to all children. Br.J Haematol. 2002;118:945–951. doi: 10.1046/j.1365-2141.2002.03670.x. [DOI] [PubMed] [Google Scholar]
- Flohr T, Schrauder A, Cazzaniga G, Panzer-Grumayer R, van d.V., V, Fischer S, Stanulla M, Basso G, Niggli FK, Schafer BW, Sutton R, Koehler R, Zimmermann M, Valsecchi MG, Gadner H, Masera G, Schrappe M, van Dongen JJ, Biondi A, Bartram CR. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008 doi: 10.1038/leu.2008.5. in press. [DOI] [PubMed] [Google Scholar]
- Flotho C, Coustan-Smith E, Pei D, Cheng C, Song G, Pui CH, Downing JR, Campana D. A set of genes that regulate cell proliferation predicts treatment outcome in childhood acute lymphoblastic leukemia. Blood. 2007;110:1271–1277. doi: 10.1182/blood-2007-01-068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotho C, Coustan-Smith E, Pei D, Iwamoto S, Song G, Cheng C, Pui CH, Downing JR, Campana D. Genes contributing to minimal residual disease in childhood acute lymphoblastic leukemia: prognostic significance of CASP8AP2. Blood. 2006;108:1050–1057. doi: 10.1182/blood-2006-01-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabert J, Beillard E, van der Velden V, Bi W, Grimwade D, Pallisgaard N, Barbany G, Cazzaniga G, Cayuela JM, Cave H, Pane F, Aerts JL, De Micheli D, Thirion X, Pradel V, Gonzalez M, Viehmann S, Malec M, Saglio G, van Dongen JJ. Standardization and quality control studies of `real-time' quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003;17:2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- Gaipa G, Basso G, Maglia O, Leoni V, Faini A, Cazzaniga G, Bugarin C, Veltroni M, Michelotto B, Ratei R, Coliva T, Valsecchi MG, Biondi A, Dworzak MN. Drug-induced immunophenotypic modulation in childhood ALL: implications for minimal residual disease detection. Leukemia. 2005;19:49–56. doi: 10.1038/sj.leu.2403559. [DOI] [PubMed] [Google Scholar]
- Gajjar A, Ribeiro R, Hancock ML, Rivera GK, Mahmoud H, Sandlund JT, Crist WM, Pui CH. Persistence of circulating blasts after 1 week of multiagent chemotherapy confers a poor prognosis in childhood acute lymphoblastic leukemia. Blood. 1995;86:1292–1295. [PubMed] [Google Scholar]
- Gaynon PS, Trigg ME, Heerema NA, Sensel MG, Sather HN, Hammond GD, Bleyer WA. Children's Cancer Group trials in childhood acute lymphoblastic leukemia: 1983–1995. Leukemia. 2000;14:2223–2233. doi: 10.1038/sj.leu.2401939. [DOI] [PubMed] [Google Scholar]
- Goulden N, Bader P, van d.V., V, Moppett J, Schilham M, Masden HO, Krejci O, Kreyenberg H, Lankester A, Revesz T, Klingebiel T, Van Dongen J. Minimal residual disease prior to stem cell transplant for childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;122:24–29. doi: 10.1046/j.1365-2141.2003.04394.x. [DOI] [PubMed] [Google Scholar]
- Goulden N, Virgo P, Grimwade D. Minimal residual disease directed therapy for childhood acute myeloid leukaemia: the time is now. Br.J.Haematol. 2006;134:273–282. doi: 10.1111/j.1365-2141.2006.06182.x. [DOI] [PubMed] [Google Scholar]
- Hong D, Gupta R, Ancliff P, Atzberger A, Brown J, Soneji S, Green J, Colman S, Piacibello W, Buckle V, Tsuzuki S, Greaves M, Enver T. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science. 2008;319:336–339. doi: 10.1126/science.1150648. [DOI] [PubMed] [Google Scholar]
- Howard SC, Pedrosa M, Lins M, Pedrosa A, Pui CH, Ribeiro RC, Pedrosa F. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA. 2004;291:2471–2475. doi: 10.1001/jama.291.20.2471. [DOI] [PubMed] [Google Scholar]
- Irish JM, Kotecha N, Nolan GP. Mapping normal and cancer cell signalling networks: towards single-cell proteomics. Nat.Rev.Cancer. 2006;6:146–155. doi: 10.1038/nrc1804. [DOI] [PubMed] [Google Scholar]
- Kerst G, Kreyenberg H, Roth C, Well C, Dietz K, Coustan-Smith E, Campana D, Koscielniak E, Niemeyer C, Schlegel PG, Muller I, Niethammer D, Bader P. Concurrent detection of minimal residual disease (MRD) in childhood acute lymphoblastic leukaemia by flow cytometry and real-time PCR. Br.J.Haematol. 2005;128:774–782. doi: 10.1111/j.1365-2141.2005.05401.x. [DOI] [PubMed] [Google Scholar]
- Knechtli CJC, Goulden NJ, Hancock JP, Grandage VLG, Harris EL, Garland RJ, Jones CG, Rowbottom AW, Hunt LP, Green AF, Clarke E, Lankester AW, Cornish JM, Pamphilon DH, Steward CG, Oakhill A. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. 1998;92:4072–4079. [PubMed] [Google Scholar]
- Krejci O, van der Velden V, Bader P, Kreyenberg H, Goulden N, Hancock J, Schilham MW, Lankester A, Revesz T, Klingebiel T, van Dongen JJ. Level of minimal residual disease prior to haematopoietic stem cell transplantation predicts prognosis in paediatric patients with acute lymphoblastic leukaemia: a report of the Pre-BMT MRD Study Group. Bone Marrow Transplant. 2003;32:849–851. doi: 10.1038/sj.bmt.1704241. [DOI] [PubMed] [Google Scholar]
- Langebrake C, Creutzig U, Dworzak M, Hrusak O, Mejstrikova E, Griesinger F, Zimmermann M, Reinhardt D. Residual disease monitoring in childhood acute myeloid leukemia by multiparameter flow cytometry: the MRD-AML-BFM Study Group. J.Clin.Oncol. 2006;24:3686–3692. doi: 10.1200/JCO.2005.05.4312. [DOI] [PubMed] [Google Scholar]
- LoCoco F, Ammatuna E. Front line clinical trials and minimal residual disease monitoring in acute promyelocytic leukemia. Curr.Top.Microbiol.Immunol. 2007;313:145–156. doi: 10.1007/978-3-540-34594-7_9. [DOI] [PubMed] [Google Scholar]
- Marcucci G, Caligiuri MA, Dohner H, Archer KJ, Schlenk RF, Dohner K, Maghraby EA, Bloomfield CD. Quantification of CBFbeta/MYH11 fusion transcript by real time RT-PCR in patients with INV(16) acute myeloid leukemia. Leukemia. 2001;15:1072–1080. doi: 10.1038/sj.leu.2402159. [DOI] [PubMed] [Google Scholar]
- Maurer U, Weidmann E, Karakas T, Hoelzer D, Bergmann L. Wilms tumor gene (wt1) mRNA is equally expressed in blast cells from acute myeloid leukemia and normal CD34+ progenitors. Blood. 1997;90:4230–4232. [PubMed] [Google Scholar]
- Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, Su X, Pui CH, Relling MV, Evans WE, Shurtleff SA, Downing JR. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- Neale GA, Coustan-Smith E, Stow P, Pan Q, Chen X, Pui CH, Campana D. Comparative analysis of flow cytometry and polymerase chain reaction for the detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2004;18:934–938. doi: 10.1038/sj.leu.2403348. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Tamaki H, Ikegame K, Soma T, Kawakami M, Tsuboi A, Kim EH, Hosen N, Murakami M, Fujioka T, Masuda T, Taniguchi Y, Nishida S, Oji Y, Oka Y, Sugiyama H. The usefulness of monitoring WT1 gene transcripts for the prediction and management of relapse following allogeneic stem cell transplantation in acute type leukemia. Blood. 2002;101:1698–704. doi: 10.1182/blood-2002-06-1831. [DOI] [PubMed] [Google Scholar]
- Panzer-Grumayer ER, Schneider M, Panzer S, Fasching K, Gadner H. Rapid molecular response during early induction chemotherapy predicts a good outcome in childhood acute lymphoblastic leukemia. Blood. 2000;95:790–794. [PubMed] [Google Scholar]
- Pongers-Willemse MJ, Seriu T, Stolz F, d'Aniello E, Gameiro P, Pisa P, Gonzalez M, Bartram CR, Panzer-Grumayer ER, Biondi A, San-Miguel JF, van Dongen JJ. Primers and protocols for standardized detection of minimal residual disease in acute lymphoblastic leukemia using immunoglobulin and T cell receptor gene rearrangements and TAL1 deletions as PCR targets. Leukemia. 1999;13:110–118. doi: 10.1038/sj.leu.2401245. [DOI] [PubMed] [Google Scholar]
- Pui CH, Campana D, Evans WE. Childhood acute lymphoblastic leukemia - Current status and future perspectives. Lancet Oncology. 2001;2:597–607. doi: 10.1016/S1470-2045(01)00516-2. [DOI] [PubMed] [Google Scholar]
- Ribeiro RC, Pui CH. Saving the children--improving childhood cancer treatment in developing countries. N.Engl.J.Med. 2005;352:2158–2160. doi: 10.1056/NEJMp048313. [DOI] [PubMed] [Google Scholar]
- Rivera GK, Pinkel D, Simone JV, Hancock ML, Crist WM. Treatment of acute lymphoblastic leukemia. 30 years' experience at St. Jude Children's Research Hospital. N.Engl.J Med. 1993;329:1289–1295. doi: 10.1056/NEJM199310283291801. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Vidriales MB, Lopez-Berges C, Diaz-Mediavilla J, Gutierrez N, Canizo C, Ramos F, Calmuntia MJ, Perez JJ, Gonzalez M, Orfao A. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood. 2001;98:1746–1751. doi: 10.1182/blood.v98.6.1746. [DOI] [PubMed] [Google Scholar]
- Sandlund JT, Harrison PL, Rivera G, Behm FG, Head D, Boyett J, Rubnitz JE, Gajjar A, Raimondi S, Ribeiro R, Hudson M, Relling M, Evans W, Pui CH. Persistence of lymphoblasts in bone marrow on day 15 and days 22 to 25 of remission induction predicts a dismal treatment outcome in children with acute lymphoblastic leukemia. Blood. 2002;100:43–47. doi: 10.1182/blood.v100.1.43. [DOI] [PubMed] [Google Scholar]
- Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig WD, Henze G, Gadner H, Odenwald E, Riehm H. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster. Leukemia. 2000;14:2205–2222. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- Sievers EL, Lange BJ, Alonzo TA, Gerbing RB, Bernstein ID, Smith FO, Arceci RJ, Woods WG, Loken MR. Immunophenotypic evidence of leukemia after induction therapy predicts relapse: results from a prospective Children's Cancer Group study of 252 acute myeloid leukemia patients. Blood. 2003;101:3398–3406. doi: 10.1182/blood-2002-10-3064. [DOI] [PubMed] [Google Scholar]
- Steinherz PG, Gaynon PS, Breneman JC, Cherlow JM, Grossman NJ, Kersey JH, Johnstone HS, Sather HN, Trigg ME, Chappell R, Hammond D, Bleyer WA. Cytoreduction and prognosis in acute lymphoblastic leukemia--the importance of early marrow response: report from the Childrens Cancer Group [see comments] J Clin Oncol. 1996;14:389–398. doi: 10.1200/JCO.1996.14.2.389. [DOI] [PubMed] [Google Scholar]
- Szczepanski T, Orfao A, van der Velden VH, San Miguel JF, van Dongen JJ. Minimal residual disease in leukaemia patients. Lancet Oncology. 2001;2:409–417. doi: 10.1016/s1470-2045(00)00418-6. [DOI] [PubMed] [Google Scholar]
- Szczepanski T, Willemse MJ, Brinkhof B, Van Wering ER, van der BM, van Dongen JJ. Comparative analysis of Ig and TCR gene rearrangements at diagnosis and at relapse of childhood precursor-B-ALL provides improved strategies for selection of stable PCR targets for monitoring of minimal residual disease. Blood. 2002;99:2315–2323. doi: 10.1182/blood.v99.7.2315. [DOI] [PubMed] [Google Scholar]
- Tobal K, Newton J, Macheta M, Chang J, Morgenstern G, Evans PA, Morgan G, Lucas GS, Liu YJ. Molecular quantitation of minimal residual disease in acute myeloid leukemia with t(8;21) can identify patients in durable remission and predict clinical relapse. Blood. 2000;95:815–819. [PubMed] [Google Scholar]
- Uzunel M, Mattsson J, Jaksch M, Remberger M, Ringden O. The significance of graft-versus-host disease and pretransplantation minimal residual disease status to outcome after allogeneic stem cell transplantation in patients with acute lymphoblastic leukemia. Blood. 2001;98:1982–1984. doi: 10.1182/blood.v98.6.1982. [DOI] [PubMed] [Google Scholar]
- van der Velden V, Bruggemann M, Hoogeveen PG, de Bie M, Hart PG, Raff T, Pfeifer H, Luschen S, Szczepanski T, Van Wering ER, Kneba M, van Dongen JJ. TCRB gene rearrangements in childhood and adult precursor-B-ALL: frequency, applicability as MRD-PCR target, and stability between diagnosis and relapse. Leukemia. 2004;18:1971–1980. doi: 10.1038/sj.leu.2403505. [DOI] [PubMed] [Google Scholar]
- van der Velden V, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER, Flohr T, Sutton R, Cave H, Madsen HO, Cayuela JM, Trka J, Eckert C, Foroni L, zur Stadt U, Beldjord K, Raff T, van der Schoot CE, van Dongen JJ. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–611. doi: 10.1038/sj.leu.2404586. [DOI] [PubMed] [Google Scholar]
- van der Velden V, Joosten SA, Willemse MJ, Van Wering ER, Lankester AW, van Dongen JJ, Hoogerbrugge PM. Real-time quantitative PCR for detection of minimal residual disease before allogeneic stem cell transplantation predicts outcome in children with acute lymphoblastic leukemia. Leukemia. 2001;15:1485–1487. doi: 10.1038/sj.leu.2402198. [DOI] [PubMed] [Google Scholar]
- van der Velden V, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003;17:1013–1034. doi: 10.1038/sj.leu.2402922. [DOI] [PubMed] [Google Scholar]
- van der Velden V, Jacobs DC, Wijkhuijs AJ, Comans-Bitter WM, Willemse MJ, Hahlen K, Kamps WA, Van Wering ER, van Dongen JJ. Minimal residual disease levels in bone marrow and peripheral blood are comparable in children with T cell acute lymphoblastic leukemia (ALL), but not in precursor-B-ALL. Leukemia. 2002;16:1432–1436. doi: 10.1038/sj.leu.2402636. [DOI] [PubMed] [Google Scholar]
- van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, Gottardi E, Rambaldi A, Dotti G, Griesinger F, Parreira A, Gameiro P, Diaz MG, Malec M, Langerak AW, San Miguel JF, Biondi A. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13:1901–1928. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- van Dongen JJ, Seriu T, Panzer-Grumayer ER, Biondi A, Pongers-Willemse MJ, Corral L, Stolz F, Schrappe M, Masera G, Kamps WA, Gadner H, Van Wering ER, Ludwig W-D, Basso G, de Bruijn MAC, Cazzaniga G, Hettinger K, van der Does-van den Berg A, Hop WCJ, Riehm H, Bartram CR. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352:1731–1738. doi: 10.1016/S0140-6736(98)04058-6. [DOI] [PubMed] [Google Scholar]
- Van Wering ER, Beishuizen A, Roeffen ET, van der Linden-Schrever BE, Verhoeven MA, Hahlen K, Hooijkaas H, van Dongen JJ. Immunophenotypic changes between diagnosis and relapse in childhood acute lymphoblastic leukemia. Leukemia. 1995;9:1523–1533. [PubMed] [Google Scholar]
- Venditti A, Maurillo L, Buccisano F, Del Poeta G, Mazzone C, Tamburini A, Del Principe MI, Consalvo MI, De FP, Cudillo L, Picardi A, Franchi A, LoCoco F, Amadori S. Pretransplant minimal residual disease level predicts clinical outcome in patients with acute myeloid leukemia receiving high-dose chemotherapy and autologous stem cell transplantation. Leukemia. 2003;17:2178–2182. doi: 10.1038/sj.leu.2403138. [DOI] [PubMed] [Google Scholar]
- Zhou J, Goldwasser MA, Li A, Dahlberg SE, Neuberg D, Wang H, Dalton V, McBride KD, Sallan SE, Silverman LB, Gribben JG. Quantitative analysis of minimal residual disease predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCI ALL Consortium Protocol 95–01. Blood. 2007;110:1607–1611. doi: 10.1182/blood-2006-09-045369. [DOI] [PMC free article] [PubMed] [Google Scholar]