Abstract
According to accepted doctrine, agonist-bound G protein-coupled receptors catalyze the exchange of GDP for GTP and facilitate the dissociation of Gα and Gβγ, which in turn regulate their respective effectors. More recently, the existence of preformed signaling complexes, which may include receptors, heterotrimeric G proteins, and/or effectors, is gaining acceptance. We show herein the existence of a preformed complex of inactive heterotrimer (Gαs·βγ) and the effector type 5 adenylyl cyclase (AC5), localized by the N terminus of AC5. GST fusions of AC5 N terminus (5NT) bind to purified G protein subunits (GDP-Gαs and Gβγ) with apparent affinities of 270 ± 21 and 190 ± 7 nM, respectively. GDP-bound Gαs and Gβγ did not compete, but rather facilitated their interaction with 5NT, consistent with the isolation of a ternary complex (5NT, Gαs, and Gβγ) by gel filtration. The AC5/Gβγ interaction was also demonstrated by immunoprecipitation and fluorescence resonance energy transfer (FRET) and the binding site of heterotrimer Gαs·βγ mapped to amino acids 60 to 129 of 5NT. Deletion of this region in full-length AC5 resulted in significant reduction of FRET between Gβγ and AC. 5NT also interacts with the catalytic core of AC, mainly via the C1 domain, to enhance Gαs- and forskolin-stimulated activity of C1/C2 domains. The N terminus also serves to constrain Gαi-mediated inhibition of AC5, which is relieved in the presence of Gβγ. These results reveal that 5NT plays a key regulatory role by interacting with the catalytic core and scaffolding inactive heterotrimeric G proteins, forming a preassembled complex that is potentially braced for GPCR activation.
cAMP is a universal second messenger produced by a family of adenylyl cyclase (AC) enzymes. Nine membrane-bound AC isoforms have been identified and characterized. The topology of mammalian ACs consists of a variable N terminus (NT) and two large cytoplasmic domains separated by two membrane-spanning domains (six transmembrane domains in each). The two cytoplasmic domains (C1 and C2) are roughly 40% identical and together form the enzyme's catalytic core at their interface (Sadana and Dessauer, 2009). Many intracellular regulators of AC activity target the catalytic domains, including kinases, RGS proteins, and heterotrimeric G proteins. For example, Gαs binds to the C2 domain to increase affinity between the domains and thus increase activity, whereas Gαi binds to the C1 domain to inhibit a subset of AC isoforms, including ACs 1, 5, and 6.
Increasing evidence suggests that the NT also plays an important role in regulating various isoforms of AC. For example, the NT can be the target of phosphorylation by protein kinases (Lai et al., 1997, 1999; Lin et al., 2002; Chou et al., 2004); anchor additional regulators of AC activity, including phosphatases (Crossthwaite et al., 2006), calmodulin (Simpson et al., 2006), or Ric8a (Wang et al., 2007); or recruit AC to larger multiprotein complexes involving AKAPs, as is the case for AC2 and Yotiao (Piggott et al., 2008) or AC5 and mAKAP (Kapiloff et al., 2009). Finally, we have shown previously that the NT of AC5 and AC6 can anchor Gβγ, facilitating Gβγ enhancement of isoproterenol-stimulated activity of AC6 (Gao et al., 2007).
Although the nine membrane-bound AC isoforms each has a unique and complex regulatory pattern, they are all stimulated by heterotrimeric G proteins via Gs-coupled receptors (Sadana and Dessauer, 2009). In the classic signaling paradigm of G protein activation, an agonist-bound G protein-coupled receptor (GPCR) promotes GDP-to-GTP exchange on Gα, converting the inactive Gα·βγ complex into an active GTP-Gα, which then dissociates from Gβγ to allow for activation of AC (Gilman, 1987). A broadened concept of “preformed complexes” that includes GPCR dimers, G protein heterotrimers, effectors, and possibly other regulators is gaining acceptance (Galés et al., 2005; Rebois et al., 2006). The evidence in support of this idea comes from biochemical and FRET/bioluminescence resonance energy transfer studies on the effector proteins AC, inwardly rectifying K+ channels (GIRK) (Rebois et al., 2006), and phospholipase Cβ (PLCβ) (Yuan et al., 2007). New models suggest a rearrangement and only a partial separation of GTP-Gα from Gβγ, such that the effector binding sites at the interface between Gα and Gβγ become exposed (Bünemann et al., 2003; Frank et al., 2005). Despite growing acceptance of this model, many key issues remain unresolved. We suggest that the effectors themselves may help to hold G protein subunits in close proximity. We show herein that the N terminus of AC5 (5NT) anchors “inactive” heterotrimeric G proteins (GDP-Gαs·βγ). The heterotrimeric G protein binding site was mapped to amino acid region 60 to 129 of 5NT. Deletion of this region in full-length AC resulted in a significant reduction of FRET between AC5 and Gβγ. Despite reduced FRET, mutant AC5 was stimulated by exogenously added GTPγS-Gαs and Gβγ, which suggests a separate activation site for Gβγ on AC5. We also show that AC5 NT interacts with its catalytic domains (5C1/5C2) to enhance the Gαs- or forskolin-stimulated activity of C1/C2. We propose that the N terminus of AC5 is a key regulatory domain that brings the inactive heterotrimeric G proteins and catalytic core in close proximity for efficient GPCR activation.
Materials and Methods
Materials.
Antibodies used were rabbit anti-Gαs (Calbiochemission, San Diego, CA), rabbit anti-Gβ (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse anti-GST (Santa Cruz Biotechnology), rabbit anti-H6 (Bethyl Laboratories, Montgomery, TX), rabbit anti-AC5 (Santa Cruz Biotechnology and Core therapeutics), and anti-flag agarose (Sigma, St. Louis, MO).
Plasmids Construction and Generation of Recombinant Viruses.
Human AC5 N terminus (5NT) was fused with GST (GST-5NT) in pGEX-CS vector (Gao et al., 2007). 6NT was generated by PCR, fusing human AC6 aa 1 to 143 to GST in pGEX-4T vector. Truncations of 5NT fused with GST (Δ60, Δ144, 1–147, 60–147, 1–129, and 60–129) were generated by PCR. AC5Δ66–137 was created using PCR, deleting aa residues 66 to 137 within full-length hAC5-pcDNA3. N-terminally tagged yellow fluorescent protein (YFP)-AC5 was created in multiple steps. YFP-tagged pcDNA3 was created by PCR, generating KpnI and BamHI sites at the 5′ and 3′ ends of YFP, respectively. Human AC5 was subcloned in two steps into the BamHI and XbaI sites of YFP-pcDNA3. YFP-AC5Δ66–137 was created by subcloning the SacII-NotI fragment from AC5Δ66–137 pcDNA3 into YFP-AC5 pcDNA3. Sequences were confirmed by nucleotide sequencing and restriction digests. N-terminal flag-tagged human AC5 and cerulean-tagged Gβ1 were generous gifts from Drs. Michael Kapiloff (University of Miami, Miami, FL) and Moritz Bünemann (University of Wuerzburg, Wuerzburg, Germany), respectively (Bünemann et al., 2003). Recombinant baculoviruses for full-length AC5 and AC5Δ66–137 were created and expressed as described previously (Chen-Goodspeed et al., 2005).
Tissue Culture and Transfection.
Human embryonic kidney (HEK) 293 cells and COS7 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum and 50 μg/ml penicillin and streptomycin. For transient expression of proteins, cells were plated 24 h before transfection. Transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or jetPEI (Genesee Scientific, San Diego, CA) according to the manufacturer's protocol. After 48 h of transfection, cells were used for the desired experiment.
Protein Purification.
Proteins 5C1(670)H6, H65C2, Gαs-H6, and myristoylated Gαi-H6 were expressed in Escherichia coli and purified as described previously (Whisnant et al., 1996; Sunahara et al., 1997; Dessauer et al., 1998). GST-tagged proteins were purified using glutathione agarose resin (Salim et al., 2003). Nontagged Gβ1γ2 was coexpressed with Gαi-H6 in Sf9 cells and purified on nickel-nitrilotriacetic acid followed by ion exchange as described previously (Kozasa and Gilman, 1995).
GST Pull-Down Assays.
GST or GST-5NT (full-length or truncations) were incubated with G protein subunits in 50 μl of binding buffer (20 mM HEPES, pH 8.0, 1 mM EDTA, 5 mM MgCl2, 1 mM DTT, 50 mM NaCl, 0.2% C12E9, and 2 μM GDP). For assays using the cytoplasmic domains (5C1 or 5C2), the binding buffer included 0.05% C12E9 and no GDP. The proteins were incubated for 30 min at 4°C followed by addition of 100 μl of 20% glutathione-agarose beads. After rotating for 2 h, the resin was washed three times with binding buffer containing 150 mM NaCl and 0.05% C12E9. For incubations containing 5C1 and 5C2, the NaCl was increased to 250 mM. Bound proteins were eluted with 15 mM glutathione, boiled with Laemmli buffer, and analyzed by SDS-PAGE and Western blotting.
Immunoprecipitation of AC5.
Human flag-tagged AC5 was transfected in HEK293 cells (10 cm dish/IP). After 48 h, HEK293 cells were rinsed with phosphate-buffered saline, resuspended in lysis buffer (20 mM HEPES, pH 8.0, 1 mM EDTA, 1 mM MgCl2, 1 mM DTT, 150 mM NaCl, 0.5% C12E10, 1 μM GDP, and protease inhibitors), and homogenized using a 23-gauge syringe. Cellular debris was removed by centrifugation, and 30 μl of anti-flag agarose was added. Samples were rotated at 4°C for 2 h, and then washed three times with lysis buffer that contained only 0.05% C12E10. Proteins were eluted with SDS-PAGE sample buffer, and analyzed by Western blotting.
Gel Filtration Chromatography.
Proteins (10 μM, 100-μl sample volume) were applied on tandem superdex 75/200 columns (GE Healthcare) and resolved in gel filtration buffer (20 mM HEPES, pH 8.0, 1 mM EDTA, 5 mM MgCl2, 1 mM DTT, 100 mM NaCl, 0.05% C12E9, and 10 μM GDP) at 0.3 ml/min, 4°C. Fractions (0.4 ml) were collected, and samples were analyzed by SDS-PAGE and immunoblotting.
Preparation of Membranes from HEK293 and Sf9 Cells.
HEK293 cells were transfected with 10 μg of total DNA (per 10-cm plate) that included 2.5 μg of plasmid encoding AC5 or AC5Δ66–137. Membranes were prepared after 48 h as described previously (Piggott et al., 2008). Membranes from Sf9 cells expressing AC5 or AC5Δ66–137 were prepared after 48 h of infection with the respective baculovirus as described by Dessauer et al. (2002).
Assay of Adenylyl Cyclase Activity.
AC activity was measured in membranes as described previously (Dessauer, 2002). In assays containing purified 5C1 and 5C2 catalytic domain proteins, limiting concentrations of the C1 domain protein were assayed with 1 μM 5C2 to promote interaction between the C1 and C2 proteins as described previously (Whisnant et al., 1996). GST or GST-5NT was preincubated with 5C1/5C2 before addition of activators.
Fluorescence Resonance Energy Transfer.
COS7 cells were plated on coverslips coated with poly-l-lysine (0.01 mg/ml) in six-well dishes at ∼10 to 15% confluence. The next day, cells were transfected with 2 μg of total DNA, which included 0.4 μg of YFP-AC5 or YFP-AC5Δ66–137, 0.9 μg of Cerulean-Gβ1, and 0.7 μg of Gγ2. Before imaging, media was exchanged with Tyrode's buffer (10 mM HEPES, pH 7.4, 145 mM NaCl, 4 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 10 mM glucose).
Fluorescence images were acquired after 48 h of transfection using a microscope (TE 2000; Nikon, Tokyo, Japan) with a DG4 xenon light source and two CoolSNAP cameras (Roper Scientific, Trenton, NJ). For FRET determinations, three images were acquired sequentially (exposure time, 200 ms) using the following filter sets: donor (CFP; excitation, 436/20 nm; emission, 465/30 nm), FRET (CFP/YFP; excitation, 436/20 nm; emission, 535/30 nm) and acceptor (YFP; excitation, 500/20 nm; em 535/30 nm). Corrected, sensitized FRET (FRETC) was calculated using the equation FRETC = IFRET − (a × ICFP) − (b × IYFP), where IFRET, ICFP, and IYFP correspond to background-subtracted images of cells expressing CFP and YFP acquired through the FRET, CFP, and YFP channels, respectively. The values a and b are the bleed-through values of CFP and YFP in FRET channel, respectively. Calibrations of bleed -through were performed in cells expressing only CFP- or YFP-tagged proteins and were calculated as 0.53 and 0.04 for CFP and YFP, respectively. In cells expressing both CFP and YFP-tagged proteins, normalized FRET values were calculated according to the following two methods (Vanderklish et al., 2000; Xia and Liu, 2001): 1) NFRET = FRETC/ and 2) FC/D = FRETC/ID, where FRETC is the mean corrected FRET as calculated above, and ID and IA are the mean intensities of the donor (CFP or Cerulean), and acceptor (YFP) fluorescence. Pseudocolor FRETC/D images were obtained using the Slidebook software (Nikon) and are displayed with deep blue indicating low values and bright red indicating high values of FRET.
Statistical Analysis.
Each experiment was repeated at least three times in duplicate or triplicate. Comparison between different experiments groups was determined with the nonpaired Student's t test. p < 0.05 is indicated with an asterisk in the figures. For fluorescence images, figures show representative images from 18 to 20 different cells from four different experiments.
Results
AC5 N Terminus Anchors Heterotrimeric G Proteins.
We reported previously that Gβ1γ2 bound to 5NT (Gao et al., 2007). Gβγ has no effect on AC activity alone, but increases the activity of AC5 and AC6 approximately 1.5- to 2-fold in the presence of Gαs or forskolin. To further examine the nature of this interaction, we sought to compete Gβγ binding to 5NT by addition of an excess of GDP-bound Gα, which should mask the Gβγ effector surface. Numerous crystallographic and mapping studies have identified an overlapping surface on Gβγ that is used for both binding to α subunits and various effectors (Wall et al., 1995). However, not only did GDP-Gαs fail to compete with binding of Gβγ to GST-5NT, but it also actually enhanced binding by more than 2-fold (Fig. 1A). In addition, the α subunit also bound to 5NT in the absence of Gβγ (Fig. 1, A and B). Gαs binding to 5NT was enhanced by Gβγ when present in the GDP-bound, aluminum fluoride-bound, and GTPγS-bound state (Fig. 1, A and B, and Supplemental Fig. S1). At the concentrations of G proteins used in these binding assays (1 μM), a complex of Gαβγ would be expected because the affinity of GDP-Gαs for Gβγ is 27 nM under the conditions used in our binding assays (Sarvazyan et al., 2002). Therefore, separate sites on Gαs and Gβγ are probably used to bind 5NT compared with the Gα-βγ interaction surface or effectors binding sites on Gαs and Gβγ.
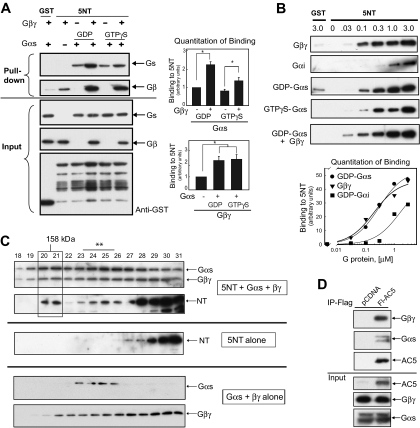
Fig. 1.
The N terminus of AC5 anchors heterotrimeric G proteins. A, GST or GST-tagged 5NT (final concentration, 2 μM) was incubated with G protein subunits Gαs (GDP or GTPγS bound) and/or Gβ1γ2 as indicated, and GST pull-down assay was performed. Western blot analysis indicated that 5NT binds GDP-Gαs and Gβγ independently and together their binding is enhanced (top). Protein input for Gαs-, Gβγ-, and GST-tagged proteins is shown below. Quantitation of binding for GDP-Gαs and Gβγ is shown (n = 3, P < 0.05). B, dose response of 5NT binding to Gβγ, GDP-Gαi, GDP-Gαs, GTPγS-Gαs, or GDP-Gαs in the presence of 100 nM Gβγ was performed by GST pull-down of 5NT (0.125 μM) and varying concentration of G protein subunits. Quantitation of binding from three independent experiments is shown (P < 0.05). C, gel filtration analysis of complex formation between 5NT and Gαs·βγ. Proteins (10 μM each) were applied on tandem Superdex 75/200 columns in buffer containing 0.05% C12E9 and 10 μM GDP and analyzed by SDS-PAGE and immunoblotting. Top, complex of 5NT/Gαs/Gβγ; middle, 5NT alone; bottom, Gαs/Gβγ. The 5NT/Gαs/Gβγ complex is boxed, whereas smaller complexes containing 5NT with Gαs or Gβγ are marked with an asterisk. D, IP of AC5-Gαs-Gβγ complex. Flag-tagged human AC5 (Fl-AC5) or pCDNA3 vector was transfected in HEK293 cells (10 cm dish/IP), immunoprecipitated with anti-Flag agarose, and subjected to Western blotting with anti-Gαs, anti-Gβ, or anti-Flag. The input represents 5% of the total used in the IP.
Apparent Affinity Measurements for G Protein Binding to AC 5NT.
To further characterize interactions with 5NT, we determined the apparent affinities for binding Gβγ or Gα. Increasing amounts of GDP-bound Gα or Gβγ were added to GST-5NT (125–150 nM), and GST-pulldown assays were performed (Fig. 1B). Binding to GST alone was tested at the highest level for each protein (3 μM). Binding data were quantitated from three independent experiments to determine apparent affinities (Fig. 1B). Gβγ (190 ± 7 nM) and GDP-Gαs (270 ± 21 nM) binding to 5NT was saturable and displayed fairly high affinity binding. GTPγS-and GDP-bound Gαs displayed similar binding to 5NT and was comparable with the affinity of GTPγS-bound Gαs for the activation site on the C2 domain of AC5 (400 nM) (Sunahara et al., 1997). GDP-bound myristoylated Gαi1 also bound to 5NT but with a much reduced apparent affinity (∼1.5 μM) compared with GDP-bound Gαs.
Isolation of AC5/Gαs·βγ Complex.
To show that it is indeed the heterotrimer that is bound to 5NT, we used gel filtration chromatography to evaluate the size and composition of potential complexes between GDP-Gαs, Gβγ, and 5NT (Fig. 1C). An approximately 90-kDa complex of Gαs·βγ can be isolated by gel filtration, which shifts in the presence of 5NT to a complex of 140 to 145 kDa. This is consistent with a 1:1:1 complex consisting of 5NT/Gαs·βγ. Smaller complexes of 5NT with either Gαs or Gβγ are also evident. Note that GST-tagged 5NT runs mainly as a monomer of 50 kDa but is also found at lower levels as a dimeric species, probably because of the tendency of GST to dimerize. Thus, our results suggest that inactive heterotrimeric Gs can bind to AC5, challenging the dogma that heterotrimeric G proteins interact with effectors only in their “activated” GTP-bound state.
Gαs·βγ can also be found in complex with full-length AC5. Endogenous Gαs and Gβγ are readily detectable in immunoprecipitations of flag-tagged AC5 from HEK293 cells (Fig. 1D). This is consistent with a model in which heterotrimeric G proteins do not release from the effector and are poised to allow for regulation of AC by both subunits.
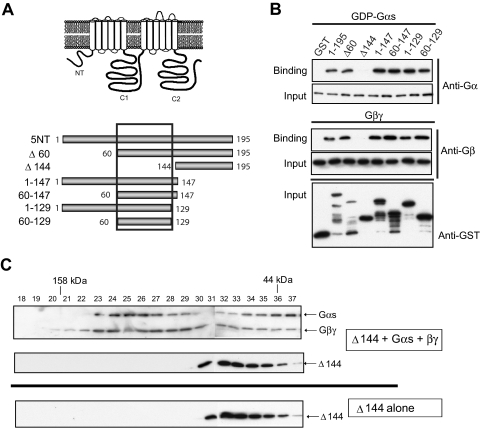
G-Protein Subunits Bind to aa 60 to 129 of 5NT.
The binding sites for Gαs and Gβγ were further mapped on 5NT. GST fusion proteins of N- or C-terminal truncations of 5NT (Fig. 2A) were tested for binding GDP-bound Gαs or Gβγ. All fragments of 5NT supported binding to both Gαs and Gβγ except the region 144 to 195 (Δ144). Both G protein subunits could be mapped to a minimal region of 69 amino acids (aa 60–129), consistent with their binding as a closely associated heterotrimeric unit (Fig. 2B). These results were confirmed by gel-filtration chromatography, where 5NT-60-129 clearly formed a complex with Gαs·βγ heterotrimer but Δ144 did not (Fig. 2C and Supplemental Fig. S2).
Fig. 2.
Mapping the G protein binding site on 5NT. A, schematic diagram of full-length AC5 and GST-tagged NT fragments. B, purified 5NT fragments (2 μM) were incubated with 1 μM G protein subunits (GDP-Gαs or Gβ1γ2) and GST pull-down assay was performed (n = 3). Immunoblots for protein input are shown. C, gel filtration analysis of 5NTΔ144 and Gαs·βγ. Proteins (10 μM) were resolved on tandem Superdex 75/200 columns and analyzed by SDS-PAGE and immunoblotting. Top, 5NTΔ144/Gαs/Gβ1γ2; bottom, 5NTΔ144 alone (n = 2).
We previously identified aa 77 to 151 in AC 6NT as necessary for Gβγ regulation (Gao et al., 2007). In general, the N-terminal domains of different AC isoforms have little similarity; however, there are small stretches of homology between aa 60 to 129 of 5NT and 77 to 151 of 6NT. We now show that both GDP-Gαs and Gβγ directly interact with 6NT as well (Supplemental Fig. S3).
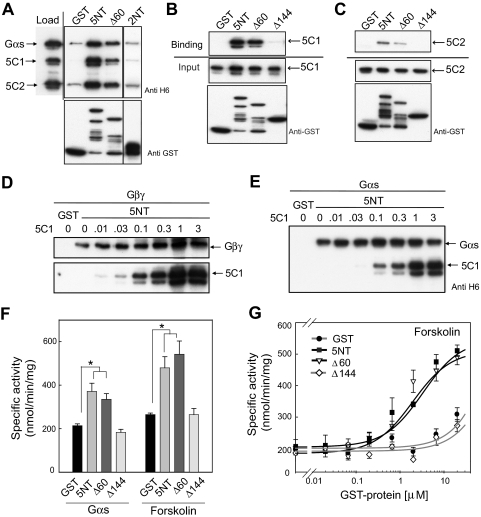
5NT Interacts with the Catalytic Core to Enhance Gs or Forskolin-Stimulated Activity.
The question remained regarding how the NT might be regulating functional properties of AC. Previous reports have suggested that AC could act as a GTPase-activating protein or possibly guanine nucleotide exchange factor for Gαs (Scholich et al., 1999); however, purified 5NT displays neither of these activities for Gαs or Gαi (Supplemental Fig. S4). Rather, the NT pulls down a complex of 5C1/5C2/Gαs in the presence or absence of forskolin and binds directly to the C1 domain of AC5 (Fig. 3, A and B, and Supplemental Fig. S5) and, to a lesser degree, the C2 domain (Fig. 3C). This interaction is isoform-specific, in that there is no interaction between the N terminus of AC2 and the C1 domain of AC5 (Fig. 3A). We have further mapped the binding site of the C1 and C2 domains on 5NT to aa 60 to 144, similar to that of the G protein subunits; however, C1 does not compete with Gαs or Gβγ for 5NT binding (Fig. 3, D and E). Computer modeling of 5NT suggests that this region is highly helical and thus different faces of this helix may be required for interactions with the C1 domain and heterotrimeric G proteins.
Fig. 3.
Interaction of 5NT with the catalytic core. A, 5NT pulls down 5C1/5C2/GTPγS-Gαs/forskolin complex. 5C1, 5C2, and GTPγS-Gαs (1 μM each) were incubated in presence of 100 μM forskolin for 30 min on ice before addition of GST, GST-5NT, or 2NT (2 μM). GST pull-down assay was performed as described under Materials and Methods. Western blot analysis of input and eluted proteins in shown. 5C1 (B) and 5C2 (C) were subjected to a GST pull-down assay with 5NT, Δ60, or Δ144 (2 μM). Western blot analysis of bound proteins and input is shown. Competition reactions between 5C1 (0–3 μM) and 200 nM Gβγ (D) or 200 nM Gαs-GDP (E) for binding to GST-5NT using GST pull-down assays. F, 5NT enhances the Gαs- or forskolin-stimulated activity of 5C1/5C2. Purified AC5 catalytic domains 5C1 (70 nM) and 5C2 (1 μM) were preincubated with GST or GST-tagged 5NT (5 μM) for 10 min before stimulation with either 400 nM GTPγS-GαS or 100 μM forskolin. G, dose-dependent enhancement of 5C1/5C2 activity by 5NT. AC activity assay was performed as described in F with varying concentrations of GST, 5NT, 5NTΔ60, or 5NTΔ144.
Most intracellular regulators of AC activity bind to the C1 and C2 domains of AC to exert their stimulatory or inhibitory affects. Thus, it is possible that the NT can also affect the enzymatic state of AC5. 5NT increased the activity of the isolated C1 and C2 domains of AC5 by 1.75 and 2 fold when stimulated by Gαs or forskolin, respectively, but displayed no effect on basal C1/C2 activity (Supplemental S6). Consistent with our mapping studies, full-length 5NT and 5NTΔ60, but not 5NTΔ144, could enhance the Gαs- or forskolin-stimulated activity of the AC5 C1/C2 domains in a dose-dependent manner (EC50 ∼2 μM, Fig. 3, F and G).
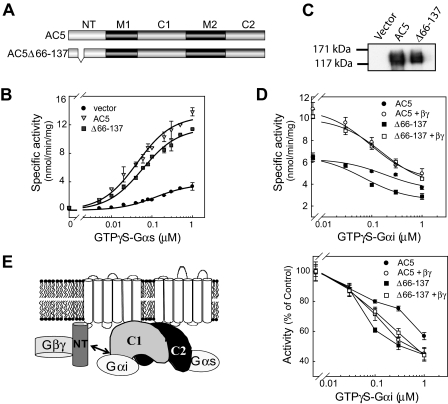
AC5Δ66–137 Is Conditionally Stimulated by Exogenously Added Gβγ.
To determine whether the Gαs·βγ binding site on NT is necessary for conditional stimulation of AC5 by Gβγ, we deleted the G protein binding site (Δ66–137) in the context of the full-length AC5 enzyme (Fig. 4A). AC5Δ66–137 can be activated by Gαs and/or forskolin when assayed in membranes from HEK293, COS7, and Sf9 cells (Figs. 4, B and D, and 5A). However, AC5Δ66–137 displayed a small but highly reproducible 38% right shift of the Gαs dose-response curve (P < 0.05, n = 6). AC5Δ66–137 was also conditionally stimulated by exogenously added Gβγ, suggesting that AC5 activation by Gβγ does not require its binding to the N terminus (Fig. 4D).
Fig. 4.
Regulation of AC5Δ66–137 by exogenously added GTPγS-Gαs, Gβγ, and GTPγS-Gαi. A, diagram of AC5 and AC5Δ66–137. B, dose response of GTPγS-Gαs stimulated AC5 and AC5Δ66–137 activity. Membranes from HEK293 cells expressing vector, AC5, or AC5Δ66–137 were stimulated with the indicated concentrations GTPγS-Gαs. C, characterization of AC5 and AC5Δ66–137 expression by Western blotting in Sf9 membranes. D, stimulation of Sf9 membranes expressing AC5 or AC5Δ66–137 by 30 nM GTPγS-Gαs in the presence or absence of 100 nM Gβ1γ2 and the indicated concentrations of GTPγS-Gαi. Bottom, each curve was normalized to the AC activity in the absence of Gαi. E, model of Gαi regulation by 5NT. GTPγS-Gαs binds to 5C2 to stimulate activity, whereas GTPγS-Gαs binds to 5C1 to inhibit AC5. 5NT also interacts with 5C1 to limit Gαi inhibition. Addition of Gβγ increases activity and relieves the influence of 5NT on Gαi inhibition. Note that although Gβγ is shown bound to 5NT, it clearly must have additional unknown activation sites.
Fig. 5.
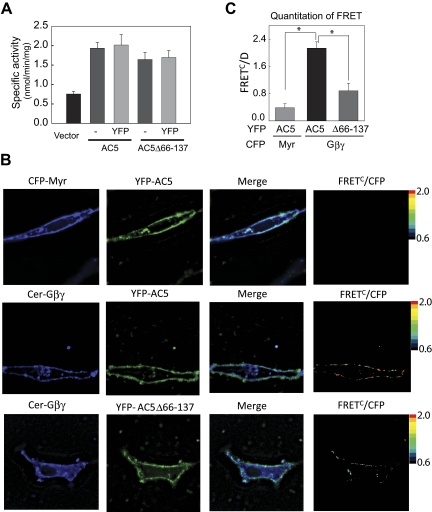
Cellular interaction of YFP-AC5 and Gβγ in COS7 cells by FRET. A, characterization of YFP-tagged proteins. Membranes from COS7 cells expressing vector, YFP-AC5, or YFPAC5Δ66–137 were stimulated with 1 μM GTPγS-Gαs. B, FRET analysis of AC5 and Gβ1γ2 in COS7 cells. Fluorescence microscopy images of COS7 cells transiently transfected with the indicated proteins were recorded using three different channels [1) donor, CFPex/CFPem; 2) FRET, CFPex/YFPem; and 3) acceptor, YFPex/YFPem]. A representative cell is shown for each combination of proteins (top, Myr-CFP and YFP-AC5; middle, Cer-Gβ1, Gγ2, and YFP-AC5; bottom, Cer-Gβ1, Gγ2, and YFP-AC5Δ66–137). Pseudocolor FRETC/D images were obtained using Slidebook software (Nikon) C, quantitative analysis of FRET by FC/D method (n = 4 using images from 18 to 20 cells, P < 0.01).
Previous studies have suggested that deletion of residues 1 to 86 of AC6 increases Gαi inhibition (Kao et al., 2004). We examined the inhibition of AC5Δ66–137 by GTPγS-Gαi in the presence and absence of Gβγ. Consistent with previous studies, deletion of aa 66 to 137 increased Gαi efficacy and the IC50 compared with wild-type AC5. This is probably due to the loss of 5NT interactions with the C1 domain, which binds GTPγS-Gαi (Fig. 4E) (Dessauer et al., 1998; Kao et al., 2004). Upon addition of Gβγ, the percentage inhibition of wild-type AC5 by Gαi increased and was nearly identical to that of AC5Δ66–137 in the presence of Gβγ. Thus, Gβγ binding seems to reverse the constraints of the N terminus on Gαi inhibition, although the overall activity is still increased by Gβγ.
Fluorescence Resonance Energy Transfer (FRET) between AC5 and Gβγ.
To explore the trafficking of AC5 to the PM, we constructed plasmids that place YFP at the N terminus of AC5 or AC5Δ66–137. Tagged proteins were tested for plasma membrane localization and activity. Upon expression in COS7 cells, YFP-tagged ACs displayed activity equal to their nontagged versions (Fig. 5A). In addition, both YFP-AC5 and YFP-AC5Δ66–137 were found to be largely localized to the PM when expressed in HEK293 or COS7 cells at low levels (Fig. 5B). Higher levels of expression generally produced a particulate cytoplasmic fluorescence that was excluded from the nucleus. Therefore, we have carefully titrated AC expression levels that give rise to a largely PM expression pattern for all fluorescence and activity assays. Although previous reports have suggested that AC5 may be present on the nuclear envelope in cardiac myocytes (Belcheva et al., 1995; Yamamoto et al., 1998; Boivin et al., 2006), we detected no such localization upon expression in COS7 cells.
To analyze G protein interactions with AC5 in living cells, we used a FRET-based approach employing YFP-tagged AC5 and N-terminal Cerulean-tagged Gβ1 (Cer-Gβ1). Cer-Gβ1 has previously been characterized for its plasma membrane localization and interactions with Gγ2 and Gαi (Bünemann et al., 2003). There is a strong inverse distance relationship between FRET and the distance separation between chromophores, such that FRET between the donor molecule CFP and acceptor molecule YFP only occurs if the two proteins are in close proximity (<100 Å). Significant FRET was observed at the plasma membrane between YFP-AC5 and Cer-Gβ1γ2 compared with myristoylated tagged CFP and YFP-AC5 (negative control; Fig. 5, B and C). In addition, no significant FRET was observed between Cer-Gβ1γ2 and the transmembrane protein YFP-Na,K-ATPase (data not shown). Numerous mathematical methods are used to quantify FRET. We compared the two most commonly used methods FC/D (Vanderklish et al., 2000) and NFRET (Xia and Liu, 2001). Using either method, a significant decrease in FRET was observed for YFP-AC5Δ66–137 and Cer-Gβ1γ2 compared with YFP-AC5 (Table 1). Despite a reduction in FRET, the deletion mutant was still stimulated by exogenously added Gβγ in membrane AC assays, consistent with a separate activation site for Gβγ on AC5.
TABLE 1.
Quantitation of FRET measurements by FRETC/D and FRETC/ method
Values are presented as mean ± S.E.M.
| Donor Proteins | Acceptor Proteins | FRETC/ Average | FRETC/D Average |
|---|---|---|---|
| CFP-Myr | YFP-AC5 | 0.38 ± 0.09 | 0.38 ± 0.05 |
| Cer-Gβγ | YFP-AC5 | 2.13 ± 0.20 | 1.61 ± 0.26 |
| Cer-Gβγ | YFP-AC5Δ66–137 | 0.88 ± 0.24 | 0.64 ± 0.13 |
Discussion
In this report, we show by various biochemical techniques that 1) preformed complexes occur between AC5 and heterotrimeric G proteins (GDP-Gαs·βγ), 2) these complexes are mediated by the N terminus of AC5, and 3) the N terminus also interacts with the catalytic core to augment Gαs-stimulatory and Gαi-inhibitory activity.
N Terminus and G Protein Interactions.
In our previous studies, we proposed a model for Gβγ activation of AC5/6 that involved the release of Gβγ upon receptor-stimulated activation of Gs, where both subunits were required for full activation by agonist. We suggested that the Gβγ released upon activation of Gs stimulates AC, serving to enhance regulation by the α-s subunit (Gao et al., 2007). Herein we show that GDP-bound Gαs also directly binds to 5NT and that GDP-Gαs and Gβγ do not compete for binding on 5NT but rather enhance binding to 5NT. Gel filtration studies confirmed the binding of G protein subunits as a heterotrimer to 5NT and endogenous Gαs and Gβγ copurified with AC5 from immunoprecipitates of HEK293 cells.
Existence of G proteins and AC as a stable complex was first proposed by Levitzki in 1988 (Levitzki, 1988; Bar-Sinai et al., 1992; Levitzki and Klein, 2002) based upon copurification of AC and G proteins from turkey erythrocyte membranes that was independent of the activation state of the G proteins. There is now additional evidence that other effectors, namely GIRK channel (Kir 3.1) (Peleg et al., 2002), PLCβ (Yuan et al., 2007), and receptor for activated C-kinase (RACK1) (Dell et al., 2002) exist as stable complexes with G protein heterotrimers. In the case of PLCβ, the activator of G protein signaling AGS8 forms a complex with both Gαi·βγ and PLCβ. Gβγ activates PLCβ through the nondissociated heterotrimer complex using an alternate interaction site, not the “hot spot” on Gβγ normally associated with effector interactions (Yuan et al., 2007; Smrcka, 2008). For the GIRK channel, the N terminus of GIRK1 anchors the inactive heterotrimer Gαi·βγ. Experimental data from several groups suggest a model in which GPCR activation of the heterotrimer bound to GIRK1 triggers partial or full separation of Gαi from Gβγ, causing Gβγ to occupy a separate activation site on GIRK1 to open the channel (Huang et al., 1995; Peleg et al., 2002; Ivanina et al., 2003; Clancy et al., 2005; Rishal et al., 2005; Riven et al., 2006; Rubinstein et al., 2009).
In an inactive Gα·βγ complex, the effector surfaces of Gα and Gβγ are masked. Only upon activation of the α subunit and subunit dissociation are effector interaction sites exposed. In our case, it is unlikely that the normal effector face of Gαs is responsible for interactions with 5NT, because it is probably masked by Gβγ binding. This is supported by the fact that addition of excess 5NT does not sequester Gαs to reduce Gαs-stimulated AC5 activity (Supplemental Fig. S7).
Mechanism of Gβγ Activation of AC5.
The binding site for Gαs·βγ on 5NT was mapped to a 69-aa region (60–129). Upon deletion of the Gαs·βγ binding site on AC5 (AC5Δ66–137), FRET between AC5Δ66–137 and Gβγ was significantly reduced compared with wild-type AC5. AC5Δ66–137 was fully functional in terms of proper localization (by fractionation and YFP fluorescence), Gαs stimulation, and surprisingly conditional Gβγ stimulation. These results indicate that the activation site of Gβγ on AC reside somewhere other than aa 66 to 137 of 5NT. The C1/C2 domains were not stimulated by Gβγ in absence or presence of 5NT (Supplemental Fig. S8), suggesting that the three cytoplasmic domains of AC5 are not sufficient for conditional stimulation by Gβγ. This is consistent with the fact that the cytoplasmic domains alone are not sufficient for conditional stimulation of AC2 by Gβγ (Dessauer and Gilman, 1996; Weitmann et al., 2001). Thus, it is likely that the AC5 membrane domains must properly orient the cytoplasmic domains for Gβγ activation.
We have previously mapped the Gβγ binding and activation site on AC6 to the NT residues 77 to 151 (Gao et al., 2007) and now show that both GDP-Gαs and Gβγ bind directly to 6NT (Supplemental Fig. S3). As opposed to AC5, deletion of the Gβγ binding site on AC6-NT abolished stimulation by exogenously added Gβγ. The NT of all nine isoforms of AC are highly variable even among the closely related isoforms AC5 and AC6. Therefore, the possibility of somewhat different mechanisms of activation by Gβγ is not completely unexpected. In fact, AC5 and AC6 also display differences in their stimulation by Gαs, inhibition by Gαi, and phosphorylation by protein kinase C (Harry et al., 1997; Lai et al., 1999; Chen-Goodspeed et al., 2005).
Role of N Terminus in Modulating Activity.
The divergence of the N terminus provides additional regulatory specificity among the nine isoforms of AC. Numerous physiological regulators bind to the NT of ACs, but in most cases, the mechanism for regulation of AC activity is unclear. Previous binding assays using in vitro-translated proteins suggested that AC6 NT contacts the C1 domain to modulate Gαi-mediated inhibition (Kao et al., 2004). We have now shown that there is a direct interaction between AC5 NT and C1/C2 domains that increases catalytic activity and, as with AC6, limits the inhibition by Gαi. Gβγ relieves the constraints of the N terminus on Gαi, although it is unclear whether this is due to direct binding to 5NT or to an allosteric effect of the Gβγ activation site. However, Gβγ binding to 5NT does not compete with 5NT-C1 binding; thus, it is unlikely to be a simple competition between interaction sites.
Post-translational modifications or direct binding to AC NT by other factors may also regulate activity by altering the interaction between NT and C1/C2 domains. For example, phosphorylation of AC6 NT by PKC δ and ε inhibits AC6 activity (Lai et al., 1997; Lai et al., 1999; Lin et al., 2002), whereas AC8 NT forms part of the calmodulin binding site that stimulates AC8 activity, although the precise mechanism for either regulation is still unclear (Simpson et al., 2006). Alternatively, the NT of ACs may simply serve as a scaffold to facilitate interactions between regulators and the catalytic domains. For example, AC5 NT also interacts with the G protein exchange factor RiC8a to suppress AC activity (Wang et al., 2007). The NT of AC2 binds to the AKAP scaffolding protein Yotiao, facilitating inhibition by an as-yet unknown regulator (Piggott et al., 2008). Finally, both AC5 and AC6 NT bind Gβγ to conditionally stimulate the enzyme, although they differ in their mechanism as discussed above (Gao et al., 2007). The possibility for NT regulation of ACs allows for even more diverse modulation of these complex enzymes.
Possible Physiological Consequences.
What is the functional role of heterotrimer binding to the N terminus of AC5? FRET measurements and immunoprecipitations confirm the stable interaction of Gβγ to AC5 in cells. Prior reports from bioluminescence resonance energy transfer studies indicate that Gβγ traffics together with AC2 (and also with GIRK) to the plasma membrane (Rebois et al., 2006). The deletion of the NT Gβγ binding site had no effect on trafficking of AC5 to the PM, and we observe no FRET between AC5 and Gβγ on intracellular sites within the cell. Thus, heterotrimer binding to the N terminus does not seem to be required for proper trafficking of AC5. Another possible effect could be on AC5 activity, as in the case of GIRK channel. Heterotrimer binding to a scaffold formed by NT and C terminus of GIRK1 lowers the basal activity of GIRK1 and predisposes the channel to GPCR-mediated activation (Rubinstein et al., 2009). Our model of heterotrimeric G protein scaffolding by AC5 resembles the activation of GIRK channels by Gβγ, because both effectors bind G protein heterotrimers at the NT, yet the Gβγ activation site is distinct from the NT at a secondary location on each effector. We hypothesize that the NT of AC5 brings catalytic core and regulators (heterotrimeric G protein subunits) in close proximity to prepare for potential GPCR mediated activation of AC.
Supplementary Material
Acknowledgments
We thank Kathryn Hassell for excellent technical support, Dr. Jim Tomlinson for the generous gift of AC5/AC6 antibody, Dr. Moritz Bünemann for cerulean tagged Gβ, and Dr. Michael Kapiloff for flag-tagged human AC5.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This research was supported by the National Institutes for Health National Institute of General Medicine [Grants GM060419, GM68493], the American Heart Association [Grant 09GRNT2200034]; and the U.S.-Israel Binational Science Foundation [Grant 01-122].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.058370
- AC
- adenylyl cyclase
- NT
- N terminus
- AKAP
- A-kinase anchoring protein
- GPCR
- G protein-coupled receptor
- FRET
- fluorescence resonance energy transfer
- GIRK
- G protein-coupled inwardly rectifying potassium channel
- PLC
- phospholipase C
- GTPγS
- guanosine 5′-O-(3-thio)triphosphate
- 5NT
- AC5 N terminus
- 6NT
- AC6 N terminus
- PCR
- polymerase chain reaction
- aa
- amino acid(s)
- GST
- glutathione transferase
- YFP
- yellow fluorescent protein
- HEK
- human embryonic kidney
- DTT
- dithiothreitol
- C12E9
- nonaethylene glycol monododecyl ether
- PAGE
- polyacrylamide gel electrophoresis
- IP
- immunoprecipitation
- CFP
- cyan fluorescent protein
- FRETC
- corrected, sensitized FRET
- Cer
- Cerulean.
References
- Bar-Sinai A, Marbach I, Shorr RG, Levitzki A. (1992) The GppNHp-activated adenylyl cyclase complex from turkey erythrocyte membranes can be isolated with its beta gamma subunits. Eur J Biochem 207:703–708 [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Gucker S, Chuang DM, Clark WG, Jefcoat LB, McHale RJ, Toth G, Borsodi A, Coscia CJ. (1995) Modulation of opioid binding associated with nuclear matrix and nuclear membranes of NG108-15 cells. J Pharmacol Exp Ther 274:1513–1523 [PubMed] [Google Scholar]
- Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hébert TE. (2006) Functional beta-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res 71:69–78 [DOI] [PubMed] [Google Scholar]
- Bünemann M, Frank M, Lohse MJ. (2003) Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc Natl Acad Sci U S A 100:16077–16082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Goodspeed M, Lukan AN, Dessauer CW. (2005) Modeling of Galpha(s) and Galpha(i) regulation of human type V and VI adenylyl cyclase. J Biol Chem 280:1808–1816 [DOI] [PubMed] [Google Scholar]
- Chou JL, Huang CL, Lai HL, Hung AC, Chien CL, Kao YY, Chern Y. (2004) Regulation of type VI adenylyl cyclase by Snapin, a SNAP25-binding protein. J Biol Chem 279:46271–46279 [DOI] [PubMed] [Google Scholar]
- Clancy SM, Fowler CE, Finley M, Suen KF, Arrabit C, Berton F, Kosaza T, Casey PJ, Slesinger PA. (2005) Pertussis-toxin-sensitive Galpha subunits selectively bind to C-terminal domain of neuronal GIRK channels: evidence for a heterotrimeric G-protein-channel complex. Mol Cell Neurosci 28:375–389 [DOI] [PubMed] [Google Scholar]
- Crossthwaite AJ, Ciruela A, Rayner TF, Cooper DM. (2006) A direct interaction between the N terminus of adenylyl cyclase AC8 and the catalytic subunit of protein phosphatase 2A. Mol Pharmacol 69:608–617 [DOI] [PubMed] [Google Scholar]
- Dell EJ, Connor J, Chen S, Stebbins EG, Skiba NP, Mochly-Rosen D, Hamm HE. (2002) The betagamma subunit of heterotrimeric G proteins interacts with RACK1 and two other WD repeat proteins. J Biol Chem 277:49888–49895 [DOI] [PubMed] [Google Scholar]
- Dessauer CW. (2002) Kinetic analysis of the action of P-site analogs. Methods Enzymol 345:112–126 [DOI] [PubMed] [Google Scholar]
- Dessauer CW, Chen-Goodspeed M, Chen J. (2002) Mechanism of Galpha i-mediated inhibition of type V adenylyl cyclase. J Biol Chem 277:28823–28829 [DOI] [PubMed] [Google Scholar]
- Dessauer CW, Gilman AG. (1996) Purification and characterization of a soluble form of mammalian adenylyl cyclase. J Biol Chem 271:16967–16974 [DOI] [PubMed] [Google Scholar]
- Dessauer CW, Tesmer JJ, Sprang SR, Gilman AG. (1998) Identification of a Gialpha binding site on type V adenylyl cyclase. J Biol Chem 273:25831–25839 [DOI] [PubMed] [Google Scholar]
- Frank M, Thümer L, Lohse MJ, Bünemann M. (2005) G Protein activation without subunit dissociation depends on a G{alpha}(i)-specific region. J Biol Chem 280:24584–24590 [DOI] [PubMed] [Google Scholar]
- Galés C, Rebois RV, Hogue M, Trieu P, Breit A, Hébert TE, Bouvier M. (2005) Real-time monitoring of receptor and G-protein interactions in living cells. Nat Methods 2:177–184 [DOI] [PubMed] [Google Scholar]
- Gao X, Sadana R, Dessauer CW, Patel TB. (2007) Conditional stimulation of type V and VI adenylyl cyclases by G protein betagamma subunits. J Biol Chem 282:294–302 [DOI] [PubMed] [Google Scholar]
- Gilman AG. (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649 [DOI] [PubMed] [Google Scholar]
- Harry A, Chen Y, Magnusson R, Iyengar R, Weng G. (1997) Differential regulation of adenylyl cyclases by Galphas. J Biol Chem 272:19017–19021 [DOI] [PubMed] [Google Scholar]
- Huang CL, Slesinger PA, Casey PJ, Jan YN, Jan LY. (1995) Evidence that direct binding of G beta gamma to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron 15:1133–1143 [DOI] [PubMed] [Google Scholar]
- Ivanina T, Rishal I, Varon D, Mullner C, Frohnwieser-Steinecke B, Schreibmayer W, Dessauer CW, Dascal N. (2003) Mapping the Gbetagamma-binding sites in GIRK1 and GIRK2 subunits of the G protein-activated K+ channel. J Biol Chem 278:29174–29183 [DOI] [PubMed] [Google Scholar]
- Kao YY, Lai HL, Hwang MJ, Chern Y. (2004) An important functional role of the N terminus domain of type VI adenylyl cyclase in Galphai-mediated inhibition. J Biol Chem 279:34440–34448 [DOI] [PubMed] [Google Scholar]
- Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, Efendiev R, Dessauer CW. (2009) A novel adenylyl cyclase-mAKAPbeta signaling complex maintains basal cAMP levels in cardiac myocytes. J Biol Chem 284:23540–23546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa T, Gilman AG. (1995) Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. Characterization of alpha 12 and inhibition of adenylyl cyclase by alpha z. J Biol Chem 270:1734–1741 [DOI] [PubMed] [Google Scholar]
- Lai HL, Lin TH, Kao YY, Lin WJ, Hwang MJ, Chern Y. (1999) The N terminus domain of type VI adenylyl cyclase mediates its inhibition by protein kinase C. Mol Pharmacol 56:644–650 [DOI] [PubMed] [Google Scholar]
- Lai HL, Yang TH, Messing RO, Ching YH, Lin SC, Chern Y. (1997) Protein kinase C inhibits adenylyl cyclase type VI activity during desensitization of the A2a-adenosine receptor-mediated cAMP response. J Biol Chem 272:4970–4977 [DOI] [PubMed] [Google Scholar]
- Levitzki A. (1988) From epinephrine to cyclic AMP. Science 241:800–806 [DOI] [PubMed] [Google Scholar]
- Levitzki A, Klein S. (2002) G-protein subunit dissociation is not an integral part of G-protein action. Chembiochem 3:815–818 [DOI] [PubMed] [Google Scholar]
- Lin TH, Lai HL, Kao YY, Sun CN, Hwang MJ, Chern Y. (2002) Protein kinase C inhibits type VI adenylyl cyclase by phosphorylating the regulatory N domain and two catalytic C1 and C2 domains. J Biol Chem 277:15721–15728 [DOI] [PubMed] [Google Scholar]
- Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. (2002) G(alpha)(i) controls the gating of the G protein-activated K(+) channel, GIRK. Neuron 33:87–99 [DOI] [PubMed] [Google Scholar]
- Piggott LA, Bauman AL, Scott JD, Dessauer CW. (2008) The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc Natl Acad Sci U S A 105:13835–13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebois RV, Robitaille M, Galés C, Dupré DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hébert TE. (2006) Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci 119:2807–2818 [DOI] [PubMed] [Google Scholar]
- Rishal I, Porozov Y, Yakubovich D, Varon D, Dascal N. (2005) Gbetagamma-dependent and Gbetagamma-independent basal activity of G protein-activated K+ channels. J Biol Chem 280:16685–16694 [DOI] [PubMed] [Google Scholar]
- Riven I, Iwanir S, Reuveny E. (2006) GIRK channel activation involves a local rearrangement of a preformed G protein channel complex. Neuron 51:561–573 [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Peleg S, Berlin S, Brass D, Keren-Raifman T, Dessauer CW, Ivanina T, Dascal N. (2009) Divergent regulation of GIRK1 and GIRK2 subunits of the neuronal G protein gated K+ channel by GalphaiGDP and Gbetagamma. J Physiol 587:3473–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadana R, Dessauer CW. (2009) Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17:5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, Sinnarajah S, Kehrl JH, Dessauer CW. (2003) Identification of RGS2 and type V adenylyl cyclase interaction sites. J Biol Chem 278:15842–15849 [DOI] [PubMed] [Google Scholar]
- Sarvazyan NA, Lim WK, Neubig RR. (2002) Fluorescence analysis of receptor-G protein interactions in cell membranes. Biochemistry 41:12858–12867 [DOI] [PubMed] [Google Scholar]
- Scholich K, Mullenix JB, Wittpoth C, Poppleton HM, Pierre SC, Lindorfer MA, Garrison JC, Patel TB. (1999) Facilitation of signal onset and termination by adenylyl cyclase. Science 283:1328–1331 [DOI] [PubMed] [Google Scholar]
- Simpson RE, Ciruela A, Cooper DM. (2006) The role of calmodulin recruitment in Ca2+ stimulation of adenylyl cyclase type 8. J Biol Chem 281:17379–17389 [DOI] [PubMed] [Google Scholar]
- Smrcka AV. (2008) G protein betagamma subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci 65:2191–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Whisnant RE, Kleuss C, Gilman AG. (1997) Interaction of Gsalpha with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem 272:22265–22271 [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Krushel LA, Holst BH, Gally JA, Crossin KL, Edelman GM. (2000) Marking synaptic activity in dendritic spines with a calpain substrate exhibiting fluorescence resonance energy transfer. Proc Natl Acad Sci U S A 97:2253–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MA, Coleman DE, Lee E, Iñiguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. (1995) The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2. Cell 83:1047–1058 [DOI] [PubMed] [Google Scholar]
- Wang SC, Lai HL, Chiu YT, Ou R, Huang CL, Chern Y. (2007) Regulation of type V adenylate cyclase by Ric8a, a guanine nucleotide exchange factor. Biochem J 406:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitmann S, Schultz G, Kleuss C. (2001) Adenylyl cyclase type II domains involved in Gbetagamma stimulation. Biochemistry 40:10853–10858 [DOI] [PubMed] [Google Scholar]
- Whisnant RE, Gilman AG, Dessauer CW. (1996) Interaction of the two cytosolic domains of mammalian adenylyl cyclase. Proc Natl Acad Sci U S A 93:6621–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Liu Y. (2001) Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys J 81:2395–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Kawamura K, James TN. (1998) Intracellular distribution of adenylate cyclase in human cardiocytes determined by electron microscopic cytochemistry. Microsc Res Tech 40:479–487 [DOI] [PubMed] [Google Scholar]
- Yuan C, Sato M, Lanier SM, Smrcka AV. (2007) Signaling by a non-dissociated complex of G Protein betagamma and alpha subunits stimulated by a receptor-independent activator of G protein signaling, AGS8. J Biol Chem 282:19938–19947 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.