Abstract
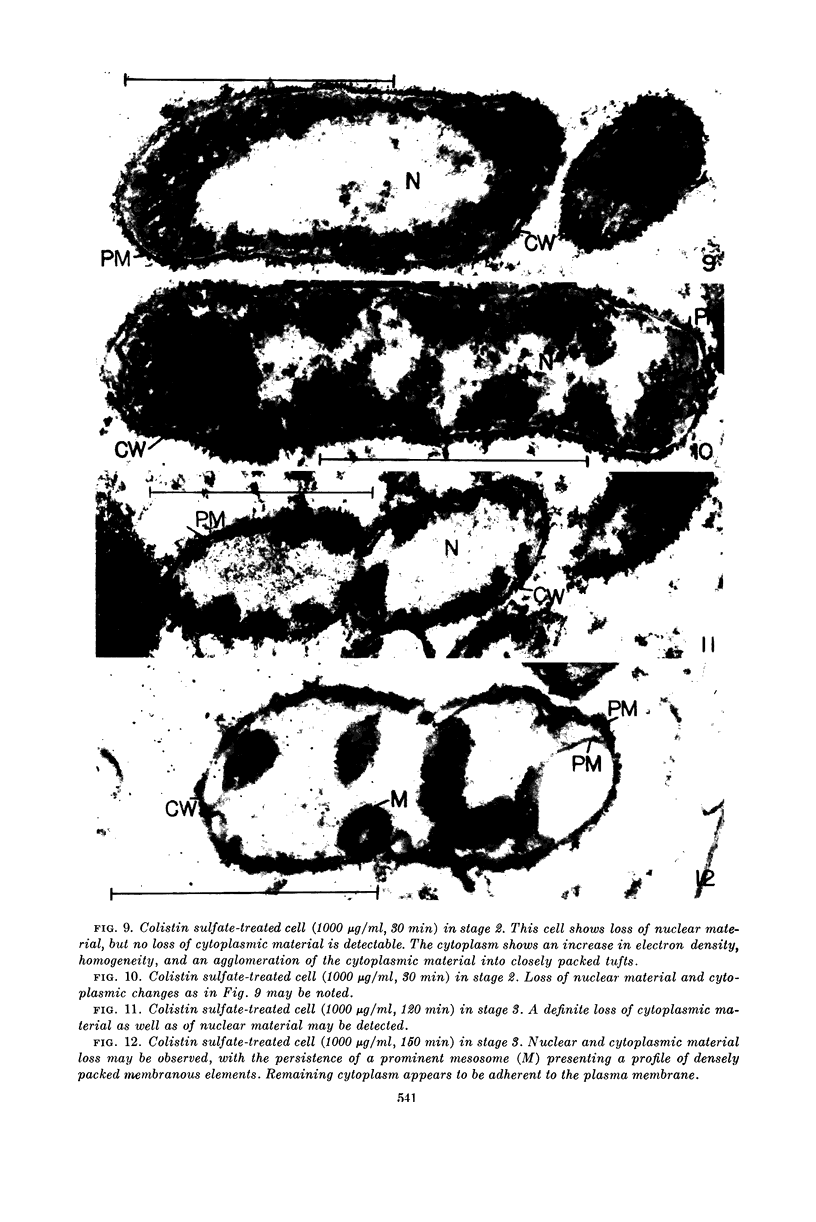
Kaye, Jeremy J. (Cornell University Medical College, New York, N.Y.) and George B. Chapman. Cytological aspects of antimicrobial antibiosis. III. Cytologically distinguishable stages in antibiotic action of colistin sulfate on Escherichia coli. J. Bacteriol. 86:536–543. 1963.—Broth cultures of Escherichia coli were subjected to a constant concentration of colistin sulfate for varying periods of time. Controls and treated cells were fixed, dehydrated, and embedded in methacrylate, and ultrathin sections were examined in an electron microscope. Three stages in the antibiotic process were discerned. Stage 1 was characterized by a disruption of the axial orientation of the nuclear material and by an invasion of nuclear areas by tufts of material presumably of cytoplasmic origin; no loss of cellular contents could be detected cytologically. Stage 2 was characterized by the loss of nuclear material and by a loss of typical cytoplasmic granularity, an increase in cytoplasmic electron density, and an agglomeration of the cytoplasm into packed tufts of material; in contrast to the nuclear material, there was no loss of cytoplasmic material in this stage. Stage 3 was characterized by the loss of the altered cytoplasmic material but with the persistence of mesosomes, plasma membrane, and cell wall. Speculation that each and all of these changes might have resulted from an altered intracellular milieu secondary to a primary effect of the antibiotic on the plasma membrane is presented.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D. Effects of magnesium ion deficiency on Escherichia coli and possible relation to the mode of action of novobiocin. J Bacteriol. 1962 Oct;84:679–682. doi: 10.1128/jb.84.4.679-682.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYSTRICKY V., LADZIANSKA K., HALASA M. Electron microscopy of the action of polymyxin on leptospirae. J Bacteriol. 1962 Oct;84:864–865. doi: 10.1128/jb.84.4.864-865.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B. Cytological aspects of antimicrobial antibiosis. I. Cytological changes associated with the exposure of Escherichia coli to colistin sulfate. J Bacteriol. 1962 Jul;84:169–179. doi: 10.1002/path.1700840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B. Cytological aspects of antimicrobial antibiosis. II. Cytological changes associated with the exposure of Pseudomonas aeruginosa and Bacillus megaterium to colistin sulfate. J Bacteriol. 1962 Jul;84:180–185. doi: 10.1128/jb.84.1.180-185.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEW A. V. Electron microscopy of disrupted bacteria treated with polymyxin E. J Gen Microbiol. 1954 Apr;10(2):304–308. doi: 10.1099/00221287-10-2-304. [DOI] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. A membranous component of the cytoplasm in Streptomyces coelicolor. J Biophys Biochem Cytol. 1959 Dec;6:515–516. doi: 10.1083/jcb.6.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. The fine structure of Streptomyces coelicolor. I. The cytoplasmic membrane system. J Biophys Biochem Cytol. 1960 Jun;7:479–488. doi: 10.1083/jcb.7.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Contribution à l'étude du noyau bactérien. Schweiz Z Pathol Bakteriol. 1955;18(5):1122–1137. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDD S., HILLIER J., BEUTNER E. H., HARTMAN P. E. Light and electron microscopic studies of Escherichia coli-coliphage interactions. II. The electron microscopic cytology of the E. coli B-T2 system. Biochim Biophys Acta. 1953 Jan;10(1):153–179. doi: 10.1016/0006-3002(53)90224-8. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., FRANCOMBE W. H., MAYALL B. H. The effect of penicillin on the structure of staphylococcal cell walls. Can J Microbiol. 1959 Dec;5:641–648. doi: 10.1139/m59-078. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. A fluorescent derivative of polymyxin: its preparation and use in studying the site of action of the antibiotic. J Gen Microbiol. 1955 Apr;12(2):226–236. doi: 10.1099/00221287-12-2-226. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. The release of soluble constituents from washed cells of Pseudomonas aeruginosa by the action of polymyxin. J Gen Microbiol. 1953 Aug;9(1):54–64. doi: 10.1099/00221287-9-1-54. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. J., HORNE R. W., COSSLETT V. E. Electron microscopy of bacteria treated with cetyltrimethylammonium bromide. J Gen Microbiol. 1951 May;5(2):405–407. doi: 10.1099/00221287-5-2-405. [DOI] [PubMed] [Google Scholar]
- SATIR P. G., PEACHEY L. D. Thin sections. II. A simple method for reducing compression artifacts. J Biophys Biochem Cytol. 1958 May 25;4(3):345–348. doi: 10.1083/jcb.4.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ B. S., WARREN M. R., BARKLEY F. A., LANDIS L. Microbiological and pharmacological studies of colistin sulfate and sodium colistinmethanesulfonate. Antibiot Annu. 1959;7:41–60. [PubMed] [Google Scholar]
- TISSIERES A., WATSON J. D. Ribonucleoprotein particles from Escherichia coli. Nature. 1958 Sep 20;182(4638):778–780. doi: 10.1038/182778b0. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITFIELD J. F., MURRAY R. G. The effects of the ionic environment on the chromatin structures of bacteria. Can J Microbiol. 1956 May;2(3):245–260. doi: 10.1139/m56-029. [DOI] [PubMed] [Google Scholar]