Abstract
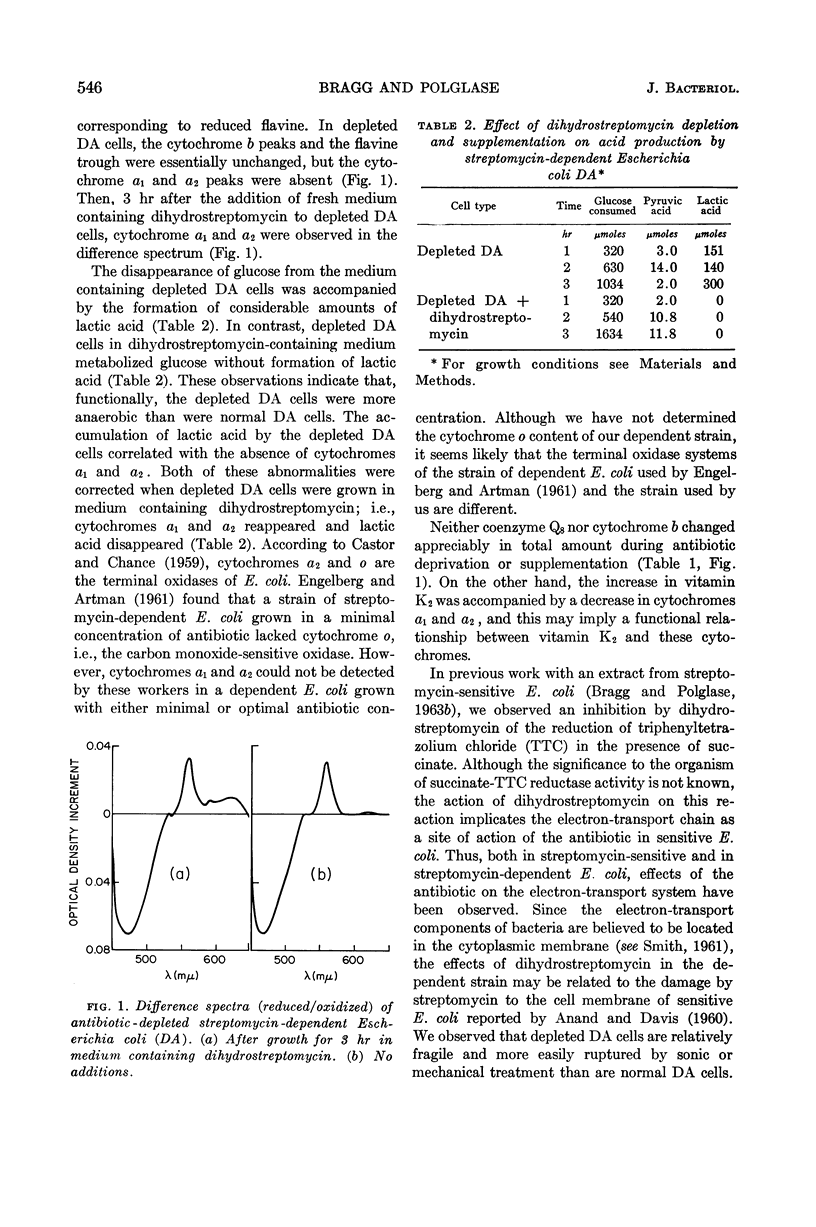
Bragg, P. D. (University of British Columbia, Vancouver, B. C, Canada) and W. J. Polglase. Electron-transport components of streptomycin-dependent Escherichia coli. J. Bacteriol. 86:544–547. 1963.—When a streptomycin-dependent strain of Escherichia coli was grown in antibiotic-free medium, the resulting streptomycin-depleted cells were found to contain relatively large amounts of vitamin K2. Cytochromes a1 and a2 were absent from depleted cells, and these cells metabolized glucose with the accumulation of lactic acid. Depleted cells were normal in their content of coenzyme Q8, total flavine, and cytochrome b. Growth of depleted cells on medium containing dihydrostreptomycin restored their ability to metabolize lactic acid concomitantly with a decrease in vitamin K2 to a normal level, and with the formation of cytochromes a1 and a2. It is concluded that streptomycin (or dihydrostreptomycin) is required by dependent E. coli for maintenance of the integrity of the electron-transport system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND N., DAVIS B. D. Damage by streptomycin to the cell membrane of Escherichia coli. Nature. 1960 Jan 2;185:22–23. doi: 10.1038/185022a0. [DOI] [PubMed] [Google Scholar]
- BISHOP D. H., PANDYA K. P., KING H. K. Ubiquinone and vitamin K in bacteria. Biochem J. 1962 Jun;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAGG P. D., POLGLASE W. J. ACTION OF DIHYDROSTREPTOMYCIN AND ANTAGONISM BY CATIONS. J Bacteriol. 1963 Mar;85:590–594. doi: 10.1128/jb.85.3.590-594.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAGG P. D., POLGLASE W. J. EFFECT OF DIHYDROSTREPTOMYCIN ON TETRAZOLIUM DYE REDUCTION IN ESCHERICHIA COLI. J Bacteriol. 1963 Apr;85:795–800. doi: 10.1128/jb.85.4.795-800.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAGG P. D., POLGLASE W. J. Extracellular metabolites of streptomycin mutants of Escherichia coli. J Bacteriol. 1962 Aug;84:370–374. doi: 10.1128/jb.84.2.370-374.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- ENGELBERG H., ARTMAN M. Studies on streptomycin dependent bacteria: effect of growth in limiting amounts of streptomycin on respiration and fermentation of a streptomycin dependent mutant of Escherichia coli. Biochim Biophys Acta. 1961 Mar 4;47:553–560. doi: 10.1016/0006-3002(61)90550-9. [DOI] [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WHITE J. R. Inhibition of polypeptide synthesis by streptomycin. Biochem Biophys Res Commun. 1962 May 11;7:385–389. doi: 10.1016/0006-291x(62)90320-0. [DOI] [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WITTING M. L., WHITE J. R. Polypeptide synthesis with ribosomes from streptomycin-resistant and dependent E. coli. Biochem Biophys Res Commun. 1962 May 11;7:390–393. doi: 10.1016/0006-291x(62)90321-2. [DOI] [PubMed] [Google Scholar]
- GALE P. H., ARISON B. H., TRENNER N. R., PAGE AC Jr FOLKERS K. Coenzyme Q. 36. Isolation and characterization of coenzyme Q10 (H-10). Biochemistry. 1963 Jan-Feb;2:196–200. doi: 10.1021/bi00901a037. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- LESTER R. L., RAMASARMA T. Chromatography of the coenzyme Q family of compounds on silicone-impregnated paper. J Biol Chem. 1959 Mar;234(3):672–676. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGER J., BENEDICT M., ARTMAN M. A common site of action of polyamines and streptomycin. Biochim Biophys Acta. 1962 Jul 30;62:202–204. doi: 10.1016/0006-3002(62)90519-x. [DOI] [PubMed] [Google Scholar]
- Miller C. P., Bohnhoff M. Development of Streptomycin-resistant Variants of Meningococcus. Science. 1947 Jun 13;105(2737):620–621. doi: 10.1126/science.105.2737.620. [DOI] [PubMed] [Google Scholar]
- PERETZ S., POLGLASE W. J. The action of streptomycin. Antibiot Annu. 1956:533–540. [PubMed] [Google Scholar]
- POLGLASE W. J., PERETZ S., ROOTE S. M. Adaptive enzyme formation by dihydrostreptomycine-dependent Escherichia coli. Can J Biochem Physiol. 1956 May;34(3):558–562. [PubMed] [Google Scholar]
- Paine T. F., Jr, Finland M. Observations on Bacteria Sensitive to, Resistant to, and Dependent upon Streptomycin. J Bacteriol. 1948 Aug;56(2):207–218. doi: 10.1128/jb.56.2.207-218.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEYER J. F., LENGYEL P., BASILIO C. Ribosomal localization of streptomycin sensitivity. Proc Natl Acad Sci U S A. 1962 Apr 15;48:684–686. doi: 10.1073/pnas.48.4.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOTTS C. R., STANIER R. Y. Mechanism of streptomycin action on bacteria: a unitary hypothesis. Nature. 1961 Nov 18;192:633–637. doi: 10.1038/192633a0. [DOI] [PubMed] [Google Scholar]
- TIRUNARAYANAN M. O., VISCHER W. A., RENNER U. Streptomycin and amino acid metabolism of bacteria. Antibiot Chemother (Northfield) 1962 Feb;12:117–122. [PubMed] [Google Scholar]
- UMBREIT W. W. The action of streptomycin. VI. A new metabolic intermediate. J Bacteriol. 1953 Jul;66(1):74–81. doi: 10.1128/jb.66.1.74-81.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]


