Abstract
Purpose
To compare retinal nerve fiber layer (RNFL) thickness assessments and the discriminating ability of Fourier-domain optical coherence tomography (FD-OCT) with time-domain optical coherence tomography (TD-OCT) for glaucoma detection.
Design
Prospective, non-randomized, observational cohort study.
Methods
Normal and glaucomatous eyes underwent complete examination, standard automated perimetry (SAP), optic disc photography, TD-OCT (Stratus™ OCT, Carl Zeiss Meditec, Dublin, CA) and FD-OCT (RTVue™, Optovue, Inc., Fremont, CA). One eye per subject was enrolled. Two consecutive scans were acquired using a 3.46-mm diameter scan with TD-OCT and a 3.45-mm diameter scan with FD-OCT. For each of 5 RNFL parameters the area under the receiver operator characteristic curve (AUROC) was calculated to compare the ability of FD-OCT and TD-OCT to discriminate between normal and glaucomatous eyes.
Results
Fifty normal (mean age 65.3 ± 9.9 years) and 50 glaucoma patients (mean age 67.7 ± 10.5 years) were enrolled. Average, superior, and inferior RNFL thickness measurements (µm) were significantly (p < 0.01) greater with FD-OCT compared with TD-OCT in normal (103.3 ± 12.6 vs. 96.3 ± 10.7, 134.5 ± 18.6 vs. 113.9 ± 16.3, and 129.7 ± 16.9 vs. 125.5 ±15.8, respectively) and glaucomatous (p < 0.001) eyes (77.6 ± 17.6 vs. 70.4 ± 18.6, 108.0 ± 26.8 vs. 86.8 ± 30.2, 82.2 ± 3.3 vs. 73.5 ± 26.1, respectively). The AUROC for RNFL thickness were similar (p > 0.05) using FD-OCT (average 0.88, superior 0.80, inferior 0.94) and TD-OCT (average 0.87, superior 0.79, inferior 0.95).
Conclusion
Cross-sectional peripapillary RNFL thickness measurements obtained using FD-OCT generated with the RTVue™ are greater than TD-OCT, and have similar diagnostic performance for glaucoma detection.
Keywords: glaucoma, retinal nerve fiber layer, time-domain OCT, Fourier-domain OCT
Introduction
Although optic disc stereophotography represents the standard for documentation of glaucomatous structural damage, advances in computerized imaging technology provide useful objective and quantitative measures that assist the clinician in glaucoma diagnosis and monitoring, and offer considerable opportunity for use as efficacy endpoints in clinical trials.1 A comprehensive recent review by the Ophthalmic Technology Assessment Committee Glaucoma Panel of the American Academy of Ophthalmology2 concluded that information obtained from imaging devices is useful in clinical practice when analyzed in conjunction with other relevant clinical parameters. Recent advances in imaging technology have resulted in instruments with greater resolution, shorter acquisition time, and three-dimensional imaging of posterior segment structures. Time-domain Optical coherence tomography (TD-OCT, Stratus OCT, Carl Zeiss Meditec, Dublin, CA) is a high-resolution, micron scale, two-dimensional imaging modality based upon low-coherence interferometry that non-invasively acquires cross-sectional depth information over time.3, 4 TD-OCT provides quantitative assessments of the retina and optic nerve by measuring the echo time delay and intensity of backscattered light from posterior segment structures, and is capable of obtaining 400 A-scans per second with a depth resolution of 10 microns.3, 5, 6 Fourier-domain OCT (FD-OCT, RTVue™ , Optovue, Inc., Fremont, CA) represents a modification of the OCT system and is capable of high-speed imaging with acquisition of 26,000 A-scans per second, greater than 60 times faster than TD-OCT.7
The technology transforms the optical spectrum into electrical signal after image acquisition and uses fast Fourier transformation of collected frequencies to improve the signal to noise ratio and registers the B-scan view with en face view of retinal surface.8 Three-dimensional assessments of the retina and optic nerve are obtainable with axial resolution of approximately 5 microns over a maximum scan area of 6×6mm.
We hypothesized that FD-OCT may have significantly better discriminating ability for glaucoma diagnosis compared with TD-OCT owing to faster speed, increased axial resolution, and reduced imaging artifact associated with saccadic eye movement during image acquisition. The purpose of the study was to compare the retinal nerve fiber layer (RNFL) thickness measurements and discriminating ability of FD-OCT with TD-OCT for glaucoma detection.
Methods
This was a prospective, non-randomized, observational cohort study of normal and glaucomatous subjects at a single institution. One eye per subject was enrolled. If both eyes met eligibility criteria, one eye was randomly selected.
Study Population
All participants signed a consent form approved by the Institutional Review Board for Human Research of the University of Miami Miller School of Medicine, which was in agreement with the provisions of the Declaration of Helsinki. All subjects underwent complete ophthalmic examination including slit lamp biomicroscopy, dilated stereoscopic examination, gonioscopy, Goldmann applanation tonometry, ultrasound pachymetry, photography of the optic disc, and RNFL imaging using TD-OCT (Stratus™ OCT, software version 4.0.7(0132), Carl Zeiss Meditec, Dublin, CA) and FD-OCT (RTVue™, software version A3,0,1,16, Optovue, Inc., Fremont, CA) on the same day. Two reliable visual field (VF) examinations were obtained using standard automated perimetry (SAP) performed with the Humphrey Field Analyzer (Carl-Zeiss Meditec, Dublin, CA) using a SITA standard strategy, program 24-2.
Inclusion criteria common to both groups consisted of age between 40 and 85 years, refractive error spherical equivalent (SE) between −8.00D and +4.00D, best corrected visual acuity of equal to or better than 20/40, reliable standard automated perimetry (SAP, <33% fixation losses, false positives and false negatives), and no prior intraocular surgery except for uncomplicated cataract extraction. Eyes with ocular disease other than glaucoma or cataract, visual acuity less than 20/40, peripapillary atrophy extending to 1.7 mm from disc center, retinal disease, or unreliable SAP were excluded.
Normal subjects consisted of volunteers such as office employees, and friends or family members of glaucoma patients. Normal subjects had no history of ocular disease except cataract, intraocular pressure (IOP) less than or equal to 21 mmHg by Goldmann applanation tonometry, normal optic disc appearance based upon clinical stereoscopic examination and review of stereodisc photography. All normal volunteers had two normal SAP examinations defined as glaucoma hemifield test (GHT) within normal limits, and mean and pattern standard deviation with a probability level of greater than 5%. Absence of glaucomatous optic neuropathy was defined as intact neuroretinal rim without peripapillary hemorrhages, notches, localized pallor, or RNFL defect. Glaucomatous optic neuropathy was defined as neural rim thinning, notching, excavation, or RNFL defect. Glaucoma patients had glaucomatous optic nerve damage and abnormal SAP defined as abnormal glaucoma hemifield test and pattern standard deviation outside 95% normal limits. Patients with SAP abnormalities had at least one confirmatory visual field examination. If both eyes satisfied inclusion criteria, one eye was randomly selected for enrolment.
Retinal Nerve Fiber Layer Imaging
TD-OCT and FD-OCT imaging of the RNFL was performed consecutively at the same session by the same experienced operator. TD-OCT imaging was performed through undilated pupils. We used a fast RNFL thickness acquisition protocol which generates an average of three peripapillary circular scans (256 A-scans per 360-degree circular path with each A-scan corresponding to a 1.406 degree arc) with a diameter of 3.46 mm centered on the optic disc and a scan acquisition time of approximately one second duration on the same day by the same examiner. Images with failure of the RNFL segmentation algorithm were excluded, as well as images that were obtained during eye movement, images that were unfocused, poorly centered or had a signal strength < 6. The average of two high quality images was used for the TD-OCT analysis.
FD-OCT imaging was performed through undilated pupils using a 3.45mm diameter circular scan acquisition protocol. This imaging protocol provides an average of four peripapillary scans acquired in 76 milliseconds (999 A-scans per 360-degree circular path with each A-scan corresponding to a 0.36 degree arc) centered on the optic disc. Images with failure of the RNFL segmentation algorithm were excluded, as well as images obtained during eye movement, images that were unfocused, poorly centered or had a scan score index (SSI) of < 30. The average of two high quality images was used for the FD-OCT analysis.
RNFL assessments were analyzed from corresponding 90-degree quadrants generated with TD-OCT (representing an average of 64 A-scans/quadrant) and FD-OCT (representing an average of 250 A-scans/quadrant). RNFL thickness parameters selected as main outcome measures included average, temporal, superior, nasal and inferior RNFL thickness (µm).
Statistical Analysis
Statistical analysis was performed using SPSS version 15.0 (SPSS Inc., Chicago, IL) and STATISTICA version 8.0 (StatSoft Inc., Tulsa, OK). Independent samples T-test, ANOVA, and Chi-square test were used to compare different measures between groups. Pearson correlation coefficients were calculated to investigate the association between OCT parameters and measures of visual function. For each of five OCT parameters the areas under the receiver operator characteristic curve (AUROC) were calculated using the RNFL thickness values to compare the discriminating ability of each imaging modality to differentiate between normal and glaucomatous eyes. Significant differences in AUROC were determined using the method of Hanley and McNeil.9
Sensitivity and specificity calculations were also generated based upon automated statistical classifications obtained from a single high quality OCT image using the commercially available TD-OCT and FD-OCT normative database. RNFL quadrants on the TD-OCT image that exceeded 99% confidence limits compared to age-matched normal controls were considered glaucomatous; quadrants within 95% confidence limits were considered normal. Automated classifications for RNFL quadrants cannot be generated using commercially available FD-OCT software. A quadrant was considered abnormal if at least one segment within the quadrant exceeded 99% confidence limits; quadrants were considered normal if all segments were within 95% confidence limits.
Sample size calculations were based upon a significance level of 5%, a type II error rate (beta) of 10%, and at least 90% power to detect a difference of 10 microns between the RNFL measurements obtained with each OCT technology. A 10 micron difference was based upon the minimum significant difference required to exceed the measurement variability between OCT instruments, taking into account other sources of variability including intra-machine variability. Previous work10 has demonstrated that inter-machine variability is an independent and important source of OCT measurement error when comparing data from one TD-OCT instrument to another and exceeds inter-operator variability. Assuming an alpha of 0.05, our study had 80% power to find a 14% difference between sensitivities (and specificities) between TD-OCT and FD-OCT.
Results
Fifty glaucoma patients (mean age 65.3 ± 9.9 years) and 50 age-matched normal volunteers (mean age 67.7 ± 10.5 years) were included. Ten subjects with unreliable visual fields were excluded. None of the eyes, or scans, was excluded due to inability to obtain high quality RNFL measurements using either OCT technology.
Table 1 demonstrates the clinical characteristics of the study population. Persons with glaucoma had significantly (p < 0.001) worse visual field mean deviation (MD) and pattern standard deviation (PSD), and consisted of more females compared with normal volunteers. Table 2 compares the mean RNFL thickness values obtained using TD-OCT and FD-OCT. Average, superior, inferior, and temporal RNFL thickness values obtained with FD-OCT were significantly greater (p < 0.05) compared with values obtained using TD-OCT in both normal and glaucomatous eyes. We explored the correlation between RNFL thickness and scan score index (SSI) for all FD-OCT parameters. Average (r = 0.28, p = 0.05), nasal (r = 0.36, p = 0.01), and temporal RNFL thickness (r = 0.35, p = 0.01) values were significantly correlated with SSI in normal subjects, and temporal RNFL thickness was significantly correlated with SSI in glaucomatous eyes (r = 0.4, p = 0.002).
Table 1.
Demographic and standard automated perimetry characteristics of the study population comprised of normal (n = 50) and glaucomatous patients (n = 50).
| Normal (n= 50) |
Glaucoma (n= 50) |
p | ||
|---|---|---|---|---|
| Age (yrs), mean ± SD (range) |
65.3±9.9 (33–81) |
67.7±10.5 (37–81) |
0.25# | |
| Visual field MD (dB) (range) |
−0.5 ±1.5 (−4.4 to 1.4) |
−9.2 ±7.1 (−30.7 to 0.5) |
P<0.001# | |
| Visual field PSD (dB) (range) |
2.4 + 5.7 (1.0 to 3.9) |
8.4 + 3.6 (2.0 to 14.7) |
P<0.001# | |
| Gender: | Male | 28 | 18 | 0.045‡ |
| Female | 22 | 32 | ||
| Race: | White non-Hispanic | 46 | 40 | 0.23‡ |
| White Hispanic | 3 | 4 | ||
| Black | 1 | 4 | ||
| Asian | 0 | 2 | ||
MD = mean deviation; PSD = pattern standard deviation
Chi-square test
ANOVA.
Table 2.
Mean±standard deviation (SD) retinal nerve fiber layer (RNFL) thickness values measured using time-domain and Fourier-domain optical coherence tomography (OCT) in normal and glaucomatous eyes.
| Normal (n=50) | Glaucoma (n=50) | |||||
|---|---|---|---|---|---|---|
| Time-domain OCT |
Fourier-domain OCT |
p* | Time-domain OCT |
Fourier-domain OCT |
p* | |
| Average RNFL (µm)±SD (range) |
96.3±10.7 (69.8–119.5) |
103.3±12.6 (70.0–127.0) |
<0.001 | 70.4±18.6 (35.1–119.7) |
77.6±17.6 (36.0–122.0) |
<0.001 |
| Superior RNFL (µm)±SD (range) |
113.9±16.3 (85.0–156.5) |
134.5±18.6 (81.0–178.0) |
<0.001 | 86.8±30.2 (39.0–167.5) |
108.0±26.8 (57.5–170.5) |
<0.001 |
| Inferior RNFL (µm)±SD (range) |
125.5±15.8 (89.0–167.5) |
129.7±16.9 (84.0–166.5) |
0.01 | 73.5±26.1 (11.5–161.5) |
82.2±23.0 (34.5–159.0) |
0.001 |
| Nasal RNFL (µm)±SD (range) |
78.7±17.7 (49.0–122.5) |
76.0–18.5 (42.0–119.0) |
0.16 | 62.6±18.8 (35.0–121.0) |
59.8±19.2 (25.5–125.0) |
0.07 |
| Temporal RNFL (µm)±SD (range) |
67.7±13.6 (45.0–97.0) |
73.0±16.3 (38.5–115.0) |
0.01 | 57.1±16.7 (25.0–94.0) |
61.0±18.5 (27.0–101.5) |
0.04 |
RNFL = retinal nerve fiber layer; SD = standard deviation; OCT = optical coherence tomography
Paired-sample T-test.
Figure 1 illustrates the optic disc photograph of a right eye with advanced glaucomatous optic neuropathy with diffuse thinning of the neural rim (top left) and corresponding double arcuate visual field depression noted on SAP (top right). TD-OCT (bottom left) and FD-OCT (bottom right) demonstrate diffuse thinning of the RNFL (average thickness 47.25μ and 48μ, respectively) outside 95% confidence limits compared with age-matched normal subjects. Note that 90-degree quadrantic RNFL assessments using TD-OCT (64 A-scans/quadrant) were generated using an average of three segments; corresponding 90-degree quadrantic RNFL assessments using FD-OCT (250 A-scans/quadrant) were generated using an average of four segments.
Figure 1.
illustrates an optic disc photograph (top left) of a right eye with advanced glaucomatous optic neuropathy and diffuse thinning of the neural rim, and corresponding double arcuate scotoma (top right) using standard automated perimetry. Time-domain (bottom left) and Fourier-domain (bottom right) optical coherence tomography images demonstrate diffuse thinning of the RNFL (average thickness 47.25μ and 48μ, respectively), outside 95% confidence limits of age-matched normal subjects. Note that 90-degree quadrantic RNFL assessments using TD-OCT (64 A-scans/quadrant) were generated using an average of three segments; corresponding 90-degree quadrantic RNFL assessments using FD-OCT (250 A-scans/quadrant) were generated using an average of four segments.
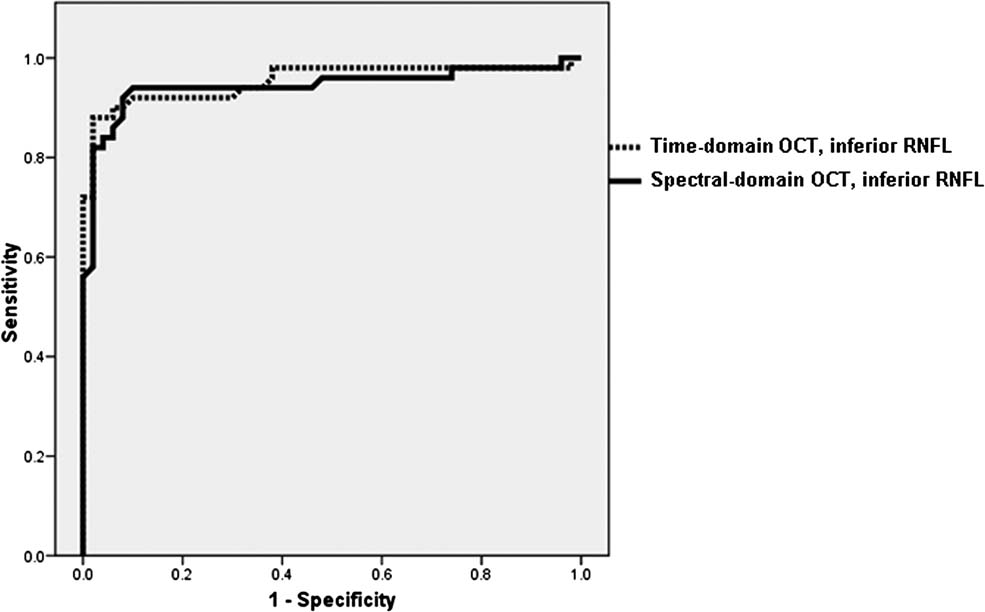
For each of five RNFL thickness parameters generated with TD-OCT and FD-OCT, the AUROC (Table 3) and sensitivity values at a fixed specificity of ≥90% (Table 4) were calculated. The ability to discriminate between normal and glaucomatous persons was similar for both OCT technologies using RNFL thickness values. Using the instrument normative database, the discriminating ability of both OCT technologies were similar for all parameters except nasal RNFL thickness, which had a significantly (p=0.04) greater AUROC value with FD-OCT (0.73 ± 0.05) compared with TD-OCT (0.62 ± 0.06). Figure 2 demonstrates the AUROC for the best parameter obtained using TD-OCT (inferior RNFL thickness, AUROC = 0.95) and FD-OCT (inferior RNFL thickness, AUROC = 0.94, p = 0.45).
Table 3.
Areas under the receiver operator characteristic (AUROC) curves using retinal nerve fiber layer (RNFL) thickness values and normative databases obtained using time-domain and Fourier-domain optical coherence tomography (OCT).
| Using RNFL Thickness | Using Normative Database | |||||
|---|---|---|---|---|---|---|
| TD-OCT AUROC±SE (95% CI) |
FD-OCT AUROC±SE (95% CI) |
p | TD-OCT AUROC±SE (95% CI) |
FD-OCT AUROC±SE (95% CI) |
p | |
| Average RNFL (µm) ± SE | 0.87±0.04 (0.82–0.96) |
0.88±0.04 (0.81–0.95) |
0.73 | 0.86 ± 0.04 (0.78–0.94) |
0.96 ± 0.02 (0.91–1.0) |
0.84 |
| Superior RNFL (µm) ± SE | 0.79±0.05 (0.69–0.88) |
0.80±0.05 (0.71–0.89) |
0.59 | 0.75 ± 0.05 (0.66–0.85) |
0.80 ± 0.05 (0.71–0.89) |
0.44 |
| Inferior RNFL (µm) ± SE | 0.95±0.02 (0.91–1.00) |
0.94±0.03 (0.89–1.00) |
0.45 | 0.90 ± 0.04 (0.83–0.97) |
0.93 ± 0.03 (0.87–0.99) |
0.35 |
| Nasal RNFL (µm) ± SE | 0.76±0.05 (0.66–0.85) |
0.74±0.05 (0.64–0.84) |
0.63 | 0.62 ± 0.06 (0.51–0.73) |
0.73 ± 0.05 (0.63–0.83) |
0.04 |
| Temporal RNFL (µm) ±SE | 0.68±0.05 (0.58–0.79) |
0.69±0.05 (0.58–0.79) |
0.95 | 0.65 ± 0.06 (0.54–0.76) |
0.76 ± 0.05 (0.67–0.86) |
0.08 |
TD-OCT = Time-domain optical coherence tomography, FD-OCT = Fourier-domain optical coherence tomography, AUROC = area under the receiver operator characteristic curve; RNFL = retinal nerve fiber layer; SE = standard error
Table 4.
Sensitivities at fixed specificities for time-domain and Fourier-domain optical coherence tomography (OCT) using retinal nerve fiber layer (RNFL) thickness values and also normative databases.
| Using RNFL Thickness | Using Normative Database | |||
|---|---|---|---|---|
| Time-domain OCT Sensitivity/Specificity (Specificity ≥ 0.90) |
Fourier-domain OCT Sensitivity/Specificity (Specificity ≥ 0.90) |
Time-domain OCT Sensitivity/Specificity (Specificity ≥ 0.90) |
Fourier-domain OCT Sensitivity/Specificity (Specificity ≥ 0.90) |
|
| Inferior RNFL (µm) | 0.92/0.90 | 0.94/0.90 | 0.80/0.98 | 0.80/1.00 |
| Average RNFL (µm) | 0.80/0.90 | 0.80/0.90 | 0.74/0.92 | 0.88/0.98 |
| Superior RNFL (µm) | 0.58/0.90 | 0.58/0.90 | 0.54/0.94 | 0.66/0.90 |
| Nasal RNFL (µm) | 0.36/0.90 | 0.48/0.090 | 0.24/1.00 | 0.52/0.92 |
| Temporal RNFL (µm) | 0.30/0.90 | 0.38/0.90 | 0.32/0.98 | 0.60/0.90 |
RNFL = retinal nerve fiber layer; OCT = optical coherence tomography
Figure 2.
demonstrates the area under the receiver operator characteristic curves (AUROC) for the best parameter obtained using time-domain optical coherence tomography (inferior RNFL thickness, AUROC = 0.95) and Fourier-domain optical coherence tomography (inferior RNFL thickness, AUROC = 0.94, p = 0.45).
We examined the relationship between RNFL thickness and visual function among glaucomatous eyes (n=50). Table 5 illustrates the association between SAP pattern standard deviation and RNFL thickness obtained with TD-OCT and FD-OCT. The superior RNFL thickness had the strongest correlation with the SAP PSD (r= −0.78 TD-OCT; r= −0.75 FD-OCT) and the temporal RNFL thickness had the weakest correlation (r= −0.35 TD-OCT; r= −0.34 FD-OCT).
Table 5.
Correlation between visual field pattern standard deviation and retinal nerve fiber layer (RNFL) thickness generated using time-domain and Fourier-domain optical coherence tomography (OCT) among glaucomatous eyes (n=50).
| Time-domain OCT | Fourier-domain OCT | Comparison between r-values |
|||
|---|---|---|---|---|---|
| r* | p-value | r* | p-value | p | |
| Inferior RNFL (µm) | −0.59 | <0.001 | −0.59 | <0.001 | 1.00 |
| Average RNFL (µm) | −0.37 | <0.001 | −0.40 | <0.001 | 0.23 |
| Superior RNFL (µm) | −0.78 | <0.001 | −0.75 | <0.001 | 0.08 |
| Nasal RNFL (µm) | −0.31 | 0.002 | −0.33 | 0.001 | 0.44 |
| Temporal RNFL (µm) | −0.35 | <0.001 | −0.34 | 0.001 | 0.70 |
RNFL= retinal nerve fiber layer; OCT = optical coherence tomography
Pearson correlation coefficient
Discussion
Glaucoma is a multifactorial optic neuropathy known to cause progressive loss of retinal ganglion cells and their axons, leading to accelerated reduction in the thickness of the RFNL. Imaging technologies such as OCT represent useful methods for objective detection and quantification of glaucomatous RNFL atrophy. TD-OCT has been demonstrated to have high levels of reproducibility,10–12 incorporates age-matched normative data,13 and provides non-invasive assessment of the peripapillary RNFL14, 15 through an undilated pupil. The FD-OCT system is a modification of TD-OCT that can generate more than 25,000 A-scans per second with an axial resolution of 5 microns over a maximum scan area of 6 mm by 6 mm.7,8 Few studies have compared the RNFL assessments generated with TD-OCT and FD-OCT.
We found that RNFL thickness measurements with FD-OCT generated with the RTvue™ were significantly greater compared with TD-OCT for all parameters, except the nasal RNFL thickness which had a mean decrease of −3.7% with FD-OCT. The increase in average RNFL thickness with FD-OCT across the entire cohort was 8.8%, and ranged from 7.5% (inferior RNFL) to 21.4% (superior RNFL). Others16 have reported similar findings comparing retinal thickness using the Cirrus FD-OCT compared with Stratus and speculated that this finding may be attributed to the increased resolution of FD-OCT enabling it detect and measure thickness corresponding to the outer band of the retinal pigment epithelium. Similarly, the increased resolution of FD-OCT may enable the FD-OCT RNFL segmentation protocol to detect and measure additional tissue that contributes to RNFL thickness. Alternatively, since SSI was significantly correlated with FD-OCT RNFL thickness, it is possible that a greater differential impact on RNFL thickness was observed using FD-OCT as compared with TD-OCT17. The nasal RNFL thickness has been reported to have significantly greater variability than other quadrants owing to the directional reflectance of the RNFL in the region.18–20 Knighton and Qian21 showed that nasal RNFL reflectance is very low, and strongly influenced by the location in the pupil of the instrument aperture. Using TD-OCT a location somewhat temporal to the center of the pupil has been suggested15 with adjustments in position in order to minimize the sinusoidal variation of the unprocessed scan. Since retinal segmentation algorithms differ across SD-OCT technologies, clinicians should be aware that RNFL thickness measurements obtained with TD-OCT and FD-OCT may not be comparable. It is unclear whether similar findings exist with other FD-OCT technologies.
In the present study, we hypothesized that by reducing the prevalence of imaging artifact associated with saccadic eye movement during image acquisition, high-speed, high resolution FD-OCT may improve the correlation between RNFL structural assessments and visual function, and improve the discriminating ability of FD-OCT for glaucoma diagnosis compared with TD-OCT. Our data suggests that peripapillary RNFL thickness measurements obtained using a 3.4 mm diameter measurement circle with FD-OCT appears to offer no advantage over TD-OCT for the diagnosis of moderate glaucoma, and provides similar correlations with standard measures of visual function. We found TD-OCT and FD-OCT to have similar AUROC values for superior, inferior, and average RNFL thickness (0.79 to 0.95), and similar sensitivity values at a fixed specificity of ≥ 90% (0.58–0.94). Our results are consistent with previously published studies involving TD-OCT, which have reported an AUROC of 0.84 to 0.94, 6, 22–28 and have generally found the inferior RNFL thickness quadrant to have the highest AUROC. 11, 29, 30 It is possible that the stage of our glaucoma cohort, which had an average visual field MD of −9dB and consisted of patients with moderate to advanced damage as judged using the Hodapp-Parrish-Anderson classification31, contributed in part to our inability to better differentiate normal eyes from glaucomatous eyes with spectral domain imaging. The ability to discriminate normal from glaucoma is directly proportional to the magnitude of disease severity across the glaucoma continuum.32,33 This observation will in part contribute to the high areas under the ROC curves identified using both TD-OCT and FD-OCT, as may other factors related to the different population sources of cases and controls in this study. Since inclusion of subjects was based on subjective disc and RNFL assessment it is possible that this may affect the agreement with OCT measures. This, however does not compromise the direct comparison between instruments.
We speculate that the high resolution scanning provided with FD-OCT may better enable detection of early structural damage as compared with TD-OCT. Further studies enriched with populations consisting of early glaucomatous damage are necessary to validate this hypothesis. We selected RNFL measurements obtained using a 3.4 mm measurement circle as the main outcome variable in order to compare similar regions of RNFL thickness obtained using FD-OCT and TD-OCT. Although peripapillary RNFL maps exist that illustrate RNFL thickness, at the time of writing limited software exists for the analysis and interpretation of these images. Newer software protocols may enhance the diagnostic performance of FD-OCT. Furthermore, commercially available FD-OCT software continues to implement cross-sectional peripapillary RNFL thickness as an integral component of RNFL assessment for glaucoma diagnosis (Cirrus HD-OCT™ , Carl-Zeiss Meditec, Dublin, CA; RTVue™ , Optovue Inc., Fremont, CA). Similar maps are generated with GDxVCC, and GDxECC and have not proven to have superior diagnostic power compared with TD-OCT.34,35
In summary cross-sectional RNFL thickness measurements obtained using FD-OCT generated with the RTvue™ are significantly greater than corresponding measurements obtained using TD-OCT in normal and glaucomatous eyes. FD-OCT and SD-OCT demonstrate similar correlations with measures of visual function, and have comparable diagnostic performance for detection of moderately advanced glaucoma.
Acknowledgments
Support: This study was supported in part by the Maltz Family Endowment for Glaucoma Research, Cleveland, Ohio; a grant from Mr. Barney Donnelley, Palm Beach, FL; The Kessel Foundation, Bergenfield, New Jersey; Source of support: NIH Grants R01-EY08684, RO1-EY013516, P30-EY14801 Bethesda, Maryland, and an unrestricted grant from Research to Prevent Blindness, New York, New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenfield DS, Weinreb RN. Role of optic nerve imaging in glaucoma clinical practice and clinical trials. Am J Ophthalmol. 2008;145:598–603. doi: 10.1016/j.ajo.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin SC, Singh K, Jampel HD, et al. Optic nerve head and retinal nerve fiber layer analysis: a report by the American Academy of Ophthalmology. Ophthalmology. 2007;114:1937–1949. doi: 10.1016/j.ophtha.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Velthoven ME, Faber DJ, Verbraak FD, van Leeuwen TG, de Smet MD. Recent developments in optical coherence tomography for imaging the retina. Prog Retin Eye Res. 2007;26:57–77. doi: 10.1016/j.preteyeres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–332. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- 6.Bowd C, Zangwill LM, Berry CC, et al. Detecting early glaucoma by assessment of retinal nerve fiber layer thickness and visual function. Invest Ophthalmol Vis Sci. 2001;42:1993–2003. [PubMed] [Google Scholar]
- 7.Lim JI, Tan O, Fawzi AA, Hopkins JJ, Gil-Flamer JH, Huang D. A pilot study of Fourier-domain optical coherence tomography of retinal dystrophy patients. Am J Ophthalmol. 2008;146:417–426. doi: 10.1016/j.ajo.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei J. High efficiency low coherence interferometry. United States Patent 7280221. 2005 [Google Scholar]
- 9.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 10.Sehi M, Guaqueta DC, Feuer WJ, Greenfield DS. A comparison of structural measurements using 2 Stratus optical coherence tomography instruments. J Glaucoma. 2007;16:287–292. doi: 10.1097/IJG.0b013e3180391a72. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros FA, Zangwill LM, Bowd C, Vessani RM, Susanna R, Jr, Weinreb RN. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139:44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 12.Kagemann L, Mumcuoglu T, Wollstein G, et al. Sources of longitudinal variability in optical coherence tomography nerve-fibre layer measurements. Br J Ophthalmol. 2008;92:806–809. doi: 10.1136/bjo.2007.129312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budenz DL, Chang RT, Huang X, Knighton RW, Tielsch JM. Reproducibility of retinal nerve fiber thickness measurements using the stratus OCT in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2005;46:2440–2443. doi: 10.1167/iovs.04-1174. [DOI] [PubMed] [Google Scholar]
- 14.Schuman JS, Hee MR, Arya AV, et al. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995;6:89–95. doi: 10.1097/00055735-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe GJ, Caprioli J. Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol. 2004;137:156–169. doi: 10.1016/s0002-9394(03)00792-x. [DOI] [PubMed] [Google Scholar]
- 16.Kiernan DF, Hariprasad SM, Chin EK, Kiernan CL, Rago J, Mieler WF. Prospective comparison of Cirrus and Stratus optical coherence tomography for quantifying retinal thickness. Am J Ophthalmol. 2009;147:267–275. doi: 10.1016/j.ajo.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Vazeen M, Varma R, Chopra V, Walsh AC, LaBree LD, Sadda SR. Factors associated with variability in retinal nerve fiber layer thickness measurements obtained by optical coherence tomography. Ophthalmology. 2007;114:1505–1512. doi: 10.1016/j.ophtha.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Huang XR, Bagga H, Greenfield DS, Knighton RW. Variation of peripapillary retinal nerve fiber layer birefringence in normal human subjects. Invest Ophthalmol Vis Sci. 2004;45:3073–3080. doi: 10.1167/iovs.04-0110. [DOI] [PubMed] [Google Scholar]
- 19.Schuman JS, Pedut-Kloizman T, Hertzmark E, et al. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology. 1996;103:1889–1898. doi: 10.1016/s0161-6420(96)30410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumenthal EZ, Williams JM, Weinreb RN, Girkin CA, Berry CC, Zangwill LM. Reproducibility of nerve fiber layer thickness measurements by use of optical coherence tomography. Ophthalmology. 2000;107:2278–2282. doi: 10.1016/s0161-6420(00)00341-9. [DOI] [PubMed] [Google Scholar]
- 21.Knighton RW, Qian C. An optical model of the human retinal nerve fiber layer: implications of directional reflectance for variability of clinical measurements. J Glaucoma. 2000;9:56–62. doi: 10.1097/00061198-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Deleon-Ortega JE, Arthur SN, McGwin G, Jr, Xie A, Monheit BE, Girkin CA. Discrimination between glaucomatous and nonglaucomatous eyes using quantitative imaging devices and subjective optic nerve head assessment. Invest Ophthalmol Vis Sci. 2006;47:3374–3380. doi: 10.1167/iovs.05-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manassakorn A, Nouri-Mahdavi K, Caprioli J. Comparison of retinal nerve fiber layer thickness and optic disk algorithms with optical coherence tomography to detect glaucoma. Am J Ophthalmol. 2006;141:105–115. doi: 10.1016/j.ajo.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 25.Kanamori A, Nagai-Kusuhara A, Escano MF, Maeda H, Nakamura M, Negi A. Comparison of confocal scanning laser ophthalmoscopy, scanning laser polarimetry and optical coherence tomography to discriminate ocular hypertension and glaucoma at an early stage. Graefes Arch Clin Exp Ophthalmol. 2006;244:58–68. doi: 10.1007/s00417-005-0029-0. [DOI] [PubMed] [Google Scholar]
- 26.Essock EA, Sinai MJ, Bowd C, Zangwill LM, Weinreb RN. Fourier analysis of optical coherence tomography and scanning laser polarimetry retinal nerve fiber layer measurements in the diagnosis of glaucoma. Arch Ophthalmol. 2003;121:1238–1245. doi: 10.1001/archopht.121.9.1238. [DOI] [PubMed] [Google Scholar]
- 27.Burgansky-Eliash Z, Wollstein G, Chu T, et al. Optical coherence tomography machine learning classifiers for glaucoma detection: a preliminary study. Invest Ophthalmol Vis Sci. 2005;46:4147–4152. doi: 10.1167/iovs.05-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114:1046–1052. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caprioli J, Nouri-Mahdavi K, Law SK, Badala F. Optic disc imaging in perimetrically normal eyes of glaucoma patients with unilateral field loss. Trans Am Ophthalmol Soc. 2006;104:202–211. [PMC free article] [PubMed] [Google Scholar]
- 30.Bourne RR, Medeiros FA, Bowd C, Jahanbakhsh K, Zangwill LM, Weinreb RN. Comparability of retinal nerve fiber layer thickness measurements of optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2005;46:1280–1285. doi: 10.1167/iovs.04-1000. [DOI] [PubMed] [Google Scholar]
- 31.Medeiros FA, Sample PA, Zangwill LM, Liebmann JM, Girkin CA, Weinreb RN. A statistical approach to the evaluation of covariate effects on the receiver operating characteristic curves of diagnostic tests in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2520–2527. doi: 10.1167/iovs.05-1441. [DOI] [PubMed] [Google Scholar]
- 32.Medeiros FA, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Influence of disease severity and optic disc size on the diagnostic performance of imaging instruments in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:1008–1015. doi: 10.1167/iovs.05-1133. [DOI] [PubMed] [Google Scholar]
- 33.Hodapp EA, Parrish RK, 2nd, Anderson DR. Clinical decisions in glaucoma. St Louis: Mosby-Year Book; 1993. [Google Scholar]
- 34.Sehi M, Ume S, Greenfield DS. Scanning laser polarimetry with enhanced corneal compensation and optical coherence tomography in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2007;48:2099–2104. doi: 10.1167/iovs.06-1087. [DOI] [PubMed] [Google Scholar]
- 35.Costa-Cunha LV, Cunha LP, Malta RF, Monteiro ML. Comparison of Fourier-Domain and Time-Domain Optical Coherence Tomography in the Detection of Band Atrophy of the Optic Nerve. Am J Ophthalmol. 147:56–63. doi: 10.1016/j.ajo.2008.07.020. [DOI] [PubMed] [Google Scholar]