Abstract
Objective
To investigate whether including a placebo arm in a clinical trial of hormone replacement therapy influenced women’s stated willingness to participate.
Design
Quasirandomised, interview based study.
Setting
10 group practices in the Medical Research Council’s General Practice Research Framework.
Participants
436 postmenopausal women aged 45-64 who had not had a hysterectomy.
Main outcome measures
Stated willingness to enter a trial and reasons for the decisions made.
Results
Of 218 women told about the trial without a placebo arm, 85 (39%) indicated their willingness to enter compared with 65 (30%) of the 218 women told about the trial with the placebo arm (P=0.06). Part of this difference was due to explicit reluctance to take a placebo. Altruism and personal benefit were the reasons most frequently given for wanting to take part in a trial. The reasons most frequently cited for not wanting to take part were reluctance to restart periods, not wanting to take unknown or unnecessary tablets, or not wanting to interfere with present good health.
Conclusion
For preventive trials the inclusion of a placebo arm may reduce patients’ willingness to participate.
Key messages
Recruitment to a clinical trial of hormone replacement therapy was lower when there was a placebo arm
The most common reasons for wanting to take part were to help medical research, to help women in the future, and personal benefit
Including potential benefits for other people in patient information, as well as personal benefits and risks, may increase recruitment
Introduction
Randomised controlled trials of new treatments often include a control group receiving no treatment. In a drug trial, a placebo is used to retain blindness and avoid bias. However, because patients often assume that a new treatment is likely to be effective, recruitment to trials with a placebo arm may be more difficult than recruitment to those of active treatments only. We investigated whether including a placebo arm in a clinical trial affects willingness to participate.
We gave postmenopausal women information about one of two trials of hormone replacement therapy: one with two active treatments only and one with two active treatments and a placebo. The main outcome measure was willingness to participate in the trial described.
The two active treatments were oestrogen only and oestrogen plus progestogen. Progestogen is added for women with a uterus to counter the increased risk of endometrial cancer with oestrogen alone.1 At the time our study was planned the balance of risks and benefits of adding progestogen was not clear since it was claimed that progestogen might also counter the cardioprotective effects of oestrogen.2 Since then the effect of oestrogen on endometrial hyperplasia has been shown to be substantial,3 and oestrogen plus progestogen seems to confer greater benefit on cardiovascular disease than oestrogen alone.3–5 The focus of our work, however, was not the specific treatments but the impact on recruitment of including a placebo arm, an important issue for clinical trials in general.
Participants and methods
Study sample
Our study was carried out through 10 group practices in the Medical Research Council’s General Practice Research Framework, a network of around 900 general practices throughout the United Kingdom. In each practice we randomly selected from the age-sex register 550 women aged 45-64 who had not had a hysterectomy. We excluded those in whom hormone replacement therapy might be contraindicated (unpublished data) and those who had had hormone replacement therapy in the previous 6 months.
Procedure
We obtained ethical approval from the local research ethics committees for the 10 general practices.
Letters were sent from each general practice inviting potentially eligible women to attend an interview at the practice. Completion of a reply slip indicated their willingness to participate in the study, and a brief question about menstrual status further established eligibility.
Women were seen at the practice on two occasions. At the first visit the nurse recorded the patient’s details and medical history. Approximately 15 minutes were spent explaining about the menopause and the potential benefits and risks of hormone replacement therapy. The benefits of oestrogen included relief of menopausal symptoms and prevention of osteoporosis and possibly heart disease, and the uncertainty of the effect of progestogen on long term benefits was described. The clear risk of endometrial cancer with oestrogen alone and a likely increased risk of breast cancer after 10 years’ continuous use were also explained, along with the uncertainty about the cardiovascular effects of progestogen. These details were recorded in an information booklet that women were given to take away. Eligible women were told about one of two proposed randomised controlled trials of hormone replacement therapy: either about a trial comparing oestrogen only and combined oestrogen and progestogen or about a trial comparing oestrogen only, combined oestrogen and progestogen, and a placebo.
The women returned for a second visit around 2 weeks later, when they were given the opportunity to ask questions about the trial and then completed a “willingness to enter” scale and an open ended question about the reasons for their decision. The importance of reporting the decision that they would make about actual participation was emphasised by the nurse.
For practical purposes nurses arranged to see patients in weekly blocks so that information about one trial was provided to all patients attending that week. Women indicated on their reply slip when they would be available, and appointments were made entirely at their convenience. Women had no prior knowledge about the trial they would be asked to consider joining, and they had no knowledge that the trial might or might not include a placebo arm. There was no difference in the days and times offered each week, ensuring no bias for one week over another. Since there was no way for the nurse to influence which week women visited, this was in effect a quasirandom assignment as each woman had an equal chance of being told about either trial.
Nurses were trained centrally to ensure standardisation of study procedures.
Outcome measure
Intention to enter the proposed trial was indicated by women selecting one of four response options: yes, definitely; yes, probably; no, probably not; and no, definitely not.
Statistical methods
We assessed differences in personal characteristics with two tailed χ2 tests between women allocated to hear about each trial, and this showed significant differences in socioeconomic group and smoking. Socioeconomic group was associated with willingness to enter the study and was adjusted for with direct standardisation.6 Smoking was not associated with willingness to enter a trial and so was not adjusted for.
Sample size calculation
Earlier feasibility studies had found that recruitment of women to a placebo controlled trial of hormone replacement therapy was 33% lower than to a trial of two active treatments, although the populations approached were different (women with and without hysterectomy in one study and only women with hysterectomy in the other), and the recruitment had taken place up to 5 years earlier over a different time period and in different general practices. To detect a 33% reduction in the proportion of women entering a trial when a placebo was included, with 85% power at the 5% significance level, would require 445 women, assuming that the proportion willing to enter a trial without a placebo arm was 40%.
Results
Case note search and response rate
We searched the case notes of 5452 women. Overall, we excluded 2981 (55%) women.
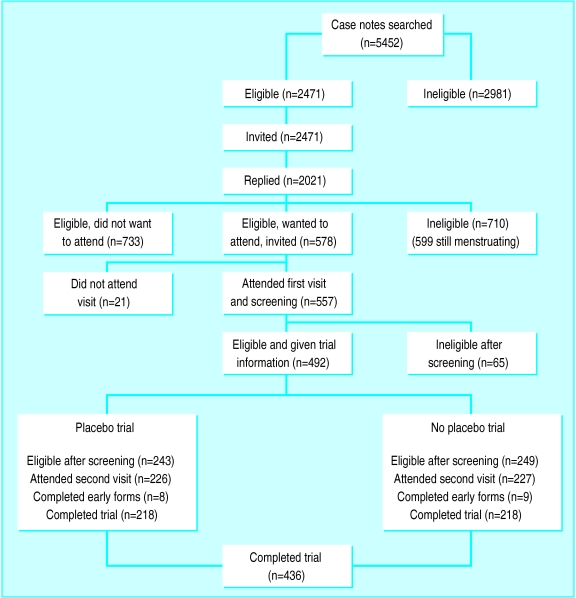
The figure shows the response rates throughout the stages of the trial. Of the 2471 women invited, 2021 (82%) completed and returned reply slips; 710 (35%) were ineligible—599 because they were still menstruating. Overall, 1311 (65%) seemed eligible and of these, 578 (44%) indicated they wanted to participate. Twenty one (4%) of these 578 women subsequently did not attend. Of the 557 women who attended the first interview, 65 (12%) were ineligible for reasons not detected at searching of the notes, 39 (7%) did not return for a second interview, and 17 (3%) were ineligible for other reasons. We used data in the analysis from the 436 women who attended both visits. Table 1 shows the characteristics of the two groups.
Table 1.
Characteristics of participants in two experimental groups. Values are numbers (percentages) of women unless stated otherwise
| Characteristic | No placebo (n=218) | Placebo (n=218) |
|---|---|---|
| Mean (SD) age (years) | 57.4 (4.7) | 57.2 (4.5) |
| Socioeconomic group:* | ||
| 1, 2, and 3 (non-manual)10 | 146 (68) | 166 (77) |
| Married or living with partner | 174 (80) | 172 (79) |
| White ethnic group | 217 (99) | 216 (99) |
| Smoking:* | ||
| Current | 54 (25) | 37 (17) |
| Ever | 47 (22) | 65 (30) |
| Never | 112 (53) | 115 (53) |
| Ever suffered from premenstrual tension | 92 (42) | 100 (46) |
| Ever used oral contraceptives | 103 (47) | 94 (43) |
| Ever used hormone replacement therapy | 37 (17) | 44 (20) |
Small amount of missing data reduced some denominators.
P<0.05.
Willingness to enter a trial
Eighty five (39%) of those women receiving information about the no placebo trial indicated their willingness to enter compared with 65 (30%) receiving information about the placebo trial (table 2). The difference of 9% (95% confidence interval 0% to 18%) is of borderline significance (χ2=3.67, df=1, P=0.06). After socioeconomic group was adjusted for, the percentages willing to enter each trial were 38% for the no placebo trial and 30% for the placebo trial (χ2=2.7, df=1, P=0.10), an 8% difference (–1% to 17%). There was no significant linear trend from “yes, probably” to “no, definitely not.”
Table 2.
Willingness to enter a trial of hormone replacement therapy with and without a placebo. Values are numbers (percentages) of women unless stated otherwise
| Variable | No placebo (n=218)
|
Placebo (n=218)
|
||
|---|---|---|---|---|
| Unadjusted | Adjusted (%)* | Unadjusted | ||
| Would participate | ||||
| Definitely | 36 (17) | 16 | 38 (17) | |
| Probably | 49 (23) | 22 | 27 (12) | |
| Total | 85 (39) | 38 | 65 (30) | |
| Would not participate | ||||
| Probably not | 41 (19) | 17 | 44 (20) | |
| Definitely not | 92 (42) | 46 | 109 (50) | |
| Total | 133 (61) | 62 | 153 (70) | |
| Did not attend second visit | 22 | — | 17 | |
Adjusted to socioeconomic distribution of those in placebo trial.
Reasons given for decision about participation
Forty two different reasons were given by women who indicated they would be willing to enter a trial compared with 83 reasons given by women who would not (some women gave more than one reason). Table 3 shows the six reasons most often given for each decision. Overall, 20 fewer women were prepared to participate in the placebo trial than the no placebo trial (table 2), of whom 55% gave not wanting to take a placebo as a reason for their decision.
Table 3.
Reasons most frequently given for decisions about likely participation in a trial.* Values are numbers (percentages) of women
| Reason | No placebo | Placebo | Total |
|---|---|---|---|
| Definitely or probably wanting to participate | n=85 | n=65 | n=150 |
| To help medical research | 21 (25) | 18 (28) | 39 (26) |
| I think it will help women in the future | 16 (19) | 18 (28) | 34 (23) |
| I will benefit from the treatment | 20 (24) | 7 (11) | 27 (18) |
| To help prevent osteoporosis | 13 (15) | 8 (12) | 21 (14) |
| Health continually assessed | 9 (11) | 8 (12) | 17 (11) |
| To help prevent heart disease | 9 (11) | 6 (9) | 15 (10) |
| Definitely or probably not wanting to participate | n=133 | n=153 | n=286 |
| Do not want periods to return | 38 (29) | 39 (26) | 77 (27) |
| Feel fine at present | 18 (14) | 17 (11) | 35 (12) |
| Won’t know which tablet I’m on | 15 (11) | 17 (11) | 32 (11) |
| Do not want to interfere with nature | 13 (10) | 9 (6) | 22 (8) |
| Do not want to take medication unnecessarily | 9 (7) | 12 (8) | 21 (7) |
| Do not want to take unnecessary risks with my health | 9 (7) | 10 (7) | 19 (7) |
| Do not want placebo | — | 11 (7) | — |
Some women gave more than one reason.
Discussion
Willingness to enter a randomised controlled trial of hormone replacement therapy seemed lower when the trial included a placebo arm, although this was not statistically significant. The sample size was set to detect a large or moderate effect and hence lacked the power to detect a small difference, so no definite conclusions can be drawn. Over half of the difference between the proportions willing to enter the two trials could be accounted for by women who stated explicitly that they did not wish to take a placebo. This shows that the inclusion of a placebo did directly influence some women’s decisions. Both proposed trials involved a treatment (oestrogen only) that would not now be recommended because of the increased risk of endometrial cancer for women with a uterus,3 but the study was underway before data from the postmenopausal estrogen/progestin interventions (PEPI) trial were published and was expected to inform the design of a trial to evaluate hormone replacement therapy that might have included unopposed as well as opposed treatment. The results are likely to provide a valid indication of the effect of including a placebo arm in a long term preventive (as distinct from therapeutic) trial.
Our study determined women’s willingness to enter rather than actual entry to the trials described. It could be argued that in response to a hypothetical situation more women might say that they were prepared to participate than actually would. Although we cannot rule out this possibility, the proportions indicating willingness to enter are similar to those for women who have already entered the Medical Research Council’s feasibility studies for a main hormone replacement therapy trial (unpublished data). As the same procedures were followed for our study, it is likely that the responses do reflect the true proportions willing to participate.
Similar proportions of patients in the two trial designs gave additional medical monitoring as their reason for wanting to take part, and this has been found in other trials.7 Women willing to participate in either trial showed a high degree of altruism and a desire to help increase scientific knowledge, again consistent with other reports.7,8 The importance of participating to provide potential benefit to others may explain why the inclusion of a placebo arm in a preventive trial may only have a slight adverse effect on recruitment. Not wanting the return of periods, not knowing which tablet they would be taking, and not wanting to take unnecessary drugs were the main reasons given for not wanting to participate. These reasons seem largely to relate to views about medical treatment and have been given by women in other trials.9
Recruitment to trials might be increased if information given to potential participants in the trial included the potential benefits for other people as well as the potential personal benefits and risks. Our study indicated that calculations for the sample size for a preventive trial may need to allow for reduced recruitment if a placebo is included, with the subsequent practical and financial implications entailed. Further work is needed to establish the generalisability of these results to other conditions.
Figure.
Flow chart of recruitment
Acknowledgments
We thank the doctors and nurses from the 10 general practices in the Medical Research Council General Practice Framework: Dr C Stubbings, Mrs L Lawrence; Dr J Wilner, Mrs E Ford; Dr W Callum, Mrs F Symes; Dr E Rule, Mrs M Couche; Dr P Meager, Mrs P Williams; Dr H Tait, Mrs B Scott, Mrs E Femandez; Dr K Barnard, Mrs J Wilkes; Dr R Roper, Mrs A Scott; Dr N Amott, Mrs E Hall; and Dr A McKay, Mrs M Mitchell. We also thank the six nurse trainers—Mrs P Allen, Mrs M Goldsborough, Mrs S Fox, Mrs L Hand, Mrs A Williams, and Mrs E Marshall; the participants who gave up their time to take part in the study; Helen Wilkes for her advice; Zaheer Islam for computer support; members of the lay panel for considering the patient information; and Sara Bordoley, Deepa Patel, and Sarah Holden for administrative help.
Footnotes
Funding: Medical Research Council. TMM is supported by the Wellcome Trust.
Competing interests: None declared.
References
- 1.Henderson BE, Pike MC, Ross RK, Mack TM, Lobo RA. Re-evaluating the role of progestogen therapy after the menopause. Fert Steril. 1988;49:9–15S. [PubMed] [Google Scholar]
- 2.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016–1041. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 3.Writing Group for the PEPI trial. Effects of estrogen/progestin regimens on heart disease risk factors in postmenopausal women: the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 4.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, et al. Postmenopausal estrogen therapy and cardiovascular disease: ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 5.Medical Research Council’s General Practice Research Framework. Randomised comparison of oestrogen versus oestrogen plus progestogen hormone replacement therapy in women with hysterectomy. BMJ. 1996;312:473–478. [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher LD, van Belle G. Biostatistics: a methodology for the health sciences. New York: John Wiley; 1993. [Google Scholar]
- 7.Mattson ME, Curb DJ, McArdle R the Aspirin Myocardial Infarction Study and Beta-blocker Heart Attack Trial Research Groups. Participation in a clinical trial: the patients’ point of view. Controlled Clin Trials. 1985;6:156–167. doi: 10.1016/0197-2456(85)90121-7. [DOI] [PubMed] [Google Scholar]
- 8.Cassileth BR, Lusk EJ, Miller DS, Hurwitz S. Attitudes towards clinical trials among patients and the public. JAMA. 1982;248:968–970. [PubMed] [Google Scholar]
- 9.Hunter MS, Liao KLM. Intentions to use hormone replacement therapy in a community sample of 45-year-old women. Maturitas. 1994;20:13–23. doi: 10.1016/0378-5122(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 10.Office of Population Censuses and Surveys. Standard occupational classifications. London: HMSO; 1993. [Google Scholar]