Abstract
Agonist stimulation of the type 5 metabotropic glutamate (mGlu5) receptor initiates robust oscillatory changes in cytosolic Ca2+ concentration ([Ca2+]i) in single cells by rapid, repeated cycles of phosphorylation/dephosphorylation of the mGlu5 receptor, involving protein kinase C and as-yet-unspecified protein phosphatase activities. An emergent property of this type of Ca2+ oscillation-generating mechanism (termed “dynamic uncoupling”) is that once a threshold concentration has been reached to initiate the Ca2+ oscillation, its frequency is largely insensitive to further increases in orthosteric agonist concentration. Here, we report the effects of positive allosteric modulators (PAMs) on the patterns of single-cell Ca2+ signaling in recombinant and native mGlu5 receptor-expressing systems. In a Chinese hamster ovary cell-line (CHO-lac-mGlu5a), none of the mGlu5 receptor PAMs studied [3,3′-difluorobenzaldazine (DFB), N-{4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl) methyl]phenyl}-2-hydroxy-benzamide (CPPHA), 3-cyano-N-(1, 3-diphenyl-1H-prazol-5-yl)benzamide (CDPPB), S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]oxadiazol-5-yl]-piperidinl-1-yl}-methanone (ADX47273)], stimulated a Ca2+ response when applied alone, but each PAM concentration-dependently increased the frequency, without affecting the amplitude, of Ca2+ oscillations induced by glutamate or quisqualate. Therefore, PAMs can cause graded increases (and negative allosteric modulator-graded decreases) in the Ca2+ oscillation frequency stimulated by orthosteric agonist. Initial data in rat cerebrocortical astrocytes demonstrated that similar effects of PAMs could be observed in a native cell background, although at high orthosteric agonist concentrations, PAM addition could much more often be seen to drive rapid Ca2+ oscillations into peak-plateau responses. These data demonstrate that allosteric modulators can “tune” the Ca2+ oscillation frequency initiated by mGlu5 receptor activation, and this might allow pharmacological modification of the downstream processes (e.g., transcriptional regulation) that is unachievable through orthosteric ligand interactions.
Glutamate, the major excitatory neurotransmitter in the central nervous system, acts on ionotropic glutamate receptors to elicit fast excitatory responses and on metabotropic glutamate (mGlu) receptors to modulate and fine tune synaptic transmission (Conn and Pin, 1997). The eight subtypes of the mammalian mGlu receptor can be divided into three subgroups, based on sequence homologies, agonist and antagonist binding profiles, and preferred coupling to signal transduction pathways. The group I mGlu receptors, mGlu1 and mGlu5, both preferentially couple via Gq/11 proteins to stimulate phospholipase C activity but are differentially localized and probably fulfill distinct physiological functions (Hermans and Challiss, 2001; Mannaioni et al., 2001; Ferraguti and Shigemoto, 2006; Kumar et al., 2008). Activation of these receptors also elicits highly distinct Ca2+ responses at a single cell level, the mGlu1 receptor primarily eliciting a peak-plateau type of Ca2+ response, whereas mGlu5 receptor activation leads to oscillatory changes in intracellular Ca2+ concentration ([Ca2+]i) in both recombinant and native (e.g., astrocyte) cell backgrounds (Kawabata et al., 1996; Nakahara et al., 1997; Nash et al., 2001, 2002; Atkinson et al., 2006).
The robust oscillatory pattern of Ca2+ signaling initiated by the mGlu5 receptor has been proposed to be a result of a “dynamic uncoupling” mechanism involving rapid cycles of receptor phosphorylation and dephosphorylation (Kawabata et al., 1996), Ser-839 being implicated most recently as the site of reversible covalent modification (Kim et al., 2005). After agonist stimulation, the mGlu5 receptor is rapidly phosphorylated by protein kinase C, disabling productive receptor-G protein coupling (Kawabata et al., 1996; Uchino et al., 2004; Kim et al., 2005). A protein phosphatase, perhaps tightly associated with the receptor, efficiently dephosphorylates Ser-839, allowing reactivation of the receptor, and through the rapid enabling and disabling of receptor activity, a Ca2+ oscillation is generated (Nash et al., 2001, 2002; Atkinson et al., 2006). This mechanism is probably similar to that reported for Ca2+ oscillations initiated by Ca2+-sensing receptor activation (Young et al., 2002), but it is clearly different from the Ca2+-induced Ca2+ release mechanism proposed to explain the majority of Ca2+ oscillatory behaviors elicited by (submaximal) agonist stimulation of a variety of G protein-coupled receptors (Berridge et al., 2000).
An intriguing property of mGlu5 receptor-stimulated Ca2+ oscillations is that once a concentration of agonist [e.g., glutamate, quisqualate, (S)-3,5-dihydroxyphenylglycine] has been reached to initiate a response, both the frequency and amplitude of the Ca2+ oscillation is essentially insensitive to further increases in agonist concentration (i.e., an “all-or-nothing” response) (Nash et al., 2002). In contrast, altering the expression level of the mGlu5 receptor has marked effects on the frequency of the Ca2+ oscillation stimulated by agonist, and Ca2+ oscillation frequency can be reduced by addition of submaximally effective concentrations of the negative allosteric modulator (NAM) MPEP (1–100 nM) (Nash et al., 2002).
Positive allosteric modulators (PAMs) of the mGlu5 receptor are thought to be of potential clinical use in a variety of neurological and psychiatric disorders, including schizophrenia (Gasparini et al., 2002; Kew, 2004). The mGlu5 receptor is known to potentiate the function of N-methyl-d-aspartate receptors in various brain regions, and so PAMs, which bind to an allosteric site on the mGlu5 receptor and increase the response of the receptor to glutamate, may be able to counteract the N-methyl-d-aspartate receptor hypofunction proposed to be associated with this condition (Lindsley et al., 2006). Several PAMs acting at the mGlu5 receptor have been identified, including DFB (O'Brien et al., 2003), CPPHA (O'Brien et al., 2004), CDPPB (Kinney et al., 2005), and ADX47273 (Liu et al., 2008). Like MPEP, these PAMs bind to seven-transmembrane domain of the mGlu5 receptor to modulate the effects of orthosteric agonists (Pagano et al., 2000; Malherbe et al., 2003; Mühlemann et al., 2006). In addition, an MPEP analog, 5MPEP, antagonizes the actions of both NAMs and PAMs at the mGlu5 receptor and thus acts as a neutral allosteric site ligand (Rodriguez et al., 2005).
Considering the mechanism used by the mGlu5 receptor to generate Ca2+ oscillations and the unusual emergent pharmacology, we have systematically investigated the effects of PAMs on orthosteric mGlu5 receptor agonist-stimulated responses at a single cell level. These new data add an important new dimension to previous investigations of the pharmacological properties of PAMs at the mGlu5 receptor, which to date have relied on signaling readouts that quantify cell population responses.
Materials and Methods
Compounds.
l-Quisqualic acid, l-glutamic acid, 2-methyl-6-(phenylethynyl)-pyridine (MPEP) and 3,3′-difluorobenzaldazine (DFB) were obtained from Tocris Cookson Ltd. (Bristol, UK). N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA), 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl) benzamide (CDPPB), S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]oxadiazol-5-yl]-piperidinl-1-yl}-methanone (ADX47273), and 5-methyl-2-(phenylethynyl)pyridine (5MPEP) were synthesized in-house by GlaxoSmithKline (Harlow, UK). 2-(2-(3-methoxyphenyl)ethynyl)-5-methylpyridine (M-5MPEP) was a kind gift from Dr. P. J. Conn (Vanderbilt Program in Drug Discovery, Nashville, TN).
Cell Culture.
Chinese hamster ovary (CHO) cells expressing the human mGlu5a receptor under the control of a lac-repressor system (Hermans et al., 1998; Nash et al., 2002) were maintained in Dulbecco's modified Eagle's medium (DMEM) containing GlutaMAX-1 with sodium pyruvate, 4.5 g/l glucose, 10% fetal bovine serum (FBS), 44 μg/ml proline, 2.5 μg/ml amphotericin B, 105 units/ml penicillin, 100 μg/ml streptomycin, and 300 μg/ml G418. Once confluent, flasks of CHO-lac-mGlu5a cells were washed twice with phosphate-buffered saline (without Ca2+/Mg2+) and harvested with 0.25% (w/v) trypsin and 0.02% (w/v) EDTA. Cells were maintained at 37°C in a humidified 5% CO2:air atmosphere. For experiments, cells were seeded on to multiwell plates in medium with dialyzed FBS (substituted for FBS) and devoid of G418. mGlu5 receptor expression was induced by incubating CHO-lac-mGlu5a cells with 100 μM isopropyl-β-d-thiogalactoside/10 mM sodium butyrate for 24 h before experimentation.
Rat Cerebrocortical Astrocyte Preparation.
Wistar rats (1–2 days of age) were decapitated, and the cortices were removed. During the dissection, cortices were placed into ice-cold Earle's balanced salt solution (EBSS; Invitrogen, Carlsbad, CA), supplemented with 3.2 mM MgSO4, 0.3% (w/v) BSA (fraction V), and 16.7 mM glucose. Tissue was cut up into small pieces and incubated at 37°C for 15 min in 10 ml of EBSS solution containing 0.025% (w/v) (bovine pancreatic) trypsin with gentle agitation. After 15 min, 10-ml modified EBSS solution [containing 50 μM MgSO4, DNase I (type IV, 150 Kunitz units), and 0.02% (w/v) trypsin inhibitor] was added, and the suspension left to settle for 5 min. The supernatant was subsequently decanted, and 2.5 ml EBSS solution containing 320 μM MgSO4, DNase I (800 Kunitz units), and 0.12% (w/v) trypsin inhibitor was added. Tissue was slowly triturated using a glass fire-polished Pasteur pipette and 2.5 ml of EBSS solution [supplemented with 0.4% (w/v) BSA and 250 μM MgSO4] added. The cell suspension was centrifuged (1000 rpm; 8 min), and pellet resuspended in DMEM containing GlutaMAX-1 with sodium pyruvate, 4.5 mg/l glucose, 15% heat-inactivated FBS, 2.5 μg/ml Fungizone, and 0.1 μg/ml gentamicin. Cells were plated into poly-d-lysine-coated cell culture flasks and incubated at 37°C in a 5% CO2, humidified air atmosphere for 7 days, with medium being replaced after 4 days. At 7 DIV, medium was replaced again and flasks were transferred to a shaking incubator overnight (37°C; 320 rpm). On the following day (8 DIV), cells were washed twice with phosphate-buffered saline (without Ca2+/Mg2+) and harvested with 0.25% (w/v) trypsin and 0.02% (w/v) EDTA. Cells were subsequently seeded onto precoated poly-d-lysine tissue-culture plates for experiments. After 24 h (DIV 9), medium was replaced with DMEM containing GlutaMAX-1 with sodium pyruvate, 4.5 mg/l glucose, 2.5 μg/ml Fungizone, and 0.1 μg/ml gentamicin and G-5 supplement. Cells were used for experiments at DIV 11 to 13.
Single-Cell Intracellular Ca2+ Concentration Assay.
CHO-lac-mGlu5a cells were seeded onto 22-mm borosilicate coverslips and grown to approximately 80% confluence. Cells were loaded with Fura-2 acetoxymethyl ester (2 μM) in Krebs-Henseleit buffer (composition: 118 mM NaCl, 4.7 mM KCl, 4 mM NaHCO3, 1.3 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 11.7 mM glucose, and 8.5 mM HEPES, pH 7.4) containing 1 mg/ml bovine serum albumin for 60–90 min at room temperature. Coverslips of astrocyte (DIV 11–13) were incubated with Fura-2 acetoxymethyl ester (2 μM) similarly to the CHO-lac cells, except that the loading period was 40 min at room temperature. Coverslips were then transferred to the stage of an inverted epifluorescence microscope (Diaphot; Nikon, Tokyo, Japan) with an oil immersion objective (40×) and a SpectraMASTER II module (PerkinElmer Life and Analytical Sciences, Waltham, MA). Cells were excited at wavelengths of 340 and 380 nm using a SpectraMASTER II monochromator, and emission was recorded at wavelengths above 520 nm. The ratio of fluorescence intensities at these wavelengths is given as an index of [Ca2+]i. All experiments were performed at 37°C; drug additions were made via a perfusion line.
Cell Population [Ca2+]i Assay.
CHO-lac-mGlu5a cells were seeded onto 96-well black-walled cell culture plates (Costar; Corning Life Sciences, Lowell, MA) and induced on the following day with isopropyl β-d-thiogalactoside (100 μM) and sodium butyrate (10 mM) for 24 h before experimentation. Cells were loaded in Tyrode's solution containing the Ca2+ sensitive fluorescent dye, calcium-3 (Calcium 3 assay kit; Molecular Devices, Sunnyvale, CA), 1.5 mM CaCl2, and 2.5 mM probenecid for 1 h. Allosteric modulators were preincubated for 30 min before the addition of the agonist on the FLIPR. Changes in fluorescence intensity are recorded as an index of [Ca2+]i.
Data Analysis.
Concentration-response relationships were analyzed by nonlinear regression using Prism 5.0 software (GraphPad Software, San Diego, CA). For statistical tests, where only two datasets were being compared an unpaired Student's t test (two-tailed) was used, where P < 0.05 was deemed statistically significant. Where more than two datasets were compared, one- or two-way analysis of variance (ANOVA) tests were used with P < 0.05 being accepted as significantly different. ANOVA tests were followed by the Bonferroni's post hoc test. All statistical analyses were performed using Prism 5.0 software.
Results
Effects of Positive Allosteric Modulators on Ca2+ Oscillation Frequency.
Each of the mGlu5 receptor PAMs studied, DFB, CPPHA, CDPPB, or ADX47273, caused significant (2–3-fold) increases in the frequency (but not the amplitude) of Ca2+ oscillations initiated by either glutamate or quisqualate in CHO-lac-mGlu5 cells (Table 1). Representative single cell traces are shown for the effects of DFB (100 μM), CPPHA (3 μM), CDPPB (10 μM), and ADX47273 (10 μM) on glutamate- and quisqualate-stimulated Ca2+ oscillations (Fig. 1). To further explore how PAMs affect the Ca2+ oscillation frequency initiated by an orthosteric agonist, we stimulated CHO-lac-mGlu5a cells with either glutamate (100 μM) or quisqualate (10 μM) and then coadded increasing concentrations of CDPPB or ADX47273 (0.01–10 μM). Analysis of these data revealed that the PAMs caused concentration-dependent increases in orthosteric agonist-stimulated Ca2+ oscillation frequency [pEC50 (M) values: for CDPPB, 6.46 ± 0.26 (+glutamate; Fig. 2B) and 6.95 ± 0.27 (+quisqualate; Fig. 2D); for ADX47273, 6.33 ± 0.13 (+ glutamate; Fig. 2F) and 6.66 ± 0.19 (+ quisqualate; Fig. 2H)].
TABLE 1.
Effects of PAMs on the frequency of the Ca2+ oscillatory response stimulated by either l-glutamate or quisqualate in CHO-lac-mGlu5a cells
CHO-lac-mGlu5a cells were stimulated with either l-glutamate (100 μM) or quisqualate (30 μM) for 300 s and then by the same concentration of orthosteric agonist plus the indicated concentration of DFB, CPPHA, CDPPB, or ADX47273. At least 20 cells were analyzed from each coverslip, and each experiment was repeated on at least 3 separate days to give the summary data shown (mean ± S.E.M.). Data are shown for the number of oscillations in a 5-min period. Data were analyzed using two-way ANOVA.
| +Glutamate | +Quisqualate | |
|---|---|---|
| DFB (100 μM) | ||
| − | 3.1 ± 0.3 | 4.3 ± 0.5 |
| + | 11.1 ± 1.4*** | 10.9 ± 0.9*** |
| CPPHA (3 μM) | ||
| − | 5.1 ± 0.7 | 5.1 ± 0.5 |
| + | 8.4 ± 1.1*** | 10.1 ± 0.8*** |
| CDPPB (10 μM) | ||
| − | 5.2 ± 0.5 | 4.2 ± 0.7 |
| + | 12.7 ± 0.9*** | 14.8 ± 1.1*** |
| ADX47273 (10 μM) | ||
| − | 5.0 ± 0.4 | 5.7 ± 0.5 |
| + | 13.4 ± 0.7*** | 14.4 ± 0.8*** |
P < 0.001, statistically significant increases in oscillation frequency in the presence versus the absence of PAM.
Fig. 1.
Effects of the PAMs DFB, CPPHA, CDPPB, and ADX47273 on the frequency of Ca2+ oscillations in CHO-lac-mGlu5a cells. Representative traces showing the response of single CHO-lac-mGlu5a cells to perfusion with l-glutamate (100 μM; A, C, E, and G) or quisqualate (30 μM; B, D, F, and H) for 5 min followed immediately by the same concentration of agonist plus DFB (100 μM; A and B), CPPHA (3 μM; C and D), CDPPB (10 μM; E and F), or ADX47273 (10 μM; G and H) perfused for a further 5 min. Data are representative of at least 50 individual cells recorded over at least 3 separate days.
Fig. 2.
Concentration-dependent effects of CDPPB and ADX47273 on the frequency of Ca2+ oscillations stimulated by l-glutamate or quisqualate in CHO-lac-mGlu5a cells. Representative traces showing the effects of increasing concentrations of CDPPB (0.01, 0.1, 1, 10 μM; A and C) or ADX47273 (0.01, 0.1, 1, 10 μM; E and G) on Ca2+ oscillations elicited by glutamate (100 μM; A and E) or quisqualate (30 μM; C and G). Concentration-response curves showing the mean number of oscillations per minute when cells were stimulated with glutamate (100 μM; B and F) or quisqualate (30 μM; D and H) plus increasing concentrations of CDPPB (B and D) or ADX47273 (F and H). Data are shown as means ± S.E.M. from 25 individual cells recorded over 4 separate days. Mean pEC50 (M) values for facilitation of glutamate and quisqualate responses were 6.46 ± 0.26 and 6.95 ± 0.27 for CDPPB and 6.32 ± 0.26 and 6.71 ± 0.39 for ADX47273, respectively.
Effects of PAMs on the Threshold for Glutamate-Evoked Ca2+ Oscillations.
After mGlu5 receptor induction (isopropyl β-d-thiogalactoside/butyrate addition for 24 h), Ca2+ oscillations were observed in the vast majority of Fura-2-loaded CHO-lac-mGlu5a cells challenged with either l-glutamate or quisqualate. In the presence of 1 μM glutamate, only a small number of cells (<15%) responded; however, increasing the glutamate concentration to 3 μM initiated baseline Ca2+ oscillations in most cells (Fig. 3, A and C) and the amplitude and frequency of the Ca2+ oscillation was not significantly altered by further increases in the concentration of glutamate (3–100 μM). Similar concentration-independent effects were observed for quisqualate, where 0.1 μM quisqualate was sufficient to initiate Ca2+ oscillations in the majority of CHO-lac-mGlu5a cells, and further increases in quisqualate concentration (0.3–10 μM) did not significantly alter either the amplitude or frequency of the agonist-stimulated Ca2+ oscillation (data not shown). We have also evaluated the effects of DFB (30 μM) on the concentration of glutamate (threshold) required to evoke Ca2+ oscillations (Fig. 3B). The presence of DFB left-shifted the threshold for the stimulation of a Ca2+ oscillation by the orthosteric agonist (glutamate or quisqualate), as well as increasing the maximal oscillation frequency achieved (Fig. 3, B and C). It should also be noted that in the presence of the PAM, it is possible to discern a more graded increase in Ca2+ oscillation frequency compared with the steep, all-or-nothing glutamate concentration-response curve seen in the absence of an allosteric ligand (see Fig. 3; Nash et al., 2002).
Fig. 3.
Effects of DFB on the threshold for glutamate evoked on Ca2+ oscillations in CHO-lac-mGlu5a cells. A, representative trace showing the effect of stimulating cells with increasing concentrations of glutamate (each concentration applied for 3 min). B, a representative trace showing responses to increasing glutamate concentrations in the presence of DFB (30 μM). C, mean data showing the changes in oscillation frequency that occur when cells were stimulated with increasing concentrations of glutamate in the absence or presence of DFB (30 μM). Data are shown as means ± S.E.M. for at least 25 individual cells over at least three experiments.
Effects of PAMs on Single-Cell Ca2+ Responses.
Although this study focuses on Ca2+ oscillatory responses, other patterns of change in [Ca2+]i can be observed in individual CHO-lac-mGlu5a cells after agonist addition. We have undertaken analyses of Ca2+ signaling patterns in CHO-lac-mGlu5a (and CHO-lac-mGlu1a) cells previously (Atkinson et al., 2006); in that study, we classified responses into four categories [nonresponders (NR); single peak (SP), oscillatory (OS), and peak-and-plateau (PP)]. Here, we undertook similar analyses to assess how PAMs alter the occurrence of the different categories of Ca2+ response. Representative data are shown for ADX47273 effects on glutamate- and quisqualate-stimulated Ca2+ signaling patterns (Fig. 4). In the absence of the PAM, orthosteric agonists stimulate a Ca2+ oscillatory response in the majority (>70%) of cells, and essentially no cells show peak-and-plateau responses. After coaddition of orthosteric agonist plus ADX47273, the number of cells that are either NR or respond with only an SP diminish toward zero, whereas the number of cells driven into a PP response increases somewhat (to ∼10–20% of all cells analyzed; Fig. 4). It is noteworthy that in both the absence and presence of the PAM, Ca2+ oscillations are the predominant response seen after orthosteric agonist addition to CHO-lac-mGlu5a cells.
Fig. 4.
Effects of the positive allosteric modulator, ADX47273, on orthosteric agonist-stimulated Ca2+ responses in CHO-lac-mGlu5a cells. Data show the percentage of the total number of cells analyzed that gave an NR, SP, OS, or PP response when stimulated with l-glutamate (100 μM; A) or quisqualate (30 μM; B) in the absence and presence of ADX47273 (10 μM). Data are shown as means ± S.E.M. from at least 50 individual cells recorded over 4 separate days. Criteria for classification of cell responses into NR, SP, OS, and PP subgroups were identical to those defined by Atkinson et al. (2006).
PAMs at the mGlu5 Receptor Are Allosteric Modulators Devoid of Intrinsic Activity.
None of the PAMs studied here stimulated a Ca2+ response per se and required the presence of orthosteric agonist to exert their positive modulator effect (Fig. 5). It should be noted that the medium over the CHO-lac-mGlu5a cells was rapidly exchanging throughout the time course (perfusion rate, 5 ml/min); if the perfusion rate was slowed or stopped, then a Ca2+ oscillatory response was quickly initiated (within a few seconds) in the presence of PAM only (data not shown). It is likely that CHO-lac-mGlu5a cells release glutamate into the medium and in static incubation systems the accumulation of glutamate is sufficient to synergize with the PAM to produce a Ca2+ response.
Fig. 5.
The positive allosteric modulators DFB, CPPHA, CDPPB, and ADX47273 possess no intrinsic agonist activity in the absence of orthosteric stimulation. Maximal concentrations of DFB (100 μM; A), CPPHA (3 μM; B), CDPPB (10 μM; C), and ADX47273 (10 μM; D) were perfused on to CHO-lac-mGlu5a cells for 5 min, followed by simultaneous perfusion of quisqualate (30 μM) plus each respective modulator. Traces are representative from at least 20 individual cells recorded over 3 separate days.
Neutral Allosteric Modulator Effects on Orthosteric/Allosteric Interactions to Regulate [Ca2+]i.
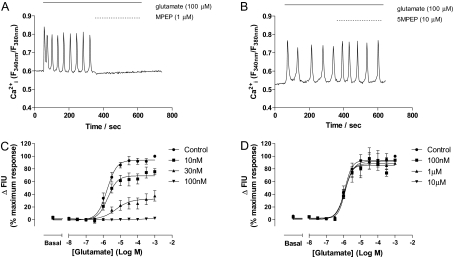
At a sufficiently high concentration (100 nM), the NAM MPEP is able to completely abolish orthosteric agonist-stimulated Ca2+ oscillations in CHO-lac-mGlu5a cells (Fig. 6A) (Nash et al., 2002), whereas the neutral allosteric modulator 5MPEP (10 μM; Rodriguez et al., 2005) was without effect (Fig. 6B). The effects of increasing concentrations of MPEP (here shown for CHO-lac-mGlu5a cells using FLIPR technology to assess population Ca2+ responses) on the glutamate concentration-response curve illustrate the noncompetitive nature of the interaction, increasing MPEP concentrations causing a progressive suppression of the maximal response with no significant effect on the glutamate EC50 value (Fig. 6C). In contrast, 5MPEP was without effect on the glutamate concentration-response curve (Fig. 6D).
Fig. 6.
Comparison of effects of MPEP and 5MPEP on Ca2+ responses in single and populations of CHO-lac-mGlu5a cells. Representative traces showing the effects of MPEP (100 nM; A) or 5MPEP (30 μM; B) on Ca2+ oscillations elicited by glutamate (100 μM) in CHO-lac-mGlu5a cells. Traces shown are representative of at least 50 cells recorded over 3 separate days. FLIPR cell population responses for glutamate-stimulated Ca2+ concentration-response curves performed in the absence or presence of 10, 30, or 100 nM MPEP (C) or 0.1, 1, or 10 μM 5MPEP (D). MPEP and 5MPEP were added 30 min before challenge with glutamate at the concentrations indicated. Data are shown as means ± S.E.M. for three separate experiments performed in duplicate.
To explore further the mechanism of action of the PAMs, we investigated whether their effects on orthosteric agonist-stimulated Ca2+ oscillatory responses are sensitive to the neutral allosteric antagonist 5MPEP. A representative trace illustrating the experimental design is shown in Fig. 7A. Addition of 5MPEP (10 μM), after sequential additions of glutamate (100 μM) and glutamate-plus-ADX47273 (10 μM), caused a complete ablation of the PAM-mediated frequency increase in the glutamate-stimulated Ca2+ oscillation. Figure 7 also shows that 5MPEP could completely reverse the positive modulator effect of ADX47273, DFB, or CDPPB (Fig. 7, B–D). In contrast, the positive modulator effect of CPPHA on glutamate-stimulated Ca2+ oscillatory responses in CHO-lac-mGlu5a cells was unaffected by 5MPEP (Fig. 8).
Fig. 7.
5MPEP abolishes the positive modulatory effects of ADX47273, DFB, or CDPPB on orthosteric agonist-stimulated Ca2+ oscillation frequency in CHO-lac-mGlu5a cells. Cells were perfused with glutamate (100 μM) for 5 min, followed by glutamate (100 μM) plus PAM for 5 min, and then glutamate (100 μM), PAM and 5MPEP (30 μM) (A). Perfusion periods with glutamate ± PAM ± 5MPEP followed on from each other without any washout between additions. A representative trace showing the effects of 5MPEP on the Ca2+ oscillation frequency elicited by glutamate (100 μM) plus ADX47273 (10 μM) is shown (A). Mean data for each PAM are also shown: ADX47273 (10 μM; B), DFB (100 μM; C), and CDPPB (10 μM; D). Histograms show means ± S.E.M. for 20 individual cells recorded over 4 separate days, with statistically significant differences (∗∗∗, P < 0.001) determined by one-way ANOVA.
Fig. 8.
5MPEP does not block the positive modulatory effect of CPPHA on orthosteric agonist-stimulated Ca2+ oscillation frequency in CHO-lac-mGlu5a cells. Cells were perfused with glutamate (100 μM) for 5 min, followed by glutamate (100 μM) plus CPPHA (3 μM) for 5 min, and then glutamate (100 μM), CPPHA (3 μM), and 5MPEP (30 μM) (A). Perfusion periods with glutamate ± CPPHA ± 5MPEP followed on from each other without any washout between additions. A representative trace showing the effects of 5MPEP on the Ca2+ oscillation frequency elicited by glutamate plus CPPHA is shown (A), whereas B presents mean data. Data are shown as means ± S.E.M. for 35 individual cells recorded over 7 separate days, with statistically significant differences (∗∗∗, P < 0.001) determined by one-way ANOVA.
A comparison of the effects of a positive (ADX47273), negative (MPEP), and neutral (5MPEP) allosteric modulator on orthosteric agonist-stimulated Ca2+ oscillation frequency in CHO-lac-mGlu5a cells is shown in Fig. 9. In addition, we have found that the previously reported mGlu5 receptor allosteric partial inverse agonist, M-5MPEP (Rodriguez et al., 2005), also causes a concentration-dependent decrease in the glutamate-evoked Ca2+ oscillations. Although this compound exhibited a lower potency with respect to inhibiting the glutamate-stimulated Ca2+ response, at a sufficiently high concentration (10 μM), M-5MPEP displayed a negative efficacy approaching that of MPEP (Fig. 9).
Fig. 9.
Allosteric modulator site pharmacology at the mGlu5 receptor. Concentration-dependent effects of ADX47273, MPEP, 5MPEP, or M-5MPEP on glutamate (100 μM) evoked Ca2+ oscillations in CHO-lac-mGlu5a cells are summarized [pEC50/IC50 (M) values: ADX47273, 6.33 ± 0.13; MPEP, 7.69 ± 0.14 M; M-5MPEP, 6.26 ± 0.21]. Data are shown are means ± S.E.M. for at least 25 cells recorded over at least 3 separate days. Note that ordinate values shown are normalized to the oscillation frequency evoked by stimulation with glutamate (100 μM) alone.
Allosteric Modulator Effects on Glutamate-Stimulated Ca2+ Responses in Astrocytes.
Addition of glutamate (100 μM) to G5-differentiated rat cerebrocortical astrocytes initiated Ca2+ oscillations that were typically of a higher frequency (≥2 oscillations/min) than observed in the CHO-lac-mGlu5a cells and occurred on a raised baseline (Fig. 10A). Addition of increasing concentrations of MPEP (0.01–1 μM) initially reduced oscillation frequency and then completely suppressed orthosteric agonist-evoked oscillations (Fig. 10, A and B). The potency of the MPEP-evoked suppression was pharmacologically indistinguishable from that seen previously in CHO-lac-mGlu5a cells (pIC50 ≈8 M; Fig. 10B). Likewise, the glutamate-stimulated Ca2+ oscillation was completely unaffected by the neutral allosteric modulator 5MPEP (Fig. 10C).
Fig. 10.
Modulatory effects of MPEP and 5MPEP on l-glutamate-stimulated Ca2+ oscillations in rat cerebrocortical astrocytes. Representative trace (A) showing the pattern of Ca2+ oscillations evoked by glutamate (100 μM) and its attenuation by coaddition of increasing concentrations of MPEP (0.01–0.3 μM). Summary data are shown (B) comparing the effects of MPEP on glutamate-stimulated Ca2+ oscillation frequency in astrocytes and CHO-lac-mGlu5a cells. Data are shown as means ± S.E.M. for at least 25 individual cells over at least three separate experiments. The lack of effect of 5MPEP (10 μM) on glutamate-stimulated Ca2+ oscillations in astrocytes is also illustrated by a representative trace (C).
Similar to their effects in CHO-lac-mGlu5a cells, the PAMs investigated here possessed no intrinsic agonist activity in cerebrocortical astrocytes (data for CDPPB shown; Fig. 11A) but were able to reduce the threshold for orthosteric agonist-evoked Ca2+ responses in astrocytes. Thus, when perfused alone, glutamate (0.3 μM) did not evoke a Ca2+ response in the vast majority of astrocytes; however, the presence of CDPPB (10 μM) resulted in the observation of Ca2+ oscillations in the majority of cells (Fig. 11, C and D).
Fig. 11.
Effects of the mGlu5 receptor PAM CDPPB on glutamate-stimulated Ca2+ responses in rat cerebrocortical astrocytes. Under the experimental conditions used here [rapidly perfused (5 ml/min) cells on coverslips], addition of CDPPB (10 μM) alone did not evoke a Ca2+ response, in contrast to addition of glutamate (100 μM) (A). Representative traces are also shown illustrating the effect of CDPPB (10 μM) on Ca2+ responses evoked by a range of glutamate concentrations (C, 0.3 μM; E, 1 μM; G, 3 μM; I, 10 μM; K, 100 μM). Mean data are shown as the percentage of the total number of cells analyzed that gave NR, SP, OS, or PP response for each condition. These latter data are determined from analysis of least 50 individual astrocytes over at least three separate experiments. Note that CDPPB reduced the threshold for glutamate-stimulated Ca2+ oscillations (C), and although this PAM increased oscillation frequency at low orthosteric agonist concentrations (E, F and G, H), it transformed the response from oscillatory to peak-plateau at high agonist concentrations (I, J and K, L).
PAMs increased the Ca2+ oscillation frequency stimulated by submaximal concentrations of orthosteric agonist (Fig. 11, G–J), whereas at maximally effective concentrations of glutamate, which already caused rapid Ca2+ oscillations, addition of the PAM most often caused glutamate-mediated Ca2+ oscillations to transition into sustained peak-plateau Ca2+ responses (Fig. 11, I–L). These data suggest that above a certain oscillation frequency (2–3 per minute), PAMs can drive orthosteric agonist-stimulated Ca2+ oscillatory responses into peak-plateau responses.
Discussion
In the present study, we have compared and contrasted the actions of orthosteric and allosteric ligands at the mGlu5 receptor using an assay readout that allows single cell responses to be evaluated. In contrast to previous studies conducted in cell populations, these new data indicate that PAMs can uniquely affect mGlu5 receptor-mediated signal transduction in ways not achievable by orthosteric agonists alone.
Stimulation of the mGlu5 receptor has been shown to elicit robust intracellular Ca2+ oscillations in astrocytes and neurons (Nakahara et al., 1997; Flint et al., 1999; D'Ascenzo et al., 2007) as well as recombinant model systems, such as the CHO-lac-mGlu5 cell-line used here (Nash et al., 2002; Atkinson et al., 2006). Considerable evidence has accrued to support the idea that this oscillatory pattern of Ca2+ signaling is brought about by a process termed “dynamic uncoupling,” which involves repetitive cycles of phosphorylation and dephosphorylation of a key residue (Ser-839) in the proximal C-terminal domain of the mGlu5 receptor, which uncouple and restore signal transduction, respectively, from mGlu5 receptor to G protein (Kawabata et al., 1996; Nash et al., 2001, 2002; Uchino et al., 2004; Kim et al., 2005). An interesting emergent pharmacological property of Ca2+ oscillations generated by the dynamic uncoupling mechanism is that once an orthosteric agonist concentration sufficient to initiate receptor-driven Ca2+ signaling has been reached, then the frequency and amplitude of the oscillatory signal changes little over a broad agonist concentration range. This “hard wiring” of the mGlu5 receptor signaling output at a single cell level might be of physiological importance, in that it should allow an invariant signal to be maintained over a wide range of glutamate concentrations.
Here we report that four previously described mGlu5 receptor-selective PAMs (DFB, CPPHA, CDPPB, and ADX47273) all significantly increase the frequency (not the amplitude) of glutamate- or quisqualate-stimulated Ca2+ oscillations in single CHO-lac-mGlu5 cells compared with stimulation with orthosteric agonist alone. None of the PAMs elicited a Ca2+ response when applied to the cells in the absence of orthosteric agonist, confirming initial reports for each of the compounds that they are true allosteric modulators possessing no intrinsic agonist activity (O'Brien et al., 2003, 2004; Kinney et al., 2005; Le Poul et al., 2005). At maximally effective concentrations in CHO-lac-mGlu5 cells, the PAMs caused 2- to 3-fold increases in the Ca2+ oscillation frequency stimulated by a maximally effective orthosteric agonist concentration. In rat cerebrocortical astrocytes, the Ca2+ oscillation frequencies observed in response to orthosteric agonist concentration were generally higher, and PAM addition could either increase Ca2+ oscillation frequency further or drive the cell into a peak-plateau response (see Fig. 11), suggesting that above a certain frequency (∼3 Ca2+ oscillations/min) dynamic uncoupling transitions to a different Ca2+ signature in this cell background.
In CHO-lac-mGlu5 cells, CDPPB and ADX47273 caused concentration-dependent changes in the Ca2+ oscillatory frequency stimulated by orthosteric agonist. The EC50 values obtained for PAM effects (l-Glu + CDPPB = 0.35 μM; l-Glu + ADX47273 = 0.47 μM; Fig. 5) were intermediate between affinity estimates determined in radioligand binding assays (e.g., Ki = 4.3 μM for ADX47273 displacing [3H]MPEP binding) (Lui et al., 2008) and functional (potentiation of Ca2+ release) assays (e.g., EC50 = 0.045 μM for CDPPB potentiation of the response to an EC20 concentration of l-Glu) (Chen et al., 2007). Furthermore, PAMs “sensitize” the mGlu5 receptor to changes in orthosteric agonist, reducing the threshold concentration of l-glutamate needed to elicit a regenerative Ca2+ oscillatory response. This was demonstrated here for DFB in CHO-lac-mGlu5 cells (Fig. 3) and for CDPPB in rat cerebrocortical astrocytes (Fig. 11). A striking feature of this sensitization is that in the presence of a PAM, Ca2+ oscillations are observed in astrocytes in the presence of extracellular l-glutamate concentrations (0.3 μM; see Fig. 11B) approaching those considered to be below that required to exert excitatory actions in the CNS (Herman and Jahr, 2007). These data suggest that mGlu5 receptor PAMs might trigger sustained Ca2+-dependent signaling at low ambient concentrations of the endogenous transmitter, as well as when extracellular glutamate is elevated by normal exocytotic release.
Therefore, mGlu5 receptor PAMs increase (and NAMs decrease; Nash et al., 2002), the Ca2+ oscillation frequency stimulated by an orthosteric agonist. This pharmacological alteration in the “hard-wired” single cell Ca2+ oscillation frequency recapitulates the effects of increasing or decreasing mGlu5 receptor expression levels (through altering induction conditions in the CHO-lac-mGlu5 cell-line) (Nash et al., 2002).
The binding sites for each of the mGlu5 PAMs have previously been characterized by other groups using site-directed mutagenesis and radioligand binding approaches. Thus, DFB and CDPPB competitively displaced binding of [3H]MPEP (or a similarly radiolabeled MPEP analog) to the mGlu5 receptor and, in the case of CDPPB, this displacement was shown to be unaltered in the presence of glutamate (O'Brien et al., 2003; Kinney et al., 2005). In addition, mutation of Ala-809 in transmembrane domain 7 of the mGlu5 receptor leads to loss of MPEP and CDPPB binding (Chen et al., 2007). ADX47273 has also been reported to bind to the MPEP site (Le Poul et al., 2005; Liu et al., 2008). In contrast, CPPHA did not displace [3H]MPEP binding to the mGlu5 receptor, and although mutation of alanine-809 had no effect on its PAM activity, mutation of phenylalanine-585 in transmembrane domain 1 of the mGlu5 receptor led to loss of the positive modulatory effect of this compound (Zhao et al., 2007; Chen et al., 2008). Taken together, these previous data indicate that DFB, CDPPB, and ADX47273 exert their PAM effects by binding to the same or an overlapping site to the NAM, MPEP, but CPPHA binds to a distinct allosteric site (Conn et al., 2009). Here, we used the mGlu5 receptor neutral allosteric modulator 5MPEP to investigate this at a single-cell level. 5MPEP binds to the MPEP site and has been shown to block both the effects of the NAM MPEP and the PAMs DFB and CDPPB (Rodriguez et al., 2005). Our initial experiments confirmed that 5MPEP had no effect on glutamate-stimulated Ca2+ responses in both single-cell and cell-population assays. Our work clearly demonstrated that the increase in orthosteric agonist-induced Ca2+ oscillation frequency caused by DFB, CDPPB, and ADX47273 was completely inhibited in the presence of 5MPEP, such that the frequency of Ca2+ oscillations was reduced to that stimulated by the orthosteric agonist alone. These data provide confirmatory evidence at a single-cell level that DFB, CDPPB, and ADX47273 all exert their modulatory activities via the MPEP/5MPEP binding site on the mGlu5 receptor. In contrast, the modulatory activity of CPPHA on orthosteric agonist-stimulated Ca2+ oscillation frequency was completely unaffected by the presence of 5MPEP, indicating that this PAM interacts with a distinct allosteric binding site on the mGlu5 receptor. Despite this difference in the locus of interaction of CPPHA, the activity of this PAM with respect to the modulation of glutamate- and quisqualate-stimulated Ca2+ responses was indistinguishable from that of the other PAMs at a single-cell level. This contrasts with previous reports that allosteric potentiation at distinct sites of the mGlu5 receptor can result in differential effects downstream in rat cortical astrocytes (Zhang et al., 2005).
This present work has clearly demonstrated that the frequency of Ca2+ oscillations initiated by glutamate, or another mGlu5 receptor orthosteric agonist, can be concentration-dependently altered by the addition of either PAMs or NAMs in a recombinant mGlu5a receptor-expressing cell line and rat cortical astrocytes. These findings have required the study of Ca2+ signaling behaviors using single-cell assays and suggest that at this level, PAMs and NAMs primarily mediate frequency rather than the amplitude modulation of the Ca2+ signal. Alteration of the Ca2+ oscillation frequency can have a number of (patho)physiological consequences, because changes in the frequency of Ca2+ oscillations have previously been shown to influence the activation of specific Ca2+-dependent enzymes (De Koninck and Schulman, 1998), the synthesis and release of various gliotransmitters (Agulhon et al., 2008) and growth factors (Jean et al., 2008), and gene transcriptional activation patterns (Dolmetsch et al., 1998; Tomida et al., 2003). Therefore, the ability of mGlu5 receptor PAMs to alter the frequency of Ca2+ oscillations in single cells has the potential to alter fundamentally the cell's interpretation of the signal initiated at the cell surface. This will require a reevaluation of how allosteric modulators are to be used to manipulate mGlu5 receptor signaling in a variety of central nervous system disorders, including schizophrenia (Conn et al., 2009).
Acknowledgments
We gratefully acknowledge the contributions of Dr. Martyn Wood, Katherine Cato, and Claire Howes (GlaxoSmithKline, Harlow, UK) at different stages in this project.
This work was supported by the Biotechnology and Biological Sciences Research Council by a CASE PhD studentship (to S.J.B.).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.059170
- mGlu
- metabotropic glutamate
- NAM
- negative allosteric modulator
- MPEP
- 2-methyl-6-(phenylethynyl)-pyridine
- PAM
- positive allosteric modulator
- DFB
- 3,3′-difluorobenzaldazine
- CPPHA
- N-{4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide
- CDPPB
- 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide
- ADX47273
- S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]oxadiazol-5-yl]-piperidinl-1-yl}-methanone
- 5MPEP
- 5-methyl-2-(phenylethynyl)pyridine
- M-5MPEP
- 2-(2-(3-methoxyphenyl)ethynyl)-5-methylpyridine
- CHO
- Chinese hamster ovary
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- EBSS
- Earle's balanced salt solution
- DIV
- day(s) in vitro
- ANOVA
- analysis of variance
- FLIPR
- fluorometric imaging plate reader
- NR
- nonresponder
- SP
- single peak
- OS
- oscillatory
- PP
- peak-and-plateau.
References
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. (2008) What is the role of astrocyte calcium in neurophysiology? Neuron 59:932–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson PJ, Young KW, Ennion SJ, Kew JN, Nahorski SR, Challiss RA. (2006) Altered expression of Gq/11α protein shapes mGlu1 and mGlu5 receptor-mediated single cell inositol 1,4,5-trisphosphate and Ca2+ signaling. Mol Pharmacol 69:174–184 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21 [DOI] [PubMed] [Google Scholar]
- Chen Y, Nong Y, Goudet C, Hemstapat K, de Paulis T, Pin JP, Conn PJ. (2007) Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol Pharmacol 71:1389–1398 [DOI] [PubMed] [Google Scholar]
- Chen Y, Goudet C, Pin JP, Conn PJ. (2008) N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol 73:909–918 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. (2009) Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci 30:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. (2007) mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A 104:1995–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. (1998) Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279:227–230 [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. (1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392:933–936 [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. (2006) Metabotropic glutamate receptors. Cell Tissue Res 326:483–504 [DOI] [PubMed] [Google Scholar]
- Flint AC, Dammerman RS, Kriegstein AR. (1999) Endogenous activation of metabotropic glutamate receptors in neocortical development causes neuronal calcium oscillations. Proc Natl Acad Sci U S A 96:12144–12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Kuhn R, Pin JP. (2002) Allosteric modulators of group I metabotropic glutamate receptors: novel subtype-selective ligands and therapeutic perspectives. Curr Opin Pharmacol 2:43–49 [DOI] [PubMed] [Google Scholar]
- Herman MA, Jahr CE. (2007) Extracellular glutamate concentration in hippocampal slice. J Neurosci 27:9736–9741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E, Challiss RA. (2001) Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J 359:465–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E, Young KW, Challiss RA, Nahorski SR. (1998) Effects of human type 1α metabotropic glutamate receptor expression level on phosphoinositide and Ca2+ signalling in an inducible cell expression system. J Neurochem 70:1772–1775 [DOI] [PubMed] [Google Scholar]
- Jean YY, Lercher LD, Dreyfus CF. (2008) Glutamate elicits release of BDNF from basal forebrain astrocytes in a process dependent on metabotropic receptors and the PLC pathway. Neuron Glia Biol 4:35–42 [DOI] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M. (1996) Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature 383:89–92 [DOI] [PubMed] [Google Scholar]
- Kew JN. (2004) Positive and negative allosteric modulation of metabotropic glutamate receptors: emerging therapeutic potential. Pharmacol Ther 104:233–244 [DOI] [PubMed] [Google Scholar]
- Kim CH, Braud S, Isaac JT, Roche KW. (2005) Protein kinase C phosphorylation of the metabotropic glutamate receptor mGluR5 on Serine 839 regulates Ca2+ oscillations. J Biol Chem 280:25409–25415 [DOI] [PubMed] [Google Scholar]
- Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, et al. (2005) A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther 313:199–206 [DOI] [PubMed] [Google Scholar]
- Kumar V, Jong YJ, O'Malley KL. (2008) Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ release. J Biol Chem 283:14072–14083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E, Bessis AS, Lutjens R, Bonnet B, Rocher JP, Epping-Jordan M, Mutel V. (2005) In vitro pharmacological characterisation of selective mGluR5 allosteric modulators (Abstract). Neuropharmacology 49:252 [Google Scholar]
- Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr., Sur C, Kinney GG. (2006) Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem 6:771–785 [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, et al. (2008) ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther 327:827–839 [DOI] [PubMed] [Google Scholar]
- Malherbe P, Kratochwil N, Zenner MT, Piussi J, Diener C, Kratzeisen C, Fischer C, Porter RH. (2003) Mutational analysis and molecular modeling of the binding pocket of the metabotropic glutamate 5 receptor negative modulator 2-methyl-6-(phenylethynyl)-pyridine. Mol Pharmacol 64:823–832 [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. (2001) Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci 21:5925–5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlemann A, Ward NA, Kratochwil N, Diener C, Fischer C, Stucki A, Jaeschke G, Malherbe P, Porter RH. (2006) Determination of key amino acids implicated in the actions of allosteric modulation by 3,3′-difluorobenzaldazine on rat mGlu5 receptors. Eur J Pharmacol 529:95–104 [DOI] [PubMed] [Google Scholar]
- Nakahara K, Okada M, Nakanishi S. (1997) The metabotropic glutamate receptor mGluR5 induces calcium oscillations in cultured astrocytes via protein kinase C phosphorylation. J Neurochem 69:1467–1475 [DOI] [PubMed] [Google Scholar]
- Nash MS, Young KW, Challiss RA, Nahorski SR. (2001) Intracellular signaling. Receptor-specific messenger oscillations. Nature 413:381–382 [DOI] [PubMed] [Google Scholar]
- Nash MS, Schell MJ, Atkinson PJ, Johnston NR, Nahorski SR, Challiss RA. (2002) Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. Receptor density versus agonist concentration. J Biol Chem 277:35947–35960 [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Lemaire W, Chen TB, Chang RS, Jacobson MA, Ha SN, Lindsley CW, Schaffhauser HJ, Sur C, Pettibone DJ, et al. (2003) A family of highly selective allosteric modulators of the metabotropic glutamate receptor subtype 5. Mol Pharmacol 64:731–740 [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Wisnoski DD, Lindsley CW, Schaffhauser HJ, Rowe B, Sur C, et al. (2004) A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 in rat forebrain. J Pharmacol Exp Ther 309:568–577 [DOI] [PubMed] [Google Scholar]
- Pagano A, Ruegg D, Litschig S, Stoehr N, Stierlin C, Heinrich M, Floersheim P, Prezèau L, Carroll F, Pin JP, et al. (2000) The non-competitive antagonists 2-methyl-6-(phenylethynyl)pyridine and 7-hydroxy-iminocyclopropan[b]chromen-1a- carboxylic acid ethyl ester interact with overlapping binding pockets in the transmembrane region of group I metabotropic glutamate receptors. J Biol Chem 275:33750–33758 [DOI] [PubMed] [Google Scholar]
- Rodriguez AL, Nong Y, Sekaran NK, Alagille D, Tamagnan GD, Conn PJ. (2005) A close structural analog of 2-methyl-6-(phenylethynyl)-pyridine acts as a neutral allosteric site ligand on metabotropic glutamate receptor subtype 5 and blocks the effects of multiple allosteric modulators. Mol Pharmacol 68:1793–1802 [DOI] [PubMed] [Google Scholar]
- Tomida T, Hirose K, Takizawa A, Shibasaki F, Iino M. (2003) NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J 22:3825–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino M, Sakai N, Kashiwagi K, Shirai Y, Shinohara Y, Hirose K, Iino M, Yamamura T, Saito N. (2004) Isoform-specific phosphorylation of metabotropic glutamate receptor 5 by protein kinase C (PKC) blocks Ca2+ oscillation and oscillatory translocation of Ca2+-dependent PKC. J Biol Chem 279:2254–2261 [DOI] [PubMed] [Google Scholar]
- Young SH, Wu SV, Rozengurt E. (2002) Ca2+-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor require negative feedback by protein kinase C. J Biol Chem 277:46871–46876 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ. (2005) Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther 315:1212–1219 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Wisnoski DD, O'Brien JA, Lemaire W, Williams DL, Jr, Jacobson MA, Wittman M, Ha SN, Schaffhauser H, Sur C, et al. (2007) Challenges in the development of mGluR5 positive allosteric modulators: the discovery of CPPHA. Bioorg Med Chem Lett 17:1386–1391 [DOI] [PubMed] [Google Scholar]