Abstract
We report herein that the TRAIL receptor DR5/FADD/caspase pathway plays a role in skeletal myoblast differentiation through modulation of the expression of the muscle regulatory transcription factor MyoD. Specifically, treatment with the selective caspase 3 inhibitor DEVD-fmk or the selective caspase 8 inhibitor IETD-fmk in growth media (GM), prior to culture in differentiation media (DM), inhibited differentiation. Further, this treatment resulted in decreased levels of MyoD message and protein. We next explored a role for the TRAIL receptor DR5/FADD pathway. We found that expression of either dominant negative (dn) FADD or dominant negative (dn) DR5 also resulted in decreased levels of MyoD mRNA and protein and blocked differentiation. This decreased level of MyoD mRNA was not a consequence of altered stability. Treatment with TSA, an inhibitor of histone deacetylases (HDACs), allowed MyoD expression in myoblasts expressing dnDR5. Finally, acetylation of histones associated with the DRR enhancer of MyoD was decreased in myoblasts expressing dnDR5. Thus, our data suggests a non-canonical role for the TRAIL receptor/FADD pathway in the regulation of MyoD expression and skeletal myoblast differentiation.
Keywords: MyoD, DR5, myoblast, differentiation
Introduction
Critical to development and homeostasis is the coordinate regulation of differentiation and apoptosis in many cell types. In some cell types, these two processes are functionally intertwined in that morphological consequences of the apoptotic process are critical components of the differentiation process. In others, these two processes result in either differentiation or apoptosis ( Raff, 1992; Lamkanfi et al., 2007). Skeletal myoblasts are a tractable model system in which to study the latter ( Sandri, 1996; Dee et al., 2002).
In vivo, myoblasts are maintained in a proliferative and undifferentiated state by certain mitogens. Differentiation is induced in response to decreasing gradients of these mitogens when myoblasts have appropriately migrated. To mimic this, the in vitro differentiation of skeletal myoblasts is initiated by switching cells from growth medium (GM:media plus 10-20% serum) to differentiation medium (DM:media with low (2%) or no serum) (Olson, 1992). Skeletal myoblast apoptosis occurs during myogenesis (Fidzianska and Goebel, 1991) and muscle regeneration (Miller and Stockdale, 1986) and has been carefully documented in vertebrate models and in cultures of primary myoblasts and established muscle cell lines induced to differentiate (Sandri, 1996; Dee et al., 2002). Our lab has reported that when skeletal myoblasts are switched from growth medium (GM) to differentiation medium (DM), roughly 30% of myoblasts undergo apoptosis within 12 hours, while the remaining 70% require at least 48 hours to complete the process of differentiation (Dee et al., 2002).
The molecules controlling the process of differentiation in skeletal myoblasts have been relatively well characterized (Berkes and Tapscott, 2005; Tapscott, 2005) while the molecules controlling the apoptotic process in skeletal myoblasts have only recently been reported (O'Flaherty et al., 2006; Shaltouki et al., 2007). Skeletal myoblast specification and differentiation during development is controlled by the muscle regulatory transcription factor family consisting of MyoD, Myf5, myogenin and MRF4. Of these, MyoD has been the most extensively studied and has emerged as a master regulatory gene of skeletal myogenesis and regeneration (Berkes and Tapscott, 2005; Tapscott, 2005). Our lab has recently reported that increased signaling by the death ligand TRAIL through the TRAIL receptor DR5, the adapter protein FADD and caspases 8 and 3 is critical to the apoptotic process that occurs in skeletal myoblasts cultured in DM (O'Flaherty et al., 2006).
The apoptosis of myoblasts is a physiological process that likely serves the necessary function of removing excess myoblasts (Miller and Stockdale, 1986) during muscle regeneration and myogenesis (Fidzianska and Goebel, 1991). While the coordinate regulation of differentiation and apoptosis is clearly important under normal physiological conditions, it is likely detrimental to the use of myoblast transfer as a treatment for a variety of diseases (Skuk et al., 2003; Bouchentouf et al., 2004; Menasche, 2004; Le Grand and Rudnicki, 2007; Price et al., 2007). Thus, a precise understanding of the molecular mechanism controlling the coordinate regulation of differentiation and apoptosis in skeletal myoblasts is paramount. To this end, we examined the differentiation of skeletal myoblasts treated with pharmacological inhibitors selective for either caspase 8 or caspase 3. Furthermore, we assessed differentiation in skeletal myoblasts stably expressing either dominant negative dnFADD or dominant negative dnDR5.
Methods
Cells and cell culture
All cells were cultured on gelatin-coated plates and maintained in growth medium (GM), which consists of basal modified Eagle's medium (BME), 10% fetal bovine serum (FBS), and a 1% combination of 10,000 I.U./ml penicillin and 10 000 μg/ml streptomycin (1%P/S). Differentiation was induced by switching cells from growth medium to differentiation medium (DM), which consists of basal modified Eagle's medium, 1% P/S and 0% FBS. Cells were incubated at 37°C in 5% CO2. 23A2 myoblasts and the creation of cell lines expressing dnFADD or dnDR5 have been described (O'Flaherty et al., 2006). The Z-DEVD-fmk and Z-IETD caspase inhibitors (Calbiochem) and TSA (Biomol) were each dissolved in DMSO. Appropriate volumes of DMSO or methanol alone were added to control cultures and did not exceed 0.15% v/v.
Immunoblot analysis
Lysates were prepared as previously described (DeChant et al., 2002). Following protein determination, lysates (50 μg of total cellular lysate for MHC, myogenin and MyoD) were denatured in 5x sample buffer (10% SDS, 50% glycerol, 10% 2-mercaptoethanol, pH 6.8) and electrophoresed through denaturing polyacrylamide gels (8% for MHC and 10% for myogenin and MyoD). Following SDS polyacrylamide gel electrophoresis (SDS-PAGE), samples were transferred electrophoretically for four amp hours to Hybond-P polyvinylidene difluoride membranes in transfer buffer containing 80% methanol and 1g/L SDS. Membranes were blocked for one hour in 1× TBS/0.1%NP40 with 10% newborn calf serum and 5% dry milk. The following primary antibodies were incubated with the appropriate membranes: anti-MyoD (each from BDPharmingen) and diluted 1:1000 and 1:250 respectively, anti-actin (Sigma) diluted 1:30,000 and monoclonal antibody MF20 that is specific for skeletal myosin heavy chain protein. Appropriate HRP-conjugated secondary antibodies, each diluted 1:1000, were incubated with the membranes for one hour. After each incubation with antibody and prior to the addition of chemiluminescent substrate, membranes were washed five times in 1×TBS (tris-buffered saline pH 7.4) with 1% NP-40. Membranes were then incubated with SuperSignal West Pico Chemiluminescent Substrate (Pierce) for 60 seconds and bands were visualized using Kodak Scientific Imaging Film.
Quantitative RT-PCR for the detection of MyoD mRNA
Analysis was performed as described (Karasarides et al., 2006). Briefly, myoblasts were plated at 4×105 and then treated the next day for 6 hours in fresh GM with or without 100 M DEVD-fmk or IETD-FMK. Total RNA was prepared using 1 mL of Trizol (Invitrogen) reagent per 100 mm plate for lysis and following the manufacturer's instructions. RNA (500 g) of was then used for a 20 μL SuperScript II RT (Invitrogen) reverse transcription reaction. Two μL of this reaction was used for quantitative PCR, with 0.3 μM each primer (Myod Forward: GACAGGACAGGACAGGGAGG, MyoD Reverse: GCACCG CAGTAGAGAAGTGT, β-actin Forward: GGTCAGAAGGATTCCTATGTTG, and β-actin Reverse: ATCACAATGCCCGTGGTACG) and SYBR green reagent (DyNAmo, MJ Research) in a total of 20 μL. Fluorescence was detected using an Opticon Monitor (MJ Research). The cycle number at which the amplification of product began linearly increasing (cycle threshold (Ct) value) was taken from the Opticon software. The difference between the Ct values determined for β-actin in an arbitrary “calibrator” reaction (untreated 23A2 cells) and that from all other samples (ΔCt(β-actin)) was subtracted from the raw Ct values for MyoD in each case (Ct(MyoD) - ΔCt(β-actin) = normalized Ct(MyoD)). The normalized Ct for the 23A2 reaction was then subtracted from itself and all other normalized Ct(MyoD) values. These ΔnormCt(MyoD) values were then taken as a negative exponent of 2 (based on the assumption that there is an exponential increase in PCR product generated in each cycle) to find the absolute amount of PCR product generated, relative to the “calibrator” sample.
Caspase activity assays
Activity assays were performed using the Caspase 3 or Caspase 8 Colorimetric Assay Kit from R&D Systems per manufacturer's instructions and as previously described (Dee et al., 2002; O'Flaherty et al., 2006). Experiments were performed within the linear range of the assay and absorbance was normalized to the protein concentration of each lysate.
Chromatin immunopreciptation
Chromatin in 1×107 cells was cross-linked with 0.5% Formaldehyde for 10 minutes at room temperature and quenched with 0.125 M glycine for 5 minutes at RT. Nuclei were then isolated for MNase digestion. Briefly, cells were washed and collected in cold PBS containing 5mM Na Butyrate and 0.5mM PMSF. Cells were pelleted by centrifugation at 1500 rpm for 5 minutes and then resuspended in 5ml cold Cell Lysis Buffer (CLB: 60 mM KCl, 15 mM NaCl, 5mM MgCl, 10 mM Tris pH 7.4, 300mM sucrose, 0.1 mM EGTA, 0.1% NP-40, 5 mM Na Butyrate, 0.5 mM PMSF). Cells were sonicated once for 10 sec to ensure lysis of the plasma membrane. Isolated nuclei were washed once in 30 ml of CLB and once in 1 ml of cold Nuclei Digestion Buffer (Cell Lysis Buffer without NP-40 and PMSF). For MNase digestion, intact nuclei were resuspended in 125 μl of Nuclei Lysis Buffer (prewarmed to 37° C), digested with MNase (50 units/ml) at 37° C for 5 minutes, and terminated by 5 mM EDTA. An aliquot from each sample was assessed for sufficient chromatin fragmentation (500-1000bp) by gel electrophoresis. Samples were sonicated twice to ensure lysis of the nuclei prior to immunoprecipitation. The remaining steps of the immunoprecipitation were performed using the EZ ChIP™ Chromatin Immunopreipitation Kit (Upstate) per manufacturer's instructions. Chromatin from 2×106 cells was immunoprecipitated with either anti-acetyl H4 (Upstate, 5 μg), anti- acetyl H3 (Upstate, 5ug), or normal Rabbit IgG (Upstate, 5μg). After washing, treatment with proteinase K, and the reversal of cross-linking, quantitative PCR was performed using primers designed to amplify -476/-379 in the MyoD promoter region (forward 5'TAGACACTGGAGAGGCTTGG 3' and reverse 5'GCAGCTGCCTAGCTAGGAAG 3') or to amplify -5445/-5135 encompassing the MyoD CArG element (forward 5'AAGTAAGAGGCCACAGGTCC 3' and reverse 5'ACTGACCTGGAGAAGCACAC 3'). Data was normalized to the signal detected from the input of each sample.
Statistics
Statistics Statistical analysis was performed using KaleidaGraph data analysis per the manufacturer's instructions (version 3.6). Briefly, for analysis of the data, a single sample t-test was used where the test value was the value assigned to untreated, parental 23A2.
Results
Myoblasts treated with either a pharmacological inhibitor of caspase 3 or caspase 8 are defective for differentiation and possess decreased levels of MyoD mRNA and protein
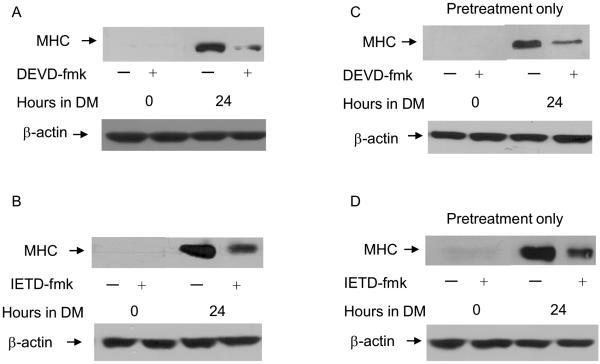
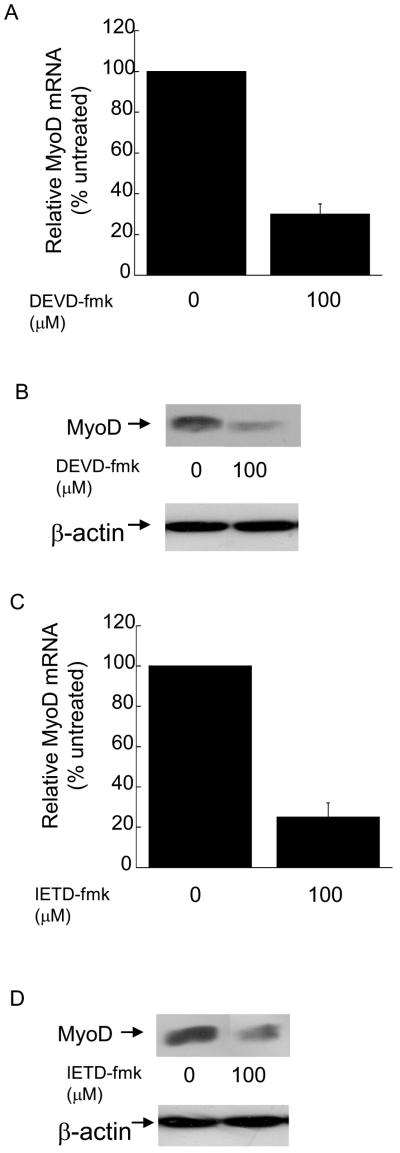
Differentiation requires 24-72 hours and is a stepwise process characterized by exit from the cell cycle, expression of muscle specific genes like myosin heavy chain (MHC) and finally fusion of individual myoblasts into multi-nucleated myotubes (Andres and Walsh, 1996). Several reports have documented a role for caspase 3 in the regulation of MyoD expression and the subsequent differentiation of skeletal myoblasts (Fernando et al., 2002; Wedhas et al., 2005; Ikeda et al., 2006; Fernando and Megeney, 2007). However, none of these studies assessed MyoD expression in the presence of pharmacological inhibitors of caspase 3 or caspase 8. We have discovered that myoblasts treated with either a pharmacological inhibitor of caspase 3 or caspase 8 are defective for differentiation (Figure 1, A-D) and possess decreased levels of MyoD mRNA and protein (Figure 2, A-D). Our results are consistent with those of others regarding the role of caspase 3 in the regulation of MyoD expression and skeletal myoblast differentiation and extend these findings to suggest a role for caspase 8 in the regulation of MyoD expression.
Figure 1.
Differentiation of skeletal myoblasts is inhibited by treatment with either the selective caspase 3 inhibitor Z-DEVD-fmk (100 μ M) or the selective caspase 8 inhibitor Z-IETD-fmk (100 μM). Cells were plated at 4×105 and the next day cultured in (A and C) GM +/- DEVD-fmk for four hours followed by culture in DM with (A) or without (C) DEVD-fmk for the times indicated or (B and D) cultured in GM +/- IETD-fmk for four hours followed by culture in DM with (B) or without (D) IETD-fmk for the times indicated. Whole cell protein lysates were prepared and equal amounts of protein were subjected to SDS-PAGE followed by Western analysis with anti-MHC or anti-actin and visualized by autoradiography as described in Materials and Methods. Shown are the results of one representative experiment that is representative of three (A and B) or two (C and D) independent experiments.
Figure 2.
Skeletal myoblasts treated with either Z-DEVD-fmk or Z-IETD-fmk express decreased levels of MyoD mRNA and protein. Cells were plated at 4×105 and the next day treated with the indicated inhibitor or solvent control for four hours. In (A and C), quantitative RT-PCR was used to assay for the relative levels of MyoD mRNA in total RNA samples. The Ct values for the MyoD PCR product were normalized to the Ct values for a b-actin product, run in parallel, as described in Materials and methods. Error bars represent mean +/- SEM from triplicates. Statistical analysis was performed as described in Materials and methods. Each value obtained after treatment with caspase inhibitor is significantly different from the corresponding value obtained after treatment with solvent (P < 0.03). In (B and D) whole cell protein lysates were prepared and equal amounts of protein were subjected to SDS-PAGE followed by Western analysis with anti-MyoD (Pharmigen) or anti-actin and visualized by autoradiogaphy as described in Materials and Methods. Shown are the results of one experiment that is representative of three independent experiments.
Myoblasts expressing either dnFADD or dnDR5 are defective for differentiation and possess decreased levels of MyoD mRNA and protein
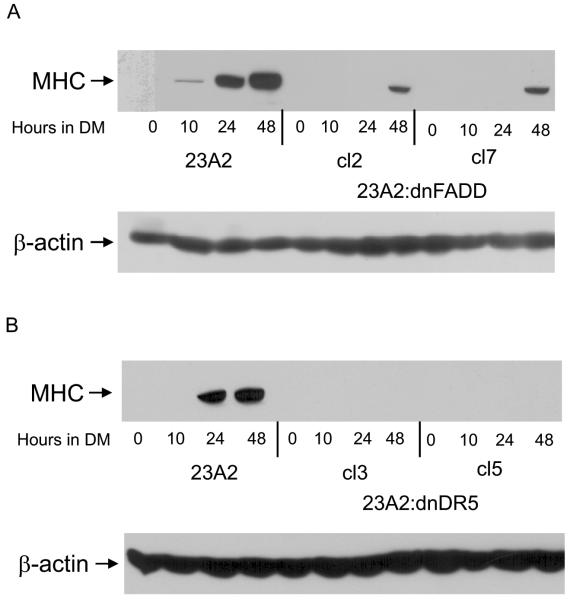
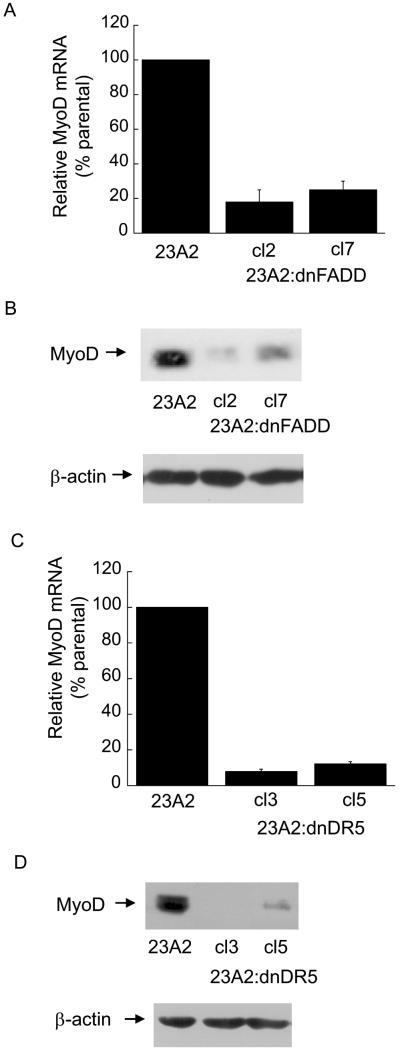
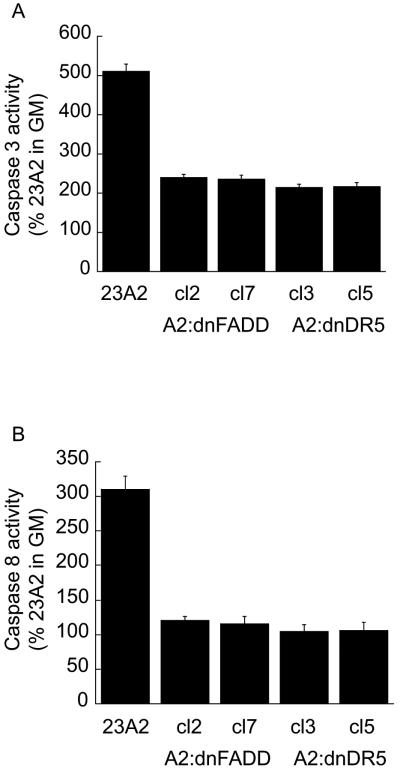
Since caspase 3 is downstream of caspase 8 and caspase 8 is activated by death ligand signaling (Thorburn, 2004), we examined a role for the death ligand adapter protein FADD or the TRAIL death ligand receptor DR5 in the differentiation of skeletal myoblasts and in the regulation of MyoD expression. The creation of these cell lines has been previously described (O'Flaherty et al., 2006). We found that myoblasts stably expressing either a dominant negative mutant of FADD (dnFADD) or dominant negative mutant of DR5 (dnDR5) are also defective for differentiation (Figure 3, A and B) and possess decreased levels of MyoD mRNA and protein (Figure 4, A and B). As expected, these cell lines display decreased levels of caspase 3 and caspase 8 activity (Figure 5, A and B). These results document a role for the TRAIL receptor DR5/FADD pathway in the regulation of MyoD expression and skeletal myoblast differentiation.
Figure 3.
Stable Expression of dnFADD or dnDR5 in skeletal myoblasts inhibits differentiation. 23A2 parental myoblasts, A2:dnFADD clones 2 and 7, and A2:dnDR5 clones 3 and 5 were plated at 4×105 and the next day switched to DM for the times indicated. Whole cell protein lysates were prepared and subjected to SDS-PAGE. Western analysis was performed using anti-MHC or anti-β-actin (Sigma) and visualized by autoradiography as described in Materials and Methods. Shown are the results of one experiment that is representative of two independent experiments.
Figure 4.
Stable expression of dnFADD or dnDR5 in skeletal myoblasts results in decreased levels of MyoD mRNA and protein. In (A and C), cells were plated at 4×105 and the next day quantitative RT-PCR was used to assay for the relative levels of MyoD mRNA in total RNA samples derived from the indicated cell lines. The Ct values for the MyoD PCR product were normalized to the Ct values for a β-actin product, run in parallel, as described in Materials and methods. Error bars represent mean +/- SEM from triplicates. Statistical analysis was performed as described in Materials and methods. Each value obtained from lysates prepared from the clones is significantly different from the corresponding value obtained from lysates prepared from parental 23A2 myoblasts (P < 0.02). In (B and D) whole cell protein lysates were prepared and equal amounts of protein were subjected to SDS-PAGE followed by Western analysis with anti-MyoD (Pharmigen) or anti-actin and visualized by autoradiogaphy as described in Materials and Methods. Shown are the results of one experiment that is representative of three independent experiments.
Figure 5.
Caspase activity is inhibited by stable expression of dnFADD or dnDR5 in skeletal myoblasts. Equal cell numbers were plated and the next day switched to DM for 3 hours prior to the determination of caspase 3 or caspase 8 activity using the respective Caspase colorimetric kits (R&D Systems) as described in Materials and Methods. Error bars represent mean +/- SEM from triplicates. Statistical analysis was performed as described in Materials and methods. Each value obtained from lysates prepared from the clones is significantly different from the corresponding value obtained from lysates prepared from parental 23A2 myoblasts (P < 0.05).
A role for acetylation in the regulation of MyoD mRNA expression by the DR5 pathway
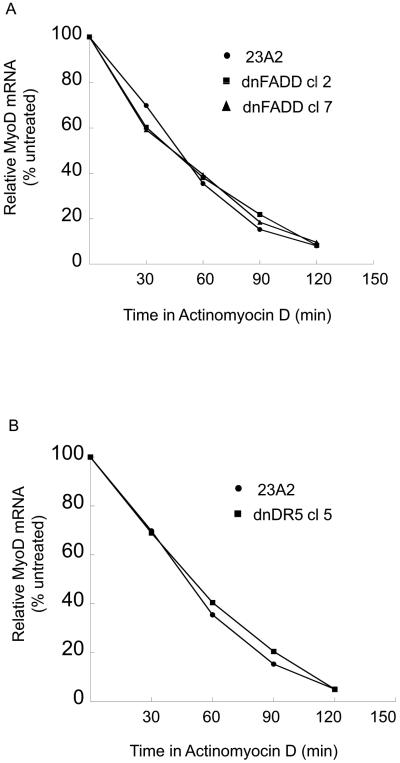
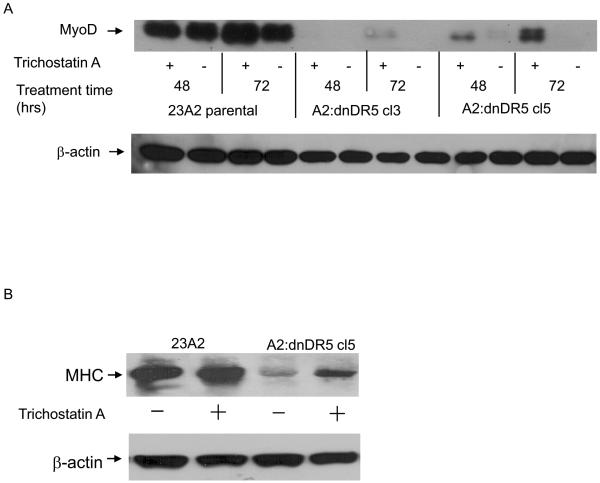
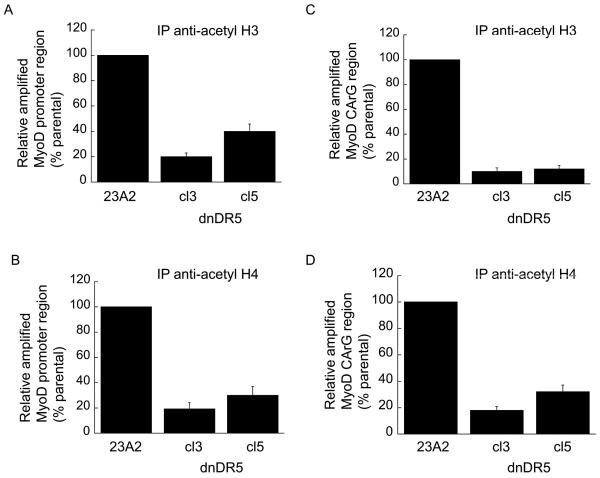
A decreased level of MyoD mRNA can result from either increased mRNA decay or decreased mRNA production. To assess the former, we compared the half-life of MyoD mRNA in parental myoblasts with that in myoblasts stably expressing either dnFADD or dnDR5. We found no appreciable affect on the half-life of MyoD as a consequence of the expression of either dnFADD or dnDR5 (Figure 6, A and B). Since the half-life of MyoD mRNA is not affected by expression of either dnFADD or dnDR5, we initiated experiments to explore the regulation of MyoD transcription. Acetylation of histones and/or transcription factors can facilitate transcription while deacetylation can inhibit transcription (Carrozza et al., 2003). Trichostatin A (TSA) is a commonly used general inhibitor of histone deacetylases (HDACs). Treatment of myoblasts expressing dnDR5 with TSA permitted the expression of MyoD and the consequent differentiation as monitored by the expression of MHC (Figure 7, A and B). To expand on our finding that HDAC inhibition allows MyoD expression in myoblasts expressing dnDR5, we compared the acetylation of histones within the MyoD promoter region in these cells with the acetylation of histones within the MyoD promoter region in parental myoblasts using chromatin immunopreciptiation (ChIP). ChIP analysis using an antibody that recognizes acetylated histone H3 revealed that the histones in the MyoD promoter region are less acetylated in myoblasts expressing dnDR5 (Figure 8A). Similar results were obtained by performing ChIP analysis using an antibody that recognizes acetylated histone H4 (Figure 8B). This is consistent with our finding that blocking HDACs with TSA allows MyoD expression. A CArG element within the MyoD distal regulatory region (DRR) enhancer is required for MyoD expression in established myoblasts and MyoD induction in satellite cells during regeneration (L'Honore et al., 2003; L'Honore et al., 2007). Thus, we compared the acetylation of histones within this CArG element in parental myoblasts and myoblasts expressing dnDR5. ChIP analysis using an antibody that recognizes acetylated histone H3 or an antibody that recognizes acetylated histone H4 revealed that the histones in the CArG element are less acetylated in myoblasts expressing dnDR5 (Figure 8, C and D).
Figure 6.
Stable expression of dnFADD or dnDR5 does not decrease the half-life of MyoD mRNA. For both (A and B), cells were plated at 4×105 and the next day treated with actinomycin D (5 μg/ml). Quantitative RT-PCR was used to assay for the relative levels of MyoD mRNA in total RNA samples collected at the indicated time points. The Ct values for the MyoD PCR product were normalized to the Ct values for a b-actin product, run in parallel, as described in Materials and methods. Shown are the results of one experiment for each that is representative of three independent experiments.
Figure 7.
Treatment with the HDAC inhibitor TSA allows MyoD expression and differentiation in myoblasts expressing dnDR5. Cells were plated at 4×105 and the next day treated with TSA (100 ng/ml). In (A) at the indicated times, whole cell protein lysates were prepared and subjected to SDS-PAGE. In (B) cells were treated with TSA and cultured for 72 hours in GM as in (A). Cells were then cultured in DM for 48 hours. Lysates were prepared and subjected to SDS-PAGE. Western analysis was performed using anti-MyoD, anti-MHC or anti-β-actin and visualized by autoradiography as described in Materials and Methods. Shown are the results of one experiment that are representative of four independent experiments for (A) and two independent experiments for (B).
Figure 8.
Decreased histone acetylation of MyoD-DRR enhancer by expression of dnDR5. For all (A, B, C, and D) chromatin from 1×107 cells was crosslinked and digested with MNase to a length between 200-1000 bp. Chromatin Immunoprecipitation was performed on each cell sample using EZ ChIPTM Chromatin Immunopreipitation Kit (Upstate) per manufacturer's instructions. Chromatin from 2×106 cells was immunoprecipitated as described in Materials and Methods. Quantitative PCR was used to assay for the relative levels of histone H3 and H4 acetylation near the MyoD promoter (A and B) and MyoD CArG element (C and D). Data was normalized to the signal detected from the input of each sample and presented as a percent of the signal obtained from parental 23A2 myoblasts. Error bars represent mean +/- SEM of triplicates. Statistical analysis was performed as described in Materials and methods. Each value obtained from lysates prepared from the clones is significantly different from the corresponding value obtained from lysates prepared from parental 23A2 myoblasts (P < 0.05).
Discussion
Herein we report a role for the TRAIL-DR5/FADD/caspase pathway in the regulation of skeletal myoblast differentiation and the regulation of MyoD expression. The MyoD transcription factor is a master regulatory gene of skeletal myogenesis and is heralded as a “pioneer” transcription factor capable of specifying the muscle lineage in multi-potential stem cells as well as converting other lineages to the muscle lineage. MyoD thus serves as a molecular paradigm, and skeletal myogenesis as a model system, for understanding the regulation of lineage specification and differentiation (Tapscott, 2005). Thus, understanding the regulation of MyoD expression has been the subject of intense scrutiny for many years. MyoD expression is regulated by two enhancers, the core enhancer (CE) and the distal regulatory region (DRR). The DRR plays a role in development as well as activation of adult muscle satellite (stem) cells during regeneration (Tapscott et al., 1992; Chen et al., 2001; Chen et al., 2002;). A CArG element in the DRR is required for MyoD expression in established myoblasts and MyoD induction in satellite cells during regeneration (L'Honore et al., 2003; L'Honore et al., 2007). Since blocking the DR5 pathway results in decreased acetylation of histones associated with the MyoD-DRR CArG element, our data suggests that the DR5 pathway facilitates acetylation. Two transcription factors, SRF and MEF2, bind to this CArG element (L'Honore et al., 2003; L'Honore et al., 2007) and transcription factors such as these are known to recruit general acetyltransferases such as CBP or p300 (Carrozza et al., 2003). The signaling pathways regulating their binding to the MyoD-DRR CArG element, however, remain unclarified. It is intriguing to speculate that the TRAILDR5/FADD/caspase pathway could regulate the binding of SRF and/or MEF2 to the MyoD CArG element.
In addition to the well-known pro-inflammatory role of caspase 1, the role of pro-apoptotic molecules in other non-apoptotic processes is an intensely exciting area of research. Caspase activation has been shown to play a role in the differentiation of several cell types. Caspases serve a positive role in the differentiation of epithelial lens cells, keratinocytes, monocytes, osteoblasts, neural stem cells and skeletal myoblasts (Fernando et al., 2002; Fernando and Megeney, 2007; Murray et al., 2008). In contrast, Fas signaling through caspase 8 functions to inhibit the differentiation of erythroblasts and megakaryocytes. Cleavage of a limited set of substrates, either through limited activation or compartmentalized activation, appears to be critical to these non-apoptotic function of caspases (Schwerk and Schulze-Osthoff, 2003). To our knowledge, other than our proposed role for the DR5/FADD pathway in the regulation of MyoD expression, the only reported non-apoptotic functions for this pathway is the induction of monocyte maturation, the proliferation of vascular endothelial cells and the differentiation of intestinal cells (Secchiero et al., 2003; Rimondi et al., 2006; Zauli and Secchiero, 2006; Secchiero and Zauli, 2008).
While our studies performed on cultured myoblasts clearly demonstrate a role for the TRAIL/DR5/FADD/caspase 8 pathway in the expression of MyoD and skeletal myoblast differentiation, no obvious muscle related phenotype has been reported for TRAIL -/- (Cretney et al., 2002), DR5 -/- (Finnberg et al., 2005), FADD -/- (Yeh et al., 1998; Zhang et al., 1998), or caspase 8 -/- mice (Varfolomeev et al., 1998). In FADD -/- or caspase 8 -/- mice, this lack of muscle phenotype in may be attributed to the fact that these mice are not viable (Varfolomeev et al., 1998; Yeh et al., 1998; Zhang et al., 1998 ). TRAIL -/- and DR5 -/- mice, however, develop normally. A defective apoptotic phenotype in any cell type in the TRAIL -/- and DR5 -/- mice is only observed in response to DNA damaging agents such as chemical carcinogen or ionizing radiation (Cretney et al., 2002; Finnberg et al., 2005). Thus, we speculate that lack of a developmental muscle phenotype could indicate either that this pathway has no role during developmental myogenesis or that compensatory signaling negates detection of such a role. Further, defective differentiation in either TRAIL -/- or DR5 -/- skeletal myoblasts might only be observed in response to specific stimuli such as conditions that lead to regeneration or in the context of myoblast transplantation (Fidzianska and Goebel, 1991; Skuk et al., 2003; Bouchentouf et al., 2004; Menasche, 2004; Le Grand and Rudnicki, 2007; Price et al., 2007). Thus, these future studies, combined with further analysis to more precisely understand the molecular mechanism linking the TRAIL:DR5/FADD caspase pathway to the regulation of MyoD expression are needed.
Acknowledgements
This work was supported by NIH grants RO1CA84212 and R15AR053857 awarded to C. M. Weyman. M. Freer-Prokop was supported by a Molecular Medicine Fellowship provided by Cleveland State University and J. A. Ross was supported a GAANN fellowship from the Department of Education.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol. 2005;16:585–595. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Bouchentouf M, Benabdallah BF, Tremblay JP. Myoblast survival enhancement and transplantation success improvement by heat-shock treatment in mdx mice. Transplantation. 2004;77:1349–1356. doi: 10.1097/01.tp.0000121503.01535.f5. [DOI] [PubMed] [Google Scholar]
- Carrozza MJ, Utley RT, Workman JL, Cote J. The diverse functions of histone acetyltransferase complexes. Trends Genet. 2003;19:321–329. doi: 10.1016/S0168-9525(03)00115-X. [DOI] [PubMed] [Google Scholar]
- Chen JC, Love CM, Goldhamer DJ. Two upstream enhancers collaborate to regulate the spatial patterning and timing of MyoD transcription during mouse development. Dev Dyn. 2001;221:274–288. doi: 10.1002/dvdy.1138. [DOI] [PubMed] [Google Scholar]
- Chen JC, Ramachandran R, Goldhamer DJ. Essential and redundant functions of the MyoD distal regulatory region revealed by targeted mutagenesis. Dev Biol. 2002;245:213–223. doi: 10.1006/dbio.2002.0638. [DOI] [PubMed] [Google Scholar]
- Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- DeChant AK, Dee K, Weyman CM. Raf-induced effects on the differentiation and apoptosis of skeletal myoblasts are determined by the level of Raf signaling: abrogation of apoptosis by Raf is downstream of caspase 3 activation. Oncogene. 2002;21:5268–5279. doi: 10.1038/sj.onc.1205648. [DOI] [PubMed] [Google Scholar]
- Dee K, Freer M, Mei Y, Weyman CM. Apoptosis coincident with the differentiation of skeletal myoblasts is delayed by caspase 3 inhibition and abrogated by MEK-independent constitutive Ras signaling. Cell Death Differ. 2002;9:209–218. doi: 10.1038/sj.cdd.4400930. [DOI] [PubMed] [Google Scholar]
- Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci U S A. 2002;99:11025–11030. doi: 10.1073/pnas.162172899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando P, Megeney LA. Is caspase-dependent apoptosis only cell differentiation taken to the extreme? Faseb J. 2007;21:8–17. doi: 10.1096/fj.06-5912hyp. [DOI] [PubMed] [Google Scholar]
- Fidzianska A, Goebel HH. Human ontogenesis. 3. Cell death in fetal muscle. Acta Neuropathol (Berl) 1991;81:572–577. doi: 10.1007/BF00310140. [DOI] [PubMed] [Google Scholar]
- Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, Kim SH, Burns TF, Ajuha H, Page R, Wu GS, et al. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol Cell Biol. 2005;25:2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Yoshida K, Ushiyama M, Yamaguchi T, Iwashita K, Futagawa T, Shibayama Y, Oiso S, Takeda Y, Kariyazono H, et al. The small heat shock protein alphaB-crystallin inhibits differentiation-induced caspase 3 activation and myogenic differentiation. Biol Pharm Bull. 2006;29:1815–1819. doi: 10.1248/bpb.29.1815. [DOI] [PubMed] [Google Scholar]
- Karasarides M, Dee K, Schulman D, Wolfman A, Weyman CM. Active Ras-induced effects on skeletal myoblast differentiation and apoptosis are independent of constitutive PI3-kinase activity. Cell Biol Int. 2006;30:308–318. doi: 10.1016/j.cellbi.2005.12.003. [DOI] [PubMed] [Google Scholar]
- L'Honore A, Lamb NJ, Vandromme M, Turowski P, Carnac G, Fernandez A. MyoD distal regulatory region contains an SRF binding CArG element required for MyoD expression in skeletal myoblasts and during muscle regeneration. Mol Biol Cell. 2003;14:2151–2162. doi: 10.1091/mbc.E02-07-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Honore A, Rana V, Arsic N, Franckhauser C, Lamb NJ, Fernandez A. Identification of a new hybrid serum response factor and myocyte enhancer factor 2-binding element in MyoD enhancer required for MyoD expression during myogenesis. Mol Biol Cell. 2007;18:1992–2001. doi: 10.1091/mbc.E06-09-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19:628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P. Skeletal myoblast transplantation for cardiac repair. Expert Rev Cardiovasc Ther. 2004;2:21–28. doi: 10.1586/14779072.2.1.21. [DOI] [PubMed] [Google Scholar]
- Miller JB, Stockdale FE. Developmental regulation of the multiple myogenic cell lineages of the avian embryo. J Cell Biol. 1986;103:2197–2208. doi: 10.1083/jcb.103.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TV, McMahon JM, Howley BA, Stanley A, Ritter T, Mohr A, Zwacka, R., Fearnhead HO. A non-apoptotic role for caspase 9 in muscle differentiation. J. Cell Sci. 2008;121:3786–3793. doi: 10.1242/jcs.024547. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J, Mei Y, Freer M, Weyman CM. Signaling through the TRAIL receptor DR5/FADD pathway plays a role in the apoptosis associated with skeletal myoblast differentiation. Apoptosis. 2006;11:2103–2113. doi: 10.1007/s10495-006-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992;154:261–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Price FD, Kuroda K, Rudnicki MA. Stem cell based therapies to treat muscular dystrophy. Biochim Biophys Acta. 2007;1772:272–283. doi: 10.1016/j.bbadis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rimondi E, Secchiero P, Quaroni A, Zerbinati C, Capitani S, Zauli G. Involvement of TRAIL/TRAIL-receptors in human intestinal cell differentiation. J Cell Physiol. 2006;206:647–654. doi: 10.1002/jcp.20512. [DOI] [PubMed] [Google Scholar]
- Sandri MC,M, Massimino ML, Geromel V, Arslan P. Myoblasts and myotubes in primary cultures deprived of growth factors undergo apoptosis. Basic Appl Myol. 1996;6:257–260. [Google Scholar]
- Schwerk C, Schulze-Osthoff K. Non-apoptotic functions of caspases in cellular proliferation and differentiation. Biochem Pharmacol. 2003;66:1453–1458. doi: 10.1016/s0006-2952(03)00497-0. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Gonelli A, Carnevale E, Milani D, Pandolfi A, Zella D, Zauli G. TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation. 2003;107:2250–2256. doi: 10.1161/01.CIR.0000062702.60708.C4. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Zauli G. Tumor-necrosis-factor-related apoptosis-inducing ligand and the regulation of hematopoiesis. Curr Opin Hematol. 2008;15:42–48. doi: 10.1097/MOH.0b013e3282f15fa6. [DOI] [PubMed] [Google Scholar]
- Shaltouki A, Freer M, Mei Y, Weyman CM. Increased expression of the pro-apoptotic Bcl(2) family member PUMA is required for mitochondrial release of cytochrome C and the apoptosis associated with skeletal myoblast differentiation. Apoptosis. 2007;12:2143–2154. doi: 10.1007/s10495-007-0135-z. [DOI] [PubMed] [Google Scholar]
- Skuk D, Caron NJ, Goulet M, Roy B, Tremblay JP. Resetting the problem of cell death following muscle-derived cell transplantation: detection, dynamics and mechanisms. J Neuropathol Exp Neurol. 2003;62:951–967. doi: 10.1093/jnen/62.9.951. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Lassar AB, Weintraub H. A novel myoblast enhancer element mediates MyoD transcription. Mol Cell Biol. 1992;12:4994–5003. doi: 10.1128/mcb.12.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Wedhas N, Klamut HJ, Dogra C, Srivastava AK, Mohan S, Kumar A. Inhibition of mechanosensitive cation channels inhibits myogenic differentiation by suppressing the expression of myogenic regulatory factors and caspase-3 activity. Faseb J. 2005;19:1986–1997. doi: 10.1096/fj.05-4198com. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Zauli G, Secchiero P. The role of the TRAIL/TRAIL receptors system in hematopoiesis and endothelial cell biology. Cytokine Growth Factor Rev. 2006;17:245–257. doi: 10.1016/j.cytogfr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]