Faced with changing food availability, organisms adapt metabolism to survive. In a recent issue of Cell, Lin et al. described the acetylation of an extranuclear enzyme being regulated by acetyl-CoA. This novel finding connects nutrient availability, energy status and survival.
Nutrient availability can have major effects on growth, development and metabolism. There are complex interactions between diet composition, caloric intake, environment and genotype in the control of health, aging and longevity. The ability of an organism to sense, acquire and efficiently process energy (nutrients) is crucial for its health and survival. Within the cell, changes in energy status (or cellular stress) alter NAD+ and AMP. These levels serve as intracellular energy indicators that lead to the modulation of metabolic pathways through a complex network of regulatory proteins that ultimately influence gene regulation in response to the existing energy status. In part, this gene regulation is driven by histone acetyltransferases (HATs) and histone deacetylases (HDACs), a family of enzymes that regulate (de)acetylation homeostasis within the nucleus. A paper published this week in Cell by Lin et al., demonstrates that elevated levels of acetyl-CoA (AcCoA) concentration favors the transcription of phosphoenolpyruvate carboxykinase (PCK1) and the stimulatory acetylation of Pck1p by the nucleosome acetyltransferase (NuA4) and, in turn, regulates yeast chronological lifespan (CLS) (Fig. 1).
Figure 1. NuA4 via PKC1 controls glucose metabolism and life span.
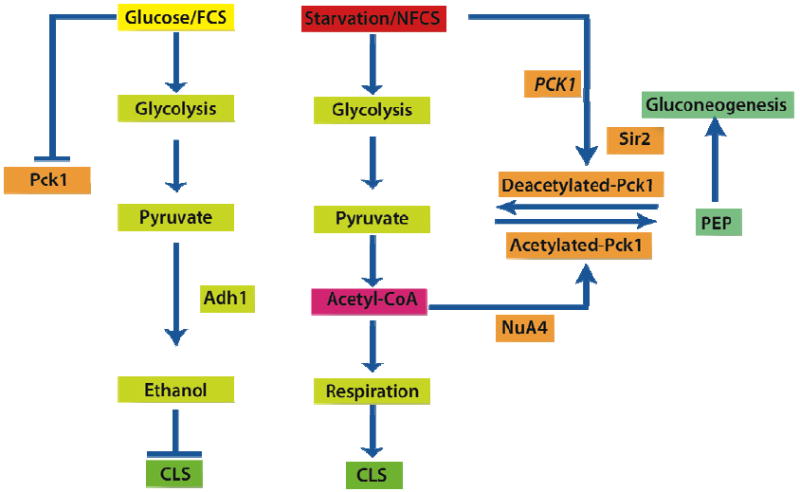
Abundance of glucose or fermentable carbon source (FCS) induces fermentation in yeast, preventing the transcription of PCK1. Ethanol accumulated in the fermentative conditions induces the inhibition of CLS (Fabrizio et al., 2005). However, under starvation or non-fermentable carbon source (NFCS) conditions both PCK1 expression and its post-transcriptional modification by acetyl-CoA- dependent acetylation are increased. Acetylation of Pck1 induces its activity and accelerates gluconeogenesis. Under these conditions, ethanol is removed and CLS increases. Acetylation of Pck1, an essential enzyme of gluconeogenesis, connects respiratory intermediates with the mechanism to maintain the supply of glucose as a physiological step for energy homeostasis.
Mounting evidence supports that many aging processes are regulated by energy metabolism, including NAD+ biosynthesis (Yang et al., 2007), NAD+-dependent sirtuin deacetylation of histones or PGC1α (Schwer and Verdin, 2008), and the transition between glycolysis and respiration by Nqr1 (Jiménez-Hidalgo et al., 2009). All these mechanisms are dependent on nutrient availability and clearly demonstrate the importance of equilibrium between the uptake and efficient processing of energy. Efficient and balanced energy metabolism leads to increases in both mean and maximum lifespan, ultimately leading to healthy aging. Most of these discoveries were made in yeast, due to the ease of genetic manipulation and ability to transit from fermentation to respiration during normal growth conditions as glucose concentrations fall in growth media. Using this model, Lin et al., report that Pck1 regulates yeast CLS. This effect is regulated by acetylation of PCK1 by the AcCoA-dependent NuA4 complex and deacetylation by NAD+-dependent Sir2. The effect of Sir2 on CLS is mediated by inactivation of alcohol dehydrogenase 2 (Adh2) that uses the ethanol produced by fermentation (Fabrizio et al., 2005).
In recent years the regulation of chromatin-associated proteins by acetylation has been reported as a key regulatory mechanism in gene regulation (Yang and Seto, 2008), but there is little to no information on the role of acetylation on cytosolic enzymes. Pck1 is unique to the gluconeogenesis pathway in that it catalyzes the formation of phosphoenolpyruvate from oxaloacetate. Gluconeogenesis is activated when yeast has access only to non-fermentable carbon sources or by starvation and results in the synthesis of glucose-6-phosphate, required for both nucleotide and aromatic amino acid biosynthesis. The balance between glycolysis and gluconeogenesis is important to maintain the bioenergetics of aerobic metabolism. In fact, it is known that transformation of pyruvate to oxalacetate by pyruvate carboxylase is activated by AcCoA and inhibited by ADP (Pronk et al., 1996).
The paper by Lin et al., indentifies Pck1 as an extranuclear, non-histone substrate of NuA4 acetylation using AcCoA, connecting nutrient availability, energy status and survival. In other words, this paper connects (de)acetylation and metabolic control via extranuclear actions of HATs and HDACs, creating a new perspective on metabolic regulation. With an elegant use of mutant strains, this group demonstrates that the acetylation of lysine 514 (K514) of Pck1 occurs by NuA4 both in vivo and in vitro and furthermore, this acetylation is reversible via Sir2 activity. Physiological experiments using PCK1 null yeast strains demonstrate the requirement of this gene product for yeast to grow in ethanol. Addition of glycerol to the media enables the cell to bypass this defect, at least the first phase of growth. K514 acetylation is required for Pck1 function, and connects Sir2 deacetylation with NuA4 acetylation as mechanisms modulating CLS in yeast (Fig. 1). This new advance puts gluconeogenesis as a key modulator of yeast longevity under starvation conditions.
The importance of these findings for mammalian aging regulation is maybe even greater. Mammals contain two isozymes: cytosolic PCK1 and mitochondrial PCK2, which share the same catalytic function as the yeast homolog but use GTP instead of ATP (Matte et al., 1997). Lin et al., demonstrates that Pck1 but not Pck2 was acetylated by TIP60, homolog for the yeast acetylase Esa1, part of the NuA4 complex. However, Pck1 was not deacetylated by Sirt1, indicating that other deacetylases must be involved. Interestingly, silencing Pck1 in mice induces a down-regulation of enzymes involved in gluconeogenesis and has a direct impact on glycemic control and energy metabolism (Gómez-Valadés et al., 2008). Furthermore, old mice over-expressing PCK1 in skeletal muscle are more active and live longer than control mice (Hakimi et al., 2007).
Burgess et al., (2007) demonstrated the importance of gluconeogenesis to maintain blood glucose during starvation to guarantee the glucose supply in mammals. The discovery of the regulation of gluconeogenesis through (de)acetylation of Pck1 connects the abundance of AcCoA induced during diauxic shift in yeast that leads to increasing respiration with the requirement of glucose in starvation conditions. Overall, the regulation of aging processes, healthspan and ultimately longevity seem to be heavily influenced by many of these basic metabolic sensors, effectors, and transcription factors, indicating that there is an intimate connection between energy homeostasis and longevity.
Acknowledgments
This work is supported by the Intramural Research Program of the National Institute on Aging.
References
- Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cell Metab. 2007;5:313–20. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Cell. 2005;123:655–67. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Gómez-Valadés AG, Méndez-Lucas A, Vidal-Alabró A, Blasco FX, Chillon M, Bartrons R, Bermúdez J, Perales JC. Diabetes. 2008;57:2199–210. doi: 10.2337/db07-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi P, Yang J, Casadesus G, Massillon D, Tolentino-Silva F, Nye CK, Cabrera ME, Hagen DR, Utter CB, Baghdy Y, Johnson DH, Wilson DL, Kirwan JP, Kalhan SC, Hanson RW. J Biol Chem. 2007;282:32844–55. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Hidalgo M, Santos-Ocaña C, Padilla S, Villalba JM, Lopez-Lluch G, Martín-Montalvo A, Minor RK, Sinclair DA, de Cabo R, Navas P. Aging Cell. 2009 doi: 10.1111/j.1474-9726.2009.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-Y, Matte A, Tari LW, Goldie H, Delbaere LT. J Biol Chem. 1997;272:8105–8. doi: 10.1074/jbc.272.13.8105. [DOI] [PubMed] [Google Scholar]
- Pronk JT, Yde Steensma H, Van Dijken JP. Yeast. 1996;12:1607–33. doi: 10.1002/(sici)1097-0061(199612)12:16<1607::aid-yea70>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schwer B, Verdin E. Cell Metab. 2008;7:104–12. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Cell. 2007;130:1095–107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Seto E. Mol Cell. 2008;31:449–61. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


