Abstract
Galantamine, a centrally acting cholinesterase (ChE) inhibitor and a nicotinic allosteric potentiating ligand used to treat Alzheimer's disease, is an effective and safe antidote against poisoning with nerve agents, including soman. Here, the effectiveness of galantamine was compared with that of the centrally active ChE inhibitors donepezil, rivastigmine, and (±)huperzine A as a pre- and/or post-treatment to counteract the acute toxicity of soman. In the first set of experiments, male prepubertal guinea pigs were treated intramuscularly with one of the test drugs and 30 min later challenged with 1.5 × LD50 soman (42 μg/kg s.c.). All animals that were pretreated with galantamine (6–8 mg/kg), 3 mg/kg donepezil, 6 mg/kg rivastigmine, or 0.3 mg/kg (±)huperzine A survived the soman challenge, provided that they were also post-treated with atropine (10 mg/kg i.m.). However, only galantamine was well tolerated. In subsequent experiments, the effectiveness of specific treatment regimens using 8 mg/kg galantamine, 3 mg/kg donepezil, 6 mg/kg rivastigmine, or 0.3 mg/kg (±)huperzine A was compared in guinea pigs challenged with soman. In the absence of atropine, only galantamine worked as an effective and safe pretreatment in animals challenged with 1.0 × LD50 soman. Galantamine was also the only drug to afford significant protection when given to guinea pigs after 1.0 × LD50 soman. Finally, all test drugs except galantamine reduced the survival of the animals when administered 1 or 3 h after the challenge with 0.6 or 0.7 × LD50 soman. Thus, galantamine emerges as a superior antidotal therapy against the toxicity of soman.
The nerve agents soman, sarin, VX, and tabun are organophosphorus (OP) compounds chemically related to, but far more toxic than OP insecticides used worldwide in agriculture and households. There are reports that some of these agents have been used as weapons of mass destruction with catastrophic results in the Second Sino-Japanese War, the 1980s Iraq-Iran conflict, and the 1990s terrorist attacks in Japan (Romano and King, 2001).
Although OP compounds interact with a number of molecular targets, acute signs of OP poisoning result primarily from the irreversible inhibition of acetylcholinesterase (AChE) (Newmark, 2007). Thus, overactivation of muscarinic receptors by accumulated acetylcholine (ACh) causes miosis, increased secretions, bronchoconstriction, hypotension, and diarrhea. Overstimulation of nicotinic receptors triggers intense skeletal muscle fasciculations and subsequent desensitization of these receptors leads to muscle weakness. Central nervous system (CNS)-related effects commonly seen in severe cases of acute OP intoxication include anxiety, restlessness, confusion, ataxia, tremors, seizures, impairment of respiratory drive, and coma (Shih et al., 2003).
Approved treatment of OP poisoning relies on the use of atropine to block muscarinic receptors, pralidoxime to reactivate OP-inhibited AChE, and benzodiazepines to control OP-induced convulsions (Newmark, 2007). However, AChE inhibited by some OPs, particularly soman, is refractory to reactivation by clinically available oximes (Kassa, 2002). In addition, reduction of the incidence and severity of OP-induced convulsions is not sufficient to prevent the development of neuropathology (Filliat et al., 1999). Consequently, the fatality rate from OP insecticide poisoning remains extremely high despite the use of these treatments, exceeding 100,000 deaths annually (Buckley et al., 2004). The prevalence of delayed neurotoxic effects is also alarming among farmers and workers who frequently handle OP pesticides and among the first responders who treated the civilian population exposed to sarin during the 1995 terrorist attack in Japan (Nishiwaki et al., 2001; Buckley et al., 2004). These reports underscore the urgent need for the development of a medical countermeasure to increase the preparedness of the first responders to attend the general population in the event of a terrorist attack and to better treat an accidental/occupational exposure to OP compounds.
Pretreatment with pyridostigmine, a reversible cholinesterase (ChE) inhibitor, prevents the OP-induced irreversible enzyme inhibition and, thereby, increases the survival of laboratory animals acutely exposed to lethal doses of nerve agents, provided that atropine and pralidoxime chloride are administered promptly after the exposure (Jones et al., 1985). The ultimate usefulness of pyridostigmine is limited by two factors. First, as a quaternary base, pyridostigmine does not cross the blood-brain barrier appreciably and, therefore, does not protect CNS AChE from the irreversible inhibition by OPs. Second, in addition to inhibiting AChE, pyridostigmine blocks butyrylcholinesterase (BuChE), an endogenous scavenger for OP compounds (Doctor et al., 1991). Thus, centrally acting reversible inhibitors that are more selective for AChE than for BuChE may offer advantages in the medical management of OP intoxication.
Potent, reversible AChE inhibitors capable of crossing the blood-brain barrier, including physostigmine and tacrine, afford protection against OP toxicity, but, in general, at doses that produce some level of CNS impairment (Deshpande et al., 1986; Fricke et al., 1994). In contrast, galantamine, a centrally acting reversible AChE inhibitor approved for treatment of mild-to-moderate Alzheimer's disease (Corey-Bloom, 2003), has been shown to counteract the acute toxicity and lethality of soman and sarin with no apparent central or peripheral toxicity (Albuquerque et al., 2006).
A number of unique actions of galantamine contribute to its effectiveness and safety as a medical countermeasure against OP poisoning. First, the selective AChE inhibition by galantamine (Thomsen and Kewitz, 1990) should help to preserve the scavenger capacity of plasma BuChE for OPs. Second, galantamine crosses the blood brain barrier readily, and, thereby, can protect brain AChE from OP-induced irreversible inhibition. Third, acting as a nicotinic allosteric potentiating ligand (APL), galantamine has neuroprotective actions (see Pereira et al., 2002; Albuqueraque et al., 2009).
The present study was designed to examine whether the centrally acting reversible ChE inhibitors donepezil and rivastigmine, both of which are currently approved for treatment of Alzheimer's disease, and (±)huperzine A can be used safely and effectively as pre- or post-treatments to curtail the acute toxicity of soman in guinea pigs, the best nonprimate model of OP poisoning (Inns and Leadbeater, 1983). Results presented herein indicate that, like galantamine, all three drugs can prevent the lethality of 1.5×LD50 soman provided that the animals are treated with atropine immediately after the nerve agent challenge. In contrast to galantamine, however, donepezil, rivastigmine, and (±)huperzine A are toxic at their respective therapeutic doses and are not effective as post-treatments in soman-intoxicated animals.
Materials and Methods
Animal Care and Treatments.
Male albino guinea pigs [Crl(HA)Br] were purchased from Charles River Laboratories (Wilmington, MA) and were 33 to 35 days old (320–350 g) on arrival at the animal-care unit. They were housed in a controlled animal-care unit with constant temperature (21 ± 0.5°C) and a 12-h light/dark cycle. Animals were handled daily and used between 5 and 7 days after arrival. Guinea pigs were chosen for this study because they are similar to humans and nonhuman primates in their sensitivity to OP compounds. Like humans and nonhuman primates, guinea pigs have low levels of circulating carboxylesterases, the enzymes that hydrolyze and inactivate OP compounds. Thus, the LD50 values for most OP nerve agents in mice and rats are much higher than in monkeys or guinea pigs. For example, the subcutaneous LD50 for the OP nerve agent soman in mice and rats is approximately 4- and 10-fold higher than in guinea pigs and monkeys, respectively (Maxwell et al., 2006). In addition, several lines of evidence indicate that guinea pigs are the best small-animal model to predict the effectiveness of medical therapies against OP intoxication in humans. Pyridostigmine bromide, a peripherally acting carbamate that reversibly inhibits ChE, provides better protection against OP toxicity in non-human primates and guinea pigs than in rats or mice (Dirnhuber et al., 1979). In addition, pralidoxime, a reactivator of OP-bound AChE, affords significant protection against soman intoxication in rats and mice, but not in primates and guinea pigs (Inns and Leadbeater, 1983). Thus, in an attempt to standardize testing of antidotes against OP intoxication, it is advised that initial studies be performed in guinea pigs and that results be confirmed subsequently in non-human primates. The use of male guinea pigs at prepubertal ages facilitates the comparison of the results obtained herein using centrally acting AChE inhibitors with those published earlier using pyridostigmine.
Donepezil, rivastigmine, or (±)huperzine A was injected intramuscularly in a hind limb in volumes not exceeding 0.5 ml/kg, before or after subcutaneous injection of soman (0.6, 1.0, or 1.5 × LD50). As reported in numerous studies (e.g., Inns and Leadbeater, 1983; Lallement et al., 1997), the reference 1.0 × LD50 for soman in prepubertal male guinea pigs is 28 μg/kg s.c. Whenever stated, atropine sulfate (10 mg/kg i.m.) was injected into the other hind limb. The subcutaneous injection of soman was made between the shoulder blades, and the injection volume did not exceed 0.5 ml/kg. Treatment of animals with nerve agents was performed according to procedures set forth by the U.S. Army Medical Research Institute of Chemical Defense.
On the day of the experiments, a vial containing an aliquot (0.3–0.5 ml) of the U.S. Army-issued stock solution of soman (1.88–1.90 mg/ml) was diluted with sterile saline to appropriate concentrations and kept on ice for the duration of the experiment. At the end of the experiments, any remaining diluted OP was decontaminated with 10% sodium hydroxide before disposal. Solutions of each test drug were prepared on the day of the experiment, and injections were made by use of disposable tuberculin syringes with 25- or 26-gauge needles. After all treatments, animals were kept warm with a heat lamp for 8 h and provided food and water ad libitum. The guinea pigs were observed every 15 min for the first hour and every 30 min for the next 7 h after the OP challenge. Onset of convulsions was the time between the OP challenge and appearance of discontinuous, involuntary skeletal muscular contractions interrupted by intervals of relaxation. A group of three investigators was in charge of observing the animals. As soon as the investigators detected that guinea pigs were showing life-threatening signs, the animals were euthanized by CO2 asphyxiation followed by decapitation according to Institutional Animal Care and Use Committee-approved protocols. Life-threatening signs included intense, unremitting motor convulsions and/or respiratory distress, manifested by gasping. Throughout the manuscript, percentage survival refers to the percentage of animals that were kept alive because they had no life-threatening symptoms.
Surviving animals were observed and weighed daily for 7 to 14 days after the treatments, and their weight on any particular day was expressed as a percentage of the weight measured on the day of the treatments. The regression coefficient of the plot of weight versus days after the treatment was used as an estimate of the rate of weight gain under a given experimental condition.
In conducting the research described in this report, the investigators complied with the regulations and standards of the Animal Welfare Act and adhered to the principles of the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Statistics.
The dose-response relationship for each test drug was analyzed individually. Probit analysis was used to derive values for the median effective doses (ED50) and their 95% confidence intervals. The software StatsDirect (StatsDirect Limited, Cheshire, UK) was used for the probit fitting. Results are presented as mean and S.E.M. and, whenever appropriate, results from multiple groups were compared with results obtained from control groups using one-way ANOVA followed by Dunnett's post test.
Chemicals.
Stock solution of soman (1.88–1.9 mg/ml) was obtained from the U.S. Army Edgewood Chemical Biological Center via an agreement with the U.S. Army Medical Research Institute of Chemical Defense. Soman was stored, handled, and disposed according to the regulations set forth by the U.S. Army Medical Research Institute of Chemical Defense. Galantamine·HBr was generously provided by Dr. Alfred Maelicke (Galantos Pharma, Mainz, Germany). Rivastigmine hydrogen tartrate and donepezil·HCl were purchased from A and A Pharmachem, Inc. (Ottawa, Ontario, Canada). (±)Huperzine A was purchased from Alexis Corporation (Lausen, Switzerland) and atropine sulfate, from Sigma-Aldrich (St. Louis, MO). The systematic names of the chemicals used are: 1) soman, methylphosphonofluoridic acid 1,2,2-trimethylpropyl ester; 2) galantamine, (4aS,6R,8aS)-5,6,9,10,11,12-hexahydro-3-methoxy-11-methyl-4aH-[1]benzofuro[3a,3,2-ef] [2] benzazepin-6-ol; 3) rivastigmine, (S)-N-Ethyl-N-methyl-3-[1-(dimethylamino)ethyl]-phenyl carbamate;4) donepezil, (RS)-2-[(1-benzyl-4-piperidyl)methyl]-5,6-dimethoxy-2,3-dihydroinden-1-one; and 5) (±)huperzine A, 5,9-methanocycloocta[b]pyridine-2(1H)-one,5-amino-11-ethylid-Ene-5,6,9,10-tetrahydro-7-methyl [5R-(5a,9,11E)].
Results
Acute Toxicity of Soman in Prepubertal Male Guinea Pigs.
Within 10 min after a single injection of 1.5 × LD50 soman (42 μg/kg s.c.) nearly 100% of prepubertal male guinea pigs presented a clear cholinergic crisis characterized by chewing, miosis, hypersecretion, diarrhea, bruxism, muscle fasciculations and tremors followed within 5 min by tonic-clonic convulsions and gasping. According to the Institutional Animal Care and Use Committee-approved protocol, animals were euthanized as soon as signs of intoxication became life-threatening. None of the guinea pigs survived for 24 h after an exposure to 1.5 × LD50.
The severity of the signs of soman-induced toxicity was defined by use of a qualitative staging system similar to that described by McLean et al. (1992). In stage 0, animals had no abnormal gross behavior. In stage 1, animals presented facial twitches, pawing at whiskers and mouth, and chewing. In stage 2, animals also showed head tremor and/or nodding and short periods of immobility. In stage 3, animals presented forelimb clonus in addition to the signs in stages 1 and 2. In stage 4, animals also showed rearing with no loss of balance, strong grinding and gnashing, or bruxism. When the animals reached this stage, they had a clear peripheral cholinergic crisis characterized by profuse secretions, muscle fasciculations with partial paralysis of the back leg muscles, and respiratory distress. In stage 5, rearing was accompanied by loss of balance, and animals reached frank convulsions. Animals in stages 0 to 3 were considered mildly intoxicated, whereas those in stages 4 and 5 were considered severely intoxicated.
Approximately 50% of the animals challenged with 1.0 × LD50 soman (28 μg/kg s.c.) presented no or only mild signs of toxicity. If motor convulsions occurred, they were self-limiting and did not progress to frank convulsions. The other 50% of the animals reached stage 4 within 45 min to 2 h after the injection. In approximately 95% of the cases, once the guinea pigs reached stage 4, their conditions quickly worsened and they entered stage 5. As described earlier, animals were euthanized as soon as they presented life-threatening signs of intoxication, in particular, unremitting convulsions and/or gasping. Guinea pigs presented no clear signs of acute intoxication and survived for the duration of the study (7–14 days) when challenged with 0.6 to 0.7 × LD50 soman.
Pretreatment of Guinea Pigs Exposed to 1.5 × LD50 Soman. Comparison of the Effectiveness And Safety of Galantamine, Donepezil, (±)Huperzine A, and Rivastigmine.
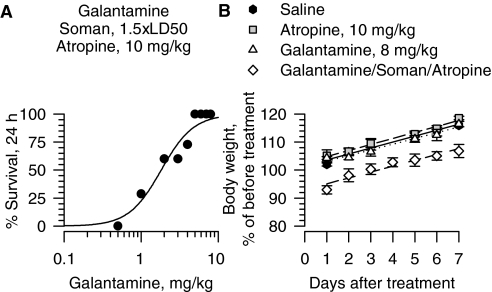
As demonstrated earlier (Albuquerque et al., 2006), all guinea pigs treated with galantamine (5–8 mg/kg i.m.) at 30 min before a challenge with 1.5 × LD50 soman survived with no apparent signs of toxicity provided that they were treated with atropine (10 mg/kg i.m.) 1 min after exposure to the nerve agent (see also Fig. 1A). Probit analysis of the dose-response relationship for galantamine to prevent the lethality of 1.5 × LD50 soman in atropine-treated guinea pigs revealed an ED50 of 1.88 mg/kg (Table 1). Under the same experimental conditions, the most effective dose of pyridostigmine, the Food and Drug Administration-approved pretreatment for soldiers at risk of exposure to soman, protected no more than 60% of the guinea pigs from the lethality of 1.5 × LD50 soman (Albuquerque et al., 2006). When used alone, atropine does not provide significant protection in guinea pigs exposed to 1.5 × LD50 soman; less than 7% of guinea pigs have been found to survive an exposure to 1.5 × LD50 soman if they are treated exclusively with 10 mg/kg atropine 1 min after the challenge (Albuquerque et al., 2006).
Fig. 1.
Pretreatment with galantamine prevents the acute toxicity of 1.5 × LD50 soman in guinea pigs post-treated with atropine. A, dose-response relationship for galantamine to afford 24-h survival of guinea pigs challenged with 1.5 × LD50 soman (42 μg/kg s.c.) and post-treated with atropine (10 mg/kg i.m.). Galantamine (0.5–8 mg/kg i.m.) was injected in one of the hind limbs of the animals 30 min before soman. Atropine was injected in the other hind limb 1 min after the nerve agent. Each group consisted of 8 to 12 animals. “% survival” represents the percentage of animals that were kept alive at 24 h after the soman challenge because they presented no life-threatening symptoms. B, body weight of animals subjected to different treatments. Body weights are expressed as percentage of the weights measured 1 h before the first treatment. Experimental groups consist of animals that were treated with 1) saline (0.5 ml/kg i.m.), 2) atropine (10 mg/kg i.m.), 3) galantamine (8 mg/kg i.m.), or 4) galantamine (8 mg/kg i.m.) followed 30 min later by 1.5 × LD50 soman (42 μg/kg s.c.), and atropine (10 mg/kg i.m.) 1 min after soman. Each experimental group consisted of five to eight animals. Data points and error bars represent mean and S.E.M., respectively. Solid, dotted, and dashed lines are the linear regression of each data set. Adapted from Albuquerque EX, Pereira EFR, Aracava Y, Fawcett WP, Oliveira M, Randall WR, Hamilton TA, Kan RK, Romano JA Jr, and Adler M (2006) Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proc Natl Acad Sci USA 103:13220–13225. Copyright © 2006. National Academy of Sciences, U.S.A.
TABLE 1.
Median effective doses (ED50 values) for galantamine, donepezil, rivastigmine, and (±)huperzine A in preventing the lethality of 1.5 × LD50 soman in guinea pigs post-treated with atropine (10 mg/kg i.m.)
As described in Materials and Methods, guinea pigs were treated intramuscularly with a given dose of the test drug. Thirty minutes later, the animals were challenged with 1.5 × LD50 soman (42 μg/kg s.c.). One minute after the soman, the animals received an injection of atropine (10 mg/kg i.m.). ED50 values were derived from the probit fitting of the resulting dose-response relationships shown in Figs. 1 to 4. Numbers in parentheses are the 95% confidence intervals.
| Test Drug | ED50 Values |
|---|---|
| Galantamine | 1.88 mg/kg |
| (1.22–2.44 mg/kg) | |
| Donepezil | 0.24 mg/kg |
| (0.11–0.42 mg/kg) | |
| (±)Huperzine A | 4.5 μg/kg |
| (0.9–11.9 μg/kg) | |
| Rivastigmine | 0.73 mg/kg |
| (0.38–1.26 mg/kg) |
Albuquerque et al. (2006) reported that guinea pigs that were pretreated with galantamine, exposed to 1.5 × LD50 soman and subsequently treated with atropine (10 mg/kg i.m.) lost approximately 7% of their body weight at 24 h after exposure to soman (Fig. 1B). They subsequently gained weight at a rate comparable with that of control (saline-injected) animals (Fig. 1B). Body weight changes correlated with food and water intake during the recovery period. At protective doses neither galantamine nor atropine alone affected the body weight or the rate of weight gain of guinea pigs (Fig. 1B).
The effectiveness of galantamine as an antidote against poisoning with 1.5 × LD50 soman was compared with that of other centrally acting, reversible ChE inhibitors under the same experimental conditions as those described above. Thus, male prepubertal guinea pigs were treated with donepezil (0.05–3 mg/kg i.m.), (±)huperzine A (1–500 μg/kg i.m.), or rivastigmine (0.1–16 mg/kg i.m.). At 30 min after the treatment, animals received an injection of 1.5 × LD50 soman (42 μg/kg s.c.) followed 1 min later by an injection of atropine (10 mg/kg i.m.). Acute (24 h) and long-term (up to 7 days) survival and weight gain were recorded for each test group.
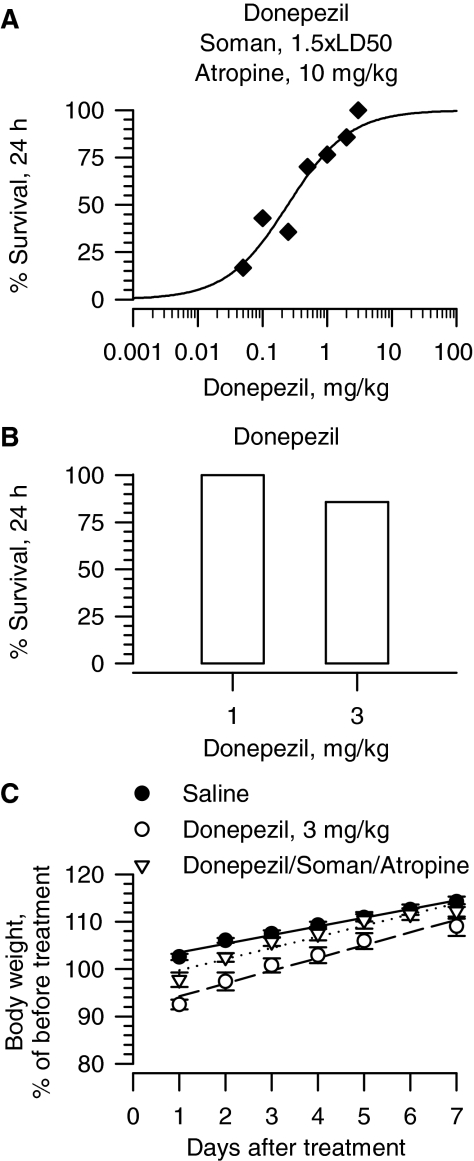
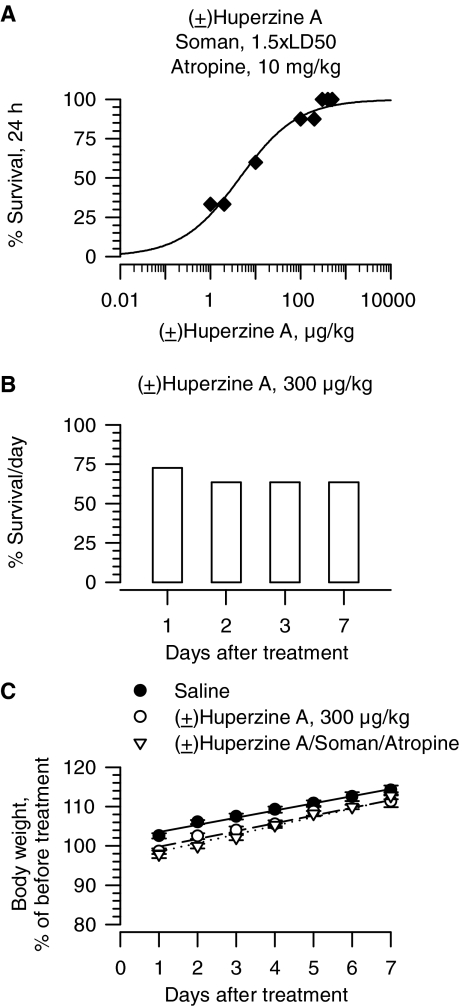
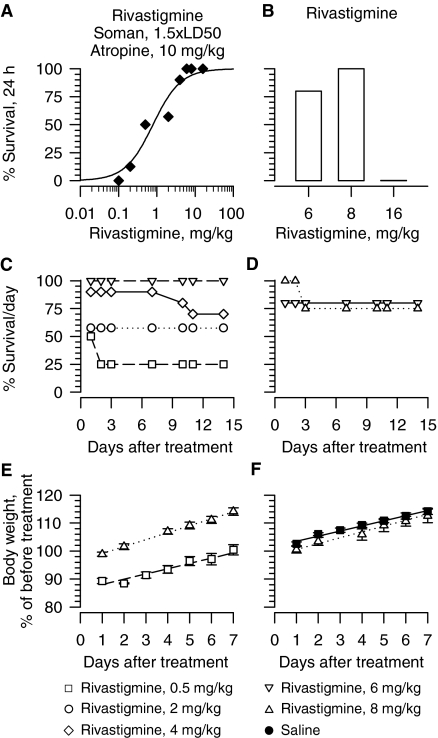
As observed with galantamine, survival of guinea pigs increased with increasing doses of drug. The lowest dose of donepezil, (±)huperzine, and rivastigmine capable of affording 100% survival of guinea pigs that were treated with atropine immediately after the soman challenge were 3 mg/kg, 300 μg/kg, and 6 mg/kg, respectively (Figs. 2A, 3A, 4A). Probit fitting of the dose-response relationships yielded the following ED50 values for donepezil, (±)huperzine A, and rivastigmine, respectively: 0.24 mg/kg, 4.5 μg/kg, and 0.73 mg/kg (Table 1).
Fig. 2.
Effectiveness of donepezil in preventing toxicity of 1.5 × LD50 soman in atropine-treated guinea pigs. A, dose-response relationship for donepezil to afford 24-h survival of guinea pigs challenged with 1.5 × LD50 soman (42 μg/kg s.c.) and post-treated with atropine (10 mg/kg i.m.). Donepezil (0.05–3 mg/kg i.m.) was injected in one of the hind limbs of the animals at 30 min before soman. Atropine (10 mg/kg i.m.) was injected in the other hind limb 1 min after the nerve agent. Each group consisted of 8 to 12 animals. “% survival” represents the percentage of animals that were kept alive at 24 h after the soman challenge because they presented no life-threatening symptoms. B, survival of guinea pigs treated with donepezil alone (1 or 3 mg/kg). C, body weight of animals subjected to different treatments. Body weights are expressed as percentage of the weights measured 1 h before the first treatment. Experimental groups consist of animals that were treated with 1) saline (0.5 ml/kg i.m.), 2) donepezil (3 mg/kg i.m.), or 3) donepezil (3 mg/kg i.m.) followed 30 min later by 1.5 × LD50 soman (42 μg/kg s.c.) and atropine (10 mg/kg i.m.). Data points and error bars represent mean and S.E.M., respectively, of results obtained from 18 animals in the saline group and 5 to 8 animals in the other two experimental groups. Solid, dotted, and dashed lines are the linear regression of each data set; r2 ranged from 0.96 to 0.98.
Fig. 3.
Effectiveness of (±)huperzine A in preventing the acute toxicity of 1.5 × LD50 soman in atropine-treated guinea pigs. A, dose-response relationship for (±)huperzine A to afford 24-h survival of guinea pigs challenged with 1.5 × LD50 soman (42 μg/kg s.c.) and post-treated with atropine (10 mg/kg i.m.). Animals were pretreated with (±)huperzine A (1–500 μg/kg i.m.), challenged with 1.5 × LD50 soman (subcutaneously) 30 min later and subsequently treated with atropine (10 mg/kg i.m.). Each group consisted of 8 to 12 animals. B, survival (1–7 days) of guinea pigs treated with (±)huperzine A alone (300 μg/kg i.m.). “% survival” represents the percentage of animals that were kept alive at 24 h after the treatment as they presented no life-threatening symptoms. C, body weight of animals subjected to different treatments. Body weights are expressed as percentage of the weights measured 1 h before the first treatment. Experimental groups consist of animals that were treated with 1) saline (0.5 ml/kg i.m.), 2) (±)huperzine A (300 μg/kg i.m.), or 3) (±)huperzine A (300 μg/kg i.m.) followed 30 min later by 1.5 × LD50 soman (42 μg/kg s.c.) and atropine (10 mg/kg i.m.). Data points and error bars represent mean and S.E.M., respectively, of results obtained from 18 animals in the saline group and from 5 to 8 animals in the other two experimental groups. Solid, dotted, and dashed lines are the linear regression of each data set; r2 ranged from 0.96 to 0.98.
Fig. 4.
Effectiveness of rivastigmine in preventing toxicity of 1.5 × LD50 soman in atropine-treated guinea pigs. A, dose-response relationship for rivastigmine to afford 24-h survival of guinea pigs challenged with 1.5 × LD50 soman (42 μg/kg s.c) and post-treated with atropine (10 mg/kg i.m.). Animals were pretreated with rivastigmine (0.1–16 mg/kg i.m.), challenged with 1.5 × LD50 soman (subcutaneously) 30 min later and subsequently treated with atropine (10 mg/kg i.m.). Each group consisted of 8 to 12 animals. B, survival (24 h) of guinea pigs treated with rivastigmine alone. “% survival” represents the percentage of animals that were kept alive at 24 h after the treatment because they presented no life-threatening symptoms. C, D, long-term survival of guinea pigs that were pretreated with rivastigmine, challenged with 1.5 × LD50 soman and treated with 10 mg/kg atropine (C) or injected with rivastigmine alone (D). Each treatment group had 8 to 10 animals. E, F, body weight of animals subjected to different treatments. Results presented in E are from animals treated with rivastigmine (0.5 or 8 mg/kg i.m.) followed 30 min later by the subcutaneous challenge with 1.5 × LD50 soman and treatment with 10 mg/kg i.m. atropine. Results in F are from animals treated with 8 mg/kg i.m. rivastigmine alone or saline. As in Fig. 3, body weights are expressed as percentage of the weights measured 1 h before the first treatment. Data points and error bars represent mean and S.E.M., respectively, of results obtained from 18 animals in the saline group and from 5 to 8 animals in the other two experimental groups. Solid, dotted, and dashed lines are the linear regression of each data set; r2 ranged from 0.96 to 0.98.
At the most effective protective doses, none of the test drugs was as safe as galantamine. For instance, guinea pigs that were treated with 1 mg/kg donepezil presented with signs of a mild cholinergic impairments, including chewing, muscle fasciculations, and tremors. The toxic signs were more severe in guinea pigs that were treated with 3 mg/kg donepezil, and 24-h survival of these animals was 85% (Fig. 2B). Likewise, 24-h survival of guinea pigs that were treated with a single injection of 300 μg/kg i.m. (±)huperzine A or 6 mg/kg i.m. rivastigmine was 75 and 80%, respectively (Figs. 3B and 4B). Increasing the dose of rivastigmine to 16 mg/kg resulted in a very poor 24-h survival (Fig. 4B). In addition, the toxicity of (±)huperzine A or rivastigmine was not always evident within the first 24 h after the treatment of the guinea pigs. For instance, long-term survival of guinea pigs treated with 300 μg/kg (±)huperzine A decreased from 75% at 24 h to 65% in subsequent days (Figs. 3B and 4D). Likewise, all guinea pigs survived for 48 h after a single injection of 8 mg/kg rivastigmine, whereas at 72 h only 8 of the 10 treated animals remained alive (Fig. 4D). Survival of guinea pigs that were pretreated with specific doses of rivastigmine, challenged with 1.5 × LD50 soman, and post-treated with atropine (10 mg/kg) also decreased with time after treatment (Fig. 4C). It is likely that, in the soman-exposed guinea pigs, post-treatment with atropine ameliorated some of the toxicity of donepezil, (±)huperzine A, or rivastigmine. Indeed, hyperlocomotion, chewing, nose rubbing, and tremors were observed during the 30-min period between the time the guinea pigs were exposed to the most effective doses of a test drug and the time they were challenged with soman. All of these signs subsided after the injection of atropine and, therefore, may have resulted from overactivation of muscarinic receptors by excessive buildup of ACh due to ChE inhibition.
The growth rates of guinea pigs, defined as the percentage of weight change per day during the first week after treatment, were significantly altered by donepezil. At 24 h after treatment, guinea pigs that were injected with donepezil (3 mg/kg) alone or followed by soman (1.5 × LD50) and atropine (10 mg/kg) lost 2.2 ± 1.5% and 7.5 ± 1.5%, respectively, of their initial body weight (Fig. 2C). In contrast, saline-injected animals had gained 2.5 ± 0.67% of their initial body weight during the same interval (Fig. 2C). In subsequent days, guinea pigs that had been injected with saline, donepezil (3 mg/kg), or donepezil followed by 1.5 × LD50 soman and atropine (10 mg/kg) gained weight at the rates of 1.83 ± 0.21%/day (n = 18), 2.36 ± 0.23%/day (n = 5), and 3.06 ± 0.15%/day (n = 7). Changes in weight gain of animals treated with donepezil were significantly different from those of control (saline-injected) animals (donepezil versus saline, p < 0.05, and donepezil/soman/atropine versus saline, p < 0.001 according to ANOVA followed by Dunnett's post test).
Unlike donepezil, (±)huperzine A and rivastigmine, at doses needed to afford full protection against the lethality of 1.5 × LD50 of soman, had no significant effect on the growth rate of guinea pigs. At 24 h after the treatment with huperzine alone, guinea pigs lost 1.5 ± 0.71% of their body weight (Fig. 3C). At the same time, a loss of 2.2 ± 0.96% of initial body weight was observed in huperzine-pretreated animals that were challenged with 1.5 × LD50 soman 30 min later and treated with atropine. However, during the first week after treatment, guinea pigs injected with 300 μg/kg (±)huperzine A gained weight at the rate of 1.98 ± 0.18%/day, which was not statistically different from that of saline-injected animals (1.83 ± 0.21%/day). Animals that had been injected with (±)huperzine A before soman and atropine also gained weight at a rate that was not statistically different from that of saline-injected animals (2.19 ± 0.10%/day versus 1.83 ± 0.21%/day, respectively).
Animals injected with any of the test doses of rivastigmine alone or with rivastigmine followed by 1.5 × LD50 soman and atropine (10 mg/kg) also showed significant weight losses at 24 h after the treatments. However, subsequently they gained weight at rates that were comparable with those of saline-injected animals (Fig. 4, E and F). The higher the dose of rivastigmine the animals received before challenge with soman followed by atropine, the lower the fractional weight loss during the first 24 h (Fig. 4, E and F).
Pretreatment of Guinea Pigs Exposed to 1.0 × LD50 Soman. Comparison of the Effectiveness of Galantamine, Donepezil, (±)Huperzine A, and Rivastigmine.
Single-drug therapies are always preferred to multiple-drug therapies, given that multiple-drug therapies incur the risk of potential untoward and, sometimes, dangerous interactions between the therapeutic agents. Low doses of atropine are generally well tolerated by humans. However, particularly for soldiers in the battlefield, hyperthermia is one of the serious complications that can develop as a result of the use of atropine. Thus, the next set of experiments was designed to assess the ability of the centrally acting ChE inhibitors to protect guinea pigs against challenge by soman in the absence of post-treatment with atropine. These tests were carried out with a lower challenge dose (1.0 × LD50) because we had already demonstrated that, in the absence of atropine, even galantamine does not afford significant protection against 1.5 × LD50 soman (Albuquerque et al., 2006).
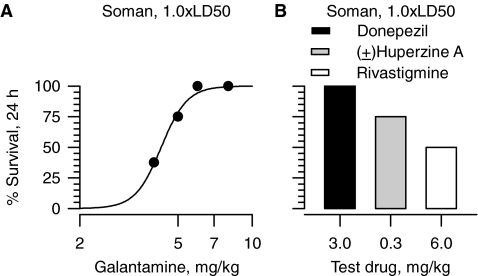
To accomplish this aim, animals were pretreated with a test drug 30 min before challenge with 1.0 × LD50 soman and 24-h survival of the animals was determined. Doses of galantamine needed to afford 100% survival of guinea pigs challenged with 1.0 × LD50 soman were similar to those required for promoting 100% survival in animals challenged with 1.5 × LD50 soman and subsequently treated with atropine (Fig. 5A). The demonstration that no adjustment of doses is necessary for galantamine to be an effective antidote against different levels of exposure to soman is clinically compelling. The subsequent tests were, therefore, designed to determine whether donepezil, (±)huperzine A, and rivastigmine would be as effective as galantamine in preventing the acute toxicity of 1.0 × LD50 soman when examined under the same experimental conditions.
Fig. 5.
Effectiveness of pretreatment with galantamine, donepezil, (±) huperzine A, or rivastigmine in preventing acute toxicity of 1.0 × LD50 soman in guinea pigs in the absence of atropine. A, dose-response relationship illustrating the ability of galantamine to afford 24-h survival of guinea pigs challenged with 1.0 × LD50 soman (28 μg/kg s.c.). Soman was injected 30 min after galantamine. Each point represents data from 8 to 12 animals. B, survival (24 h) of guinea pigs pretreated with donepezil (3 mg/kg i.m.), (±)huperzine A (0.3 mg/kg i.m.), or rivastigmine (6 mg/kg i.m.), and challenged 30 min later with 1.0 × LD50 soman. Each experimental group consisted of eight guinea pigs. Ordinates in A and B represent the percentage of animals that were kept alive at 24 h after the treatment because they presented no life-threatening symptoms.
As with galantamine, treatment of guinea pigs with 3 mg/kg donepezil at 30 min before challenge with 1.0 × LD50 soman resulted in survival of 100% of the animals (Fig. 5B). However, in contrast with galantamine, signs of excessive activation of the cholinergic system (sialorrhea, chewing, nose rubbing, and tremors) were evident in the 30-min period between the time the animals were treated with 3 mg/kg donepezil and the time they were challenged with 1.0 × LD50 soman. The signs became more severe, although not life threatening, when the animals were challenged with soman. Within 30 min after the soman challenge, the signs of toxicity subsided. The response of guinea pigs to the pretreatment with 0.3 mg/kg (±)huperzine A or 6 mg/kg rivastigmine was similar to that observed with 3 mg/kg donepezil. However, after challenge with soman, signs of toxicity became life threatening in some of the animals, and 24-h survival was less than 100% (Fig. 5B).
Post-treatment of Guinea Pigs Exposed to ≤1.0 × LD50 Soman. Comparison of the Effectiveness of Galantamine, Donepezil, (±)Huperzine A, and Rivastigmine.
In another set of experiments, the efficacy of galantamine was compared with that of donepezil, (±)huperzine A, or rivastigmine as the only treatment drug administered after 1.0, 0.7, or 0.6 × LD50 soman. Under this experimental condition each test drug was examined at the lowest dose that afforded 100% survival when administered to guinea pigs before their challenge with 1.5 × LD50 soman.
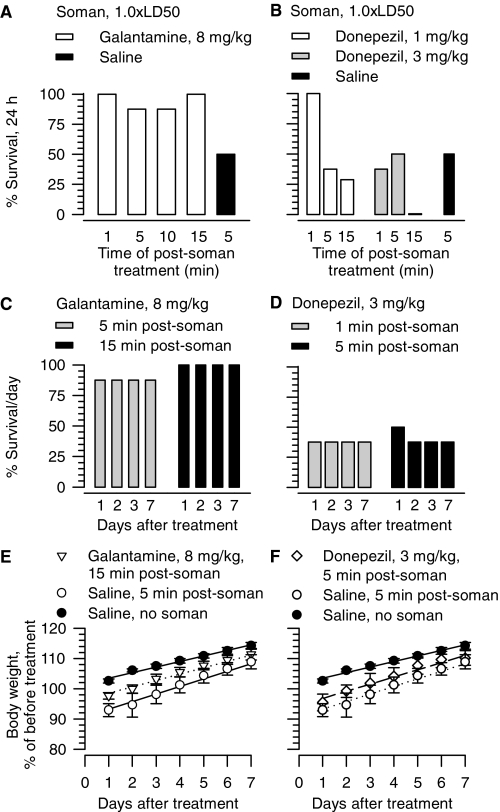
Acute (24-h) survival of guinea pigs challenged with 1.0 × LD50 soman increased markedly when the animals were treated with galantamine (8 mg/kg i.m.) up to 15 min after challenge by soman relative to animals injected with vehicle or with the other test drugs (Figs. 6A and 7, A and B). The level of survival attained at 24 h after galantamine treatment of soman (1.0 × LD50)-challenged guinea pigs was sustained throughout the 7-day observation period (Fig. 6C). Although control guinea pigs gained approximately 2% of their initial body weight at 24 h, guinea pigs injected with 1.0 × LD50 soman and post-treated with saline 5 min later lost approximately 8% of their initial body weight during the first 24 h (Fig. 6E). Soman-challenged guinea pigs that were post-treated with galantamine (8 mg/kg) lost approximately 4% of their initial body weight (Fig. 6E). Soman-challenged guinea pigs and control (saline-injected) animals subsequently gained weight at comparable rates: 1.83 ± 0.21%/day (n = 18) and 2.19 ± 0.22%/day (n = 7), respectively (Fig. 6E). In contrast, untreated guinea pigs that were challenged with soman gained weight at the rate of 2.46 ± 0.23%/day (n = 7), which is significantly faster than the rate of weight gain of control animals (p < 0.05, ANOVA followed by Dunnett's post test).
Fig. 6.
Effectiveness of post-treatment with galantamine or donepezil in counteracting the acute toxicity of 1.0 × LD50 soman in guinea pigs. A, B, survival (24 h) of guinea pigs treated with 8 mg/kg i.m. galantamine (A) or 1 to 3 mg/kg i.m. donepezil (B) at the indicated times after the challenge with soman (28 μg/kg s.c.). C, D, seven-day survival of guinea pigs challenged with 1.0 × LD50 soman and 5 or 15 min later treated im with 8 mg/kg galantamine or 1 or 5 min later with 3 mg/kg donepezil. “% Survival/day” represents the percentage of animals that were kept alive at days 1–7 after treatment because they presented no life-threatening symptoms. Each experimental group in A to D consisted of 8 to 12 animals. E, F, body weight of animals subjected to different treatments. Body weights are expressed as percentage of the weights measured 1 h before the first treatment. Experimental groups consist of animals that were treated with 1) saline (0.5 ml/kg i.m.) alone, 2) saline (0.5 ml/kg i.m.) injected 5 min after soman (1.0 × LD50, s.c.), 3) galantamine (8 mg/kg i.m.) injected 15 min after the soman, or 4) donepezil (3 mg/kg i.m.) injected 5 min after the soman challenge. In E and F, data points and error bars represent mean and S.E.M., respectively, of results obtained from 18 animals in the control (saline) group and from 4 to 8 animals in the other experimental groups. Solid, dotted, and dashed lines are the linear regression of each data set; r2 ranged from 0.96 to 0.98.
Fig. 7.
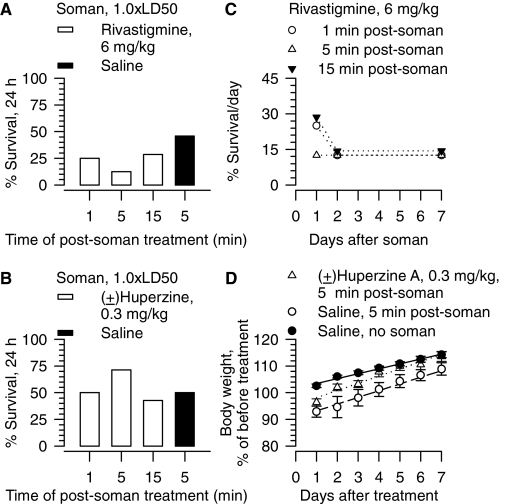
Effectiveness of post-treatment with rivastigmine or (±)huperzine A in counteracting the acute toxicity of 1.0 × LD50 soman in guinea pigs. A and B, survival (24 h) of guinea pigs treated im with 6 mg/kg rivastigmine (A) or 0.3 mg/kg (±)huperzine A (B) at the indicated times after the challenge with soman (28 μg/kg s.c.). Each experimental group consisted of 10 to 12 animals. C, seven-day survival of guinea pigs challenged with 1.0 × LD50 soman and 1, 5, or 15 min later treated im with 6 mg/kg rivastigmine. “% survival” represents the percentage of animals that were kept alive at any given day after the treatment because they presented no life-threatening symptoms. D, body weight of animals subjected to different treatments. Body weights are expressed as percentage of the weights measured 1 h before the first treatment. Experimental groups consisted of animals that were treated with 1) saline (0.5 ml/kg i.m.) alone, 2) saline (0.5 ml/kg i.m.) at 5 min after the soman (1.0 × LD50, sc), or 3) (±)huperzine A (0.3 mg/kg i.m.) at 5 min after the soman. Data points and error bars represent mean and S.E.M., respectively, of results obtained from 18 animals in the control (saline) group and from 4 to 6 animals in the other experimental groups. Solid, dotted, and dashed lines are the linear regression of each data set; r2 ranged from 0.96 to 0.98.
The 24-h survival of guinea pigs that were challenged with 1.0 × LD50 soman and subsequently treated with 3 mg/kg i.m. donepezil, 6 mg/kg i.m. rivastigmine, or 0.3 mg/kg i.m. (±)huperzine A was generally less than that observed in untreated, soman-challenged animals (Figs. 6B and 7, A and 7B). Moreover, for donepezil, as the time between the exposure to soman and the post-treatment with the test drug increased from 1 to 15 min, the 24-h survival of the animals decreased markedly. For some of the treatment regimens, percentage of survival of soman-challenged guinea pigs continued to decline in subsequent days (Figs. 6D and 7C). Furthermore, post-treatment of the soman-challenged guinea pigs with donepezil (Fig. 6F), (±)huperzine A (Fig. 7D), or rivastigmine (data not shown) did not counteract the weight loss after soman.
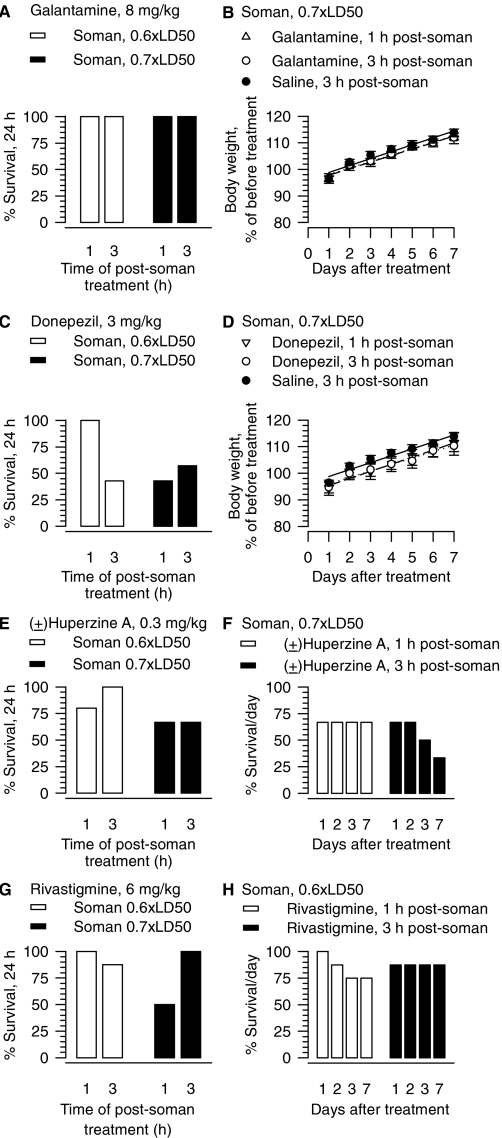
As stated before, guinea pigs presented no acute signs of intoxication and survived the subcutaneous challenge with 0.6 or 0.7 × LD50 soman. No signs of acute toxicity and 100% survival were also observed among guinea pigs that were challenged with 0.6 or 0.7 × LD50 soman and treated with galantamine (8 mg/kg) 1 to 3 h later (Fig. 8A). The results obtained when donepezil, (±)huperzine A, or rivastigmine was used as the treatment drug differed considerably. Treatment with any of these test drugs at 1 or 3 h after the challenge with soman led to signs of acute toxicity and resulted in a poor 24-h survival outcome (Fig. 8, C, E, and G). Survival of the animals subjected to specific treatment regimens with (±)huperzine A or rivastigmine after the soman challenge continued to decline in subsequent days (Fig. 8, F and H).
Fig. 8.
Effectiveness of post-treatment with galantamine, donepezil, (±)huperzine A, or rivastigmine administered 1 or 3 h after an acute challenge of guinea pigs with sublethal doses of soman. Survival (24 h) of guinea pigs treated im with 8 mg/kg galantamine (A), 3 mg/kg donepezil (C), 0.3 mg/kg (±)huperzine A (E), or 6 mg/kg rivastigmine (G) at 1 or 3 h after challenge with sublethal doses of soman (16.8 or 19.6 μg/kg s.c.). “% survival” represents the percentage of animals that were kept alive at any given day after the treatment because they presented no life-threatening symptoms. Each experimental group consisted of 10 to 12 animals. B, D, body weight of animals subjected to different treatments. Body weights are expressed as percentage of the weights measured 1 h before the first treatment. Experimental groups consisted of animals that were challenged with 0.7 × LD50 soman (19.6 μg/kg s.c.) and treated 1 or 3 h later with 8 mg/kg galantamine, 3 mg/kg donepezil, or saline. Data points and error bars represent mean and S.E.M., respectively, of results obtained from four to six animals per treatment. Solid, dotted, and dashed lines are the linear regression of each data set; r2 ranged from 0.96 to 0.98. F, seven-day survival of the guinea pigs that were challenged with 0.7 × LD50 soman and 1 or 3 h later treated with 0.3 mg/kg (±)huperzine A. H, seven-day survival of the guinea pigs that were challenged with 0.6 × LD50 soman and 1 or 3 h later treated with 6 mg/kg rivastigmine.
At 24 h after the soman challenge, saline-treated guinea pigs had lost approximately 4% of their initial body weight (Fig. 8A). In subsequent days they gained weight at the rate of 2.21 ± 0.15% (n = 6), which is comparable with that of control (saline-injected) animals (1.83 ± 0.21%, n = 18). Post-treatment with galantamine or donepezil affected neither the initial weight loss nor the rate of weight gain of the soman-challenged guinea pigs (Fig. 8, B and D).
Discussion
This study demonstrates that galantamine, a centrally acting AChE inhibitor currently used for treatment of Alzheimer's disease, is more effective and safer than the centrally acting AChE inhibitors donepezil, (±)huperzine A or rivastigmine as an antidote against soman intoxication.
Galantamine Versus Donepezil, (±)Huperzine A, or Rivastigmine as a Medical Countermeasure against Soman Poisoning.
As reported previously (Albuquerque et al., 2006), pretreatment of guinea pigs with galantamine (8 mg/kg) prevented the acute toxicity of 1.5 × LD50 soman, provided that the animals were also post-treated with atropine (10 mg/kg i.m.). In the present study, donepezil (3 mg/kg), (±)huperzine A (0.3 mg/kg), or rivastigmine (6 mg/kg) also afforded 100% survival of guinea pigs challenged with 1.5 × LD50 soman and treated with atropine. Other studies have also reported that pretreatment with galantamine, donepezil, or (±)huperzine A in combination with post-treatment with a muscarinic antagonist effectively prevents the lethality of high doses of other nerve agents and OP pesticides (Lallement et al., 1997; Albuquerque et al., 2006; Haug et al., 2007; Hilmas et al., 2009). However, as shown here, at therapeutic doses only galantamine is devoid of untoward side effects.
Variable degrees of survival have been observed among animals pretreated with (±)huperzine A and subsequently challenged with soman. For instance, Karasova et al. (2009) reported that rats pretreated with (±)huperzine A (0.5 mg/kg i.p.) did not survive the subcutaneous challenge with 1.5 × LD50 soman. In the study by Tonduli et al. (2001), 93% of mice survived the subcutaneous exposure to 1.0 × LD50 soman when they were pretreated with (±)huperzine A (0.5 mg/kg i.p.). Finally, Lallement et al. (1997) reported that an acute pretreatment with (±)huperzine A (0.5 mg/kg i.p.) protected 100% of guinea pigs against the lethality of 1.3 × LD50 soman. These apparently paradoxical results could be reconciled by 1) species-dependent responsiveness to OP antidotal therapies (Inns and Leadbeater, 1983), 2) use of different doses of soman, 3) varied routes of administration of the antidote (intramuscular in the present study versus intraperitoneal in other studies), and/or 4) use of additional supportive therapies.
Of the four centrally active compounds examined in this study, galantamine was the only one that, in the absence of atropine, proved to be effective and safe against 1.0 × LD50 soman. Pretreatment with galantamine (8 mg/kg i.m.) afforded 100% survival of guinea pigs that were challenged with 1.0 × LD50 soman. In line with the reported effectiveness of donepezil in preventing the acute toxicity of diisopropyl fluorophosphate (Janowsky et al., 2005), pretreatment with donepezil (3 mg/kg i.m.) resulted in 100% survival of guinea pigs challenged with 1.0 × LD50 soman. However, donepezil-treated guinea pigs showed signs of toxicity that could be attributed to excessive AChE inhibition. Animals that were pretreated with (±)huperzine A (0.3 mg/kg i.m.) or rivastigmine (6 mg/kg i.m.) before exposure to 1.0 × LD50 soman also developed signs of cholinergic hyperactivity. These signs became more severe after the soman challenge and, as a result, the 24-h survival of the animals that had been pretreated with (±)huperzine A or rivastigmine was only 50 to 75% (Fig. 5B).
Galantamine was also superior to the other test drugs as a therapy against 1.0 × LD50 soman. Significant survival (90–100%) was attained when guinea pigs were treated with galantamine (8 mg/kg) up to 15 min after an acute challenge with 1.0 × LD50 soman. Under the same conditions, the use of donepezil (3 mg/kg), (±)huperzine A (0.3 mg/kg), or rivastigmine (6 mg/kg) as the post-treatment resulted in very poor survival outcome.
Examining the safety of a potential post-treatment that can be used after an exposure to sublethal doses of OP agents is a critical step for the subsequent assessment of the effectiveness of such treatment in preventing delayed OP toxicity. As demonstrated herein, all guinea pigs that were challenged with 0.6 or 0.7 × LD50 soman and treated 1 or 3 h later with galantamine (8 mg/kg) presented no signs of acute intoxication. In contrast, a large percentage of the guinea pigs that were challenged with either dose of soman and post-treated with donepezil (3 mg/kg), (±)huperzine A (0.3 mg/kg), or rivastigmine (6 mg/kg) presented severe signs of toxicity, including tonic-clonic convulsions and gasping, and had to be euthanized (Figs. 6 and 7).
Why Is Galantamine Superior to Other Centrally Acting AChE Inhibitors as a Medical Countermeasure against Soman Poisoning?
The effectiveness of an antidotal therapy based on reversible AChE inhibitors against the acute toxicity of OPs seems to depend on the generation of a reversible inhibitor-AChE complex that 1) protects the enzyme in the periphery and CNS from irreversible inhibition by OPs, and 2) rapidly dissociates in the presence of excess ACh, thereby releasing active enzyme to hydrolyze the substrate surplus (Tonkopii, 2009). It is also essential that the reversible inhibitors are more selective toward AChE than BuChE, given that the latter is an endogenous OP scavenger (Fricke et al., 1994). Within this framework, the pseudo-irreversible nature of ChE inhibition by rivastigmine, in addition to the high selectivity of this carbamate toward BuChE (Darvesh et al., 2004), could explain its low overall effectiveness and high toxicity as an OP antidote.
In contrast to rivastigmine, donepezil, and (±)huperzine A are several orders of magnitude more potent inhibitors of AChE than BuChE (Darvesh et al., 2004). Galantamine is less potent than donepezil or (±)huperzine A as an AChE inhibitor (Darvesh et al., 2004), although it still shows some degree of selectivity toward AChE compared with BuChE (Thomsen and Kewitz, 1990). Therefore, donepezil, (±)huperzine A, and galantamine are able to protect AChE from irreversible inhibition by soman, while sparing the OP scavenger activity of BuChE. A major difference among these three drugs lies in their kinetics and mode of AChE inhibition. Although (±)huperzine A and galantamine are competitive inhibitors of AChE, donepezil is a noncompetitive inhibitor (Wilkinson, 1999). Thus, excess ACh does not play a significant role in accelerating the reversibility of the complex donepezil-AChE. The inhibition by (±)huperzine A of AChE follows a time-dependent, second-order kinetics typical of slowly reversible and irreversible inhibitors (Darvesh et al., 2004). The thermodynamic stability of the complex AChE-(±)huperzine A prevents its rapid dissociation (Ashani et al., 1992; Raves et al., 1997). Because galantamine competitively blocks AChE with kinetics typical of reversible inhibitors, the galantamine-AChE complex can be readily dissociated by excess ACh (Tonkopii et al., 1975).
A high degree of selective reversible inhibition of peripheral AChE is necessary to prevent the non-CNS toxic effects of OPs. Doses of galantamine and other reversible AChE inhibitors required to prevent acute OP poisoning inhibit blood AChE by approximately 70% (see Albuquerque et al., 2006, and references therein). In contrast, the effective doses of galantamine inhibit brain AChE by 25%. Thus, it has been proposed that a low degree of reversible inhibition of brain AChE may be sufficient to protect a significant pool of the enzyme from OP-induced irreversible inhibition, while limiting the occurrence of unwanted central side effects (Albuquerque et al., 2006).
It is generally believed that protection of AChE from OP-induced irreversible inhibition is an important determinant of the effectiveness of antidotes to counteract the lethality of OPs. However, interactions of an OP with targets other than AChE can contribute to its acute toxicity (Albuquerque et al., 1985; Silveira et al., 1990; Jett et al., 1994). Consequently, AChE-unrelated mechanisms may also determine how effective a drug is as an OP antidote. For example, at concentrations that are likely to be achieved after the therapeutic doses used herein, donepezil binds to and activates σ1 receptors (Maurice et al., 2006). Activation of these receptors facilitates NMDA responses (Monnet et al., 1990) and increases ACh concentrations in specific brain regions (Kobayashi et al., 1996). Considering that increased brain cholinergic and glutamatergic activities underlie the neurotoxicity of soman (Solberg and Belkin, 1997), activation of σ1 receptors may be a detriment to the effectiveness of donepezil as an OP antidote. In addition, at concentrations that may be attained with the high doses necessary to prevent the lethality of soman, (±)huperzine A acts as a noncompetitive NMDA receptor antagonist (Lallement et al., 1997). The NMDA receptor inhibition by (±)huperzine A could be beneficial to attenuate the excitotoxicity triggered by soman (Solberg and Belkin, 1997).
The AChE-unrelated, nicotinic APL action of galantamine can potentially contribute to its effectiveness as an OP antidote. In particular, as a nicotinic APL, galantamine acts as a neuroprotectant in vitro and in vivo (Takada-Takatori et al., 2006; Wang et al., 2007; Albuquerque et al., 2009). In fact, neurodegeneration is absent in the brains of galantamine-treated guinea pigs that are exposed to 1.5 × LD50 soman (Albuquerque et al., 2006). The ability of galantamine to counteract soman-induced changes in nAChR activity known to regulate the rhythms of hippocampal neuronal networks may also serve as a mechanism to control soman-induced convulsions (Alkondon et al., 2009).
In summary, the greater effectiveness and safety of galantamine compared with that of other centrally acting AChE inhibitors as an OP antidote could be accounted for by 1) the competitive mode of interaction of galantamine with AChE, 2) the rapid dissociation of the complex galantamine-AChE, 3) the preservation of some of the endogenous OP scavenger BuChE, 4) the differential degree of inhibition of blood and brain AChE, and 5) the nicotinic APL action.
Acknowledgments
We thank Mabel Zelle for technical assistance.
This work was supported by the Army Research Office [Contract W911NF-06-1-0098]; and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant UO1NS059344] (CounterACT Program).
Disclosures: Opinions or assertions contained herein are the private views of the authors and should not be construed as official or as reflecting the views of the U.S. Army, the Department of Defense or the federal government. The use of galantamine as an antidote against OP poisoning is protected under Albuquerque EX, Adler M, and Pereira EFR (2005) inventors; University of Maryland, Baltimore, Assignee. International Patent Application PCT/US05/33789. 2005 Sept 23.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.160028
- ACh
- Acetylcholine
- AChE
- acetylcholinesterase
- APL
- allosteric potentiating ligand
- BuChE
- butyrylcholinesterase
- ChE
- cholinesterase
- OP
- organophosphorus
- CNS
- central nervous system
- ANOVA
- analysis of variance
- NMDA
- N-methyl-d-aspartate.
References
- Albuquerque EX, Deshpande SS, Kawabuchi M, Aracava Y, Idriss M, Rickett DL, Boyne AF. (1985) Multiple actions of anticholinesterase agents on chemosensitive synapses: molecular basis for prophylaxis and treatment of organophosphate poisoning. Fundam Appl Toxicol 5:S182–S203 [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Aracava Y, Fawcett WP, Oliveira M, Randall WR, Hamilton TA, Kan RK, Romano JA, Jr, Adler M. (2006) Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proc Natl Acad Sci USA 103:13220–13225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Aracava Y, Pereira EF, Albuquerque EX. (2009) A single in vivo application of cholinesterase inhibitors has neuron type-specific effects on nicotinic receptor activity in guinea pig hippocampus. J Pharmacol Exp Ther 328:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashani Y, Peggins JO, III, Doctor BP. (1992) Mechanism of inhibition of cholinesterases by huperzine A. Biochem Biophys Res Commun 184:719–726 [DOI] [PubMed] [Google Scholar]
- Buckley NA, Karalliedde L, Dawson A, Senanayake N, Eddleston M. (2004) Where is the evidence for treatments used in pesticide poisoning? Is clinical toxicology fiddling while the developing world burns? J Toxicol Clin Toxicol 42:113–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey-Bloom J. (2003) Galantamine: a review of its use in Alzheimer's disease and vascular dementia. Int J Clin Pract 57:219–223 [PubMed] [Google Scholar]
- Darvesh S, Arora RC, Martin E, Magee D, Hopkins DA, Armour JA. (2004) Cholinesterase inhibitors modify the activity of intrinsic cardiac neurons. Exp Neurol 188:461–470 [DOI] [PubMed] [Google Scholar]
- Deshpande SS, Viana GB, Kauffman FC, Rickett DL, Albuquerque EX. (1986) Effectiveness of physostigmine as a pretreatment drug for protection of rats from organophosphate poisoning. Fundam Appl Toxicol 6:566–577 [PubMed] [Google Scholar]
- Dirnhuber P, French MC, Green DM, Leadbeater L, Stratton JA. (1979) The protection of primates against soman poisoning by pretreatment with pyridostigmine. J Pharm Pharmacol 31:295–299 [DOI] [PubMed] [Google Scholar]
- Doctor BP, Raveh L, Wolfe AD, Maxwell DM, Ashani Y. (1991) Enzymes as pretreatment drugs for organophosphate toxicity. Neurosci Biobehav Rev 15:123–128 [DOI] [PubMed] [Google Scholar]
- Filliat P, Baubichon D, Burckhart MF, Pernot-Marino I, Foquin A, Masqueliez C, Perrichon C, Carpentier P, Lallement G. (1999) Memory impairment after soman intoxication in rat: correlation with central neuropathology. Improvement with anticholinergic and antiglutamatergic therapeutics. Neurotoxicology 20:535–549 [PubMed] [Google Scholar]
- Fricke RF, Koplovitz I, Scharf BA, Rockwood GA, Olson CT, Hobson DW, Blank JA. (1994) Efficacy of tacrine as nerve agent pretreatment. Drug Chem Toxicol 17:15–34 [DOI] [PubMed] [Google Scholar]
- Haug KH, Myhrer T, Fonnum F. (2007) The combination of donepezil and procyclidine protects against soman-induced seizures in rats. Toxicol Appl Pharmacol 220:156–163 [DOI] [PubMed] [Google Scholar]
- Hilmas CJ, Poole MJ, Finneran K, Clark MG, Williams PT. (2009) Galantamine is a novel post-exposure therapeutic against lethal VX challenge. Toxicol Appl Pharmacol doi: 10.1016/j.taap.2009.07.029 [DOI] [PubMed] [Google Scholar]
- Inns RH, Leadbeater L. (1983) The efficacy of bispyridinium derivatives in the treatment of organophosphonate poisoning in the guinea-pig. J Pharm Pharmacol 35:427–433 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources ( 1996) Guide for the Care and Use of Laboratory Animals, 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Janowsky DS, Davis JM, Overstreet DH. (2005) Anticholinesterase (DFP) toxicity antagonism by chronic donepezil: a potential nerve agent treatment. Pharmacol Biochem Behav 81:917–922 [DOI] [PubMed] [Google Scholar]
- Jett DA, Fernando JC, Eldefrawi ME, Eldefrawi AT. (1994) Differential regulation of muscarinic receptor subtypes in rat brain regions by repeated injections of parathion. Toxicol Lett 73:33–41 [DOI] [PubMed] [Google Scholar]
- Jones DE, Carter WH, Jr, Carchman RA. (1985) Assessing pyridostigmine efficacy by response surface modeling. Fundam Appl Toxicol 5:S242–S251 [DOI] [PubMed] [Google Scholar]
- Karasova JZ, Bajgar J, Novotny L, Kuca K. (2009) Is a high dose of Huperzine A really suitable for pretreatment against high doses of soman? J Appl Biomed 7:93–99 [Google Scholar]
- Kassa J. (2002) Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol 40:803–816 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Matsuno K, Nakata K, Mita S. (1996) Enhancement of acetylcholine release by SA4503, a novel σ1 receptor agonist, in the rat brain. J Pharmacol Exp Ther 279:106–113 [PubMed] [Google Scholar]
- Lallement G, Veyret J, Masqueliez C, Aubriot S, Burckhart MF, Baubichon D. (1997) Efficacy of huperzine in preventing soman-induced seizures, neuropathological changes and lethality. Fundam Clin Pharmacol 11:387–394 [DOI] [PubMed] [Google Scholar]
- Maurice T, Meunier J, Feng B, Ieni J, Monaghan DT. (2006) Interaction with σ1 protein, but not N-methyl-d-aspartate receptor, is involved in the pharmacological activity of donepezil. J Pharmacol Exp Ther 317:606–614 [DOI] [PubMed] [Google Scholar]
- Maxwell DM, Brecht KM, Koplovitz I, Sweeney RE. (2006) Acetylcholinesterase inhibition: does it explain the toxicity of organophosphorus compounds? Arch Toxicol 80:756–760 [DOI] [PubMed] [Google Scholar]
- McLean MJ, Gupta RC, Dettbarn WD, Wamil AW. (1992) Prophylactic and therapeutic efficacy of memantine against seizures produced by soman in the rat. Toxicol Appl Pharmacol 112:95–103 [DOI] [PubMed] [Google Scholar]
- Monnet FP, Debonnel G, Junien JL, De Montigny C. (1990) N-Methyl-d-aspartate-induced neuronal activation is selectively modulated by σ receptors. Eur J Pharmacol 179:441–445 [DOI] [PubMed] [Google Scholar]
- Newmark J. (2007) Nerve agents. Neurologist 13:20–32 [DOI] [PubMed] [Google Scholar]
- Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Minami M, Omae KSarin Health Effects Study Group ( 2001) Effects of sarin on the nervous system in rescue team staff members and police officers 3 years after the Tokyo subway sarin attack. Environ Health Perspect 109:1169–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX. (2002) Unconventional ligands and modulators of nicotinic receptors. J Neurobiol 53:479–500 [DOI] [PubMed] [Google Scholar]
- Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL. (1997) Structure of acetylcholinesterase complexed with the nootropic alkaloid, (−)-huperzine A. Nat Struct Biol 4:57–63 [DOI] [PubMed] [Google Scholar]
- Romano JA, Jr, King JM. (2001) Psychological casualties resulting from chemical and biological weapons. Mil Med 166( Suppl 12): 21–22 [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH. (2003) Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol 188:69–80 [DOI] [PubMed] [Google Scholar]
- Silveira CL, Eldefrawi AT, Eldefrawi ME. (1990) Putative M2 muscarinic receptors of rat heart have high affinity for organophosphorus anticholinesterases. Toxicol Appl Pharmacol 103:474–481 [DOI] [PubMed] [Google Scholar]
- Solberg Y, Belkin M. (1997) The role of excitotoxicity in organophosphorous nerve agents central poisoning. Trends Pharmacol Sci 18:183–185 [DOI] [PubMed] [Google Scholar]
- Takada-Takatori Y, Kume T, Sugimoto M, Katsuki H, Niidome T, Sugimoto H, Fujii T, Okabe S, Akaike A. (2006) Neuroprotective effects of galanthamine and tacrine against glutamate neurotoxicity. Eur J Pharmacol 549:19–26 [DOI] [PubMed] [Google Scholar]
- Thomsen T, Kewitz H. (1990) Selective inhibition of human acetylcholinesterase by galanthamine in vitro and in vivo. Life Sci 46:1553–1558 [DOI] [PubMed] [Google Scholar]
- Tonduli LS, Testylier G, Masqueliez C, Lallement G, Monmaur P. (2001) Effects of Huperzine used as pre-treatment against soman-induced seizures. Neurotoxicology 22:29–37 [DOI] [PubMed] [Google Scholar]
- Tonkopii V. (2009) New approaches to treatment of poisoning by soman, in Counteraction to Chemical and Biological Terrorism in East European Countries. NATO Science for Peace and Security Series A: Chemistry and Biology ( Dishovsky C, Pivovarov A. eds), pp 117–121, Springer, Dordrecht, The Netherlands [Google Scholar]
- Tonkopii VD, Prozorovskii VB, Konstorum MG. (1975) A simple method of assessing competitive interaction of reversible inhibitors with cholinesterases. Biull Eksp Biol Med 80:993–995 [PubMed] [Google Scholar]
- Wang D, Noda Y, Zhou Y, Mouri A, Mizoguchi H, Nitta A, Chen W, Nabeshima T. (2007) The allosteric potentiation of nicotinic acetylcholine receptors by galantamine ameliorates the cognitive dysfunction in beta amyloid25–35 i.c.v.-injected mice: involvement of dopaminergic systems. Neuropsychopharmacology 32:1261–1271 [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. (1999) The pharmacology of donepezil: a new treatment of Alzheimer's disease. Expert Opin Pharmacother 1:121–135 [DOI] [PubMed] [Google Scholar]