Abstract
Human aldehyde oxidase 1 (AOX1) has been subcloned into a vector suitable for expression in Escherichia coli, and the protein has been expressed. The resulting protein is active, with sulfur being incorporated in the molybdopterin cofactor. Expression levels are modest, but 1 liter of cells supplies enough protein for both biochemical and kinetic characterization. Partial purification is achieved by nickel affinity chromatography through the addition of six histidines to the amino-terminal end of the protein. Kinetic analysis, including kinetic isotope effects and comparison with xanthine oxidase, reveal similar mechanisms, with some subtle differences. This expression system will allow for the interrogation of human aldehyde oxidase structure/function relationships by site-directed mutagenesis and provide protein for characterizing the role of AOX1 in drug metabolism.
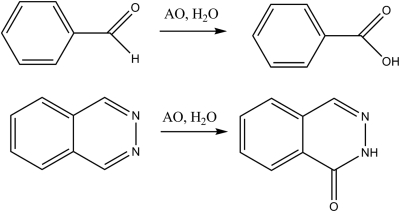
Over the past decade there has been a growing awareness that metabolism can play a major role in drug development and toxicity (O'Brien and de Groot, 2005; Rettie and Jones, 2005). In particular, drug design has evolved in the direction of more metabolically stable molecules, often with the major metabolite being oxidation of an aromatic ring by cytochrome P450 enzymes. This leads to reasonable pharmacokinetics; however, the oxidation products can show toxicity. One method to slow aromatic oxidation is incorporation of a nitrogen to make a heteroaromatic ring (Dowers et al., 2004). The electron-withdrawing characteristics of nitrogen slow the electrophilic chemistry of the cytochrome P450 enzymes. The net result can lead to a change in the metabolic pathways to nucleophilic addition by the molybdenum iron-sulfur flavoproteins, xanthine oxidase (XO) and aldehyde oxidase (AO) (Obach and Walsky, 2005). Both XO and AO can oxidize aldehydes and nitrogen-containing aromatic compounds (Fig. 1) (Beedham et al., 1990; Panoutsopoulos and Beedham, 2004). At present more than 40 drugs, nutritional supplements, and xenobiotics are metabolized to some extent by AO (Kitamura et al., 2006). Given that the changes in structural characteristics to decrease P450 metabolism have only been happening over the past 10 years we should see a significant increase in the importance of XO and AO in drug metabolism over the next decade as these drug design efforts result in drugs (Obach et al., 2004).
Fig. 1.
Typical oxidations by xanthine and aldehyde oxidases.
XO has a reasonably well defined physiological role in the metabolism of purines (Garattini et al., 2008), whereas the physiological role of AO has only recently been defined in mammals. The role of these enzymes in plants has received more attention (Mendel, 2007). Knockdown experiments on AOX1 (the mouse ortholog of the only active human enzyme) in mice indicate that this enzyme plays a functional role in adipogenesis (Weigert et al., 2008), whereas knockout of aldehyde oxidase homolog 2 in mice causes a decrease in retinoid-dependent genes and alteration of the epidermis (Terao et al., 2009).
One confounding feature in both drug metabolism and in understanding the physiological roles of AO enzymes is that significant interspecies variations exist in both the number and activity of mammalian enzymes. For example, the major species used in early drug development (mice, rats, and dogs) have a number of AO enzymes, whereas humans only have a single AO enzyme. Dogs in general have low activity, and two active AO enzymes, whereas rats and mice have four enzymes and show a marked interspecies variation in metabolism, whereas humans have a single highly active AO (AOX1) (Kitamura et al., 2006; Terao et al., 2006; Garattini et al., 2008). These interspecies differences mean that standard animal models will underestimate clearance, potentially leading to ineffective drugs due to rapid clearance when the compounds enter clinical trials. Human AOX1 has been partially purified from human liver and substrate specificity has been assessed and compared with that of guinea pig, rabbit, and baboon (Beedham et al., 1995).
Another interesting feature of AO is that the end electron acceptor is oxygen, resulting in the production of reactive oxygen species. These reactive oxygen species have been implicated in amyotrophic lateral sclerosis, otherwise known as Lou Gehrig's disease (Berger et al., 1995), and in ethanol-induced liver injury (Moriwaki et al., 1997). At this time, it is not known whether increasing turnover of AO leads to pathology as a result of increased reactive oxygen species. Another concern is that drugs that are substrates for AOX1 will alter adipose tissue homeostasis (Weigert et al., 2008).
Currently, if drugs are even screened, the major tool used in early drug discovery to identify the role of XO or AOX1 is human liver cytosol (Obach et al., 2004). Because both XO and AO metabolize similar substrates, inhibitors specific for each enzyme are used to determine which enzyme plays the major role in metabolism (Rashidi et al., 1997). Although the expression of human XO in Escherichia coli has been reported (Yamaguchi et al., 2007), to our knowledge no expression system is available for human AO. To this end, we have expressed and partially purified human AO in E. coli. Partial purification is done by incorporation of six histidines onto the amino terminus followed by affinity chromatography. Mechanistic comparisons using substituent effects and kinetic isotope effects with bovine XO confirm the speculation that AO and XO have relatively similar chemical and kinetic mechanisms.
Materials and Methods
Materials.
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) at reagent grade purity or finer unless specified otherwise. Anthranilic acid was from Mallinckrodt Baker (Phillipsburg, NJ), and 2-amino-5-nitrobenzoic acid was from Alfa Aesar (Ward Hill, MA).
Construction of Expression Vector.
The vector was constructed using a strategy similar to that described by Tanaka and coworkers (Hoshino et al., 2007). The protein coding region of human aldehyde oxidase cDNA in a pCMV6-XL4 mammalian plasmid was PCR-amplified using PfuUltra Hotstart PCR Master Mix (Stratagene, La Jolla, CA). The primers used for amplification were 5′-TAC CAT ATC GGT ACC ATG GAC CGG GCG-3′ (forward), with an introduced Acc65I restriction site, and 5′-TAC CAT ATC GTC GAC TCA GAT GGG TAC-3′ (reverse), with an introduced SalI restriction site. PCRs contained 8.6 ng of template and 16.5 μg (10 μl, 20 μM) of forward primer and 16.3 μg (10 μl, 20 μM) of reverse primer in a 25-μl volume, which was mixed with 25 μl of PCR Master Mix with a final volume of 50 μl. The thermocycling program used was as follows: predenaturation at 95°C for 5 min then 35 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 5 min and final extension at 72°C for 10 min. The resulting 4.1-kilobase PCR product was then purified using QIAquick columns (QIAGEN, Valencia, CA). The PCR fragment and pQE-30 Xa (QIAGEN) were then digested with Acc65I and SalI (New England Biolabs, Ipswich, MA), and the DNA fragments were gel-purified using low-melt SeaPlaque Agarose (Lonza Walkersville, Inc., Walkersville, MD). The DNA bands were visualized using SYBR Safe dye (Invitrogen, Carlsbad, CA) and a Dark Reader blue-light transilluminator (Clare Chemical Research Inc., Dolores, CO) and subsequently were excised. The gels slabs were melted at 68°C and in-gel ligation was performed using T4 ligase (New England Biolabs). Ligation reactions included a human AO PCR product (10 μl, 69 ng), pQE-30 Xa (2.5 μl, 22 ng), ligation buffer (2 μl, 1× final concentration), sterile double-distilled H2O (4.5 μl), and T4 ligase (1 μl, 400 units) in a final volume of 20 μl. The ligation reaction was performed at room temperature for 1.5 h and then placed at 4°C overnight. The following morning the ligation reaction was removed from the 4°C and allowed to stand for an additional 1 h at room temperature. The ligation reaction was melted at 68°C and diluted to 100 μl with sterile double-distilled H2O. Then 25 μl of the diluted ligation reaction was transformed into 50 μl of DH5α subcloning efficiency competent cells (Invitrogen). Transformants were plated on LB agar plates containing 100 μg/ml ampicillin and grown overnight at 37°C. Plasmid DNA from several colonies was isolated using a QIAprep Spin Miniprep Kit (QIAGEN) and screened by restriction digestion with Acc65I and SalI. A positive colony was replated and grown over night at 37°C. Then a single colony was picked and grown up overnight in LB broth containing 100 μg/ml ampicillin, and plasmid DNA was isolated using a Wizard II midi prep kit (Promega, Madison, WI).
Sequencing of AO-Containing Plasmid.
Sequence analysis was performed using a model 3730 DNA analyzer from Applied Biosystems (Foster City, CA). The Applied Biosystems BigDye sequencing reaction was used to sequence the DNA. The cDNA (A1717G/K573E mutant) was sequenced in the forward (5′) and reverse directions (3′) until the forward and reverse data overlapped. This process provided the entire sequence for the gene. Individual directions (forward and reverse) were sequenced with overlapping data between analyses. Blast searches were used to compare sequencing data with the known sequence of the gene.
Expression of AO in E. coli.
Aldehyde oxidase was overexpressed as an N-terminal 6x-His-tag with a factor Xa protease recognition site fusion in TP-1000 cells (a gift from John Enemark's laboratory, University of Arizona). The pQE-30 Xa plasmid containing AO cDNA was transformed into TP-1000 cells and grown on LB agarose plates containing 100 μg/ml ampicillin. A single colony was picked and grown overnight in 100 ml of supplemented LB broth (100 μg/ml ampicillin, 1 μg/ml riboflavin, and 50 μM sodium molybdate). The overnight culture (10 ml) was used to inoculate 500 ml of supplemented Terrific broth [100 μg/ml ampicillin, 250 μl of trace element solution containing 2.7 g of FeCl3 · 6H2O, 0.2 g of ZnCl2 · 4H2O, 0.2 g of CoCl2 · 6H2O, 0.2 g Na2MoO4 · 2H2O, 0.2 g of CaCl · 2H2O, 0.1 g of CuCl2, 0.05 g of H3BO3, and 10 ml of HCl (concentrated) autoclaved in a total volume of 100 ml of di-H2O, 1 μg/ml riboflavin, and additional 50 μM sodium molybdate]. Cultures were grown at 37°C and 250 rpm until an absorbance of 0.4 at 600 nm was reached for 60 to 90 min; isopropyl β-d-thiogalactoside (1 mM) was added for induction, and cells were allowed to continue growing at room temperature for 72 h at 150 rpm. Cells were harvested by centrifugation at 3500g at 4°C for 30 min. Cell paste was collected and resuspended in equal volumes of 1 g of paste to 1 ml of buffer 1 [100 mM potassium phosphate buffer, 300 mM NaCl, 0.5 μl/ml protease inhibitor cocktail (Sigma P8849)] and frozen at −80°C.
Mutagenesis.
Human AO cDNA in the pQE-30 Xa expression plasmid was mutagenized using the QuikChange II site-directed mutagenesis kit (Stratagene). The mutagenesis was performed using the manufacturer's suggested protocol. Primers were designed using the online tool provided by Stratagene. High-performance liquid chromatography-purified primers were purchased from Invitrogen. The primers used to generate the wild-type plasmid (E573K) were 5′-aagtaccagaatataggcccaaagcagcatcctgaa-3 (forward) and 5′-ttcaggatgctgctttgggcctatattctggtactt-3′ (reverse).
Purification of His-Tagged AO.
Cell suspension was thawed and lysed by adding lysozyme (5 mg/ml), MgCl2 (28.6 μg/ml), RNase (10 μg/ml), and DNase (10 μg/ml) and stirring at 4°C for 60 min. The cell suspension was disrupted by sonication. After centrifugation for 40 min at 100,000g, the supernatant was loaded onto a 1-ml HiTrap Chelating HP column (GE Healthcare, Little Chalfont, Buckinghamshire, UK) charged with Ni2+ equilibrated with buffer 1. The column was washed with 5 ml of buffer 1 and 5 ml of buffer 2 (100 mM potassium phosphate buffer, pH 7.4, 20 mM imidazole, and 0.5 μl/ml protease inhibitor cocktail). The human AO was eluted with 3 ml of 100 mM potassium phosphate buffer at pH 7.4 and 500 mM imidazole. Protein concentrations were determined by the Bradford assay using bovine serum albumin as a standard and by quantifying flavin adenine dinucleotide content (Massey et al., 1970).
Gel Electrophoresis.
SDS-PAGE was performed using the Phast System (GE Healthcare) with PhastGel Homogeneous 7.5 gels (GE Healthcare) using the protocol reported in Separation Technique Files 111 (GE Healthcare Life Sciences, 1998b). Proteins were stained with PhastGel Blue using Development Technique File 200 (GE Healthcare Life Sciences, 1998a).
Tryptic Digestion of Protein.
AO (300 μl, 2.84 μM) was concentrated by trichloroacetic acid precipitation. The protein was resuspended in 8 M urea with 100 mM NH4HCO3 follow by addition of dithiothreitol to a 5 mM final concentration and incubated at 37°C for 30 min. Iodoacetamide was then added (1 μl, 25 mM final), and the mixture was incubated at 37°C for an additional 30 min. The solution was diluted to 100 μl with 100 mM NH4HCO3 (pH 7.5–8.0) and subsequently digested overnight with trypsin (50 μl, 1 μg) at 37°C.
Mass Spectrometry.
Tryptic digests were analyzed on an Esquire HCT electrospray ion-trap mass spectrometer (Bruker Daltonics, Billerica, MA) coupled to an LC Packings Ultimate Nano high-performance liquid chromatography system (instrument). Chromatography was performed with an LC Packings monolithic column using a binary solvent system containing 0.1% formic acid with 3% acetonitrile (solvent A) and 0.1% formic acid with 95% acetonitrile (solvent B). The sample was eluted using a five-step linear gradient program of 5% buffer B at 3 min, 15% buffer B at 15 min, 30% buffer B at 60 min, 65% buffer B at 95 min, and 100% buffer B at 95.1 min and held at 100% until 115 min with a flow rate of 800 nl/min (Weigert et al., 2008).
Ferricyanide Activity Assay.
Spectrophotometric activity assays using ferricyanide as a single electron acceptor were performed as described by Krenitsky et al. (1972). The reaction buffer for assays contained 1 mM potassium ferricyanide, 0.13 mM EDTA, and 100 mM potassium phosphate buffer (pH 6.8) in a final volume of 1 ml and was incubated at room temperature. Enzyme and substrate concentrations were of adequate concentration to obtain linear initial rates. Substrate concentrations of 6.25, 12.5, 25, 50, 100, 200, and 400 μM were used with 19.12 pmol of AOX1 and 82.15 pmol of XO. Reaction progress for the single electron reduction of ferricyanide was monitored by UV absorption at 420 nm, and rates were calculated using an extinction coefficient of 2.1 mM−1 cm−1. The extinction coefficient for the single electron transfer to ferricyanide is 1.040 mM−1 cm−1; however, because the stoichiometry for oxidation of a single substrate is 1 to 2 mol of electrons transferred, the extinction coefficient was multiplied by 2. Kinetic parameters were obtained by fitting data to a hyperbola using GraphPad Prism.
Activity Assays Using Quinazolinones and Direct UV Measurement.
Quinazolinone activities were measured using methods similar to those described by Skibo et al. (1987). The oxidation of quinazolinones was monitored by UV at 270 nm. Assays were performed in buffer containing 100 mM potassium phosphate buffer (pH 7.4), at room temperature in a final volume of 1 ml. Enzyme and substrate concentrations were of adequate concentration to obtain linear initial rates. For the quinazolinones we used five substrates concentrations 12.5, 25, 50, 100, and 200 μM and 118 pmol of partially purified AOX1. Absorption changes were measured for 2 to 3 min, depending on the linearity of the absorption change. Kinetic parameters were obtained by fitting data to a hyperbola using GraphPad Prism.
Synthesis of Quinazolinones.
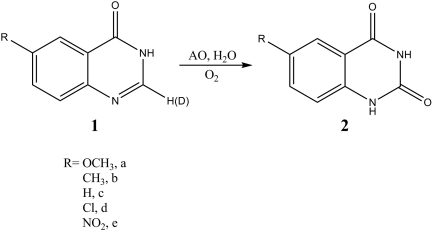
Substituted quinazolinones were synthesized using a microwave-assisted Niementowski reaction (Alexandre et al., 2002). A general procedure is given with the synthesis of compound 1a (Fig. 3). Anthranilic acid (200 mg, 1.45 mmol) and formamide (5 ml, reagent grade) were combined in a 24 × 200-mm test tube equipped with a ceramic funnel. This tube was irradiated using a standard kitchen microwave oven for a total of 3 min in 1-min intervals with 5 min of cooling between irradiation. Before the open-tube method of preparation was used, a 15-ml heavy walled pressure tube equipped with a Teflon plug and O-ring was used for the synthesis, and the reaction was irradiated for 30 min in 5-min intervals with 5 min of cooling (preparation of 1c and 1d) (Fig. 2). However, we found that under these conditions the reaction was prone to explosion. Thus, we suggest using the open tube method, not the capped tube method. After irradiation and upon cooling, excess formamide was removed by rotary evaporation and the resulting crude quinazolinones were crystallized from hot ethanol, affording a solid. The yields for quinazolinones after crystallization were 41% (1a), 61% (1b), 91% (1c and 1d), and 34% (1e). The 1H NMR spectra of the products were consistent with literature values (Orfi et al., 2004; Domarkas et al., 2006). Deuterium exchanges in the C-2 position of 4(3H)-quinazolinones (1a–1e) were accomplished using an uncatalyzed microwave-assisted method (de Keczer et al., 2004). A typical exchange reaction is given with the synthesis of deuterated 1c. In a 15-ml heavy walled pressure tube equipped with a Teflon plug and O-ring 1c (100 mg, 0.684 mmol) was combined with D2O (2 ml) and CD3OD (2 ml). The reactions were irradiated using a standard kitchen microwave oven with irradiation times of 2 min (15-s intervals at 50% power with 2–3 min of cooling) and 15 min. Upon cooling, the solvent was removed by rotary evaporation, and the reaction was repeated until deuterium incorporation was complete as monitored by 1H NMR. In general, three cycles were necessary to achieve deuterium incorporation at the C-2 position of 95% as determined by mass spectral analysis.
Fig. 3.
Oxidation of 6-substututed quinazolinones.
Fig. 2.
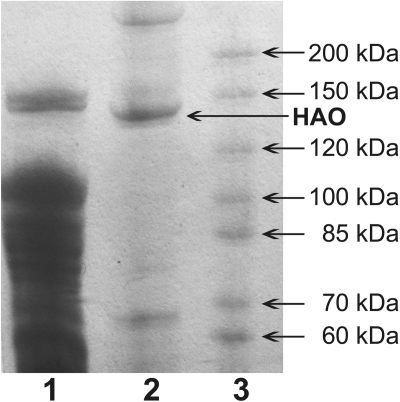
SDS-PAGE gel. Lane 1, 10 μl of precolumn lysate. Lane 2, 10 μl of purified human AO (HAO). Lane 3, 10 μl of EZ-Run Rec Protein Ladder (Fisher BioReagents, Houston, TX).
Results
The human AO-containing vector was constructed by PCR, amplifying the protein coding region of AO cDNA from the pCMV6-XL4 plasmid with the addition of flanking Acc65I and SalI restriction sites. The PCR product was then subcloned into a pQE-30 Xa expression vector. The resulting vector construct was sequenced and a single nucleotide mutation, A1818G (the mutation is A1717G using numbering starting from the start codon of the protein encoding region of the cDNA), was found, translating into a single amino acid mutation, K573E. Comparison of this region in the bovine xanthine oxidase crystal structure (pdb1V97) shows that this residue is on the exterior of the protein (Okamoto et al., 2004). Site-directed mutagenesis was used to correct this mutation to wild-type, and 100% of the coding region is in agreement with the GenBank sequence.
The enzyme was expressed in TP-1000 cells and purified by affinity chromatography. In general, 20 g of cell paste was obtained from 1 liter of growth medium with ∼5 to 15 nmol in 1 ml of purified enzyme using our procedures. Figure 2 shows an SDS-PAGE gel of AO before and after purification using a nickel column. The significantly purified protein ran close to the 150-kDa molecular mass marker and appears to be full-length based on this and the tandem mass spectrometry sequencing.
Purified human AO was reduced, alkylated, and subsequently digested with trypsin. The tryptic digest was analyzed by liquid chromatography-tandem mass spectrometry and the following tryptic peptides were observed and confirmed: ASELLFYVNGR (4–14), YGCGGGGCGACTVMISR (42–58), HHPANACLIPICSLYGAAVTTVEGIGSTHTR (68–98), FKYPQAPVIMGNTSVGPEVK (257–276), VFFGEGDGIIR (447–457), VFCVGQLVCAVLADSEVQAK (668–687), RVGGAFGGK (802–810), GFGFPQAALITESCITEVAAK (922–942), FPVGLGSR (1014–1021), and GLHGPLTLNSPLTPEK (1295–1310). The peptides observed only account for 12% sequence coverage, but the last peptide covers up to the last 28 amino acids, whereas the first covers up to the first 4 amino acids of the 1338 amino acid protein, consistent with full-length expression.
We measured the activity of the 6-substituted quinazolinones (Fig. 3) in our human AO preparation, and the data are given in Table 1. Overall, V/K values increase as the substituents become more electron withdrawing with the exception of R = CH3. The kinetic isotope effects (KIE) on V/K were determined for substitution of deuterium at the site of oxidation and are given in Table 1. The KIE is significant for all the compounds and increases for the slower reactions.
TABLE 1.
Kinetic parameters for human AO metabolism of 6-substituted quinazolinones
| R = | KMa | Vmaxb | V/Kc | KIEd |
|---|---|---|---|---|
| μM | nmol/s/mg enzyme | |||
| OCH3 | 293 | 135 | 461 | N.D. |
| CH3 | 142 | 22 | 155 | 7.6 |
| H | 399 | 240 | 601 | 6.3 |
| Cl | 27 | 113 | 4185 | 4.5 |
N.D., not determined.
The concentration at half-maximal velocity. Errors are approximately 10% for each value.
The maximum velocity estimated from the asymptote for the hyperbola. Errors are approximately 10% for each value.
V/K multiplied by 1000.
The isotope effect on V/K determined by taking the ratio of V/K for the proton/deuterium substitution at the site of oxidation.
We repeated these experiments using commercial preparations of bovine xanthine oxidase for comparison with our results for human aldehyde oxidase and the results are shown in Table 2. We chose bovine XO to compare with human AO because it is available commercially and has been studied extensively. Overall, although the values are not in perfect agreement, the trends are similar. The kinetic isotope effects we measured for AO are larger than those of Skibo et al. (1987); however, without further study we do not know if this is a significant difference. Uncertainties arise from the fact that the values are not corrected for substrate inhibition or sulfur incorporation in the cofactor (Schumann et al., 2009), both of which could affect Vmax estimates.
TABLE 2.
Kinetic parameters for bovine XO metabolism of 6-substituted quinazolinones
| R = | KMa | Vmaxb | V/Kc | KIEd |
|---|---|---|---|---|
| μ M | nmol/s/mg enzyme | |||
| OCH3 | 85 | 9 | 101 | N.D. |
| CH3 | 56 | 121 | 2161 | 5.2 |
| H | 31 | 17 | 548 | N.D. |
| Cl | 11 | 164 | 14,909 | 3.0 |
N.D., not determined.
The concentration at half-maximal velocity. Errors are approximately 10% for each value.
The maximum velocity estimated from the asymptote for the hyperbola. Errors are approximately 10% for each value.
V/K multiplied by 1000.
The isotope effect on V/K determined by taking the ratio of V/K for the proton/deuterium substitution at the site of oxidation from Skibo et al. (1987).
One exception to the general agreement between the kinetic trends in AO and XO is that when we attempted to obtain kinetic parameters for 6-nitroquinazolinone we found that aldehyde oxidase was unable to catalyze this reaction, whereas under the same conditions we could obtain product with bovine xanthine oxidase. Presently, we are trying to characterize why 6-nitroquinazoinone is not a substrate for AO.
Discussion
Human AOX1 is at present responsible for the metabolism of a number of drugs containing aldehydes and the more prevalent nitrogen heterocycles (Kitamura et al., 2006; Torres et al., 2007). The fraction of drugs metabolized by AOX1 is likely to increase over the next decade. However, the availability of an in vitro system for distinguishing between XO and AOX1 metabolism in humans is lacking. The most commonly used preparation is human liver cytosol, which contains both XO and AOX1. The expression of AOX1 in humans will allow researchers in the field to answer a number of questions that have not been approachable to this point. Our E. coli expression system should allow for the exploration of the protein structure-function relationship by site-directed mutagenesis and the use of biophysical methods to explore catalysis and binding. In this study, we report the expression and partial purification of AOX1 and use the expressed enzyme to characterize the kinetics of the enzyme without interference from XO.
Human AOX1 cDNA was subcloned into a pQE-30 Xa vector for expression, relying on the ground-breaking work of Tanaka and coworkers in subcloning of monkey AOX1 (Hoshino et al., 2007). We included a 6x-His-tag on the amino terminus to aid in purification. No attempt was made to remove the His-tag to determine whether these amino acids alter activity. The resulting plasmid, when sequenced, was consistent with the 6x-His wild-type AOX1 with a single mutation at A1717G relative to the start codon, translating into a single amino acid mutation, K573E, in the expressed protein. Site-directed mutagenesis was used to restore the wild-type sequence.
Expression was achieved in TP-1000 E. coli cells (Palmer et al., 1996) using standard expression protocols. The TP-1000 E. coli strain does not have the mobA and mobB genes that put GMP onto the molybdopterin cofactor. Although GMP molybdopterin cofactor is required for binding to E. coli molybdoenzymes, mammalian enzymes use the GMP-free cofactor. This expression resulted in between 5 and 15 nmol of partially purified protein per liter of medium based on the flavin optical absorbance at 450 nm. Experiments using cyanide to remove sulfur from the molybdopterin cofactor in AO resulted in catalytically inactive protein, as expected from previous results with XO (Massey et al., 1970). It is obvious that a sulfurase from E. coli is incorporating sulfur into the cofactor, although the specific sulfurase is unknown. Attempts to increase activity by chemically incorporating sulfur using established protocols were not successful (Wahl and Rajagopalan, 1982). By using this procedure, we were able to recover cyanide-inactivated XO. This led us to conclude that sulfur incorporation is high in the expressed enzyme, although methods for determining the amount of sulfur in the cofactor were not sensitive enough in our hands to determine the sulfur content of the enzyme. One caveat to this conclusion is that we found that the human AO enzyme is not particularly stable to manipulation and that dialysis, for example, can inactivate the enzyme.
Purification was accomplished by affinity chromatography using a nickel chelate column, after addition of six histidine residues to the amino terminus. The histidine tags did not appear to alter trends in activity and binding affinity; however, because this enzyme has never been purified and evaluated some caution is required. Comparison of phthalazine activities (an established AO probe) (Beedham et al., 1990; Obach et al., 2004) in the partially purified enzyme and in human cytosol gave Km values of 29 and 3.7 μM, respectively. Although this is not a large difference, it is outside of the normal 10 to 15% error we see in determining Km values for AO. This difference could be a result of the histidine affinity tag or the fact that XO is present in the human cytosol. Although to our knowledge, the Km for human XO for phthalazine has not been reported, we measured a 40 μM Km for bovine XO. If human XO has a lower Km, it could account for the lower apparent Km in the cytosolic mixture.
We used 6-substituted quinazolinones (Fig. 2) to probe the kinetic and chemical mechanism of human AO. We used this series of substrates because previous work by Skibo et al. (1987) established that electron-withdrawing groups increase the rates of reaction and that significant kinetic isotope effects were observed when deuterium replaces hydrogen at the site of oxidation (the 2-position). Although it has been speculated that AO and XO have similar mechanisms, almost all mechanistic studies have been done on XO, and no direct comparison has been made. At present two very similar mechanisms are supported by the literature, both involving nucleophilic attack of a deprotonated water molecule coordinated to the molybdenum with either a concerted hydride transfer or initial tetrahedral intermediate formation followed by hydride transfer. The results presented in Tables 1 and 2 are consistent with either mechanism with some caveats (Alfaro and Jones, 2008; Doonan et al., 2008), although the hydride transfer is required to be rate-determining at least to some extent (Schimerlik et al., 1977; Northrop, 1981; Ray, 1983). The results for AO provide a limited free energy relationship, although for both XO and AOX1 the methyl-substituted quinazolinones show very different nonlinear behavior. However, in general, the electron-withdrawing group seems to increase the catalytic efficiency as measured by V/K. The positive slope relative to the Hammett parameters indicates that either a negative charge is developed in the transition state or that equilibrium addition of the nucleophile increases the concentration of the tetrahedral intermediate, increasing the flux of the next step, hydride transfer (Ilich and Hille, 1999; Okamoto et al., 2004; Doonan et al., 2005; Hille, 2005). To fit the isotope effect data, hydride transfer must be rate-limiting, and the trends in kinetic isotope effect support the concerted mechanism (Alfaro and Jones, 2008).
The results presented in Tables 1 and 2 also appear to support the conclusion that for substituted quinazolinone substrates the mechanisms for AO and XO are similar but not identical. Two major differences are observed: 1) the 6-nitroquinazolinone is not a substrate for AO and 2) the kinetic isotope effects appear to be larger for AO than for XO. The contrast between the behavior of the nitro compound in XO, for which it is a very good substrate as reported by Skibo et al. (1987), and in AO, which does not turn over the compound is surprising and unexplained. However, the larger KIE values for AO than for XO can be explained in that the KIE of XO may be masked by other partially rate-determining steps, whereas AO hydride transfer is more rate-limiting, unmasking the intrinsic KIE value (Northrop, 1981; Ray, 1983). Studies are ongoing to elaborate on these possible differences in mechanism.
In conclusion, we have expressed human AO in E. coli and have been able to purify protein that is kinetically active. To illustrate the usefulness of this construct, we have done a series of kinetic experiments and compared the kinetic mechanisms of AO and XO, concluding that they share a similar but not identical mechanism. The availability of pure enzyme that can be manipulated by site-directed mutagenesis allows us to compare and contrast human AO with other species. Furthermore, phenotypic variation in human metabolism has not been explored even though more than eight phenotypic variants have been identified (Mwenifumbo and Tyndale, 2009). This construct will allow us to understand whether any of these variants have functional consequences.
Acknowledgments.
We thank Professor John Enemark for sending us TP-1000 E. coli cells, Tom Rushmore for providing the human cDNA and for helpful discussions, and Professor Yorihisa Tanaka for providing monkey AO, which we used to optimize expression conditions.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM84546].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.029520
- XO
- xanthine oxidase
- AO
- aldehyde oxidase
- AOX1
- aldehyde oxidase 1
- PCR
- polymerase chain reaction
- PAGE
- polyacrylamide gel electrophoresis
- MS/MS
- mass spectrometry
- KIE
- kinetic isotope effect.
References
- Alexandre FR, Berecibar A, Besson T. (2002) Microwave-assisted Niementowski reaction: back to the roots. Tetrahedron Lett 43:3911–3913 [Google Scholar]
- Alfaro JF, Jones JP. (2008) Studies on the mechanism of aldehyde oxidase and xanthine oxidase. J Org Chem doi:10.1021/jo801053u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beedham C, Bruce SE, Critchley DJ, Rance DJ. (1990) 1-Substituted phthalazines as probes of the substrate-binding site of mammalian molybdenum hydroxylases. Biochem Pharmacol 39:1213–1221 [DOI] [PubMed] [Google Scholar]
- Beedham C, Critchley DJ, Rance DJ. (1995) Substrate specificity of human liver aldehyde oxidase toward substituted quinazolines and phthalazines: a comparison with hepatic enzyme from guinea pig, rabbit, and baboon. Arch Biochem Biophys 319:481–490 [DOI] [PubMed] [Google Scholar]
- Berger R, Mezey E, Clancy KP, Harta G, Wright RM, Repine JE, Brown RH, Brownstein M, Patterson D. (1995) Analysis of aldehyde oxidase and xanthine dehydrogenase/oxidase as possible candidate genes for autosomal recessive familial amyotrophic lateral sclerosis. Somat Cell Mol Genet 21:121–131 [DOI] [PubMed] [Google Scholar]
- de Keczer SA, Lane TS, Masjedizadeh MR. (2004) Uncatalyzed microwave deuterium exchange labeling of bleomycin A2. J Labelled Compd Radiopharm 47:733–740 [Google Scholar]
- Domarkas J, Dudouit F, Williams C, Qiyu Q, Banerjee R, Brahimi F, Jean-Claude BJ. (2006) The combi-targeting concept: synthesis of stable nitrosoureas designed to inhibit the epidermal growth factor receptor (EGFR). J Med Chem 49:3544–3552 [DOI] [PubMed] [Google Scholar]
- Doonan CJ, Rubie ND, Peariso K, Harris HH, Knottenbelt SZ, George GN, Young CG, Kirk ML. (2008) Electronic structure description of the cis-MoOS unit in models for molybdenum hydroxylases. J Am Chem Soc 130:55–65 [DOI] [PubMed] [Google Scholar]
- Doonan CJ, Stockert A, Hille R, George GN. (2005) Nature of the catalytically labile oxygen at the active site of xanthine oxidase. J Am Chem Soc 127:4518–4522 [DOI] [PubMed] [Google Scholar]
- Dowers TS, Rock DA, Rock DA, Perkins BN, Jones JP. (2004) An analysis of the regioselectivity of aromatic hydroxylation and N-oxygenation by cytochrome P450 enzymes. Drug Metab Dispos 32:328–332 [DOI] [PubMed] [Google Scholar]
- Garattini E, Fratelli M, Terao M. (2008). Mammalian aldehyde oxidases: genetics, evolution and biochemistry. Cell Mol Life Sci 65:1019–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GE Healthcare Life Sciences (1998a) Development Technique File 200: fast Coomassie staining GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK: [Google Scholar]
- GE Healthcare Life Sciences (1998b) Separation Technique File 111: SDS-PAGE in homogeneous media GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK: [Google Scholar]
- Hille R. (2005) Molybdenum-containing hydroxylases. Arch Biochem Biophys 433:107–116 [DOI] [PubMed] [Google Scholar]
- Hoshino K, Itoh K, Masubuchi A, Adachi M, Asakawa T, Watanabe N, Kosaka T, Tanaka Y. (2007) Cloning, expression, and characterization of male cynomolgus monkey liver aldehyde oxidase. Biol Pharm Bull 30:1191–1198 [DOI] [PubMed] [Google Scholar]
- Ilich P, Hille R. (1999) Mechanism of formamide hydroxylation catalyzed by a molybdenum-dithiolene complex: a model for xanthine oxidase reactivity. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys 103:5406–5412 [Google Scholar]
- Kitamura S, Sugihara K, Ohta S. (2006) Drug-metabolizing ability of molybdenum hydroxylases. Drug Metab Pharmacokinet 21:83–98 [DOI] [PubMed] [Google Scholar]
- Krenitsky TA, Neil SM, Elion GB, Hitchings GH. (1972) A comparison of the specificities of xanthine oxidase and aldehyde oxidase. Arch Biochem Biophys 150:585–599 [DOI] [PubMed] [Google Scholar]
- Massey V, Komai H, Palmer G, Elion GB. (1970) On the mechanism of inactivation of xanthine oxidase by allopurinol and other pyrazolo[3,4-d]pyrimidines. J Biol Chem 245:2837–2844 [PubMed] [Google Scholar]
- Mendel RR. (2007) Biology of the molybdenum cofactor. J Exp Bot 58:2289–2296 [DOI] [PubMed] [Google Scholar]
- Moriwaki Y, Yamamoto T, Higashino K. (1997) Distribution and pathophysiologic role of molybdenum-containing enzymes. Histol Histopathol 12:513–524 [PubMed] [Google Scholar]
- Mwenifumbo JC, Tyndale RF.2009. Molecular genetics of nicotine metabolism. Handb Exp Pharmacol 192:235–259 [DOI] [PubMed] [Google Scholar]
- Northrop DB. (1981) Minimal kinetic mechanism and general equation for deuterium isotope effects on enzymic reactions: uncertainty in detecting a rate-limiting step. Biochemistry 20:4056–4061 [DOI] [PubMed] [Google Scholar]
- O'Brien SE, de Groot MJ. (2005) Greater than the sum of its parts: combining models for useful ADMET prediction. J Med Chem 48:1287–1291 [DOI] [PubMed] [Google Scholar]
- Obach RS, Huynh P, Allen MC, Beedham C. (2004) Human liver aldehyde oxidase: inhibition by 239 drugs. J Clin Pharmacol 44:7–19 [DOI] [PubMed] [Google Scholar]
- Obach RS, Walsky RL. (2005) Drugs that inhibit oxidation reactions catalyzed by aldehyde oxidase do not inhibit the reductive metabolism of ziprasidone to its major metabolite, S-methyldihydroziprasidone: an in vitro study. J Clin Psychopharmacol 25:605–608 [DOI] [PubMed] [Google Scholar]
- Okamoto K, Matsumoto K, Hille R, Eger BT, Pai EF, Nishino T. (2004) The crystal structure of xanthine oxidoreductase during catalysis: implications for reaction mechanism and enzyme inhibition. Proc Natl Acad Sci U S A 101:7931–7936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfi L, Wáczek F, Pató J, Varga I, Hegymegi-Barakonyi B, Houghten RA, Kéri G. (2004) Improved, high yield synthesis of 3H-quinazolin-4-ones, the key intermediates of recently developed drugs. Curr Med Chem 11:2549–2553 [DOI] [PubMed] [Google Scholar]
- Palmer T, Santini CL, Iobbi-Nivol C, Eaves DJ, Boxer DH, Giordano G. (1996) Involvement of the narJ and mob gene products in distinct steps in the biosynthesis of the molybdoenzyme nitrate reductase in Escherichia coli. Mol Microbiol 20:875–884 [DOI] [PubMed] [Google Scholar]
- Panoutsopoulos GI, Beedham C. (2004) Kinetics and specificity of guinea pig liver aldehyde oxidase and bovine milk xanthine oxidase towards substituted benzaldehydes. Acta Biochim Pol 51:649–663 [PubMed] [Google Scholar]
- Rashidi MR, Smith JA, Clarke SE, Beedham C. (1997) In vitro oxidation of famciclovir and 6-deoxypenciclovir by aldehyde oxidase from human, guinea pig, rabbit, and rat liver. Drug Metab Dispos 25:805–813 [PubMed] [Google Scholar]
- Ray WJ., Jr (1983) Rate-limiting step: a quantitative definition: application to steady-state enzymic reactions. Biochemistry 22:4625–4637 [DOI] [PubMed] [Google Scholar]
- Rettie AE, Jones JP. (2005) Clinical and toxicological relevance of CYP2C9: drug-drug interactions and pharmacogenetics. Annu Rev Pharmacol Toxicol 45:477–494 [DOI] [PubMed] [Google Scholar]
- Schimerlik MI, Grimshaw CE, Cleland WW. (1977) Determination of the rate-limiting steps for malic enzyme by the use of isotope effects and other kinetic studies. Biochemistry 16:571–576 [DOI] [PubMed] [Google Scholar]
- Schumann S, Terao M, Garattini E, Saggu M, Lendzian F, Hildebrandt P, Leimkühler S. (2009) Site directed mutagenesis of amino acid residues at the active site of mouse aldehyde oxidase AOX1. PLoS One 4:e5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibo EB, Gilchrist JH, Lee CH. (1987) Electronic probes of the mechanism of substrate oxidation by buttermilk xanthine oxidase: role of the active-site nucleophile in oxidation. Biochemistry 26:3032–3037 [DOI] [PubMed] [Google Scholar]
- Terao M, Kurosaki M, Barzago MM, Fratelli M, Bagnati R, Bastone A, Giudice C, Scanziani E, Mancuso A, Tiveron C, et al. (2009) Role of the molybdoflavoenzyme aldehyde oxidase homolog 2 in the biosynthesis of retinoic acid: generation and characterization of a knockout mouse. Mol Cell Biol 29:357–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao M, Kurosaki M, Barzago MM, Varasano E, Boldetti A, Bastone A, Fratelli M, Garattini E. (2006) Avian and canine aldehyde oxidases. Novel insights into the biology and evolution of molybdo-flavoenzymes. J Biol Chem 281:19748–19761 [DOI] [PubMed] [Google Scholar]
- Torres RA, Korzekwa KR, McMasters DR, Fandozzi CM, Jones JP. (2007) Use of density functional calculations to predict the regioselectivity of drugs and molecules metabolized by aldehyde oxidase. J Med Chem 50:4642–4647 [DOI] [PubMed] [Google Scholar]
- Wahl RC, Rajagopalan KV. (1982) Evidence for the inorganic nature of the cyanolyzable sulfur of molybdenum hydroxylases. J Biol Chem 257:1354–1359 [PubMed] [Google Scholar]
- Weigert J, Neumeier M, Bauer S, Mages W, Schnitzbauer AA, Obed A, Gröschl B, Hartmann A, Schäffler A, Aslanidis C, et al. (2008) Small-interference RNA-mediated knock-down of aldehyde oxidase 1 in 3T3-L1 cells impairs adipogenesis and adiponectin release. FEBS Lett 582:2965–2972 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Matsumura T, Ichida K, Okamoto K, Nishino T. (2007) Human xanthine oxidase changes its substrate specificity to aldehyde oxidase type upon mutation of amino acid residues in the active site: roles of active site residues in binding and activation of purine substrate. J Biochem 141:513–524 [DOI] [PubMed] [Google Scholar]