Abstract
Although the activation of cannabinoid receptor-1 (CB1) receptors by cannabinoids is known to inhibit neuronal hyperexcitability and reduce excitotoxic cell death, the mechanistic links between these two actions remain elusive. We tested the hypothesis that activation of CB1 receptors inhibits N-methyl-d-aspartic acid (NMDA)-mediated calcium influx and cell death via the inositol triphosphate (IP3) signaling pathway in both primary dorsal root ganglia neurons and a cultured neuronal cell line (F-11 cells). These cells were pretreated with the cannabinoid agonist (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de)-1,4-benzoxazin-6-yl]-1-napthalenylmethanone (R-(+)-WIN 55,212-2; WIN) before exposure to NMDA. Concentrations of cytosolic calcium were measured with the ratiometric calcium indicator, Fura-2, and cell death was determined by a cell viability test. WIN dose-dependently attenuated both the calcium influx and cell death induced by NMDA. These effects were blocked by selective cannabinoid CB1 receptor antagonists N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) or N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251), but not CB2 receptor antagonist N-[(1S)-endo-1,3,3,-trimethylbicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methyl-benzyl)-pyrazole-3-carboxamide (SR144528). It is interesting to note that a transient Ca2+ signal was observed after the acute application of WIN. This Ca2+ increase was blocked by a CB1 receptor antagonist AM251, IP3 receptor antagonist 2- aminoethyl diphenylborinate, or by depleting intracellular Ca2+ stores with the endoplasmic reticulum Ca2+ pump inhibitor thapsigargin. Removal of extracellular Ca2+, on the other hand, had no effect on the CB1 receptor-induced Ca2+ increase. These data suggest that WIN triggers a cascade of events: it activates the CB1 receptor and the IP3 signaling pathway, stimulates the release of Ca2+ from intracellular stores, raises the cytosolic Ca2+ levels, and inhibits the NMDA-mediated Ca2+ influx and cell death through a process that remains to be determined.
Cannabinoid receptors are members of the superfamily of Gi/Go-coupled receptors and include at least two subtypes, Cannabinoid receptor-1 (CB1) and CB2 receptors. The CB1 receptor is expressed primarily in the central nervous system (Matsuda et al., 1990) and peripheral nociceptors (Agarwal et al., 2007), whereas the CB2 receptor is predominantly expressed in immune cells (Munro et al., 1993) and is also detectable in brainstem neurons (Van Sickle et al., 2005) and spinal cord (Zhang et al., 2003). More recently, another cannabinoid receptor GPR55 was identified that appears to be highly expressed in large dorsal root ganglion neurons (Lauckner et al., 2008). The CB1 receptor is involved in the integration of signals from both lipid- and peptide-derived signaling molecules. The two lipid endogenous agonists, anandamide and 2-arachidonoylglycerol, are well characterized and are derived from cell membrane lipids (Boyd, 2006; Di Marzo and Petrosino, 2007). In contrast, the two peptide endogenous agonists, RVD-Hpα and VD-Hpα, are newly discovered (Gomes et al., 2009) and are derived from the precursor α-hemoglobin, which is extensively expressed in many cell types including neurons and glia (Richter et al., 2009), in addition to erythrocytes. Previous studies have shown that cannabinoid receptor activation leads to the inhibition of adenylyl cyclase activity, inhibition of calcium channels, and D-type potassium channels, increases in the phosphorylation of mitogen-activated protein kinases, and activation of A-type and inwardly rectifying potassium channels (Pertwee, 1997; Howlett and Mukhopadhyay, 2000; Pertwee and Ross, 2002). The endocannabinoid system is proposed to have important roles in many pathophysiological processes including Parkinson's disease, Alzheimer's disease, atrophic lateral sclerosis, multiple sclerosis, obesity, depression, inflammation, and neuropathic pain (Iversen and Chapman, 2002; Boyd, 2006; Di Marzo and Petrosino, 2007).
CB1 receptor agonists protect neurons from N-methyl-d-aspartic acid (NMDA)-induced excitotoxicity in in vitro experiments (van der Stelt and Di Marzo, 2005). The cellular mechanisms underlying the CB1 receptor-mediated neuroprotection may include inhibition of the presynaptic release of glutamate (Shen and Thayer, 1998), inhibition of NMDA-induced intracellular Ca2+ release (Zhuang et al., 2005), antioxidant activity (Marsicano et al., 2002), protein kinase A (PKA) signaling, and nitric oxide generation (Kim et al., 2006). Activation of CB1 receptors also protects cultured spinal neurons from the cytotoxic effects of excitatory amino acids (Abood et al., 2001). The cannabinoid-mediated neuroprotection was used as a therapeutic approach to manage neurodegenerative conditions such as multiple sclerosis (Docagne et al., 2007). However, the mechanisms by which the cannabinoids protect spinal neurons remain elusive.
It is well established that excessive Ca2+ influx through NMDA channels triggers cytotoxic cell death in neurons, and suppression of the Ca2+ influx can protect cells from NMDA-induced cytotoxicity. In addition, the roles of NMDA receptor activation in the development of persistent neuropathic pain have been demonstrated previously (Woolf, 1983; Woolf and Salter, 2000). Cannabinoids may modulate NMDA-induced Ca2+ influx in different neurons through different mechanisms. The NMDA-induced Ca2+ increase was inhibited by a CB1 receptor-mediated membrane hyperpolarization in rat cortical and cerebellar slices (Hampson et al., 1998). In primary hippocampal cells, CB1 receptor activation was capable of inhibiting the calcium release, in a cAMP/PKA-dependent manner, from ryanodine-sensitive intracellular store (Zhuang et al., 2005). In contrast, CB1 receptor activation increased NMDA-evoked Ca2+ release from inositol triphosphate (IP3)-sensitive intracellular store in cerebellar granule neurons (Netzeband et al., 1999). Therefore, cannabinoids may enhance or inhibit the NMDA-induced increase in Ca2+ levels depending on the cell type, the type of cannabinoid ligands, and the subsequent signaling pathways activated.
In this study, we examined the effects of cannabinoid R-(+)-WIN 55,212-2 (WIN) on NMDA-induced cytotoxicity and NMDA-evoked Ca2+ influx. These experiments were carried out in dorsal root ganglia (DRG) neurons and murine F-11 cells (spinal DRG × neuroblastoma hybrid), both of which express CB1 receptors and NMDA receptors. We tested the hypothesis that activation of cannabinoid CB1 receptors inhibits NMDA-mediated calcium influx and cell death via the IP3 signaling pathway in both primary DRG neurons and cultured F-11 cells. We chose these two types of cells with different pharmacology for the release of calcium from intracellular stores so that we could identify the signaling pathways of WIN-induced calcium increase. Although both cell types are able to produce IP3 receptor-mediated Ca2+ signals, only DRG neurons are sensitive to stimulation with ryanodine receptor activator, caffeine, and express functional ryanodine receptors. The F-11 cells do not respond to caffeine, indicating that these cells lack endogenous ryanodine receptors (Yankura et al., 2003).
Materials and Methods
Cell Culture.
All procedures used in the animal experiments were approved by the Institutional Animal Care and Use Committee of Cleveland Clinic Foundation. DRG neurons were obtained as described earlier (Dedov et al., 2001). In brief, DRG isolated from adult Sprague-Dawley rats (200–300 g) were incubated in Hanks' balanced saline solution (Invitrogen, Carlsbad, CA) with 0.05% collagenase and 0.25% trypsin for 25 min at 37°C. Neurons were dissociated by trituration of ganglia with fire-polished Pasteur pipettes of decreasing diameter, and afterward the cellular suspension was washed twice in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 2 mM l-glutamine. Freshly isolated neurons were plated onto collagen-coated coverslips and cultured in Dulbecco's modified Eagle's medium/F-12 Ham media containing 2 mM l-glutamine supplemented with 10% fetal bovine serum, HAT supplement (100 μM hypoxanthine, 400 nM aminopterin, 16 μM thymidine), 50 ng/ml nerve growth factor, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were kept under 5% CO2 at 37°C and passed twice a week using nonenzymatic cell dissociation solution.
F-11 cells (purchased from Dr. Mark C. Fishman, Massachusetts General Hospital, MA) were cultured in F-12 Ham media containing 2 mM l-glutamine supplemented with 15% fetal bovine serum, HAT supplement, 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were kept under 5% CO2 at 37°C and passed twice a week using nonenzymatic cell dissociation solution.
Cells were visualized by using bright-field illumination at 20× magnification on a Leica DMIRB inverted microscope (Leica Microsystems, Inc., Deerfield, IL). The images of the cells were captured 24 h before and after drug treatment by using a CCD video camera and QCapture Pro imaging software from QImaging (Surrey, BC, Canada).
Compound Preparation.
WIN 55,212-2 and AM251 were purchased from BIOMOL International LP (Plymouth Meeting, PA). SR141716A and SR144528 were provided by the National Institute on Drug Abuse. Compounds were dissolved in 100% dimethyl sulfoxide (DMSO) as a 10 mM stock and diluted with Dulbecco's phosphate-buffered saline (DPBS; Invitrogen) to their final concentrations. Final concentrations of 10, 50, 100, and 500 nM of WIN 55,212-2 were used to activate CB1 receptors. SR141716A and AM251 were used at a final concentration of 500 nM to block CB1 receptor activation, and SR144528 was used at a final concentration at 500 nM to block CB2 receptor activation. NMDA (Sigma-Aldrich, St. Louis, MO) was dissolved in DPBS as a 10 mM stock. The final concentration of 100 μM NMDA was used to elicit Ca2+ signals. Thapsigargin (TG) (Research Biochemical, Natick, MA) was prepared as a 10 mM stock solution in DMSO. Final concentration of 150 nM TG was used in the experiments. 2-Aminoethyl diphenylborinate (2-APB) (Sigma-Aldrich) was prepared as a 1 mM stock solution in DMSO, and a final concentration of 100 μM was used in the experiments. Stock solutions were stored at −20°C. All dilutions of stock were prepared fresh before addition to the culture medium. All vehicles were confirmed to have no biological effects.
Cytotoxicity Analysis.
Cells were plated in poly-d-lysine-coated 96-well plates at a density of 104 cells/well in their respective culture media. Cells were allowed to adhere for 12 h before the medium was replaced with Mg2+ and serum-free medium: DME/F-12 supplemented with 2% B27 (Sigma-Aldrich). Cell death was induced by incubating with 0.001 to 10 mM NMDA for 20 ± 2 h. To determine the effects of cannabinoids, cells were incubated with or without NMDA (100 μM) in presence or absence of WIN 55,212-2 (10, 50, 100, 500 nM) for 24 h. To determine the cannabinoid receptor subtype specificity, cells were treated with SR141716A (500 nM) for 20 to 30 min before incubating with NMDA and WIN 55, 212-2. In experiments where the effects of cannabinoids on intracellular calcium levels and cytotoxicity were investigated, cells were pretreated with 150 nM TG for 10 min, before cells were coincubated with cannabinoids and NMDA. The cell viability was assayed by measuring the conversion of a tetrazolium salt, 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), into its formazan derivative by cellular dehydrogenases. The assay was performed according to the manufacturer's instruction (Trevigen TACS XTT Cell Proliferation Assay; Trevigen, Gaithersburg, MD). The absorbance was read on a VICTOR3V plate reader by using a 490-nm filter (PerkinElmer Life and Analytical Sciences, Waltham, MA). For each condition, four wells of cells were measured and the experiments were repeated three times (n = 12 for each condition). The cytotoxicity index (CI) was calculated as follows: CI = (control well absorbance − treatment well absorbance)/(control well absorbance − Triton-treated well absorbance).
Calcium Imaging.
Cytosolic Ca2+ was monitored with the ratiometric indicator Fura-2 (InCyt Im2 Dual-wavelength Fluorescence Imaging System; Intracellular Imaging, Cincinnati, OH). Cells were grown on glass coverslips coated with collagen (MatTek Corporation, Ashland, MA) and then washed twice in DPBS before incubation in 2 ml of DPBS containing 2.0 to 3.0 μM Fura-2 AM and 0.066% Pluronic F-127 (Invitrogen). After incubating for 60 to 75 min at 37°C in darkness, cultures were washed twice in DPBS to remove extracellular dye and kept at room temperature in the dark for more than 30 min before use in the experiments. All measurements were performed in DPBS or, where specified, in Ca2+-free DPBS. Drugs were added in a volume of 200 μl to cells in 3 ml of DPBS to make the final volume less than 4 ml in the Petri dishes. The dishes with dye-loaded cells were mounted on the stage of Nikon TS-100 fluorescence inverted microscope with a Cohu model 4915 charge-coupled device (CCD) camera (Nikon, Melville, NY). Fluorescent images were captured alternately at the excitation wavelengths of 340 and 380 nm with an emission wavelength of 520 nm, which were analyzed with InCyt Im2 version 4.62 imaging software (Intracellular Imaging).
A standard curve was used to derive experimental [Ca2+]i values. The standard curve was generated by using various concentrations of Ca2+ (Calcium Calibration Buffer Kit) in the presence of indicator dye Fura-2 free acid (Invitrogen). During each experiment, background fluorescence was estimated for a region without cells, and this value was automatically subtracted from the measured emission of each channel. The F340/F380 ratios of cell emissions were compared with the standard curve stored in the computer, and both the ratio and [Ca2+]i were displayed on screen. Preliminary measurement of [Ca2+]i was taken on various cells in the field before any drug application. Only cells with basal [Ca2+]i in the range of 90 to 120 nM were chosen for the experiments described here.
Experimental Paradigm.
All pharmacological agents were dissolved in DPBS and applied by brief microperfusion from micropipettes placed near the cells of interest. The concentration and duration (<2 s) of application were adjusted under control conditions for each experiment to produce Ca2+ signals with peak amplitude (150–350 nM) that could be easily quantified.
Ca2+ levels in the presence of TG and cannabinoids were typically measured 5 to 20 min after the initial drug exposure. NMDA was added 10 min after the responses returned to baseline. For a majority of the experiments, the bath saline (e.g., DPBS) used during control recordings contained DMSO concentration equivalent to that used in the presence of thapsigargin or cannabinoid agents. Separate vehicle control experiments showed that DMSO (<0.15%) did not affect the measurements under study.
In general, Ca2+ levels at rest or in response to challenges were measured simultaneously for 10 to 30 cells within a microscopic field, with three to five microscopic fields measured per condition. One microscopic field was measured in each Petri dish. Each cell was tested under only one condition. Resting Ca2+ levels were subtracted from amplitude measurements for individual cells to yield peak Ca2+ values.
Data Analysis.
A between-cell comparison was used to determine the effects of the tested compounds on Ca2+ levels or cytotoxicity. For each group of studies, data from at least five individual Petri dishes were pooled for summary analysis. Each drug was tested on at least two different days, with concurrent interleaved controls. Averages are reported as the mean ± S.E.M., and the number of cells and/or cultures studied is given. Raw data were analyzed with appropriate parametric tests: paired or unpaired t test or analysis of variance (performed with SPSS software; SPSS Inc., Chicago, IL). When analysis of variance was used, post hoc analysis for group differences was performed by using Scheffe's F test or Dunn's test for unequal sample sizes. Statistical significance was determined at a significance level of p < 0.05.
Results
Cannabinoid R-(+)-WIN Inhibited NMDA-Induced Cytotoxicity in Both DRG and F-11 Cells.
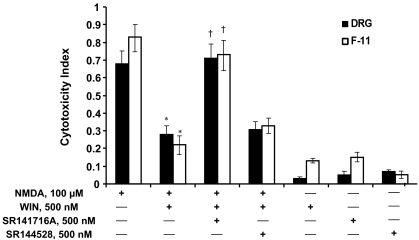
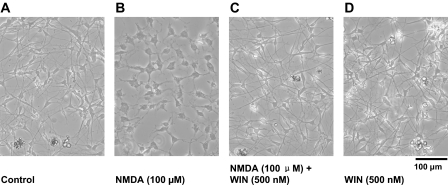
Long-term treatment of both DRG and F-11 cells with NMDA (100 μμM) produced significant cytotoxic effects. A 24-h exposure to NMDA reduced the viability of the DRG neurons by ∼70% and the viability of F-11 cells by ∼80% (Fig. 1). These cytotoxic effects were blocked by adding the cannabinoid receptor agonist R-(+)-WIN (500 nM) to the culture medium (F(1,19) = 21.25 for DRG neurons, F(1,13) = 12.16 for F-11 cells, p < 0.05). The protective effects of WIN were reversed by CB1 receptor antagonist SR141716A (500 nM) (F(1,18) = 16.73 for DRG neurons, F(1,14) = 9.61 for F-11 cells, p < 0.05) but not by CB2 receptor antagonist SR144528 (500 nM) (p > 0.05), suggesting a specific CB1 receptor-mediated effect. Figure 2 shows representative microphotographs of DRG neuron cultures in control (Fig. 2A), treated with NMDA (100 μM) (Fig. 2B), treated with the combination of NMDA (100 μM) and WIN (500 nM) (Fig. 2C), or treated with WIN (500 nM) alone. After 24 h of drug treatment, DRG neurons exposed to NMDA exhibited signs of cytotoxicity that was prevented by treatment with WIN. The cytotoxicity of NMDA and the protective effects of WIN were quantified by using a commercial assay for cell viability and proliferation (see Materials and Methods).
Fig. 1.
Activation of CB1 receptors inhibits NMDA-induced cell death in DRG neurons and F-11 cells. Cells were exposed to NMDA (100 μM) for 24 h in the absence or presence of cannabinoid receptor agonist R-(+)-WIN 55,212-2 (500 nM). NMDA reduced the viability of cells by approximately 70% for DRG neurons and 80% for F-11 cells in the absence of WIN. The NMDA-induced cytotoxicity was reduced significantly in the presence of WIN. This WIN-mediated protective effect was blocked by the CB1 receptor antagonist SR141716A (500 nM), but not the CB2 receptor antagonist SR144528 (500 nM). Cell survival was not affected by either WIN, SR141716A, or SR144528 alone. Cell death was measured by the cytotoxicity index, as defined under Materials and Methods. Data shown are means ± S.E.M. for 20 wells per condition. *, p < 0.05 compared to treatment with NMDA alone, Scheffe's tests. †, p < 0.05 compared to NMDA + WIN condition.
Fig. 2.
WIN reduced NMDA-induced cytotoxicity. Representative examples of DRG neurons cultured in control medium without drugs (A), medium containing NMDA (100 μM) (B), medium containing both NMDA (100 μM) and WIN (500 nM) (C), and medium containing WIN (500 nM) (D) for 24 h.
WIN Dose-Dependently Inhibited the Ca2+ Rise Induced by NMDA.
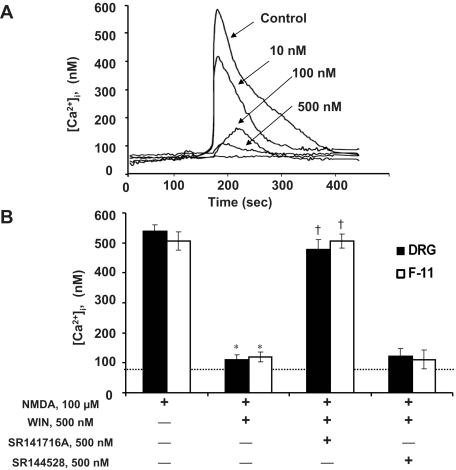
Many toxic effects of NMDA are mediated by increases in cytoplasmic Ca2+ levels ([Ca2+]i). Therefore, we examined the effects of R-(+)-WIN 55,212-2 on the NMDA-induced increase in [Ca2+]i. Figure 3A (Control) shows that NMDA (100 μM) elicited an increase in [Ca2+]i in DRG neurons. The NMDA-induced change in [Ca2+]i was characterized by a relatively rapid initial rise in intracellular Ca2+ to a peak amplitude of ∼600 nM that was followed by a slow recovery to baseline. All of the cells tested responded to NMDA (100 μM). Ca2+ levels increased approximately 400% above baseline for both the DRG neurons and F-11 cells (Fig. 3B). Resting Ca2+ levels in DRG neurons and F-11 cells were fairly consistent within and between experimental trials and typically ranged from 85 to 105 nM.
Fig. 3.
CB1 receptor activation reduces NMDA-induced Ca2+ influx in DRG neurons and F-11 cells. Neurons were pretreated with WIN in the presence or absence of CB1 receptor antagonist SR141716A for 5 min before exposure to NMDA (100 μM). A, representative Ca2+ signal recordings from DRG neurons show a dose-dependent inhibition of NMDA-induced Ca2+ signals by WIN pretreatment (10, 100, and 500 nM). The control is from cells treated with NMDA (100 μM) alone. Ratios of wavelengths 340:380 nm were converted into calcium concentrations (nM) by using a standard curve as described under Materials and Methods. B, calcium concentrations increased by ∼400% in response to NMDA (100 μμM) in both cell types. Coapplication of NMDA with WIN reduced Ca2+ signals by ∼80%. The inhibition was reversed by CB1 receptor antagonist, SR141716A. A CB2 receptor antagonist SR144528 (500 nM) did not reduce the WIN-induced inhibition. The dotted line indicates the baseline calcium level. Values are averages from 10 to 15 cells ± S.E.M. *, p < 0.05 compared to treatment with NMDA alone, Scheffe's tests. †, p < 0.05 compared to NMDA + WIN condition.
The effects of WIN on the NMDA-elicited [Ca2+]i increase were studied by pretreating cells with 10, 100, and 500 nM WIN 5 min before the application of NMDA (100 μM). WIN produced a dose-dependent depression of the calcium rise elicited by NMDA over the entire testing period (Fig. 3A). Likewise, WIN also depressed NMDA-evoked [Ca2+]i increase in F-11 cells in a dose-dependent manner (data not shown). Figure 3B shows pooled data for the effects of 500 nM WIN on the NMDA-evoked Ca2+ increase. The depression of NMDA-induced Ca2+ rise by WIN was observed in both DRG neurons and F-11 cells (F(2,34) = 7.69 for DRG neurons, F(2,18) = 8.14 for F-11 cells, p < 0.05 versus NMDA alone; Fig. 3B). The effects of WIN were reversed by pretreatment with the selective CB1 receptor antagonist, SR141716A (500 nM) (F(1,34) = 6.39 for DRG neurons F(1,21) = 5.82 for F-11 cells, p < 0.05 versus NMDA + WIN), but not by the CB2 antagonist SR144528 (500 nM) (Fig. 3B), suggesting that the effects of WIN were mediated by the CB1 receptors.
WIN-Evoked a Dose-Dependent Calcium Rise in Primary DRG Neurons and F-11 Cells.
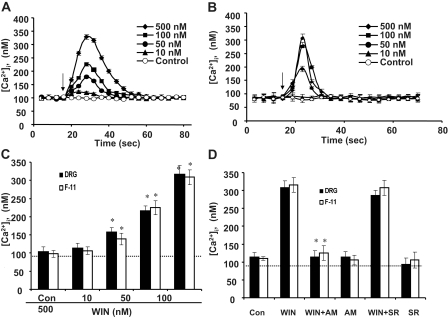
Cells were challenged with different concentrations of WIN (10, 50, 100, and 500 nM) while the intracellular Ca2+ levels were monitored. WIN-evoked a dose-dependent intracellular calcium rise in both DRG neurons (Fig. 4A) and F-11 cells (Fig. 4B). Ca2+ signals increased upon WIN application, peaked within 30 s, and returned to baseline within 2 min (Fig. 4, A and B). This transient calcium increase was observed in 92% DRG neurons and 87% F-11 cells. Figure 4C shows that the average peak amplitudes of WIN-induced calcium rise was dose-dependent (F(3,34) = 8.07 for DRG neurons, F(3,25) = 6.14 for F-11 cells, p < 0.05 versus Control; Fig. 4C). The calcium rise induced by WIN was blocked by the CB2 antagonist AM251 (500 nM) but not the CB2 antagonist SR144528 (500 nM; Fig. 4D), suggesting that it was mediated by CB1 receptors in both DRG neurons and F-11 cells [F(1,33) = 9.73 for DRG neurons, F(1,22) = 6.58 for F-11 cells, p < 0.05 versus WIN treatment (500 nM); Fig. 4D]. AM251 and SR144528 did not significantly change [Ca2+]i for either cell type (p > 0.05).
Fig. 4.
Cannabinoid agonist WIN induces increases in [Ca2+]i. WIN produced a transient rise in [Ca2+]i that began within seconds after application (arrows) and returned to baseline within 1 min in a dose-dependent manner for both DRG neurons (A) and F-11 cells (B). Each Ca2+ trace represents the mean ± S.E.M. from 25 DRG neurons or 21 F-11 cells measured individually. Error bars are smaller than the corresponding symbols in many cases. Data were acquired at 4-s intervals throughout the entire experiments. C, average peak amplitudes in the [Ca2+]i produced by WIN in both cell types. *, p < 0.05, compared to control (Con) by post hoc Scheffe's tests. D, CB1 receptor antagonist AM251 (AM; 500 nM) inhibited WIN-induced increase in [Ca2+]i, whereas the CB2 receptor antagonist SR144528 (SR; 500 nM) had little effect. AM251 alone had little effect on Ca2+ levels. *, p < 0.05 compared to WIN alone, Scheffe's tests. All experiments were conducted in normal DPBS. The dotted line indicates the baseline calcium level.
Sources of the WIN- and NMDA-Induced Calcium Increase.
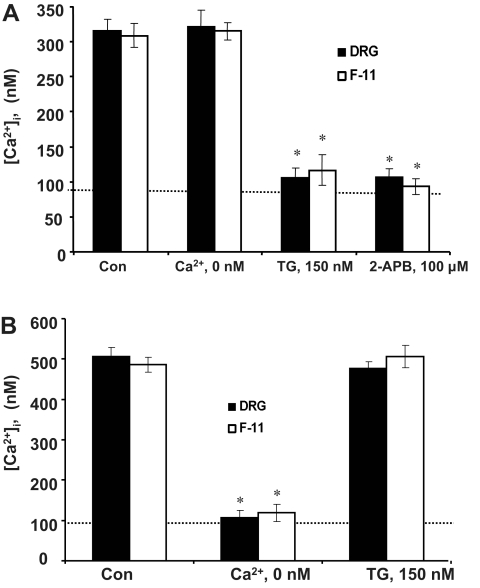
The WIN- and NMDA-induced increase in [Ca2+]i could come from either an extracellular calcium source or from intracellular calcium stores such as the endoplasmic reticulum (ER). Influx of extracellular calcium was assessed by using a calcium-free medium (0 Ca2+ DPBS). Release from intracellular calcium store was determined by depleting ER stores with a sarco/endoplasmic reticulum Ca2+ (SERCA) pump inhibitor, TG, before NMDA or WIN challenge. WIN-induced [Ca2+]i increase (Fig. 5A, Con) was significantly reduced by pretreatment of both DRG neurons and F-11 cells with TG [F(1,18) = 10.56 for DRG neurons, F(1,16) = 9.33 for F-11 cells, p < 0.05 versus WIN treatment (500 nM)], whereas removal of extracellular calcium (Ca2+, 0 nM) did not affect the signals (p > 0.05; Fig. 5A). These data suggest that Ca2+ released from the ER contributes to the WIN-induced calcium increase. Pretreating cells with TG for 15 min in 0 Ca2+ DPBS has been shown to be sufficient to deplete TG-sensitive intracellular calcium stores (Chen et al., 2005). The ability of thapsigargin to deplete the intracellular Ca2+ stores was tested by stimulating Ca2+ release from IP3-sensitive stores with a type 1 muscarinic receptor agonist, carbachol (Cruzblanca et al., 1998). We found that the same thapsigargin treatment almost abolished the Ca2+ increase produced by 1 mM carbachol (data not shown). In contrast, the NMDA-evoked [Ca2+]i increase (Fig. 5B, Con) was not affected by thapsigargin (Fig. 5B). Instead, it was significantly reduced by removal of extracellular calcium (Ca2+, 0 nM), suggesting that the [Ca2+]i evoked by NMDA comes from an influx of extracellular calcium, at least initially (F(1,17) = 5.89 for DRG neurons, F(1,14) = 7.24 for F-11 cells, p < 0.05 versus NMDA alone; Fig. 5B).
Fig. 5.
Sources of cytosolic calcium rise. A, WIN-induced Ca2+ signals are from release of intracellular calcium stores in both DRG and F-11 cells. Cells were treated with thapsigargin (150 nM) or 2-APB (100 μM) for 5 min to deplete or block release from intracellular Ca2+ stores. The application of TG or 2-APB before WIN abolished the calcium rise. In contrast, the removal of extracellular Ca2+ (“′Ca2+, 0 nM”) from DPBS did not affect the WIN-induced Ca2+ signals. Values are averages from 15 to 20 cells ± S.E.M. B, NMDA-induced Ca2+ signals are from extracellular calcium sources. Removal of extracellular Ca2+ (Ca2+, 0 nM) from DPBS abolished the NMDA-induced Ca2+ rise, whereas pretreatment with thapsigargin did not reduce the Ca2+ signals. *, p < 0.05 compared to control. The dotted line indicates the baseline calcium level.
We further tested the role of IP3 signaling pathway in the cannabinoid-evoked intracellular calcium release. Cells were pretreated with IP3 receptor blocker, 2-APB (100 μM), for 5 min before they were exposed to WIN (500 nM). Pretreatment with 2-APB reduced the WIN-evoked calcium release in both DRG neurons and F-11 cells (F(1,17) = 6.47 for DRG neurons, F(1,12) = 5.83 for F-11 cells, p < 0.05 versus WIN treatment; Fig. 5A). These results suggest that the cannabinoid-induced increase in [Ca2+]i was mediated by IP3 receptors.
WIN-Induced Calcium Release Is Related to Its Inhibition of the NMDA-Mediated Ca2+ Influx.
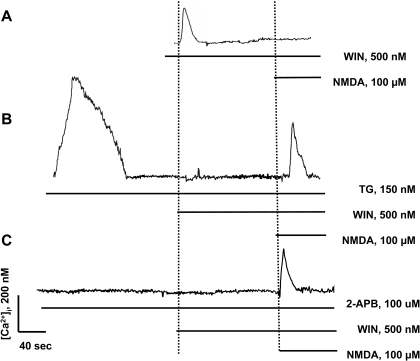
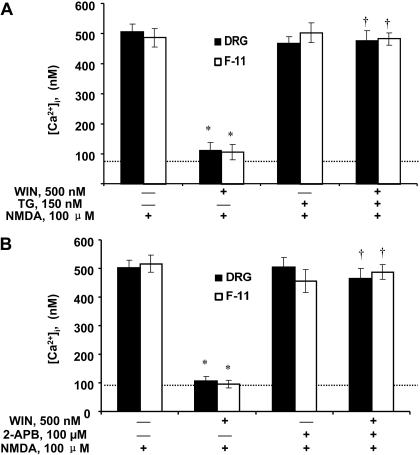
We tested whether the WIN-induced [Ca2+]i increase was necessary to depress the NMDA-induced Ca2+ influx. DRG neurons and F-11 cells were preincubated with TG or 2-APB to inhibit the WIN-evoked increase in [Ca2+]i, before exposure to NMDA. Application of thapsigargin (150 nM) caused a robust transient rise in [Ca2+]i in DRG neurons, which is indicative of intracellular calcium store depletion (Fig. 6B). After the [Ca2+]i returned to baseline, application of 500 nM WIN did not produce rises in [Ca2+]i in DRG neurons, whereas the subsequent application of NMDA produced near normal Ca2+ increase. Similar results were seen in F-11 cells (data not shown). The differential effects of thapsigargin on the modulation of Ca2+ levels by WIN and NMDA are summarized in Fig. 7A. For both DRG neurons and F-11 cells, thapsigargin inhibited the WIN-induced Ca2+ increase (F(1,34) = 9.66 for DRG neurons, F(1,21) = 7.45 for F-11 cells, p < 0.05 versus NMDA alone; Fig. 7A), but not the NMDA (100 μM)-elicited Ca2+ rise (F(1,24) = 8.74 for DRG neurons, F(1,15) = 6.27 for F-11 cells, p < 0.05 versus NMDA + WIN; Fig. 7A). These results suggest that TG abolished the ability of WIN to inhibit the NMDA-induced Ca2+ influx and that Ca2+ release from intracellular stores was therefore required for this effect.
Fig. 6.
WIN-induced intracellular calcium release is required to block NMDA-mediated calcium influx in DRG neurons. A, WIN blocked NMDA-induced calcium rise. B, depletion of intracellular calcium store with TG inhibited the ability of WIN to block the NMDA-induced calcium rise. C, inhibition of intracellular calcium release by 2-APB-mediated inactivation of IP3 receptors also inhibited the ability of WIN to block the NMDA-induced calcium rise. These representative examples of Ca2+ signals were recorded continuously during pharmacological challenges of DRG neurons in DPBS (n = 17 cells). The vertical and horizontal lines indicate the beginning and duration of the drug applications.
Fig. 7.
Depression of intracellular calcium release prevents WIN from blocking NMDA-induced calcium influx in both DRG neurons and F-11 cells. A, pretreatment with SERCA pump inhibitor TG prevented WIN from blocking NMDA-induced calcium rise in both DRG neurons and F-11 cells. B, pretreatment with IP3 receptor inhibitor 2-APB prevented WIN from blocking NMDA-induced Ca2+ signals in both cell types. Note, neither thapsigargin nor 2-APB alone produced any effect on NMDA-induced Ca2+ increase. *, p < 0.05 compared to NMDA treatment alone. †, p < 0.05 compared to NMDA + WIN treatment condition. The dotted line indicates the baseline calcium level.
Likewise, the WIN-induced depression of NMDA-mediated calcium influx was blocked by the IP3 receptor blocker, 2-APB (100 μM), in DRG neurons and F-11 cells (Figs. 6C and 7B). Pretreatment with 2-APB before WIN application abolished the WIN-evoked intracellular Ca2+ rise (F(1,28) = 7.53 for DRG neurons, F(1,18) = 6.92 for F-11 cells; p < 0.05 versus WIN alone; Fig. 7B). In addition, 2-APB blocked the WIN-induced inhibition of NMDA-mediated Ca2+ influx (F(1,23) = 11.32 for DRG neurons, F(1,19) = 9.53 for F-11 cells, p < 0.05 versus NMDA + WIN; Fig. 7B). These data suggest that WIN-induced [Ca2+]i release is mediated by the IP3 signaling pathway and is responsible for the inhibition of NMDA-mediated Ca2+ influx.
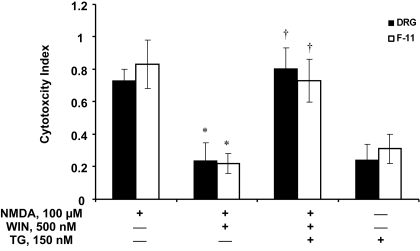
WIN-Induced Ca2+ Release Is Responsible for the Inhibition of NMDA-Induced Cytotoxicity.
We tested whether WIN-induced Ca2+ release is necessary for the protective effects of WIN on NMDA-evoked cytotoxicity in both DRG and F-11 cells. Coincubation of WIN (500 nM) with NMDA (100 μM) blocked the NMDA-induced cytotoxicity in both DRG and F-11 cells (F(1,34) = 33.96 for DRG neurons, F(1,24) = 19.55 for F-11 cells, p < 0.05 versus NMDA alone; Figs. 1 and 8). This protective effect was reversed by thapsigargin (150 nM) for both cell types (F(1,42) = 41.66 for DRG neurons, F(1,27) = 34.19 for F-11 cells, p < 0.05 versus NMDA + WIN; Fig. 7). These data suggest that WIN-induced [Ca2+]i rise is required for WIN to protect cells from NMDA-induced cytotoxicity. Thapsigargin alone did not produce any significant cell viability (<30% for both cell types, p > 0.05).
Fig. 8.
The neuroprotective effects of CB1 activation are dependent on calcium release from intracellular stores. Depletion of intracellular calcium stores with SERCA pump inhibitor TG prevented WIN from inhibiting NMDA-induced cytotoxicity in both DRG neurons and F-11 cells. Note, thapsigargin alone did not affect the survival of cells. Data are means ± S.E.M. from 23 wells per condition. *, p < 0.05 compared to NMDA alone. †, p < 0.05 compared to NMDA + WIN condition.
Discussion
The data from this study showed that the cannabinoid R-(+)-WIN 55,212-2 produced a CB1-mediated neuroprotection against NMDA-induced cytotoxicity in both primary DRG neurons and F-11 cells. These effects were blocked by a SERCA pump inhibitor, thapsigargin, and an IP3 receptor inhibitor, 2-APB, suggesting that Ca2+ release from intracellular stores via the IP3 signaling pathway is critical for the CB1-mediated neuroprotection (Fig. 8). This work is the first demonstration that the IP3 signaling pathway has been implicated in the cannabinoid-induced neuroprotection of spinal neurons.
The biochemical mechanisms of cannabinoid protection from NMDA-induced neuronal cytotoxicity are just beginning to be understood. It has been shown that WIN protects neurons against NMDA toxicity by activation of CB1 receptor and downstream inhibition of PKA signaling and nitric oxide generation in cultured cortical neurons (Kim et al., 2006). A natural extract from cannabis plant, Δ9-tetrahydrocannabinol, functions as an antioxidant to increase cell survival in NMDA-induced neurotoxicity in a mesencephalic cell line (Chen et al., 2005). In contrast, the data of this study indicate that the cannabinoid-mediated neuroprotection from NMDA-induced cytotoxicity in spinal neurons is largely attributable to its suppression of NMDA-induced Ca2+ influx through the IP3 signaling pathway. We demonstrated that blocking CB1 receptor activation or the subsequent increase in [Ca2+]i abolished the cannabinoid-mediated inhibition of Ca2+ influx and cytotoxicity induced by NMDA in primary DRG neurons. We also confirmed these findings for the F-11 cells.
The NMDA-induced cytosolic Ca2+ increase in spinal neurons could come from either an influx of extracellular Ca2+ through NMDA receptor ion channels and voltage-gated calcium channels (Reichling and MacDermontt, 1993) or from release of intracellular Ca2+ stores (Qiu et al., 1995). The data of this study suggest that activation of CB1 receptors primarily inhibits the influx of extracellular Ca2+. Removal of extracellular Ca2+ significantly reduced NMDA-mediated cytosolic Ca2+ signals, whereas the SERCA pump inhibitor thapsigargin, in contrast, did not affect the NMDA-induced calcium increase. Therefore, the CB1-mediated inhibition of the Ca2+ increase in these neurons is probably due to inactivation of NMDA receptor channels and/or voltage-gated calcium channels. It has been shown that cannabinoids inhibit NMDA-elicited Ca2+ signals by blocking voltage-gated calcium channels in rat brain cerebellar and cortical slices (Hampson et al., 1998). However, it remains to be determined whether this is the case in the DRG neurons.
An interesting finding of this study is that the cannabinoid-mediated suppression of NMDA-induced Ca2+ influx depended on Ca2+ release from intracellular stores. CB1-mediated Ca2+ release from intracellular stores has been demonstrated in hippocampal neurons (Lauckner et al., 2005) and other cell types. The Ca2+ release was thought to be mediated through either Gi/o βγ or Gq-coupled phospholipase C signaling pathway, depending on the cell types and drug concentrations. For example, WIN induced Ca2+ signals from IP3-sensitive ER stores in hippocampal neurons and transfected HEK293 cells (Lauckner et al., 2005). The data of this study are consistent with this finding. The short duration of Ca2+ signal surge (<40 s) observed in these experiments, compared with the reported long-lasting Ca2+ rise (>100 s), may be due to differences in WIN concentrations. A relatively low concentration (500 nM) was used in the experiments of this study, whereas high doses (5 μM) were used by others.
We demonstrated in this study that the CB1-induced calcium release from intracellular stores attenuated NMDA-induced Ca2+ influx, and subsequently the NMDA-induced cytotoxicity in DRG neurons. This finding is consistent with the reports that increases in [Ca2+]i inactivates NMDA receptors in spinal dorsal horn neurons (Kyrozis et al., 1996) and hippocampal neurons (Kotecha and MacDonald, 2003). The NMDA receptor inactivation in response to increase in [Ca2+]i was mediated by activation of Ca2+-dependent signaling proteins, such as calmodulin and calcineurin. However, it should be noted that cytosolic calcium increase can also activate NMDA receptors via a Ca2+-dependent Src kinase pathway (Nishizuka, 1988; Ben-Ari et al., 1992; Lu et al., 1999). Therefore, one signaling molecule (e.g., Ca2+) can have opposite effects on the same receptors, possibly depending on the intensity and/or timing of the calcium transient. For example, it has been shown that [Ca2+]i > 400 nM will result in excitotoxicity in neural crest-derived sensory neurons, whereas [Ca2+]i between 200 to 400 nM will promote survival in these cells (Johnson et al., 1992). Consistent with the previously published literature, we found that an ∼550 nM increase in the [Ca2+]i evoked by NMDA was toxic to both DRG neurons and F-11 cells, but a smaller increase produced by WIN (∼350 nM) was not cytotoxic and even protected cells from NMDA-induced cytotoxicity. The temporal pattern of the elevation in the [Ca2+]i can also produce different survival effects. In addition, synaptic transmission in hippocampal neurons could either be enhanced (long-term potentiation) by a brief increase of [Ca2+]i with relatively high magnitude or suppressed (long-term depression) by a prolonged modest rise of [Ca2+]i (Yang et al., 1999). Consistent with this argument is our finding that NMDA-evoked Ca2+ influx was inhibited by a short duration (<30 s) and low amplitude increase in [Ca2+]i (<300 nM) induced by WIN but not by a more sustained (>100 s) and higher amplitude (>400 nM) [Ca2+]i rise triggered by thapsigargin. The temporal and spatial characteristics of the Ca2+ signals may be related to the differential sensitivities of distinct signaling pathways. For example, short duration and low amplitude Ca2+ signals preferably stimulate calcineurin and lead to dephosphorylation of various signaling proteins and subsequent inactivation of NMDA receptors (Tong et al., 1995). In contrast, sustained and spatially diffuse and homogenous Ca2+ signals may simultaneously activate both “′potentiation” and “′depression” pathways that converge upon NMDA receptors, resulting in a cancellation effect (Harvey and Collingridge, 1992; Simpson et al., 1993). The temporal and spatial characteristics of calcium signaling may also be determined by the types of cannabinoid ligands. It has been recently demonstrated that two distinct “′endogenous” ligands that differ in their chemical nature (lipid versus peptide) activate the same receptor to initiate distinct signaling pathways (Gomes et al., 2009). It is known that CB1 receptors can couple to different G proteins (Lauckner et al., 2005) and that the same G protein-coupled receptors can activate distinct signaling pathways after activation by different ligands (Drake et al., 2008).
Taken together, the data of this study provided significant insight into the interactions between the cannabinoid system and glutamate receptor activation in DRG neurons through the modulation of cytosolic calcium dynamics and the IP3 signaling pathway (Fig. 9). Given the fact that the DRG neurons are critical for sensory-motor integration, understanding the mechanisms underlying the cannabinoid protection of these neurons and the mechanisms underlying the cannabinoid-mediated antinociception is necessary for the design of specific and efficacious therapies. Recent studies have demonstrated the neuroprotective potential of cannabinoids in various neurodegenerative diseases, such as multiple sclerosis (Pertwee, 2007) and amyotrophic lateral sclerosis, which are characterized by selective death of spinal neurons (Centonze et al., 2007). Also important is the recent recognition of the role of cannabinoids in antinociception and the cannabinoid system as an emerging target for chronic pain pharmacotherapy (Walker and Hohmann, 2005; Pacher et al., 2006).
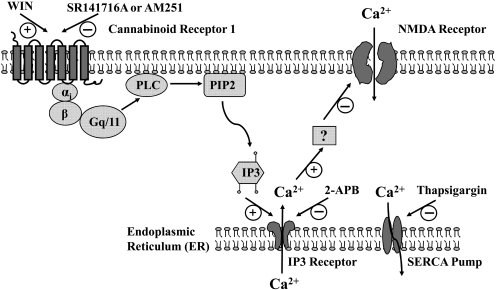
Fig. 9.
Schematic illustration of the calcium signaling pathways that are modulated by the cannabinoid system. Activation CB1 receptors mobilizes the IP3 pathway through a G protein-coupled process. Activation of IP3 receptors triggers calcium release from intracellular calcium stores, which in turn inhibits calcium influx mediated by activated NMDA receptor channels or by voltage-dependent calcium channels (data not shown). Depletion of intracellular calcium stores by SERCA pump inhibitor or blocking calcium release from intracellular calcium stores by IP3 receptor inhibitors prevents the cannabinoid from inhibiting the calcium influx related to the excitotoxicity of neurons and development of neuropathic pain. How the calcium release from intracellularly stores leads to the inhibition of NMDA receptors and/or voltage-dependent calcium channels remains to be determined.
Acknowledgments
We thank Dr. Lyle Fox for editorial assistance.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant 1 R01-NS052372] (to J.C.).
Parts of this work were previously presented in abstract form as follows: Cheng J, Liu Q, and Bhat M (2008) Cannabinoid R-(+)-WIN 55,212-2 inhibits NMDA-induced Ca2+ influx and cell death in dorsal root ganglion neurons. 6th Forum of European Neurosciences; 2008 Jul 12–16; Geneva, Switzerland. Abstract, vol. 4, 115.2, The Swiss Society for Neuroscience, Geneva, Switzerland; Cheng J, Liu Q, Bhat BM, and Bowen WD (2007) Attenuation of NMDA-induced Ca2+ rise by cannabinoid receptors in primary sensory neurons; 2007 Nov 3–7; San Diego, CA. Abstract 33, 821.11, Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.156216
- CB1
- cannabinoid receptor-1
- NMDA
- N-methyl-d-aspartic acid
- PKA
- protein kinase A
- IP3
- inositol triphosphate
- R-(+)-WIN 55,212-2
- (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de)-1,4-benzoxazin-6-yl]-1-napthalenylmethanone
- WIN
- R-(+)-WIN 55,212-2
- DRG
- dorsal root ganglia
- AM251
- N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- SR141716A
- N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- SR144528
- N-[(1S)-endo-1,3,3,-trimethylbicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methyl-benzyl)-pyrazole-3-carboxamide
- DMSO
- dimethyl sulfoxide
- TG
- thapsigargin
- DPBS
- Dulbecco's phosphate-buffered saline
- 2-APB
- 2-aminoethyl diphenylborinate
- XTT
- 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
- ER
- endoplasmic reticulum
- SERCA
- sarco/endoplasmic reticulum Ca2+ pump
- HEK
- human embryonic kidney.
References
- Abood ME, Rizvi G, Sallapudi N, McAllister SD. (2001) Activation of the CB1 cannabinoid receptor protects cultured mouse spinal neurons against excitotoxicity. Neurosci Lett 309:197–201 [DOI] [PubMed] [Google Scholar]
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, et al. ( 2007) Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 10:870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Aniksztejn L, Bregestovski P. (1992) Protein kinase C modulation of NMDA currents: an important link for LTP induction. Trends Neurosci 15:333–339 [DOI] [PubMed] [Google Scholar]
- Boyd ST. (2006) The endocannabinoid system. Pharmacotherapy 26:218S–221S [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Finazzi-Agrò A, Bernardi G, Maccarrone M. (2007) The (endo)cannabinoid system in multiple sclerosis and amyotrophic lateral sclerosis. Int Rev Neurobiol 82:171–186 [DOI] [PubMed] [Google Scholar]
- Chen J, Lee CT, Errico S, Deng X, Cadet JL, Freed WJ. (2005) Protective effects of Delta(9)-tetrahydrocannabinol against N-methyl-d-aspartate-induced AF5 cell death. Brain Res Mol Brain Res 134:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzblanca H, Koh DS, Hille B. (1998) Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc Natl Acad Sci U S A 95:7151–7156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedov VN, Mandadi S, Armati PJ, Verkhratsky A. (2001) Capsaicin-induced depolarisation of mitochondria in dorsal root ganglion neurons is enhanced by vanilloid receptors. Neuroscience 103:219–226 [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Petrosino S. (2007) Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol 18:129–140 [DOI] [PubMed] [Google Scholar]
- Docagne F, Muñetón V, Clemente D, Ali C, Loría F, Correa F, Hernangómez M, Mestre L, Vivien D, Guaza C. (2007) Excitotoxicity in a chronic model of multiple sclerosis: neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol Cell Neurosci 34:551–561 [DOI] [PubMed] [Google Scholar]
- Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. (2008) β-Arrestin-biased agonism and β2-adrenergic receptor. J Biol Chem 283:5669–5676 [DOI] [PubMed] [Google Scholar]
- Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, Heimann AS, Ferro ES, Scarlata S, Fricker LD, Devi LA. (2009) Novel endogenous peptide agonists of cannabinoid receptors. FASEB J 23:3020–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson AJ, Bornheim LM, Scanziani M, Yost CS, Gray AT, Hansen BM, Leonoudakis DJ, Bickler PE. (1998) Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J Neurochem 70:671–676 [DOI] [PubMed] [Google Scholar]
- Harvey J, Collingridge GL. (1992) Thapsigargin blocks the induction of long-term potentiation in rat hippocampal slices. Neurosci Lett 139:197–200 [DOI] [PubMed] [Google Scholar]
- Howlett AC, Mukhopadhyay S. (2000) Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chem Phys Lipids 108:53–70 [DOI] [PubMed] [Google Scholar]
- Iversen L, Chapman V. (2002) Cannabinoids: a real prospect for pain relief? Curr Opin Pharmacol 2:50–55 [DOI] [PubMed] [Google Scholar]
- Johnson EM, Jr, Koike T, Franklin J. (1992) A “calcium set-point hypothesis” of neuronal dependence on neurotrophic factor. Exp Neurol 115:163–166 [DOI] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Mao XO, Jin K, Greenberg DA. (2006) Molecular mechanisms of cannabinoid protection from neuronal excitotoxicity. Mol Pharmacol 69:691–696 [DOI] [PubMed] [Google Scholar]
- Kotecha SA, MacDonald JF. (2003) Signaling molecules and receptor transduction cascades that regulate NMDA receptor-mediated synaptic transmission. Int Rev Neurobiol 54:51–106 [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Albuquerque C, Gu J, MacDermott AB. (1996) Ca2+-dependent inactivation of NMDA receptors: fast kinetics and high Ca2+ sensitivity in rat dorsal horn neurons. J Physiol 495:449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner JE, Hille B, Mackie K. (2005) The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Natl Acad Sci U S A 102:19144–19149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. (2008) GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A 105:2699–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. (1999) G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci 2:331–338 [DOI] [PubMed] [Google Scholar]
- Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C. (2002) Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem 80:448–456 [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564 [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65 [DOI] [PubMed] [Google Scholar]
- Netzeband JG, Conroy SM, Parsons KL, Gruol DL. (1999) Cannabinoids enhance NMDA-elicited Ca2+ signals in cerebellar granule neurons in culture. J Neurosci 19:8765–8777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. (1988) The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 334:661–665 [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. (1997) Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 74:129–180 [DOI] [PubMed] [Google Scholar]
- Pertwee RG. (2007) Cannabinoids and multiple sclerosis. Mol Neurobiol 36:45–59 [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Ross RA. (2002) Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids 66:101–121 [DOI] [PubMed] [Google Scholar]
- Qiu Z, Parsons KL, Gruol DL. (1995) Interleukin-6 selectively enhances the intracellular calcium response to NMDA in developing CNS neurons. J Neurosci 15:6688–6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, MacDermott AB. (1993) Brief calcium transients evoked by glutamate receptor agonists in rat dorsal horn neurons: fast kinetics and mechanisms. J Physiol 469:67–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter F, Meurer BH, Zhu C, Madvedeva VP, Chesselet MF. (2009) Neurons express hemoglobin α and β chains in rat and human brains. J Comp Neurol 515:538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Thayer SA. (1998) Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol Pharmacol 54:459–462 [DOI] [PubMed] [Google Scholar]
- Simpson PB, Challiss RA, Nahorski SR. (1993) Involvement of intracellular stores in the Ca2+ responses to N-methyl-d-aspartate and depolarization in cerebellar granule cells. J Neurochem 61:760–763 [DOI] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. (1995) Synaptic desensitization of NMDA receptors by calcineurin. Science 267:1510–1512 [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. (2005) Cannabinoid receptors and their role in neuroprotection. Neuromolecular Med 7:37–50 [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, et al. ( 2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329–332 [DOI] [PubMed] [Google Scholar]
- Walker JM, Hohmann AG. (2005) Cannabinoid mechanisms of pain suppression. Handb Exp Pharmacol. 168:509–554 [DOI] [PubMed] [Google Scholar]
- Woolf CJ. (1983) Evidence for a central component of post-injury pain hypersensitivity. Nature 306:686–688 [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. (2000) Neuronal plasticity: increasing the gain in pain. Science 288:1765–1769 [DOI] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. (1999) Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol 81:781–787 [DOI] [PubMed] [Google Scholar]
- Yankura K, Zhang HY, Bhat MB. (2003) Role of vanilloid receptor in the capacitative calcium entry in sensory neurons. Anesthesiology 99:A764 [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. (2003) Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci 17:2750–2754 [DOI] [PubMed] [Google Scholar]
- Zhuang SY, Bridges D, Grigorenko E, McCloud S, Boon A, Hampson RE, Deadwyler SA. (2005) Cannabinoids produce neuroprotection by reducing intracellular calcium release from ryanodine-sensitive stores. Neuropharmacology 48:1086–1096 [DOI] [PubMed] [Google Scholar]