Abstract
pQDLDHVFLRFamide is a highly conserved crustacean neuropeptide with a structure that places it within the myosuppressin subfamily of the FMRFamide-like peptides. Despite its apparent ubiquitous conservation in decapod crustaceans, the paracrine and/or endocrine roles played by pQDLDHVFLRFamide remain largely unknown. We have examined the actions of this peptide on the cardiac neuromuscular system of the American lobster Homarus americanus using four preparations: the intact animal, the heart in vitro, the isolated cardiac ganglion (CG), and a stimulated heart muscle preparation. In the intact animal, injection of myosuppressin caused a decrease in heartbeat frequency. Perfusion of the in vitro heart with pQDLDHVFLRFamide elicited a decrease in the frequency and an increase in the amplitude of heart contractions. In the isolated CG, myosuppressin induced a hyperpolarization of the resting membrane potential of cardiac motor neurons and a decrease in the cycle frequency of their bursting. In the stimulated heart muscle preparation, pQDLDHVFLRFamide increased the amplitude of the induced contractions, suggesting that myosuppressin modulates not only the CG, but also peripheral sites. For at least the in vitro heart and the isolated CG, the effects of myosuppressin were dose-dependent (10−9 to 10−6 mol l−1 tested), with threshold concentrations (10−8−10−7 mol l−1) consistent with the peptide serving as a circulating hormone. Although cycle frequency, a parameter directly determined by the CG, consistently decreased when pQDLDHVFLRFamide was applied to all preparation types, the magnitudes of this decrease differed, suggesting the possibility that, because myosuppressin modulates the CG and the periphery, it also alters peripheral feedback to the CG.
Keywords: cardiac ganglion (CG), FMRFamide-like peptide (FLP), heart, Homarus americanus, lobster, myosuppressin, neurohormone, neuropeptide
INTRODUCTION
The crustacean cardiac neuromuscular system is one of the premier models for understanding the basic principles that underlie the modulatory control of rhythmic motor programs (Cooke, 2002). This system, which is composed of the cardiac ganglion (CG) and the heart musculature, is one of the simplest central pattern generator (CPG)-effector systems known. For example, in the American lobster Homarus americanus, the cardiac CPG consists of just nine neurons, four small pacemaker neurons and five larger motor neurons, all of which are contained within a nerve trunk located on the dorsal surface of the heart lumen (reviewed by Cooke, 2002).
In decapod crustaceans, the cardiac CPG receives modulatory input from several sources, including ones intrinsic to the cardiac neuromuscular system and others extrinsic to it (Cooke, 2002). The best-documented intrinsic source of modulation is nitric oxide, which appears to be synthesized in the cardiac muscle (Mahadevan et al., 2004) and diffuses to the CG, where it causes a decrease in cycle frequency. A second intrinsic modulator, Homarus americanus calcitonin-like diuretic hormone (Homam-CLDH), has recently been identified (Christie et al., 2010). The transcript for this peptide hormone, which strongly activates cardiac activity, is present within the motor neurons of the CG. It has also been suggested that acetylcholine may act as an intrinsic modulator (reviewed in Cooke, 2002), since the ganglion can synthesize this transmitter (Sullivan and Miller, 1990) and is sensitive to it (Freschi and Livengood, 1989). In addition, the CG is thought to receive sensory feedback from the heart musculature through stretch-sensitive dendrites on the neurons of the ganglion itself (Cooke, 2002). Extrinsic modulators of the cardiac neuromuscular system include substances released onto the CPG by axons that project to the CG from somata located within the central nervous system (e.g. Fort et al., 2004; Delgado et al., 2000; Freschi and Livengood, 1989; Sullivan and Miller, 1990), as well as hemolymph-borne agents, including a variety of peptide hormones (e.g. Trimmer et al., 1987; Christie et al., 1995; Cruz-Bermúdez and Marder, 2007). Although many endocrine sites have been identified in crustaceans, two neuroendocrine organs, the sinus gland (typically located in the eyestalk) and the pericardial organ (PO; present in the pericardial chamber surrounding the heart), and the midgut and its associated caeca are probably the major contributors to the complement of circulating peptides (Cooke and Sullivan, 1982; Christie et al., 2007).
Although the crustacean cardiac neuromuscular system is a relatively simple system, its modulation has the potential to be quite complex, with locally-released and/or circulating substances theoretically capable of simultaneously influencing multiple sites within the system. Interestingly, although many studies have examined the modulatory actions of substances on the isolated CG or CG neurons (e.g. Cruz-Bermúdez et al., 2006; Cruz-Bermúdez and Marder, 2007; Saver et al., 1999), on the in vitro heart (e.g. Mercier and Russenes, 1992; Worden et al., 1995; Saver and Wilkens, 1998; Dickinson et al., 2007a; Christie et al., 2008; Dickinson et al., 2009), or on other properties of the cardiac system, such as contraction amplitude, elasticity or resistance to flow (e.g. Wilkens and Taylor, 2002; Wilkens et al., 2005), relatively few have examined the actions of a substance at multiple sites within the cardiac CPG-effector system (Fort et al., 2004; Fort et al., 2007a; Fort et al., 2007b). We have cloned the mRNA coding for the FMRFamide-like peptide pQDLDHVFLRFamide (Stemmler et al., 2007; Ma et al., 2008), a member of the myosuppressin subfamily, to confirm its sequence assignment. We then examined its effects on the lobster cardiac neuromuscular system, using several distinct preparations (the heart in the intact animal, the heart in vitro, the isolated CG and a stimulated heart muscle preparation), to allow for assessment of modulation at multiple sites, as well as for assessment of the potential integration of these modulatory effects.
Some of these data have appeared previously in abstract form (Dickinson et al., 2007b; Stevens et al., 2008).
MATERIALS AND METHODS
Animals
American lobsters Homarus americanus Milne-Edwards were purchased from local (Bar Harbor and Brunswick, ME, USA) seafood retailers. All animals were housed in flow-through or recirculating natural seawater aquaria at 10-12°C.
Molecular cloning and peptide prediction
cDNA library construction and generation of ESTs
The construction and normalization of the H. americanus cDNA library used here has been described in detail elsewhere (Towle and Smith, 2006). In brief, multiple tissues (including the supraesophageal ganglion, commonly referred to as the brain, and the heart) from four individuals were collected; total RNA samples were prepared individually from each tissue, checked for quality, then pooled for construction and normalization of a cDNA library by the Invitrogen Corporation (Carlsbad, CA, USA). Plasmids were isolated and inserts single-pass sequenced from their 5′ end using SP6 primer (5′-ATTTAGGTGACACTATAG-3′) at the Marine DNA Sequencing and Analysis Facility at Mount Desert Island Biological Laboratory (Salisbury Cove, ME, USA). Sequence traces were processed for submission to dbEST (National Center for Biotechnology Information; Bethesda, MD, USA) using the trace2dbest component of PartiGene software (University of Edinburgh, Edinburgh, UK). Prior to submission, all expressed sequence tags (ESTs) were subjected to BLASTX analysis, i.e. translated nucleotide sequence versus protein sequence (Altschul et al., 1997), and annotated accordingly.
cDNA sequence analysis
To characterize cDNA clones identified by BLASTX analysis, a sample of Escherichia coli possessing the insert-containing vector was cultured overnight in LB (Luria broth) medium at 37°C. Plasmid containing the cDNA was subsequently isolated using a Purelink Quick Plasmid Miniprep kit (Invitrogen). The vector insert was then sequenced on an ABI 3100 16-capillary sequencer (Applied Biosystems Foster City, CA, USA) using both vector- and insert-specific forward and reverse sequencing primers (Integrated DNA Technologies, Coralville, IA, USA; Table 1). The sequence trace files resulting from each round of sequencing were analyzed using Chromas 2.31 software (Technelysium Pty, Tewantin, Queensland, Australia), and the high quality nucleotide sequences were aligned using SeqMan 2.6 software (DNASTAR Inc., Madison, Wisconsin, USA).
Table 1.
Vector- and insert-specific primers used for sequencing the Homarus americanus prepro-myosuppressin cDNA GQ303179
| Primer name | Direction | Sequence |
| Vector-specific primers | ||
| SP6 | Forward | ATTTAGGTGACACTATAG |
| T-Party | Reverse | TTTTTTTTTTTTTTTTTTTV * |
| Insert-specific primers | ||
| Hoam-MS F1 | Forward | TGTTCGTGGTCTTGCCTTCT |
| Hoam-MS F2 | Forward | CAGGGATCATGGAAGGAAGA |
| Hoam-MS F3 | Forward | GTCAACACCTCCTGGCTGAT |
V in T-Party represents A, T or G.
Peptide prediction
Full-length cDNA sequences were translated using the Translate tool of ExPASy (Swiss Institute of Bioinformatics, Basel, Switzerland; http://www.expasy.ch/tools/dna.html). Signal peptide and signal peptide cleavage was predicted using the online program SignalP 3.0, with both Neural Networks and Hidden Markov Models algorithms (Center for Biological Sequence Analysis, Technical University of Denmark, Lyngby, Denmark; http://www.cbs.dtu.dk/services/SignalP/) (Bendtsen et al., 2004). Pro-hormone cleavage sites were predicted based on the information presented by Veenstra (Veenstra, 2000). Prediction of the sulfation state of tyrosine residues was done using the online program Sulfinator (Swiss Institute of Bioinformatics, Geneva, Switzerland; http://www.expasy.org/tools/sulfinator/) (Monigatti et al., 2002). Other post-translational modifications (i.e. N-terminal glutamine cyclization and C-terminal amidation) were predicted by homology to known myosuppressin isoforms.
Immunohistochemistry
For whole-mount immunohistochemistry, lobsters were anesthetized by packing them in ice for 30-60 min, after which the heart and foregut were removed. The CG and stomatogastric ganglion (a positive control for FMRFamide-like immunoreactivity) (Kilman et al., 1999) were separated from the associated muscle by manual microdissection in chilled (approximately 4°C) physiological saline (composition in mmol l−1: 479.12 NaCl, 12.74 KCl, 13.67 CaCl2, 20.00 MgSO4, 3.91 Na2SO4, 11.45 Trizma base and 4.82 maleic acid; pH 7.45). Isolated tissues were fixed for 12-24 h in a solution of freshly prepared 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA; catalog no. 15710) in 0.1 mol l−1 sodium phosphate (P) buffer (pH 7.4). Following fixation, tissues were rinsed five times over approximately 5 h in a solution of P containing 0.3% Triton X-100 (P-Triton) and then incubated for approximately 72 h with a rabbit anti-FMRFamide primary antibody (ImmunoStar Inc., Hudson, WI, USA; catalog no. 20091), which was diluted to a final working dilution of 1:1000 using P-Triton containing 10% normal goat serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA; catalog no. 005-000-121) to block non-specific staining. After incubation in primary antibody, tissues were again rinsed hourly, five times, in P-Triton and then incubated for 12-24 h in a 1:300 dilution of Rhodamine Red X-AffiniPure goat anti-rabbit IgG (Jackson ImmunoResearch; catalog no. 111-295-144). Secondary antibody, like primary antibody, was diluted in P-Triton containing 10% normal goat serum. After incubation in secondary antibody, tissues were rinsed hourly, five times, in P and then mounted on a glass microscope slide using Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA; catalog no. H-1000). All antibody incubations were done at 4°C, and all rinses were done at room temperature (approximately 20°C). Incubation in secondary antibody was conducted in the dark, as was all subsequent processing. Slides were stored in the dark at 4°C until examined using an Olympus Model BX51 fluorescent microscope system (Melville, NY, USA).
Electrophysiology
In the intact animal
We monitored the electrical activity of the heart in the intact animal using Teflon-coated silver wire electrodes that were implanted in or just over the heart. To implant the electrodes, we drilled two small holes, approximately 1 cm apart, in the dorsal carapace directly over the heart. The electrodes were inserted into the holes and glued in place with epoxy resin, then secured to the carapace with dental wax. A third hole, located anteriorly and to one side of the heart, was covered with a septum that allowed us to inject myosuppressin directly into the hemocoel. Animals were returned to their tank and allowed to recover for at least 24 h before recordings were taken.
For recordings, the lobster was placed in a small container of seawater, with the temperature maintained between 10-12°C. The seawater was aerated with a small aquarium air pump, and a covering of black plastic was placed over the lobster's eyes to prevent vision-related changes in heart activity.
The signal from the heart was amplified with a Model 1700 AM Systems differential AC amplifier (Sequim, WA, USA) and a Model 410 Brownlee Precision Instrumentation amplifier (San Jose, CA, USA). The amplified signal was recorded directly onto a computer via a CED Micro 1401 digitizer and Spike2 v5.15 (Cambridge Electronic Design, Cambridge, UK). To assess the effects of myosuppressin, we injected 500 μl of a 10−5 mol l−1 solution of the peptide into the hemocoel surrounding the pericardial cavity. Based on measurements of the volume of this region (made by filling the cavity around the heart with clay in several lobsters), we estimated that this would result in an initial concentration of approximately 10−7 mol l−1 in this part of the hemocoel, so that the initial concentration in the pericardial cavity would be 10−7 mol l−1 or lower.
In the in vivo heart
Lobsters were anaesthetized by packing them in ice for 30-60 min before dissection. The heart and the thoracic carapace onto which it was connected were removed from the posterior dorsal side of the lobster and pinned to the bottom of a small Sylgard 184 (KR Anderson, Santa Clara, CA, USA)-lined dish. The dorsal part of the heart was left attached to the carapace, so that the extent to which it was stretched was identical to that in the intact animal.
The posterior artery was cannulated with a short piece of polyethylene tubing drawn out to fit the artery, and was continuously perfused with chilled physiological saline. A second perfusion line was directed across the top of the heart to help maintain temperature, which was monitored continuously and kept between 10 and 12°C using an in-line temperature control system (CL-100 bipolar temperature controller and SC-20 solution heater/cooler; Warner Instruments, Hamden, CT, USA). Because isolated hearts continue to contract only when adequately stretched (Alexandrowicz, 1932; Maynard, 1960; Cooke, 2002), flow rate through the heart was kept at approximately 2.5 ml min−1. Under our recording conditions, stable heart activity could be recorded for at least 8 h.
To record heart contractions, the anterior arteries were tied off with 6/0 Suture Silk and attached to a Grass FT03 force-displacement transducer (Astro-Med, West Warwick, RI, USA) at an angle of approximately 30 deg. from the horizontal plane. The output of the transducer was amplified using a Model 410 Brownlee Precision Instrumentation amplifier, and recorded using the same instrumentation described for the intact animal.
One ostium on the ventral surface of the heart was extended to allow a suction electrode to enter the heart chamber. An en passant suction electrode was attached to one of the motor nerves and extracellular activity was recorded as in the intact animal. Preparations were allowed to stabilize for 1-2 h before the first application of the peptide. Saline containing the peptide was directed into the heart cavity through the perfusion cannula.
In isolated cardiac ganglion
To determine the effects of myosuppressin on neural activity in the CG without the possibility of peripheral feedback, we recorded the electrical activity of neurons in the isolated ganglion. The heart was removed from the animal as described above, then detached from the carapace and pinned in a Sylgard-coated dish. The CG was exposed by opening the heart ventrally and then pinning back the heart tissue overlying the ganglion. The main trunk, including the posterior region with all the small pacemaker neurons, as well as the anterolateral and posterolateral nerves were cut out and pinned in a clear Sylgard-lined dish filled with physiological saline.
To record motor output extracellularly, petroleum jelly wells were placed on the postero- and anterolateral nerves; stainless steel pin electrodes were put in the wells to record electrical activity on the nerves. These extracellular recordings were amplified and acquired using the same equipment described for the intact animal.
Intracellular recordings were made using microelectrodes filled with 0.6 mol l−1 K2SO4 with 20 mmol l−1 KCl, having resistances between 10-40 MΩ. The membrane potential was recorded in the somata of anterior motor neurons and amplified using an AxoClamp 2B (Axon Instruments) and a Model 410 Brownlee Precision Instrumentation amplifier. Data were recorded to a PC via a CED Micro 1401 USB interface and the data acquisition program Spike2 v5.15.
In stimulated heart muscle preparations
To determine whether myosuppressin had effects directly on the neuromuscular junction or the muscle fibers, we removed the portion of the CG that included the endogenous bursting capability of the system, then elicited contractions using controlled trains of stimuli delivered to the remaining motor nerves.
The cardiac network produces a reliable patterned output from the small and the large cells in the CG. In the large cells, impulse initiation zones are located on the axons of neurons rather than in the axon-hillock, sometimes as much as 1-2 mm away from the soma (Hartline, 1967). Impulse generation occurs first in the most posterior motor cells, and progresses anteriorly (Hartline, 1967). Thus, to eliminate any spontaneous activity, we stimulated the anterior portions of the anterolateral nerves of the CG where there were no cell bodies.
To accomplish this, the heart was dissected out of the animal, cannulated, and attached to a force transducer. The posterior region of the CG was transected on both sides, removing small pacemaking cells as well as the large motor cells that drive contraction. The motor end of one of the two motor nerves was then pulled into a suction electrode and stimulated with trains of impulses of 0.5 ms duration, at a frequency of 60 Hz. Bursts of 300 ms duration were delivered at 50-60 bursts min−1. Although the isolated whole heart can continue contracting regularly for many hours, we found that the force generated in response to stimulations frequently decreased over time with continual stimulation. Thus, we stimulated the nerve in bouts of bursts delivered once every 2 min. Each bout included 13 bursts, which was sufficient to reach a stable level of facilitation. Electrical impulses were generated by a Model S88 stimulus generator (Grass Medical Instruments, Quincy, MA, USA) and a Model SIU5 stimulus isolation unit (Grass Instruments), and then sent through the suction electrode to the motor nerve. The force of contraction generated in response to controlled bursts was recorded as previously described.
Data analysis
Heart contraction parameters were analyzed in Spike2 using locally written scripts and the built-in analysis functions of the program. Extracellular recordings of bursts were also analyzed in Spike2 using scripts adapted from ones created by Dr Dirk Bucher, which are available at: http://www.whitney.ufl.edu/BucherLab/Spike2_Scripts2_box.htm.
Both contraction and burst characteristics were plotted as a function of time, and 200 s sections were chosen to determine baseline and effect averages. The values for 200 s before the onset of peptide application were averaged to serve as the baseline. The 200 s average to examine peptide effects was taken where maximal effects were seen. In most preparations, this region was 200-400 s into peptide application. Myosuppressin caused delayed modulation of some parameters, so averages were taken at different time points, as detailed in the Results.
To examine the time courses of changes in all preparations, values for each individual preparation were divided into bins of 120 s, adjusted to time equals zero at the onset of peptide application, then averaged across preparations. Thus, our time resolution for temporal effects is only in the order of minutes.
To account for different baseline parameters, the percentage change from baseline was calculated for each preparation, and averages across different days were combined to find the mean ± s.e.m. for each peptide. To determine whether any treatment caused a change in activity of any of the parameters that was significantly different from zero (P<0.05), one-sample t-tests were performed comparing percentage change to zero. All data analysis was done using Prism v4.0 or v5.0 (GraphPad Software, San Diego, CA, USA).
RESULTS
Molecular confirmation of the myosuppressin sequence pQDLDHVFLRFamide
Previously, a peptide with structural similarity to members of the myosuppressin family, pQD(L/I)DHVF(L/I)RFamide, was identified by mass spectrometry from the nervous system of H. americanus (Stemmler et al., 2007; Ma et al., 2008). Owing to the nature of the mass spectral platforms used for its characterization, i.e. matrix-assisted laser desorption/ionization Fourier transform mass spectrometry (MALDI-FTMS) or electrospray ionization quadrupole time-of-flight tandem mass spectrometry (ESI-Q-TOF MS/MS), the position 3 and 8 amino acids of this peptide could not be assigned conclusively; leucine and isoleucine are isobaric, and thus cannot be distinguished from one another by either MALDI-FTMS or ESI-Q-TOF MS/MS (Stemmler et al., 2007; Ma et al., 2008). However, during an ongoing H. americanus EST project (Towle and Smith, 2006), a lobster transcript (accession no. FD699285) with homology to a prepro-myosuppressin from the cockroach Diploptera punctata (accession no. AAB39926) (Donly et al., 1996) was serendipitously identified by BLASTX analysis (Bit score, 67.4; E-value, 6e-10). Using a combination of vector- and insert-specific primers (Table 1), we sequenced the full-length clone from which FD699285 was derived. As is shown in Fig. 1A, this cDNA (accession no. GQ303179) was 1634 base pairs (bp) in length, and consisted of a 140 bp 5′ untranslated region (UTR), a 303 bp open reading frame (ORF; including the stop codon) and a 1191 bp 3′ UTR, which contained numerous polyadenylation signal sequences located upstream of a 49 bp poly(A)+ tail.
Fig. 1.
Nucleotide and deduced amino acid sequences of Homarus americanus prepro-myosuppressin. (A) Nucleotide sequence of Homarus americanus prepro-myosuppressin (accession no. GQ303179). The open reading frame of the cDNA, including the stop codon, is shown in bold font, with the 3′ polyadenylation signal sequences indicated by underline in black. (B) Deduced amino acid sequence of Homarus americanus prepro-myosuppressin. The signal peptide is shown in grey, with prohormone convertase cleavage loci shown in black. The encoded myosuppressin isoform is shown in red, with additional precursor-related peptides in blue. The asterisk indicates the position of the stop codon. (C) Putative processing scheme resulting in the production of myosuppressin from its precursor protein. The first line of sequence shows the full-length prepro-hormone with the locus of cleavage locus for signal peptidase underlined. The second line of sequence shows the cleaved pro-hormone with the loci for prohormone convertase underlined. The third line of sequence shows the immature myosuppressin peptide with the site of action for carboxypeptidase underlined. The fourth line of sequence shows the peptide resulting from carboxypeptidase activity with the Gly destined for a-amidation underlined. The fifth line of sequence shows the amidated peptide with the Gln destined for cyclization underlined. The final line of sequence shows the putative, mature myosuppressin after full post-translational processing.
Translation of the ORF of GQ303179 predicted a 100 amino acid prepro-peptide, named here Homam-prepro-myosuppressin (accession no. GQ303179; Fig. 1B,C). SignalP analysis of this deduced protein predicted the presence of a signal peptide within the prepro-hormone, although the length of the hypothetical signal peptide differed between the Neural Networks and the Hidden Markov Models algorithms, i.e. 21 amino acids (cleavage between Gly21 and Val22) versus 25 amino acids (cleavage between Gly25 and Val26), respectively (Fig. 1B). Regardless of the length of the signal sequence, two dibasic and one monobasic prohormone convertase processing sites were identified within the putative pro-hormone(s), enzymatic processing at which, followed by carboxypeptidase activity and α-amidation at an exposed Gly, would liberate four peptides (listed in their order of appearance within the pro-hormone; Fig. 1B,C): VCVGVGETMPPPICLSQQVPLSPFA or VGETMPPPICLSQQVPLSPFA, depending on which signal sequence is used, LCSALINISEFSRAMEEYLGAQAIERSMPVNEPEV, QDLDHVFLRFamide, and SQQ. Based on homology to the peptide detected by mass spectrometry, the Glu1 residue in QDLDHVFLRFamide is predicted to become cyclized, either spontaneously or enzymatically, resulting in the mature structure pQDLDHVFLRFamide (Fig. 1C), which is identical to the mass spectrally identified H. americanus myosuppressin (Stemmler et al., 2007; Ma et al., 2008), but with the ambiguous Leu/Ile position 3 and 8 residues conclusively assigned as Leu. Sulfinator analysis of LCSALINISEFSRAMEEYLGAQAIERSMPVNEPEV suggests that the position 18 Cys in this peptide is sulfated (E-value, 2.4), resulting in a putative mature structure of LCSALINISEFSRAMEEY(SO3H)LGAQAIERSMPVNEPEV. No additional post-xtranslational modifications are predicted for any of the other peptides.
FMRFamide-like peptides, including myosuppressin, are probably delivered to the cardiac neuromuscular system hormonally
Although several extended FMRFamide-related peptides have been shown to modulate the output of the cardiac neuromuscular system, it remains unknown whether or not members of this peptide family reach this system solely via hormonal routes or via a combination of hormonal and local delivery. To directly assess the mode of peptide delivery to the heart, we immunolabeled the CG with an antibody known to cross-react broadly with members of the FMRFamide family, including myosuppressin (A.E.C., unpublished data). In none of the three CGs subjected to FMRFamide immunoprocessing was there any evidence of labeling (data not shown). By contrast, in the stomatogastric ganglion, one of four ganglia that constitute the stomatogastric nervous system, isolated from the same animals and immunoprocessed simultaneously with the CGs, extensive FMRFamide-like immunoreactivity was present (data not shown). Thus, modulation of the lobster cardiac neuromuscular system by FMRFamide-like peptides, including myosuppressin, must be achieved solely via hormonal routes.
Effects of myosuppressin on the heart in the intact lobster
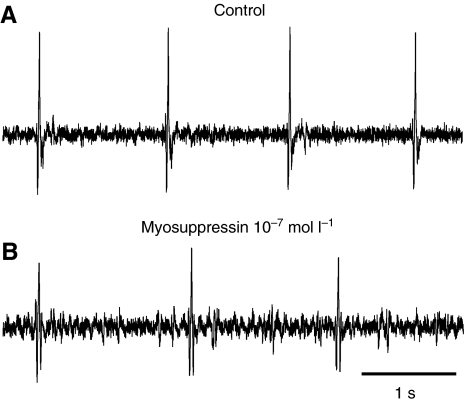
To examine the effects of myosuppressin on cardiac activity, we first injected the peptide into the hemolymph of intact animals, while monitoring cardiac activity with electrodes implanted through the carapace just over the heart. Heart rate in the resting lobsters before peptide injection ranged from 46 to 90 beats min−1, with an average of 70.7±6.2 beats min−1 (i.e. 1.18 Hz), a value similar to that recorded using non-invasive methods in intact animals in previous studies [e.g. heart rate of 82.5 beats min−1 (Reiber et al., 1997)]. Within 4 min of the injection, the heart rate had decreased significantly (P=0.0245, N=4; Fig. 2), reaching its lowest value, an average decrease of 15.4±3.7%, 6 min after the injection. The effects of myosuppressin injections into intact animals were long lasting, with a complete return to baseline taking from 25 to more than 60 min.
Fig. 2.
Myosuppressin decreased heart rate when injected into intact lobsters. Heart rate was monitored with extracellular electrodes implanted over the heart. (A) Control heart rate was approximately 60 beats min−1. (B) Recording taken 6 min after injection of 500 μl of 5×10−3 mol l−1 myosuppressin, calculated to bring the initial concentration of peptide in the pericardial cavity to approximately 10−7 mol l−1. Heart rate slowed to ~40 beats min−1.
Effects of myosuppressin on the lobster heart in vitro
To understand how myosuppressin caused changes in the cardiac activity of the intact animal, we examined the effects of the peptide in the isolated heart, where we were able to monitor the contractile behavior of the heart using a force transducer and the electrical activity of the ganglion using extracellular recordings made with a suction electrode introduced into the heart. Baseline heart rate was considerably lower than in the intact animal, averaging 0.64±0.04 Hz (38.1±2.3 beats min−1) in control saline. It was, however, stable for up to 8 h or longer when unperturbed.
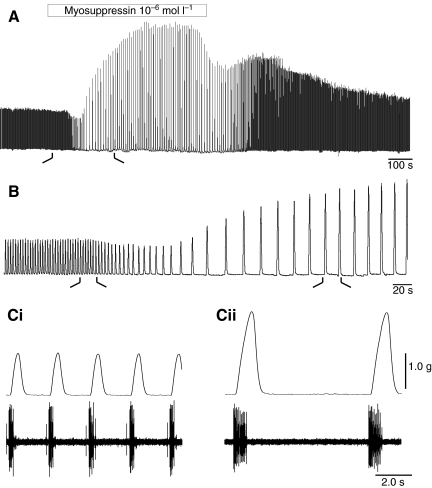
When perfused through the whole heart, myosuppressin caused a complex modulation of cardiac activity (Fig. 3). At 10−7 mol l−1, myosuppressin caused a decrease in cycle frequency, an increase in burst duration and in the number of spikes per burst, and a biphasic modulation of contraction amplitude (Fig. 3Ci,Cii). Changes in number of spikes per burst closely paralleled those in burst duration, with spike frequency therefore remaining relatively constant. Changes in number of spikes per burst can thus be attributed largely to the recorded changes in burst duration. The effects on cycle frequency as well as on both contraction and burst duration began rapidly and reached a peak within 8 min after the perfusion began. The effects of myosuppressin on amplitude were more complex, with an initial attenuation followed by a more pronounced potentiation, which took approximately 16 min to reach peak levels. The effects of myosuppressin on frequency and amplitude were long-lasting, with effects significantly different from zero persisting 14 min into the wash.
Fig. 3.
Myosuppressin decreased heart rate and had complex effects on amplitude when perfused through an isolated whole heart at 10−7 mol l−1. (A) Recording on a slow time base illustrates a typical response to myosuppressin: heart rate decreased, amplitude of contractions first decreased slightly (~10% in the example shown), then dramatically increased to over 200% of its original value. (B) Higher speed recording of initial response, taken from time demarcated in A by the black lines. (C) Higher speed recordings of heartbeat and associated neural activity, as recorded extracellularly on the anterior lateral nerve in control (Ci) and 4 min after the onset of myosuppressin perfusion (Cii), as demarcated in B. Cycle frequency decreased, contraction amplitude increased, and both burst and contraction duration increased in the presence of 10−7 mol l−1 myosuppressin.
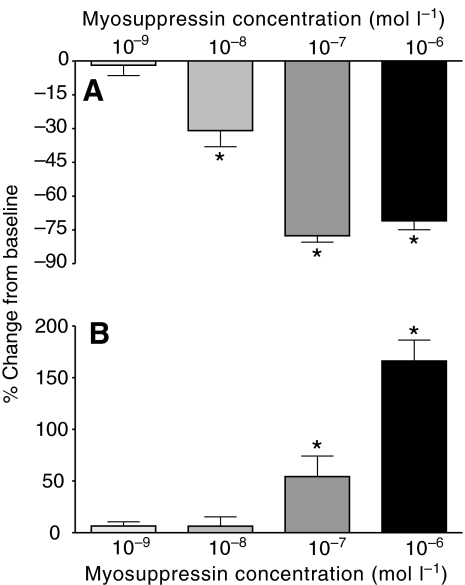
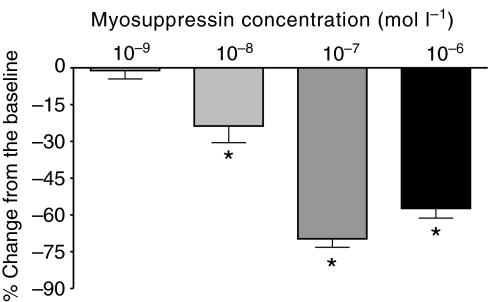
To understand the potency of myosuppressin as a modulator, we perfused a range of concentrations (10−9, 10−8, 10−7 and 10−6 mol l−1) of the peptide through the heart (N=6, 6, 11 and 14, respectively). To compare quantitatively the effects myosuppressin had on amplitude across preparations and concentrations, we averaged the heart beat parameters during a 200 s period at the time of maximal effects (decreased frequency and increased amplitude) for each preparation, and found that myosuppressin modulated the whole heart in a dose-dependent manner. Specifically, myosuppressin caused dose-dependent decreases in cycle frequency and increases in the amplitude of heart contractions (Fig. 4). The threshold concentration for these effects, as defined by the concentration at which the percentage change is significantly different from zero (P<0.05), was 10−8 mol l−1 for frequency (P=0.7004, 0.0076, <0.0001, <0.0001) and 10−7 mol l−1 for amplitude (P=0.1705, 0.5074, 0.0209, <0.0001, for concentrations of 10−9, 10−8, 10−7 and 10−6 mol l−1, respectively). At the maximal concentration, myosuppressin produced large changes in both of these parameters (frequency: −70.97±3.97%; amplitude: 166.1±20.3%).
Fig. 4.
Both heartbeat frequency and contraction amplitude in the whole heart preparation changed in a dose-dependent manner in the presence of myosuppressin. Pooled data from preparations exposed to concentrations of myosuppressin ranging from 10−6 to 10−9 mol l−1 showed that heart rate decreased (A) and contraction amplitude increased (B), with larger effects at higher concentrations. Threshold for cycle frequency, defined as the concentration needed to produce a change significantly different from zero, was between 10−8 and 10−9 mol l−1; threshold for changes in amplitude was between 10−7 and 10−8 mol l−1. *Significantly different from zero, one-sample, two-tailed t-test, P<0.05. N=6, 6, 11, 14 for 10−9, 10−8, 10−7 and 10−6 mol l−1 myosuppressin applications. Error bars represent standard errors.
Additionally, myosuppressin altered the duration of heart contractions, with increasing concentrations causing larger increases in duration (Fig. 5A,B). The increased duration resulted from increases in both rise time (Fig. 5B,C) and fall time (Fig. 5B,D), with the largest effect on the time to the peak of the contraction (rise time). To compare visually the durations of recorded contractions in myosuppressin to those in control saline, we scaled the amplitude of a single control contraction to that of a myosuppressin contraction (Fig. 5B); a longer rise time is clearly the largest contributor to the lengthened contraction duration. Thresholds for changes in these three parameters were all 10−8 mol l−1 (contraction duration: P=0.4195, 0.0025, 0.0002, <0.0001; rise time: P=0.6671, 0.0027, 0.0065, <0.0001; fall time: P=0.2544, 0.0032, <0.0001, <0.0001; for 10−9, 10−8, 10−7 and 10−6 mol l−1, respectively). The increases in contraction duration in turn were, at least in part, the result of increases in the duration of the burst recorded in the motor nerve (Fig. 3C and Fig. 5B,E). Threshold for these changes was, however, slightly higher than the effects on contraction duration, with percentage change being significantly different from zero only at concentrations of 10−7 mol l−1 or higher (burst duration: P=0.1758, 0.5180, 0.0091, 0.0024 for 10−9, 10−8, 10−7 and 10−6 mol l−1, respectively). This suggested the possibility that factors other than burst duration might also be involved. Like the effects on cycle frequency and amplitude, the changes in both contraction duration and burst duration were substantial, with changes at the highest concentrations of 57.1±5.4% for contraction duration, 88.2±9.6% for rise time, 34.9±5.0% for fall time, and 104.9±26.8% for burst duration.
Fig. 5.
Myosuppressin caused dose-dependent increases in contraction duration in whole heart preparations, with similar effects on a range of parameters related to duration. (A) Duration of contractions, measured at half-amplitude, increased with increasing myosuppressin concentration. (B) Recordings of a single contraction in control saline (black) and in myosuppressin (red) illustrate the difference in time course and amplitude of heart beats. The recording in control saline has been scaled to the amplitude of the contraction in myosuppressin (blue), showing that the biggest change is in the rate and duration of the rising phase of the contraction. (C) Rise time, measured as the time from the onset of contraction to the maximum contraction, increased with increasing myosuppressin concentration. (D) Fall time, measured as the time from the peak of contraction to the return to baseline tension, increased to a lesser extent. (E) Burst duration, measured on the anterolateral nerves, likewise increased with increasing myosuppressin concentration. A,C—E are all shown on the same vertical scale to facilitate comparisons between graphs. (F) Number of spikes per burst similarly increased with increasing myosuppressin concentration; spike frequency (not shown) thus remained approximately constant. Threshold for contraction duration, rise time and fall time was between 10−8 and 10−9 mol l−1; threshold for burst duration and number of spikes per burst was between 10−7 and 10−8 mol l−1. *Significantly different from zero, one-sample, two-tailed t-test, P<0.05. N=6, 6, 11, 14 for 10−9, 10−8, 10−7 and 10−6 mol l−1 myosuppressin, respectively. Error bars represent standard errors.
As burst duration increased, the number of spikes per burst recorded on the motor nerves likewise increased, with the same threshold (10−7 mol l−1) for differences significantly different from zero (Fig. 5F; P=0.1013, 0.0566, 0.0135, 0.0007 for 10−9, 10−8, 10−7 and 10−6 mol l−1, respectively). Thus, as can be seen in the recordings in Fig. 3 and Fig. 5B, average spike frequency within bursts changed very little, with a maximal change of 16.6±7.4% when the heart was perfused with 10−6 mol l−1 myosuppressin.
Finally, we asked whether myosuppressin altered the duty cycle for contractions. Although both burst duration and cycle period increased, there was a larger increase in cycle period than in duration; thus, duty cycle decreased in the presence of myosuppressin. The threshold concentration at which the percentage change in this parameter was significantly different from zero, like that for cycle frequency, was 10−8 mol l−1, with the effect appearing to saturate at a concentration of 10−7 mol l−1 (Fig. 6; duty cycle: P=0.7646, 0.0174, <0.0001, <0.0001 for 10−9, 10−8, 10−7 and 10−6 mol l−1, respectively).
Fig. 6.
Duty cycle, calculated as the contraction duration per cycle period, decreased as myosuppressin concentration increased to 10−7 mol l−1, after which it remained relatively constant. Both contraction duration and cycle period increased, but the increase in cycle period was greater, leading to a decrease in duty cycle. Threshold was between 10−8 and 10−9 mol l−1; * Significantly different from zero, one-sample, two-tailed t-test, P<0.05. N=6, 6, 11, 14 for 10−9, 10−8, 10−7 and 10−6 mol l−1 myosuppressin, respectively. Error bars represent standard errors.
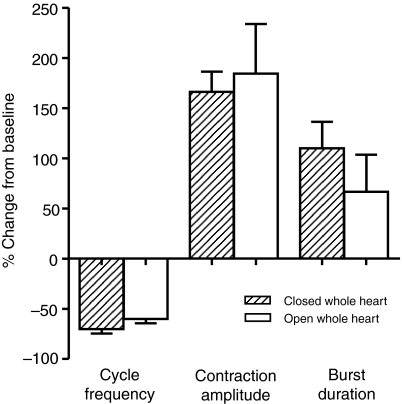
One explanation for the observed increase in amplitude is that it was an indirect effect of the decreased heart rate that resulted from myosuppressin perfusion. Because the heartbeat slowed significantly when myosuppressin was applied, the amount of time between contractions increased. Thus, since the perfusion rate was constant (2.5 ml min−1), the increased time between heartbeats might have allowed the heart to become more distended and stretched. Consequently, the observed increase in amplitude could have been due to an activation of the stretch reflex in the heart. To determine whether this was the case, we examined the effects myosuppressin had on a whole heart preparation that did not allow pressure to build up. After having recorded the response of the whole heart to myosuppressin, a midline incision was made on the ventral surface of the heart (open whole heart) to prevent perfusion pressure from building up. We then directly compared the effects of myosuppressin on the closed whole heart and on the open whole heart. The results of this experiment indicated that a stretch feedback loop did not explain the changes in amplitude in the whole heart. Cycle frequency, contraction amplitude and burst duration were all changed similarly in the two preparations by 10−6 mol l−1 myosuppressin (Fig. 7; cycle frequency: −70.26±4.52%, N=16; −60.08±4.34%, N=3; contraction amplitude: 166.1±20.26%, N=16; 184.4±49.36%, N=3; burst duration: 110.0±26.32%, N=12; 66.50±37.09, N=3; closed and open, respectively), with no significant differences between these two groups (paired t-test; P=0.3595, 0.7655 and 0.4555 for cycle frequency, contraction amplitude, and burst duration, respectively).
Fig. 7.
Eliminating the stretch caused by the build-up of perfusion fluid in the heart as heart rate slowed did not alter heart rate, contraction amplitude or the duration of bursts of action potentials recorded on the anterolateral nerves within the whole heart. Stretch was eliminated by cutting a slit in the ventral wall of the heart (open whole heart). There were no significant differences between any of the parameters measured in the open whole heart and the more intact preparation (closed whole heart), t-test, P>0.05, N=12. Error bars represent standard errors.
These experiments suggested that at least some of the effects of myosuppressin were direct effects on the CG. Both the decrease in cycle frequency and the increase in burst duration, which could be at least partially responsible for the increase in contraction amplitude, seemed likely to be direct effects of myosuppressin on the CG. To examine these mechanisms more closely, we extended our examination of modulation by myosuppressin to the CG in isolation.
Effects of myosuppressin on the isolated cardiac ganglion
To examine the effects that myosuppressin exerts on the CG when isolated from the muscle tissue, the ganglion was removed from the heart and the bursting activity of the network was monitored extracellularly using pin electrodes on the anterolateral nerves. The activity of individual motor neurons was simultaneously recorded with intracellular microelectrodes in some preparations.
Control activity in the isolated CG consisted of very regular bursts of action potentials (Fig. 8A), with burst durations, as recorded extracellularly, averaging 0.47±0.07 s, and cycle frequency averaging 0.45±0.04 Hz (26.8±2.7 beats min−1). The global effects of superfusing myosuppressin (10−7 mol l−1) over the isolated CG (Fig. 9B) were similar to those of perfusing it through the whole heart. Cycle frequency of the bursting activity recorded on the anterolateral nerves decreased dramatically, with peak effects occurring approximately 8 min after the onset of peptide application. In addition, burst duration increased, with the maximal increase slightly later, at about 10 min after the onset of peptide application. Both cycle frequency and burst duration returned to baseline values when the isolated CG was returned to saline, with significant differences from baseline lasting approximately 18 and 10 min into the wash for heart beat frequency and burst duration, respectively.
Fig. 8.
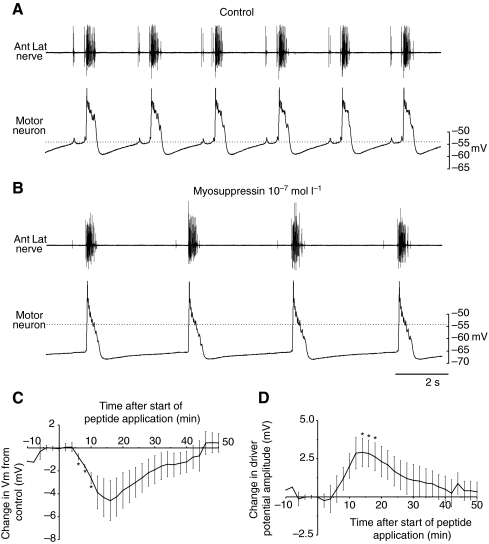
Myosuppressin superfused over the isolated cardiac ganglion (CG) decreased cycle frequency. (A) Recording of extracellular activity on the anterolateral nerve (Ant lat nerve) and a simultaneous intracellular recording from a motor neuron. Dotted line shows membrane potential just before the driver potential, at about −55 mV. (B) In 10−7 mol l−1 myosuppressin, cycle frequency decreased, and the motor neuron hyperpolarized, in this case by approx. 12 mV. It can also be seen that the duration of the depolarizing slow wave (driver potential) in the motor neuron increased in myosuppressin. Dotted line indicates the membrane potential in control saline for comparison. (C,D) Pooled data from five preparations, illustrating the time course of the changes in membrane potential (C) and driver potential amplitude (D) after the start of myosuppressin superfusion (at time=0). Myosuppressin was superfused for 8 min before the preparation was returned to control saline. Error bars represent standard errors.
Fig. 9.
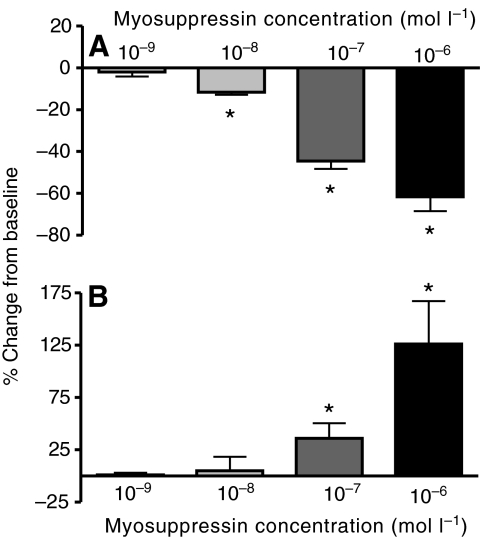
Both cycle frequency and burst duration in the isolated CG changed in a dose-dependent manner in the presence of myosuppressin. Pooled data from preparations exposed to concentrations of myosuppressin ranging from 10−6 to 10−9 mol l−1 showed that cycle frequency decreased (A) and burst duration increased (B), with larger effects at higher concentrations. Threshold for cycle frequency was between 10−8 and 10−9 mol l−1; threshold for the increase in burst duration was between 10−7 and 10−8 mol l−1. *Significantly different from zero, one-sample, two-tailed t-test, P<0.05, N=3, 3, 10, 6 for 10−9, 10−8, 10−7 and 10−6 mol l−1, respectively. Error bars represent standard errors.
In preparations in which we recorded intracellularly from the motor neurons, it could be seen that, in addition to decreasing the frequency of the bursting activity of the ganglion, superfusion of 10−7 mol l−1 myosuppressin caused a hyperpolarization of the membrane potential accompanied by an increase in the driver potential amplitude of motor neurons (Fig. 8). Consequently, the peak membrane potential was actually somewhat hyperpolarized from control.
To gain better insight into the time course of these effects, the changes in the resting membrane potential and in the driver potential amplitude were averaged for all preparations (N=5) and represented as a function of time after the onset of application of 10−7 mol l−1 myosuppressin (Fig. 8C,D). The peptide was applied for 8 min, after which the preparation was again superfused with control saline. During the superfusion of myosuppressin, the resting membrane potential hyperpolarized slowly, peaking approximately 16 min after the onset of myosuppressin superfusion, that is, about 8 min into the saline wash, with an average maximum hyperpolarization of 4.9±1.6 mV (Fig. 8C). Because of variability between preparations, with maximum hyperpolarizations ranging from 2-11 mV, the change was significantly different from 0 mV only during the rapidly changing phase from 6-10 min after the onset of myosuppressin superfusion (P<0.05), although P-values remained less than 0.07 from 10-18 min after the onset of perfusion. The driver potential amplitude increased with a similar time course, peaking 14-16 min after the start of superfusion; the change was significantly different from 0 mV from 14-18 min (P<0.05), with a maximum increase of 3.2 ±0.9 mV (Fig. 8D). Both parameters returned to baseline levels after approximately 30 min of the saline wash.
To understand the sensitivity of the isolated CG to modulation by myosuppressin, we superfused a range of concentrations of the peptide (N=3, 3, 10, 6 for 10−9, 10−8, 10−7 and 10−6 mol l−1, respectively) over the isolated CG and recorded the resultant changes in activity extracellularly on the anterolateral nerves. Myosuppressin caused dose-dependent decreases in cycle frequency and increases in the duration of bursts recorded from the anterolateral nerves (Fig. 9), with threshold concentrations of 10−8 mol l−1 for frequency and 10−7 mol l−1 for burst duration (frequency: P=0.4509, 0.0106, <0.0001, 0.0003; duration: P=0.6912, 0.7489, 0.0357, 0.0272; for 10−9, 10−8, 10−7 and 10−6 mol l−1, respectively). At the maximal concentration used, myosuppressin produced large changes in both parameters (frequency: −61.6±6.8%; duration: 126.3±40.9%).
That myosuppressin altered the bursting activity of the isolated CG suggested the possibility that changes at this level alone might explain the effects of myosuppressin on the whole heart and in the intact animal. However, the hyperpolarization of the motor neuron membrane potential also suggested that motor neuronal drive might not be sufficient to account for the large (>150%) increases in contraction amplitude recorded in the whole heart. The changes in the isolated ganglion likewise do not clearly explain the complex changes recorded in whole heart contraction amplitude, with an initial decrease followed by an increase in contraction force. Thus, we considered whether or not some of the effects of myosuppressin on the whole heart might be mediated by effects that occur at the level of either the neuromuscular junction or the muscle itself. To investigate this possibility, we examined the effects of myosuppressin on cardiac muscle, with contractions driven by controlled stimuli.
Effects of myosuppressin on stimulated heart muscle
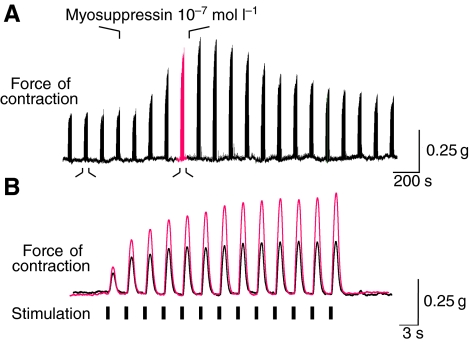
By removing all endogenous inputs from the CG, we were able to drive heart muscle contractions with a controlled stimulus that mimicked the endogenous bursting activity of the CG. We applied 300 ms bursts of short stimuli (0.5 ms each) at 60 Hz, with a burst frequency of 0.43 Hz (i.e. equivalent to 26 beats min−1). However, because hearts continued contracting consistently for many hours when controlled by the CG, but generally for less than 2 h when stimulated, we delivered these stimuli in bouts of bursts, each bout lasting 30 s (i.e. 13 bursts), once every 2 min. With these stimulation parameters, consistent contractions could be maintained for several hours. In control saline, we saw marked facilitation of contraction in response to repeated bursts of stimuli. That is, within each bout of bursts, contraction amplitude increased gradually to a peak by the eleventh or twelfth burst. The force of driven contractions was potentiated when myosuppressin was perfused through the heart at 10−7 mol l−1 (Fig. 10). The amplitude of each contraction in the stimulation series increased in the presence of 10−7 mol l−1 myosuppressin (Fig. 10B), but the extent of facilitation was not altered consistently across preparations.
Fig. 10.
Myosuppressin perfused through the heart at 10−7 mol l−1 caused an increase in the amplitude of nerve-evoked contractions. (A) One of the anterolateral nerves was stimulated with 300 ms bursts of 60 Hz stimuli. Each burst caused a single contraction of the heart. Bursts were delivered in bouts of 13 bursts once every 2 min. (A) On a slow time base, each bout is compressed to form a single peak; the amplitude of these peaks more than doubled when 10−7 mol l−1 myosuppressin was perfused through the heart. (B) Faster time scale illustrates one bout of bursts in control (black, smaller contractions) and in myosuppressin (red, larger amplitude contractions). In this example, there was a substantial increase in facilitation in myosuppressin, but statistically, facilitation did not increase across multiple preparations.
To quantify these changes, the amplitudes of the last two contractions in each series (contractions 12 and 13) were averaged and a percentage change from baseline was calculated for each preparation (N=7). Application of myosuppressin at 10−7 mol l−1 significantly increased the amplitude of contractions, by 61.4±19.1%, beginning 12 min after the onset of peptide application (P=0.0184). The modulatory effects of myosuppressin on contraction amplitude lasted 24 min into the wash. These results indicate that the effects of myosuppressin on cardiac activity are not limited to the CG, but also extend into the periphery.
Comparative analyses of the effects of myosuppressin at multiple sites in the lobster cardiac neuromuscular system
To examine the multilayered modulation of the lobster cardiac neuromuscular system by myosuppressin, we next compared the extent and timecourse of the peptide's effects on cycle frequency, contraction amplitude, and motor neuron burst duration across different preparation types at a single concentration (10−7 mol l−1) of the peptide. For these comparisons, values for the 200 s around the time of peak change were used to calculate percentage change from baseline (200 s before the onset of peptide application). In addition, we compared the effects of myosuppressin on the duration of single spontaneous contractions in the whole heart to those of single stimulated contractions (Fig. 11).
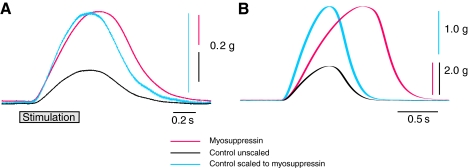
Fig. 11.
Comparison of the effects of 10−7 mol l−1 myosuppressin on the time courses of single heart contractions evoked by controlled nerve stimulation (A) with those generated by spontaneous neuronal activity (B). Control contractions are shown in black; myosuppressin are in red; control contractions scaled to the amplitude of myosuppressin contractions are in blue. Myosuppressin increased the duration of contractions elicited by controlled stimulation, but to a much smaller extent than those generated by spontaneous activity of the CG. Only the falling phase of stimulation-evoked contractions was increased by myosuppressin; both the rising and falling phases of CG-evoked contractions were slowed by myosuppressin.
Contraction duration
A comparison of contraction durations suggests that the alterations in both the overall duration and the detailed timecourse of individual heart contractions result largely from changes in the bursts that drive the contractions. Myosuppressin does cause a small increase in the duration of stimulation-evoked contractions (Fig. 11A), indicating that myosuppressin has some effects on duration at the level of the muscle or neuromuscular junction. However, the changes in duration evoked during ongoing spontaneous activity are considerably larger (Fig. 11B). Moreover, it is interesting to note that the recorded force rises more slowly during spontaneous contractions in myosuppressin than in control saline, whereas the rates of rise in response to nerve stimulation are virtually the same in myosuppressin and in control saline.
Motor neuron burst duration
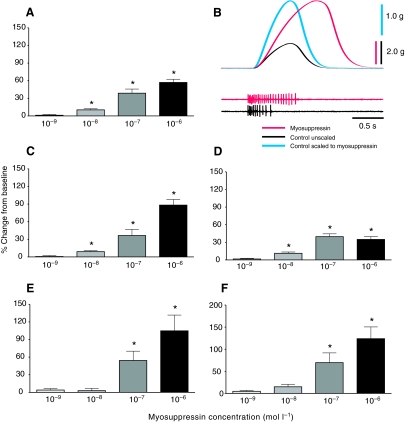
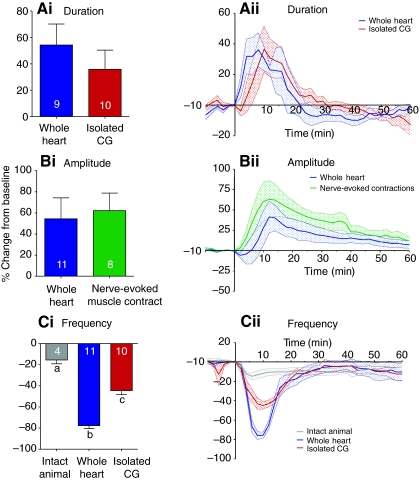
We were able to compare the effects of myosuppressin on motor neuron burst duration in two preparation types, the whole heart and the isolated CG. The effects it evoked in burst duration were similar in the two preparation types (Fig. 12Ai). Myosuppressin caused increases in the duration of bursts recorded extracellularly from the anterolateral nerve in the whole heart (54.3±16.0%; P=0.0091, N=9) and in the isolated CG (35.8±14.5%; P=0.0357, N=10), with no significant differences in these two averages (P=0.4026). The time courses of effects on burst duration were also similar in these two preparations (Fig. 12Aii). Burst duration began increasing almost immediately in both preparations, and reached peak effects at 8 min in the whole heart and 10 min in the isolated CG. Myosuppressin caused changes that were significantly different from zero from 4-12 min in the whole heart and from 10-18 min in the isolated CG.
Fig. 12.
Comparisons of the extents and time courses of the effects of 10−7 mol l−1 myosuppressin on burst duration, contraction amplitude and heart beat frequency in the four preparation types. (A) Burst duration was increased to similar extents (Ai) and with similar time courses (Aii) in the whole heart and the isolated CG. (B) Contraction amplitude was increased to similar extents (Bi) and with similar time courses (Bii) in the whole heart and in the heart stimulated with controlled pulses (nerve-evoked muscle contraction). (C) The effects of myosuppressin on heartbeat frequency differed significantly among preparation types (ANOVA, P<0.05). Post-hoc t-tests indicated that the values for all three types of preparation, i.e. the intact animal, the whole heart and the isolated CG, differed from each other (P<0.05), with significantly larger decreases in the whole heart than in the isolated CG, and larger decreases in the isolated CG than in the intact animal. Comparison of time courses in Ai, Bii and Cii suggests that the changes in cycle frequency peaked sooner than did changes in contraction amplitude. Different letters over bars indicate values that were significantly different from one another. N values are indicated on each bar in Ai, Bi and Ci. Stippled shading in Aii, Bii and Cii indicates standard errors.
Contraction amplitude
The changes in amplitude caused by application of myosuppressin were remarkably similar across preparation types (Fig. 12Bi). In data pooled from multiple preparations, the only significant changes in amplitude were the delayed increases in force recorded in both the whole heart (54.33±19.84%; P=0.0209, N=11) and in the stimulated heart (62.10±16.58%; P=0.0072, N=7). A two-tailed unpaired t-test revealed that the changes in amplitude of these two preparations were not significantly different (P=0.8137). Because the initial decrease in contraction amplitude in the whole heart was smaller than the later increase, and varied somewhat in its duration, this decrease was not obvious in the pooled data; however, a clear initial decrease was seen in all eleven hearts examined at this concentration of myosuppressin. By contrast, an initial decrease in amplitude was seen in only half of the stimulated hearts. This may be partly explained by the fact that we stimulated the heart in bouts of bursts every 2 min; thus, it is possible that there was in fact an initial decrease, but that it was over or largely over by the time the preparation was stimulated.
The peptide elicited similar effects on contraction amplitude in spontaneously active and nerve-stimulated preparations; the time courses for these effects were also similar. Modulation of amplitude began within 1-2 min of the onset of peptide perfusion and reached a maximum at 16 and 12 min for the whole heart and stimulated heart, respectively. The effects of myosuppressin on contraction amplitude were significantly different from zero from 16-22 min in the whole heart and from 10-32 min in the stimulated heart. Similar to the changes seen in motor neuron burst duration, these changes in contraction amplitude were similar at different levels of the system.
Heartbeat frequency
By contrast, although myosuppressin reliably decreased the cycle frequency of the heart contractions and bursting activity of the motor neurons in the intact animal, whole heart, and isolated CG, the extents of these effects were not consistent across preparation types (Fig. 12Ci). The effects were smallest in the intact animal (−15.4±3.7%, N=4), largest in the whole heart (−77.6±2.8%, N=11) and moderate in the isolated CG (−44.5±3.8%, N=10). A one-way ANOVA revealed significant group differences (P<0.0001) and a Tukey's post-hoc test revealed that the effects of myosuppressin on cycle frequency were significantly different for all group comparisons (Fig. 12Ci; all P<0.05).
Although the time courses of the effects of myosuppressin on cycle frequency were similar among the three preparation types (Fig. 12Cii), these effects appeared to occur sooner than the effects on amplitude, particularly in the whole heart. Specifically, heartbeat frequency peaked at about 8 min, whereas maximum amplitude in the whole heart was not reached until about 16 min, after the onset of myosuppressin perfusion. This may reflect the biphasic effect of myosuppressin on amplitude: changes in frequency appear to peak at about the same time that the amplitude reaches its minimum value before beginning to increase towards its peak.
DISCUSSION
Confirmation of the structure of Homarus americanus myosuppressin
The myosuppressin subfamily of FMRFamide-like peptides is characterized by the C-terminal motif —HVFLRFamide (Vilaplana et al., 2004). Previously, a peptide with the sequence pQD(L/I)DHVF(L/I)RFamide was identified by mass spectrometry from the nervous system of H. americanus (Stemmler et al., 2007; Ma et al., 2008), however, the position 3 and 8 amino acids remained ambiguous as the mass spectral platforms used for the identification of the peptide could not distinguish leucine from isoleucine, because the two residues are isobaric. In the study described here, we cloned the precursor for this peptide, deducing the two ambiguous residues to be leucines. Thus, the sequence of the peptide can now be conclusively assigned as pQDLDHVFLRFamide, which contains the consensus motif that defines the myosuppressin family.
Delivery of myosuppressin to the lobster heart appears to be hormonal
H. americanus possesses an extensive collection of FMRFamide-like peptides (Trimmer et al., 1987; Dickinson et al., 2007a; Stemmler et al., 2007; Ma et al., 2008), including the myosuppressin isoform studied here (Stemmler et al., 2007; Ma et al., 2008). Previous immunohistochemical and/or biochemical/mass spectral mapping of the distribution of this peptide family have shown that these peptides are broadly distributed within the nervous system of the lobster (e.g. Kobierski et al., 1987; Schmidt, 1997; Schmidt and Ache, 1997; Kilman et al., 1999; Pulver and Marder, 2002; Stemmler et al., 2007; Ma et al., 2008); they are present in the PO (Pulver and Marder, 2002), one of the major neuroendocrine organs of this species (Cooke and Sullivan, 1982), as well as in regions of synaptic neuropil, e.g. the brain and stomatogastric nervous system. Because the antibodies used to map the distribution of FMRFamide-like labeling in H. americanus appear to cross-react broadly with members of this peptide family (A.E.C., unpublished data), it is not possible to ascertain which of the various family members are responsible for the labeling in any given structure. Mass spectral studies, however, have detected pQDLDHVFLRFamide in the PO, confirming that it is, at least in part, responsible for the labeling present there (Ma et al., 2008). In this study, we examined the CG for evidence of intrinsic FMRFamide-like peptides using the same anti-FMRFamide antibody previously used to map the distribution of the peptide family in other portions of the lobster nervous system. As no FMRFamide-like labeling was detected in the CG, it appears unlikely that any member of the family, including myosuppressin, could be released within the ganglion itself. Given the lack of peptide in the CG, but its presence in the PO, it would appear that mode of delivery of pQDLDHVFLRFamide, and in fact all FMRFamide-like peptides, to the cardiac neuromuscular system must be via the hemolymph from endocrine sites, including the PO. Thus, the physiological actions we report for the peptide on this system must be hormonal in origin.
The effects of pQDLDHVFLRFamide on the lobster cardiac neuromuscular system involve modulation at multiple sites
Interestingly, most of the numerous peptides and amines that have been shown to modulate the intact or semi-intact decapod heart, e.g. proctolin (Kuramoto and Ebara, 1984; Wilkens et al., 2005; Wilkens and McMahon, 1992; Wilkens and McMahon, 1994; Worden and Goy, 1995; Saver and Wilkens, 1998; Saver et al., 1998), serotonin (Battelle and Kravitz, 1978; Wilkens and McMahon, 1992; Florey and Rathmayer, 1978), octopamine (Grega and Sherman, 1975; Florey and Rathmayer, 1978; Battelle and Kravitz, 1978), calcitonin-like diuretic hormone (Christie et al., 2010), crustacean cardioactive peptide (CCAP) (Wilkens and McMahon, 1992; Fort et al., 2007b), and a number of FMRFamide-like peptides (McGaw and McMahon, 1995; Wilkens et al., 2005; Mercier and Russenes, 1992; Fort et al., 2007a; Worden et al., 1995; Wilkens and McMahon, 1992; McGaw et al., 1995; Krajniak, 1991; Dickinson et al., 2007a), are generally cardioexcitatory, causing increases in both amplitude and frequency of the heartbeat. This group of excitatory modulators includes all of the other FMRFamide-like peptides that have been examined. Only a C-type allatostatin-like peptide (Dickinson et al., 2009) and two other isoforms of myosuppressin, PDVDHVFLRFamide (SchistoFLRFamide) (Mercier and Russenes, 1992) and pQDVDHVFLRFamide (leucomyosuppressin) (Mercier and Russenes, 1992) have consistently been shown to decrease contraction frequency in the whole heart.
When their effects are examined on the isolated CG, most modulators that have been examined are likewise excitatory [e.g. proctolin (Miller and Sullivan, 1981; Sullivan and Miller, 1984; Freschi, 1989; Saver et al., 1999) serotonin (Cooke and Hartline, 1975; Cooke, 1966; Saver et al., 1999; Lemos and Berlind, 1981) and acetylcholine (Freschi and Livengood, 1989)]. However, several substances, including gamma-amino-butyric acid (GABA) (Kerrison and Freschi, 1992; Cruz-Bermúdez and Marder, 2007) and an A-type allatostatin in Cancer borealis (Cruz-Bermúdez and Marder, 2007) decrease cycle frequency in the isolated cardiac ganglion. The effects reported for the FMRFamide-like peptides have been contradictory. When applied to the isolated CG of C. borealis (Cruz-Bermúdez and Marder, 2007), the FMRFamide-like peptide SDRNFLRFamide was excitatory, evoking an increase in both cycle frequency and number of spikes per burst. Similarly, Saver and colleagues found that SDRNFLRFamide, when pressure ejected onto localized portions of the isolated CG or onto motor neurons isolated and cultured from the blue crab C. sapidus, caused an increase in burst rate (Saver et al., 1999). However, in another study (Fort et al., 2007a), bath application of the same peptide, as well as application of two other FMRFamide-like peptides, TNRNFLRFamide and the native C. sapidus isoform GYNRSFLRFamide, caused a decrease rather than an increase in cycle frequency in the isolated C. sapidus CG. Surprisingly, when applied to the whole heart, the effects of the same three FMRFamide-like peptides were excitatory, causing increases in both amplitude and frequency of contraction in C. sapidus. Thus, there appear to be differences between the effects of peptides in this family, either between species or as a result of methodological differences. In addition, even within a single species, there are differences in the effects of the peptides when they are applied to the isolated CG and when the heart is intact. Fort et al. (Fort et al., 2007a) suggested that these contradictory effects of the three FMRFamide-like-peptides result from a combination of direct effects on the ganglion, direct effects on the periphery, and the resultant feedback to the CG from the enhanced contraction, probably via the stretch-sensitive dendrites of the CG neurons.
The effects of myosuppressin on the whole heart recorded in our study resembled those induced by SchistoFLRFamide and leucomyosuppressin in the crayfish (Mercier and Russenes, 1992), with a few exceptions. Specifically, both of these myosuppressins caused a decrease in heart rate, as did myosuppressin in our study. SchistoFLRFamide evoked first a decrease, then a dramatic increase in contraction amplitude, as was seen here. By contrast, leucomyosuppressin caused only a modest increase in contraction amplitude. However, neither of these peptides, both of which were identified in insects, is the native crayfish peptide. Instead, the native isoform of myosuppressin in the crayfish appears to be the same as that used in this study, i.e. pQDLDHVFLRFamide (Stemmler et al., 2007). The physiological effects of pQDLDHVFLRFamide on the crayfish heart have not yet been tested.
By examining the effects of myosuppressin in the intact animal, the whole heart, the isolated CG and on cardiac muscle/neuromuscular junction, we were able to determine that the global effects of myosuppressin represent the integration of specific effects on a number of different sites. Moreover, our data suggest that this peptide may also modulate one or more of the feedback systems that exist between the cardiac muscle and the neural circuit that generates the heartbeat.
Modulation of contraction amplitude and duration
Our data suggest that the increases in contraction amplitude evoked by myosuppressin result largely from effects on the periphery, either at the neuromuscular junction or in the cardiac muscle itself, but that increases in contraction duration are due largely to effects on the CG. We saw similar increases in motor neuron burst duration in the isolated CG and in the whole heart, suggesting that the increase in the neuronal drive was largely responsible for the increased contraction duration. However, a close comparison of individual contractions generated by stimulating the heart with identical inputs showed that myosuppressin also caused the duration of this stimulation-evoked contraction to increase slightly, suggesting that there are also peripheral contributions to the increased contraction duration. Interestingly, most of the increase in contraction duration was due to a prolongation and slowing of the rising phase of the contraction. This contrasts with the increased contraction duration seen in the lobster heart in nitric oxide, in which the relaxation rate of muscle contraction is altered to change contraction duration (Mahadevan et al., 2004). Similarly, modulation of contraction duration in several Aplysia californica muscles is due primarily to changes in relaxation rate (e.g. Evans et al., 1999; Hurwitz et al., 2000; Vilim et al., 2000; Weiss et al., 1992). However, in at least one of the same A. californica muscles in which relaxation rate is altered by neuropeptides, application of serotonin alters the rate at which tension is developed; this effect appears to occur largely at the level of the muscle itself, since tension developed in response to single action potentials rises more quickly in serotonin. The mechanism responsible for the decreased rate of contraction and the prolongation of the burst in the lobster heart is more likely to be a result of changes in the motor neuron burst characteristics, since the rate of tension development did not change when contraction was controlled by motor neuron stimulation.
The observed increases in burst duration might also be responsible for some of the increase in contraction amplitude, but duration increases were relatively small, and are thus unlikely to account entirely for the large increases in contraction amplitude. Another factor that could affect the extent of contraction amplitude is the amplitude of the driver potential in the motor neurons of the CG, which also increased slightly. This increase can be explained largely by the increased driving force that would result from the hyperpolarization of motor neuron membrane potential recorded in myosuppressin. Part of this increased driver potential amplitude might also be due to the longer delay between motor neuron depolarizations, as was shown by Tazaki and Cooke (Tazaki and Cooke, 1990). In spite of the slight increase in driver potential amplitude we observed, the peak of the driver potential was hyperpolarized relative to control, suggesting that increased motor neuron drive was not likely to be responsible for the increase in contraction amplitude.
We considered that the very large increase in contraction amplitude recorded in the presence of myosuppressin might result from the activation of a stretch feedback loop (reviewed by Cooke, 2002). Because the heart was perfused at a constant rate, the increased delay between beats as the heart rate slowed would be expected to result in an increased stretch of the heart itself. This could in turn, via the stretch-sensitive dendrites of CG neurons (Alexandrowicz, 1932; Sakurai and Wilkens, 2003), lead to enhanced ganglionic activity and thus increased contraction amplitude. Alternatively, increased stretch might directly, via a Frank—Starling type mechanism, lead to increased contraction force. To determine whether one of these mechanisms might be responsible for the increased contraction amplitude, we eliminated the increased stretch by cutting open the heart so that no pressure could build up. Under these conditions, the effects of myosuppressin on contraction amplitude, as well as on heartbeat frequency, were not different from those recorded in the intact whole heart, suggesting that stretch feedback is not an important contributor to the modulatory effects of myosuppressin.
These data, together with the observed similarity of the effects of myosuppressin on the whole heart and on the stimulated muscle preparation, where neuronal input was constant, suggested that myosuppressin exerted its effects on contraction amplitude largely by modulating at the periphery. We do not currently have data to determine whether the peripheral modulation is at the neuromuscular junction or whether myosuppressin alters the contractility of the cardiac muscle directly. A number of previous studies have shown that many modulators act by altering transmission at the neuromuscular junction in both cardiac muscle [e.g. CCAP (Fort et al., 2007b)] and other skeletal muscles [e.g. TNRNFLRFamide, SDRNFLRFamide, serotonin, proctolin, dopamine and several A-type allatostatins (Mercier et al., 1993; Weiss et al., 2003; Worden et al., 1995; Jorge-Rivera et al., 1998; Jorge-Rivera and Marder, 1996)], suggesting this as a probably site for the peripheral modulation. Studies examining cardiac muscle itself have found that at least some modulators have direct effects on the muscle, largely by altering calcium-dependent processes (e.g. proctolin and SDRNFLRFamide) (Wilkens et al., 2005). Further experimentation will be required to determine if the site of peripheral modulation by myosuppressin is the muscle itself or the synapse, and if the latter, whether myosuppressin is altering transmission pre-synaptically, post-synaptically, or both.
The peripheral changes do not, however, readily explain the initial decrease in amplitude that we observed in every preparation. Although there were decreases in the amplitude of nerve-evoked contractions in a few preparations, these were small and not consistent between preparations, so direct effects of myosuppressin at the periphery seem unlikely to explain the initial decrease in contraction amplitude. One possible explanation for this decrease is a change in facilitation at the neuromuscular junction. Specifically, as the burst frequency decreased, facilitation between bursts would likewise decrease, leading to smaller contractions in response to each motor nerve burst. Changes in facilitation have similarly been proposed as a part of the explanation for some of the effects of other modulators (e.g. Mahadevan et al., 2004; Fort et al., 2007a; Fort et al., 2007b). If facilitation is indeed responsible for the initial decrease in amplitude, with the subsequent increase being due to effects on the periphery, then we would predict that the decrease in cycle frequency, mediated by the CG, would occur sooner than the increases in amplitude, both those evoked by nerve stimulations and those recorded in the whole heart. A comparison of the time courses in Fig. 12 shows this to be the case, suggesting that facilitation is sufficient to explain the early decrease in contraction amplitude.
Modulation of heart rate
In contrast to its effects on amplitude and duration, the effects of myosuppressin on cycle frequency differed significantly across preparation types. The smaller effect on heart rate in the whole animal could be due to any of several factors. First, concentrations of the peptide might have differed from our estimate of 10−7 mol l−1, which was based on dilution into a volume of 15 ml, the average volume we measured for the hemolymph cavity just outside the heart. However, it is difficult to measure the volume of the hemolymph cavity precisely. Moreover, hemolymph in this region would be rapidly diluted by ongoing cardiac activity. Additionally, proteases present in the hemolymph may cause rapid peptide breakdown; similarly, other proteins could bind to the peptide and render it inactive. Finally, myosuppressin certainly has other effects within the lobster; it is possible that it triggers the activation of neural inputs to the heart or the release of other neuroactive substances that counteract its effects in the intact animal. Interestingly, at least several of the peptides that are excitatory on isolated hearts are inhibitory when injected into the intact animal [e.g. CCAP (McGaw et al., 1994) FMRFamide-like peptides TNRNFLRFamide and SDRNFLRFamide (McGaw et al., 1995)], suggesting that these peptides may alter cardiac function indirectly as well as directly, as suggested here for myosuppressin. Our whole animal data do suggest, in any case, that myosuppressin released hormonally in the whole animal could have the effect of temporarily decreasing heart rate.
The fact that heart rate is slowed significantly more in the whole heart than in the isolated ganglion is intriguing. Because contractions are driven directly by the CG, and because cardiac muscle in H. americanus is neurogenic (Alexandrowicz, 1932), we had predicted that the effects of myosuppressin on heart rate would be determined solely by its effects on the CG. One possible explanation, which has been suggested for a similar observation in which nitric oxide causes a larger decrease in frequency in the whole heart than in the isolated CG, is that the initial frequency in the whole heart is greater; thus, the percentage change is likely to be greater as well (Mahadevan et al., 2004). However, although we, too, found that the control frequency was higher in the whole heart than in the isolated CG, this factor seems unlikely to account for the greater effect of myosuppressin on frequency in the whole heart than in the isolated CG. In nitric oxide, the frequency of both preparation types decreased to about the same final value (Mahadevan et al., 2004), whereas in myosuppressin, the absolute frequency in myosuppressin was, like the percentage change, significantly lower in the whole heart than in the isolated CG (average 0.14±0.02 Hz in the whole heart versus 0.28±0.05 Hz in the isolated CG).
There are a number of documented cases in which non-oscillatory neurons that are electrotonically coupled to pacemaker neurons can alter the cycle frequency of the pacemaker (e.g. Hooper and Marder, 1987; Kepler et al., 1990; Marder et al., 1992); however, the pacemaker neurons in the cardiac system are not connected electrically to the muscle. There is electrical coupling between motor neurons within the cardiac ganglion (Watanabe, 1958; Hagiwara et al., 1959; Watanabe and Takeda, 1963; Connor, 1969), and there is feedback, either electrotonic, chemical, or both, from the large motor neurons to the pacemakers, which has been shown to alter cycle frequency (Watanabe, 1958; Connor, 1969; Mayeri, 1973; Watanabe and Bullock, 1960). However, the motor neurons appear to synapse onto the cardiac muscle using conventional chemical synapses (Anderson and Cooke, 1971).
Fort and colleagues have recently documented the importance of feedback in the blue crab C. sapidus cardiac neuromuscular system, and have pointed to the role it plays in shaping the modulatory effects of dopamine (Fort et al., 2004), CCAP (Fort et al., 2007b) and several different FLRFamide-like peptides [TNRNFLRFamide, SDRNFLRFamide and GYNRSFLRFamide (Fort et al., 2007a)]. They recorded effects that differed in whole heart preparations, the isolated CG and stimulated heart muscle preparations, and were able to explain these only as a combination of effects on more than one site, together with the secondary consequences of those changes that were induced by the feedback systems within the heart (Fort et al., 2004; Fort et al., 2007a; Fort et al., 2007b). We have similarly seen complex effects that can only be explained by multiple effects and the resultant activation of feedback systems.
Two feedback systems have been described in the lobster cardiac neuromuscular system, one involving stretch, as discussed earlier, the second involving the gaseous messenger, nitric oxide (NO). In our experiments in which the heart was opened, so that changes in baseline stretch were minimized, we saw no difference in frequency, suggesting that stretch feedback plays a minimal role in the effects of myosuppressin. However, the periodic stretch of each contraction was still potentiated by the myosuppressin, and it has been shown the isopod Ligia pallasii that periodic stretches can alter heart rate by phase advancing or delaying the next beat (Sakurai and Wilkens, 2003), so we cannot entirely rule out this possibility.
Cardiac muscle in H. americanus contains high levels of nitric oxide synthase (NOS); NO produced in the muscle could readily diffuse to the CG, where it has been shown to decrease heart rate (Mahadevan et al., 2004). Thus, if by activating muscle contraction, myosuppressin were to increase the activity of NOS, the increased NO production could help to account for the larger decrease in heart rate when the cardiac muscle is present than when it is absent. Although NO also decreases contraction amplitude, this is an indirect consequence of the slower frequency and the resultant decrease in facilitation at the neuromuscular junction (Mahadevan et al., 2004). Any such decrease in facilitation in our preparations would probably have been overwhelmed by the large increase in contraction amplitude that appears to be a direct effect of the myosuppressin. Whether the increased amplitude of contraction is sufficient to activate NOS to the extent required for NO to be responsible for the additional decrease in frequency remains to be tested. Alternatively, myosuppressin may modulate the feedback mechanisms from the cardiac muscle to the CG, thus potentiating the effect of the peptide on the CG.
Overall, myosuppressin appeared to cause a unique suite of effects on the cardiac neuromuscular system, effects that can only be explained by the integration of multiple effects that occur at different sites. Contraction frequency decreased, while amplitude first decreased, then increased substantially (to more than double the initial value in 10−7 mol l−1 myosuppressin). To achieve these effects, myosuppressin acted on the CG to cause an increase in cycle frequency and burst duration; it acted on the muscle or the neuromuscular junction to cause an increase in contraction amplitude; and it is possible that it acts on one of the feedback systems (possibly NO) to amplify the effects of the peptide on the ganglion itself.
This study was supported by grants from the National Center for Research Resources' INBRE Program (NIH P20 RR-016463 to Patricia Hand), the Paller Fund of Bowdoin College, National Science Foundation grant IBN 01140, the Howard Hughes Medical Institute Undergraduate Science Program, and institutional funds provided by MDIBL and by Bowdoin College. Deposited in PMC for release after 12 months.
- CCAP
- crustacean cardioactive peptide
- CG
- cardiac ganglion
- CPG
- central pattern generator
- ESI-Q-TOF MS/MS
- electrospray ionization quadrupole time-of-flight tandem mass spectrometry
- EST
- expressed sequence tag
- Homam-CLDH
- Homarus americanus calcitonin-like diuretic hormone
- MALDI-FTMS
- matrix assisted laser desorption/ionization Fourier transform mass spectrometry
- NO
- nitric oxide
- NOS
- nitric oxide synthase
- P
- 0.1 mol l−1 sodium phosphate buffer
- PO
- pericardial organ
- P-Triton
- 0.1 mol l−1 sodium phosphate buffer containing 0.3% Triton X-100
REFERENCES
- Alexandrowicz J. S. (1932). Innervation of the heart of the Crustacea. I. Decapoda. Q. J. Microsc. Sci. 75, 182-249 [Google Scholar]
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389-3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M., Cooke I. M. (1971). Neural activation of the heart of the lobster Homarus americanus. J. Exp. Biol. 55, 449-468 [DOI] [PubMed] [Google Scholar]
- Battelle B. A., Kravitz E. A. (1978). Targets of octopamine action in the lobster: cyclic nucleotide changes and physiological effects in hemolymph, heart and exoskeletal muscle. J. Pharmacol. Exp. Ther. 205, 438-448 [PubMed] [Google Scholar]
- Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004). Improved prediction of signal peptides: SignalP 3. 0. J. Mol. Biol. 340, 783-795 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Skiebe P., Marder E. (1995). Matrix of neuromodulators in neurosecretory structures of the crab Cancer borealis. J. Exp. Biol. 198, 2431-2439 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Kutz-Naber K. K., Stemmler E. A., Klein A., Messinger D. I., Goiney C. C., Conterato A. J., Bruns E. A., Hsu Y. W., Li L., et al. (2007). Midgut epithelial endocrine cells are a rich source of the neuropeptides APSGFLGMRamide (Cancer borealis tachykinin-related peptide Ia) and GYRKPPFNGSIFamide (Gly1-SIFamide) in the crabs Cancer borealis, Cancer magister and Cancer productus. J. Exp. Biol. 210, 699-714 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Cashman C. R., Stevens J. S., Smith C. M., Beale K. M., Stemmler E. A., Greenwood S. J., Towle D. W., Dickinson P. S. (2008). Identification and cardiotropic actions of brain/gut-derived tachykinin-related peptides (TRPs) from the American lobster Homarus americanus. Peptides 29, 1909-1918 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Stevens J. S., Bowers M. R., Chapline M. C., Jensen D. A., Schegg K. M., Goldwaser J., Kwiatkowski M. A., Pleasant T. K., Shoenfeld L., et al. (2010). Identification of a calcitonin-like diuretic hormone that functions as an intrinsic modulator of the American lobster, Homarus americanus, cardiac neuromuscular system. J. Exp. Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. (1969). Burst activity and cellular interaction in the pacemaker ganglion of the lobster heart. J. Exp. Biol. 50, 275-295 [DOI] [PubMed] [Google Scholar]
- Cooke I. M. (1966). The sites of action of pericardial organ extract and 5-hydroxytryptamine in the decapod crustacean heart. Am. Zool. 6, 107-121 [DOI] [PubMed] [Google Scholar]
- Cooke I. M. (2002). Reliable, responsive pacemaking and pattern generation with minimal cell numbers: the crustacean cardiac ganglion. Biol. Bull. 202, 108-136 [DOI] [PubMed] [Google Scholar]
- Cooke I. M., Hartline D. K. (1975). Neurohormonal alteration of integrative properties of the cardiac ganglion of the lobster Homarus americanus. J. Exp. Biol. 63, 33-52 [DOI] [PubMed] [Google Scholar]
- Cooke I. M., Sullivan R. E. (1982). Hormones and neurosecretion. In The Biology of Crustacea, Vol. 3 (ed. Bliss D., Atwood H., Sandeman D.), pp. 205-290 New York: Academic Press; [Google Scholar]
- Cruz-Bermúdez N. D., Marder E. (2007). Multiple modulators act on the cardiac ganglion of the crab, Cancer borealis. J. Exp. Biol. 210, 2873-2884 [DOI] [PubMed] [Google Scholar]
- Cruz-Bermúdez N. D., Fu Q., Kutz-Naber K. K., Christie A. E., Li L., Marder E. (2006). Mass spectrometric characterization and physiological actions of GAHKNYLRFamide, a novel FMRFamide-like peptide from crabs of the genus Cancer. J. Neurochem. 97, 784-799 [DOI] [PubMed] [Google Scholar]
- Delgado J. Y., Oyola E., Miller M. W. (2000). Localization of GABA- and glutamate-like immunoreactivity in the cardiac ganglion of the lobster Panulirus argus. J. Neurocytol. 29, 605-619 [DOI] [PubMed] [Google Scholar]
- Dickinson P. S., Stevens J. S., Rus S., Brennan H. R., Goiney C. C., Smith C. M., Li L., Towle D. W., Christie A. E. (2007a). Identification and cardiotropic actions of sulfakinin peptides in the American lobster Homarus americanus. J. Exp. Biol. 210, 2278-2289 [DOI] [PubMed] [Google Scholar]
- Dickinson P. S., Stevens J. S., Polistena C., Bors E., Christie A. E. (2007b). Peptides with similar C-terminal endings (M/LRFamide) have different effects on pattern generators in the American lobster, Homarus americanus. Program No. 288. 11. 2007 Neuroscience Meeting Planner San Diego, CA: Society for Neuroscience, 2007 http://sfn.org/index.aspx?pagename=abstracts_ampublications [Google Scholar]
- Dickinson P. S., Wiwatpanit T., Gabranski E. R., Ackerman R. J., Stevens J. S., Cashman C. R., Stemmler E. A., Christie A. E. (2009). Identification of SYWKQCAFNAVSCFamide: a broadly conserved crustacean C-type allatostatin-like peptide with both neuromodulatory and cardioactive properties. J. Exp. Biol. 212, 1140-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donly B. C., Fuse M., Orchard I., Tobe S. S., Bendena W. G. (1996). Characterization of the gene for leucomyosuppressin and its expression in the brain of the cockroach Diploptera punctata. Insect Biochem. Mol. Biol. 26, 627-637 [DOI] [PubMed] [Google Scholar]
- Evans C. G., Vilim F. S., Harish O., Kupfermann I., Weiss K. R., Cropper E. C. (1999). Modulation of radula opener muscles in Aplysia. J. Neurophysiol. 82, 1339-1351 [DOI] [PubMed] [Google Scholar]
- Florey E., Rathmayer M. (1978). The effects of octopamine and other amines on the heart and on neuromuscular transmission in decapod crustaceans: further evidence for a role as neurohormone. Comp. Biochem. Physiol. 61C, 229-237 [DOI] [PubMed] [Google Scholar]
- Fort T. J., Brezina V., Miller M. W. (2004). Modulation of an integrated central pattern generator-effector system: dopaminergic regulation of cardiac activity in the blue crab Callinectes sapidus. J. Neurophysiol. 92, 3455-3470 [DOI] [PubMed] [Google Scholar]
- Fort T. J., Brezina V., Miller M. W. (2007a). Regulation of the crab heartbeat by FMRFamide-like peptides: multiple interacting effects on center and periphery. J. Neurophysiol. 98, 2887-2902 [DOI] [PubMed] [Google Scholar]
- Fort T. J., García-Crescioni K., Agricola H. J., Brezina V., Miller M. W. (2007b). Regulation of the crab heartbeat by crustacean cardioactive peptide (CCAP): central and peripheral actions. J. Neurophysiol. 97, 3407-3420 [DOI] [PubMed] [Google Scholar]
- Freschi J. E. (1989). Proctolin activates a slow, voltage-dependent sodium current in motoneurons of the lobster cardiac ganglion. Neurosci Lett. 106, 105-111 [DOI] [PubMed] [Google Scholar]
- Freschi J. E., Livengood D. R. (1989). Membrane current underlying muscarinic cholinergic excitation of motoneurons in lobster cardiac ganglion. J. Neurophysiol. 62, 984-995 [DOI] [PubMed] [Google Scholar]
- Grega D. S., Sherman R. G. (1975). Responsiveness of neurogenic hearts to octopamine. Comp. Biochem. Physiol. 52C, 5-8 [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Watanabe A., Saito N. (1959). Potential changes in syncytial neurons of lobster cardiac ganglion. J. Neurophysiol. 22, 554-572 [DOI] [PubMed] [Google Scholar]
- Hartline D. K. (1967). Impulse identification and axon mapping of the nine neurons in the cardiac ganglion of the lobster Homarus americanus. J. Exp. Biol. 47, 327-340 [DOI] [PubMed] [Google Scholar]
- Hooper S. L., Marder E. (1987). Modulation of the lobster pyloric rhythm by the peptide proctolin. J. Neurosci. 7, 2097-2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz I., Cropper E. C., Vilim F. S., Alexeeva V., Susswein A. J., Kupfermann I., Weiss K. R. (2000). Serotonergic and peptidergic modulation of the buccal mass protractor muscle (I2) in Aplysia. J. Neurophysiol. 84, 2810-2820 [DOI] [PubMed] [Google Scholar]
- Jorge-Rivera J. C., Marder E. (1996). TNRNFLRFamide and SDRNFLRFamide modulate muscles of the stomatogastric system of the crab Cancer borealis. J. Comp. Physiol. A 179, 741-751 [DOI] [PubMed] [Google Scholar]
- Jorge-Rivera J. C., Sen K., Birmingham J. T., Abbott L. F., Marder E. (1998). Temporal dynamics of convergent modulation at a crustacean neuromuscular junction. J. Neurophysiol. 80, 2559-2570 [DOI] [PubMed] [Google Scholar]
- Kepler T. B., Marder E., Abbott L. F. (1990). The effect of electrical coupling on the frequency of model neuronal oscillators. Science 248, 83-85 [DOI] [PubMed] [Google Scholar]
- Kerrison J., Freschi J. E. (1992). The effects of gamma-aminobutyric acid on voltage-clamped motoneurons of the lobster cardiac ganglion. Comp. Biochem. Physiol. 101C, 227-233 [DOI] [PubMed] [Google Scholar]
- Kilman V., Fénelon V. S., Richards K. S., Thirumalai V., Meyrand P., Marder E. (1999). Sequential developmental acquisition of cotransmitters in identified sensory neurons of the stomatogastric nervous system of the lobsters, Homarus americanus and Homarus gammarus. J. Comp. Neurol. 408, 318-334 [DOI] [PubMed] [Google Scholar]
- Kobierski L. A., Beltz B. S., Trimmer B. A., Kravitz E. A. (1987). FMRFamide-like peptides of Homarus americanus: distribution, immunocytochemical mapping, and ultrastructural localization in terminal varicosities. J. Comp. Neurol. 266, 1-15 [DOI] [PubMed] [Google Scholar]
- Krajniak K. G. (1991). The identification and structure-activity relations of a cardioactive FMRFamide-related peptide from the blue crab Callinectes sapidus. Peptides 12, 1295-1302 [DOI] [PubMed] [Google Scholar]
- Kuramoto T., Ebara A. (1984). Neurohormonal modulation of the cardiac outflow through the cardioarterial valve in the lobster. J. Exp. Biol. 111, 123-130 [Google Scholar]
- Lemos J. R., Berlind A. (1981). Cyclic adenosine monophosphate mediation of peptide neurohormone effects on the lobster cardiac ganglion. J. Exp. Biol. 90, 307-326 [Google Scholar]
- Ma M., Chen R., Sousa G. L., Bors E. K., Kwiatkowski M. A., Goiney C. C., Goy M. F., Christie A. E., Li L. (2008). Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. Gen. Comp. Endocrinol. 156, 395-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan A., Lappé J., Rhyne R. T., Cruz-Bermúdez N. D., Marder E., Goy M. F. (2004). Nitric oxide inhibits the rate and strength of cardiac contractions in the lobster Homarus americanus by acting on the cardiac ganglion. J. Neurosci. 24, 2813-2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E., Abbott L. F., Kepler T. B., Hooper S. L. (1992). Modification of oscillator function by electrical coupling to nonoscillatory neurons. In Induced Rhythms in the Brain (ed. Baar E., Bullock T. H.), pp. 287-296 Boston: Birkhauser; [Google Scholar]
- Mayeri E. (1973). Functional organization of the cardiac ganglion of the lobster, Homarus americanus. J. Gen. Physiol. 62, 448-472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard D. M. (1960). Circulation and heart function. In The Physiology of Crustacea (ed. Waterman T. H.), pp. 161-226 New York: Academic Press,. [Google Scholar]
- McGaw I. J., McMahon B. R. (1995). The FMRFamide-related peptides Fl and F2 alter hemolymph distribution and cardiac output in the crab Cancer magister. Biol. Bull. 188, 186-196 [DOI] [PubMed] [Google Scholar]
- McGaw I. J., Airriess C. N., McMahon B. R. (1994). Peptidergic modulation of cardiovascular dynamics in the Dungeness crab Cancer magister. J. Comp. Physiol. 164B, 103-111 [Google Scholar]
- McGaw I. J., Wilkens J. L., McMahon B. R., Airriess C. N. (1995). Crustacean cardioexcitatory peptides may inhibit the heart in vivo. J. Exp. Biol. 198, 2547-2550 [DOI] [PubMed] [Google Scholar]
- Mercier A. J., Russenes R. T. (1992). Modulation of crayfish hearts by FMRFamide-related peptides. Biol. Bull. 182, 333-340 [DOI] [PubMed] [Google Scholar]
- Mercier A. J., Orchard I., TeBrugge V., Skerrett M. (1993). Isolation of two FMRFamide-related peptides from crayfish pericardial organs. Peptides 14, 137-143 [DOI] [PubMed] [Google Scholar]
- Miller M. W., Sullivan R. E. (1981). Some effects of proctolin on the cardiac ganglion of the Maine Lobster, Homarus americanus (Milne Edwards). J. Neurobiol. 12, 629-639 [DOI] [PubMed] [Google Scholar]
- Monigatti F., Gasteiger E., Bairoch A., Jung E. (2002). The Sulfinator: predicting tyrosine sulfation sites in protein sequences. Bioinformatics 18, 769-770 [DOI] [PubMed] [Google Scholar]
- Pulver S. R., Marder E. (2002). Neuromodulatory complement of the pericardial organs in the embryonic lobster, Homarus americanus. J. Comp. Neurol. 451, 79-90 [DOI] [PubMed] [Google Scholar]
- Reiber C., McMahon B., Burggren W. (1997). Cardiovascular functions in two macruran decapod crustaceans (Procambarus clarkii and Homarus americanus) during periods of inactivity, tail flexion and cardiorespiratory pauses. J. Exp. Biol. 200, 1103-1113 [DOI] [PubMed] [Google Scholar]
- Sakurai A., Wilkens J. L. (2003). Tension sensitivity of the heart pacemaker neurons in the isopod crustacean Ligia pallasii. J. Exp. Biol. 206, 105-115 [DOI] [PubMed] [Google Scholar]
- Saver M. A., Wilkens J. L. (1998). Comparison of the effects of five hormones on intact and open heart cardiac ganglionic output and myocardial contractility in the shore crab Carcinus maenas. Comp. Biochem. Physiol. 120A, 301-310 [Google Scholar]
- Saver M. A., Wilkens J. L., Airriess C. N. (1998). Proctolin affectsthe activity of the cardiac ganglion, myocardium, and cardioarterialvalves in Carcinus maenas hearts. J. Comp. Physiol. B 168, 473-482 [Google Scholar]
- Saver M. A., Wilkens J. L., Syed N. I. (1999). In situ and in vitro identification and characterization of cardiac ganglion neurons in the crab, Carcinus maenas. J. Neurophysiol. 81, 2964-2976 [DOI] [PubMed] [Google Scholar]
- Schmidt M. (1997). Distribution of presumptive chemosensory afferents with FMRFamide- or substance P-like immunoreactivity in decapod crustaceans. Brain Res. 746, 71-84 [DOI] [PubMed] [Google Scholar]
- Schmidt M., Ache B. W. (1997). Immunocytochemical analysis of glomerular regionalization and neuronal diversity in the olfactory deutocerebrum of the spiny lobster. Cell Tissue Res. 287, 541-563 [DOI] [PubMed] [Google Scholar]
- Stemmler E. A., Cashman C. R., Messinger D. I., Gardner N. P., Dickinson P. S., Christie A. E. (2007). High-mass-resolution direct-tissue MALDI-FTMS reveals broad conservation of three neuropeptides (APSGFLGMRamide, GYRKPPFNGSIFamide and pQDLDHVFLRFamide) across members of seven decapod crustaean infraorders. Peptides 28, 2104-2115 [DOI] [PubMed] [Google Scholar]
- Stevens J. S., Taylor E., Stemmler E. A., Christie A. E., Dickinson P. S.(2008). Complex modulation of cardiac activity by the FMRFamide-related peptide hormones SGRNFLRFamide and myosuppressin in the American lobster, Homarus americanus. Program No. 574. 9. 2008 Neuroscience Meeting Planner Washington DC: Society for Neuroscience, 2008 http://sfn.org/index.aspx?pagename=abstracts_ampublications [Google Scholar]
- Sullivan R. E., Miller M. W. (1984). Dual effects of proctolin on the rhythmic burst activity of the cardiac ganglion. J. Neurobiol. 15, 173-196 [DOI] [PubMed] [Google Scholar]
- Sullivan R. E., Miller M. W. (1990). Cholinergic activation of the lobster cardiac ganglion. J. Neurobiol. 21, 639-650 [DOI] [PubMed] [Google Scholar]
- Tazaki K., Cooke I. M. (1990). Characterization of Ca current underlying burst formation in lobster cardiac ganglion motorneurons. J. Neurophysiol. 63, 370-384 [DOI] [PubMed] [Google Scholar]
- Towle D. W., Smith C. M. (2006). Gene discovery in Carcinus maenas and Homarus americanus via expressed sequence tags. Int. Comp. Biol. 46, 912-918 [DOI] [PubMed] [Google Scholar]
- Trimmer B. A., Kobierski L. A., Kravitz E. A. (1987). Purification and characterization of FMRFamidelike immunoreactive substances from the lobster nervous system: isolation and sequence analysis of two closely related peptides. J. Comp. Neurol. 266, 16-26 [DOI] [PubMed] [Google Scholar]
- Veenstra J. A. (2000). Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch. Insect Biochem. Physiol. 43, 49-63 [DOI] [PubMed] [Google Scholar]
- Vilaplana L., Castresana J., Bellés X. (2004). The cDNA for leucomyosuppressin in Blattella germanica and molecular evolution of insect myosuppressins. Peptides 25, 1883-1889 [DOI] [PubMed] [Google Scholar]
- Vilim F. S., Cropper E. C., Price D. A., Kupfermann I., Weiss K. R. (2000). Peptide cotransmitter release from motorneuron B16 in Aplysia californica: costorage, corelease, and functional implications. Neurosci. 20, 2036-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A. (1958). The interaction of electrical activity among neurons of lobster cardiac ganglion. Jpn. J. Physiol. 8, 305-318 [DOI] [PubMed] [Google Scholar]
- Watanabe A., Bullock T. H. (1960). Modulation of activity of one neuron by subthreshold slow potentials in another in lobster cardiac ganglion. J. Gen. Physiol. 43, 1031-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Takeda K. (1963). The spread of excitation among neurons in the heart ganglion of the stomatopod, Squillia oratoria. J. Gen. Physiol. 46, 773-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss K. R., Brezina V., Cropper E. C., Hooper S. L., Miller M. W., Probst W. C., Vilim F. S., Kupfermann I. (1992). Peptidergic co-transmission in Aplysia: functional implications for rhythmic behaviors. Experientia 48, 456-463 [DOI] [PubMed] [Google Scholar]
- Weiss T, Kreissl S., Rathmayer W. (2003). Localization of a FMRFamide-related peptide in efferent neurons and analysis of neuromuscular effects of DRNFLRFamide (DF2) in the crustacean Idotea emarginata. Eur. J. Neurosci. 17, 239-248 [DOI] [PubMed] [Google Scholar]
- Wilkens J. L., McMahon B. R. (1992). Intrinsic properties and extrinsic neurohormonal control of crab cardiac hemodynamics. Experientia 48, 827-834 [Google Scholar]
- Wilkens J. L., McMahon B. R. (1994). Cardiac performance in semi-isolated heart of the crab Carcinus maenas. Am. J. Physiol. 266, R781-R789 [DOI] [PubMed] [Google Scholar]
- Wilkens J. L., Taylor H. H. (2003). The control of vascular resistance in the southern rock lobster, Jasus edwardsii (Decapoda: Palinuridae). Comp. Biochem. Physiol. 135A, 369-376 [DOI] [PubMed] [Google Scholar]
- Wilkens J. L., Shinozaki T., Yazawa T., ter Keurs H. E. (2005). Sites and modes of action of proctolin and the FLP F2 on lobster cardiac muscle. J. Exp. Biol. 208, 737-747 [DOI] [PubMed] [Google Scholar]
- Worden M. K., Kravitz E. A., Goy M. F. (1995). Peptide F1, an N-terminally extended analog of FMRFamide, enhances contractile activity in multiple target tissues in lobster. J. Exp. Biol. 198, 97-108 [DOI] [PubMed] [Google Scholar]