Abstract
Transmission of prions between mammalian species is thought to be limited by a “species barrier,” which depends on differences in the primary structure of prion proteins in the infecting inoculum and the host. Here we demonstrate that a strain of hamster prions thought to be nonpathogenic for conventional mice leads to prion replication to high levels in such mice but without causing clinical disease. Prions pathogenic in both mice and hamsters are produced. These results demonstrate the existence of subclinical forms of prion infection with important public health implications, both with respect to iatrogenic transmission from apparently healthy humans and dietary exposure to cattle and other species exposed to bovine spongiform encephalopathy prions. Current definitions of the species barrier, which have been based on clinical end-points, need to be fundamentally reassessed.
The prion diseases include Creutzfeldt-Jakob disease (CJD) and kuru in humans and scrapie and bovine spongiform encephalopathy (BSE) in animals. They are all transmissible to the same species by inoculation with, or dietary exposure to, infected tissues. According to the protein-only hypothesis (1), prions are composed principally or entirely of abnormal isoforms of a host-encoded glycoprotein, prion protein (PrP) (2). The disease-related isoform, PrPSc, is derived from its normal cellular precursor, PrPC, by a posttranslational process that involves a conformational change and is distinguished biochemically by its partial protease resistance and detergent insolubility. PrPSc is hypothesized to act as a conformational template, promoting the conversion of PrPC to further PrPSc.
Prion diseases are both naturally and experimentally transmissible between different mammalian species but such transmission, as judged by appearance of clinical signs, is limited by a so-called “species barrier” (3). This barrier may be of sufficient magnitude that transmissions, even when attempted by the most efficient, intracerebral, route of inoculation with high titer tissues, are extremely infrequent or absent. In contrast, same-species transmission of prions is typically highly efficient. Transmission is dose-dependent, with increasing mean incubation periods and a decreasing fraction of animals succumbing to the disease as increasing dilutions of inoculum are used. However, at higher titers, 100% of inoculated animals succumb to disease with a constant and remarkably consistent incubation period, which is not reduced by further increase of inoculum titer.
The biological effect of a species barrier is to increase mean incubation periods, increase the range of incubation periods, and reduce the fraction of animals succumbing to disease. Prion transmission across species appears to involve a stochastic process in as much as only a fraction of animals succumb, and with highly variable incubation periods. Second and subsequent passages of infectivity to the same species are associated with transmission parameters more closely resembling same-species transmissions. Species barriers have been quantified by this fall in mean incubation period on primary and second passage in the same species or by comparative end-point titration in the two species concerned.
The appearance of a novel human prion disease, variant CJD, in the United Kingdom from 1995 onward, and the experimental evidence that this disease is caused by the same prion strain as that causing BSE in cattle (4–6), has raised the possibility that a major epidemic of variant CJD will occur in the United Kingdom and other countries as a result of dietary or other exposure to BSE prions (7). Understanding the molecular basis of barriers to intermammalian transmission of prions is therefore of major public health importance.
The most intensively studied species barrier is the substantial barrier limiting transmission of prion diseases between hamsters and mice. In particular, the hamster scrapie strain Sc237 (8), which is similar to the strain classified as 263K (9, 10), is regarded as nonpathogenic for mice (with no clinical disease in mice observed for up to 735 days postinoculation; ref. 10) and was used in studies of species barriers in transgenic mice (8, 11, 12). It was demonstrated that transgenic mice expressing hamster PrP (in addition to endogenous mouse PrP), in sharp contrast to conventional mice, were highly susceptible to Sc237 hamster prions with consistent short incubation periods that were inversely correlated to hamster PrP expression levels (8, 12). The prions propagated in the transgenic mice were only pathogenic for hamsters and not for conventional mice. Importantly, however, these studies defined transmission by using clinical criteria and did not report PrPSc levels and types (4) or prion titers in the brains of clinically unaffected animals. Such studies argued that species barriers resided in differences in the primary structure of the PrP in the inoculum and host, prion propagation proceeding most efficiently when these sequences were identical.
However, it has been recognized for many years that prion strain type has an important influence on ease of transmission of prion disease between species (13). Prion strains are associated with distinct PrPSc types that can be distinguished by Western blot analysis with distinct cleavage sites to proteinase K, implying distinct PrPSc conformations (4, 14–18), and by differences in glycoform ratios of protease-digested PrPSc (4). Although conventionally the primary structure of a single polypeptide chain is thought to specify a single defined fold, in the case of PrP several distinct folds appear to be possible, accounting for these distinct PrPSc conformers. The importance of PrP primary structure homology to species barriers would be expected therefore to be only one factor in determining the efficiency of the interactions between PrP isoforms that determine prion propagation. With PrP, primary structure does not fully specify tertiary structure, but rather may influence which conformations, among the full range seen in mammalian prion diseases, are thermodynamically preferred. Inoculated prions preferentially convert PrPC into one of these conformers. According to this model, species barriers may be determined by the degree of overlap between the subset of PrPSc conformers allowed by the PrP in the host with that represented in the donor species (7).
A striking example of the strain effect to species barriers has been provided by analysis of BSE prions. Classical CJD prions, propagated in humans expressing wild-type human PrP, transmit highly efficiently to mice expressing only human PrP with transmission characteristics consistent with complete absence of a species barrier (19). Variant CJD prions, also propagated in humans expressing wild-type PrP of identical primary structure, have transmission properties completely distinct from classical human prions (as assessed either in transgenic or wild-type mice) but indistinguishable from those of cattle BSE (5, 6) and consistent with the presence of a transmission barrier.
Here we have investigated conventional mice inoculated with Sc237 hamster prions in more detail. Although, in agreement with earlier studies, no clinical signs of scrapie developed in such mice, neuropathological, molecular, and passage studies reveal the presence of subclinical prion infection in such animals with high prion titers in brain. These results necessitate a re-evaluation and definition of prion transmission barriers.
Materials and Methods
Inoculation of CD-1 Mice with Hamster Scrapie Strain Sc237.
Strict biosafety protocols were followed. Animal care was in accordance with institutional guidelines. Mice were inoculated in a class I microbiological safety cabinet and maintained in an animal microbiological containment level II facility. Preparation of inocula and removal of tissues was performed in a microbiological containment level III facility. All animals were examined twice weekly for clinical signs of scrapie. At onset of signs animals were examined daily and culled if exhibiting any signs of distress. Criteria for clinical diagnosis of scrapie in mice were as described (20). Animals were anaesthetized with halothane/O2 and intracerebrally inoculated into the right parietal lobe with 30 μl of a 1% brain homogenate in PBS. Sc237 hamster scrapie was provided by S. Prusiner (University of California, San Francisco) and passaged once in Syrian hamsters. Initial challenge of CD-1 mice with Sc237 used purified PrPSc, which was prepared as described (21) but omitting the final proteinase K step. Approximately 8.5 × 106 LD50 units of purified Sc237 PrPSc diluted in PBS were inoculated intracerebrally in a volume of 30 μl into Swiss CD-1 mice.
Titration of Infectivity from Sc237-Inoculated CD-1 Mice.
Serial 10-fold dilutions of mouse brain homogenate were prepared from 10-2 to 10-9, and each dilution was inoculated into six Tg20 mice and six hamsters. Prion titers were determined as described (22).
Immunoblotting Analysis.
Tissue was homogenized in 9 vol of PBS, and proteinase K was added to 50 μg/ml with incubation at 37°C for 60 min. Western blotting of brain homogenates was as described (4).
Neuropathology.
Mice were culled by using CO2 asphyxiation. Brains were fixed in 10% buffered formaldehyde and immersed in 98% formic acid for 1 h and paraffin-embedded sections (6 μm) stained with Harris's hematoxylin and eosin. Serial sections of 4 μm were examined for abnormal PrP immunohistochemistry by using the mAbs 3F4 (23) and ICSM18 [raised against recombinant-derived human PrP (); ref. 24]. Sections were pretreated with autoclaving, formic acid, and 4 M guanidine thiocyanate followed by a standard avidin-biotin complex with diaminobenzedine tetrachloride as the chromagen (25). All methods were performed by using appropriate positive controls. Negative controls for immunohistochemistry involved omitting the primary antibody.
Results
Challenge of CD-1 Mice with Sc237 Syrian Hamster Prions.
Conventional CD-1 mice were intracerebrally inoculated with ≈ 8.5 × 106 LD50 units of Sc237 prions or vehicle (PBS) alone. No scrapie-like clinical signs were observed in any animals from either group. All mice were carefully observed until death or until they developed other, intercurrent disease, which necessitated culling according to normal animal care criteria (Table 1). The observation periods for the Sc237- and PBS-inoculated mice [638 ± 28 days and 649 ± 48 days (means ± SEM), respectively] were not significantly different (P = 0.84, unpaired t test).
Table 1.
Challenge of CD-1 mice with Sc237 Syrian hamster prions (38)
| Animal no. | Inoculum | Clinical signs of scrapie | Observation period, days* | Western blot, R073† | Western blot, 3F4‡ |
|---|---|---|---|---|---|
| 7007 | Sc237 | No | 408 | − | − |
| 7009 | Sc237 | No | 463 | − | − |
| 5531 | Sc237 | No | 483 | − | − |
| 5554 | Sc237 | No | 492 | − | − |
| 6967 | Sc237 | No | 530 | − | − |
| 5553 | Sc237 | No | 554 | − | − |
| 6969 | Sc237 | No | 641 | − | − |
| 5532 | Sc237 | No | 655 | − | − |
| 5528 | Sc237 | No | 659 | + | − |
| 7008 | Sc237 | No | 694 | + | − |
| 5538 | Sc237 | No | 703 | + | − |
| 5537 | Sc237 | No | 706 | + | − |
| 5539 | Sc237 | No | 706 | + | − |
| 6968 | Sc237 | No | 726 | + | − |
| 5540 | Sc237 | No | 730 | + | − |
| 7010 | Sc237 | No | 745 | + | − |
| 5530 | Sc237 | No | 769 | + | − |
| 5526 | Sc237 | No | 828 | + | − |
| 6954 | PBS | No | 436 | − | − |
| 6956 | PBS | No | 521 | − | − |
| 6953 | PBS | No | 586 | − | − |
| 6952 | PBS | No | 630 | − | − |
| 5533 | PBS | No | 683 | − | − |
| 5536 | PBS | No | 707 | − | − |
| 5535 | PBS | No | 780 | − | − |
| 5525 | PBS | No | 849 | − | − |
Mice either died from natural causes at advanced age or were culled because of intercurrent illness.
Detects both mouse and hamster PrP.
Detects hamster PrP only.
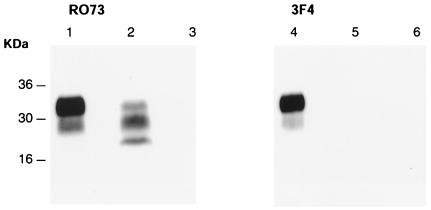
Western blot analysis was performed on all brains. PrPSc was demonstrated in approximately 50% of the Sc237-inoculated mice, but none of the PBS-inoculated controls. The observation periods for the PrPSc-positive mice ranged from 659 to 828 days postinoculation, whereas PrPSc-negative Sc327-inoculated mice were observed for 408–655 days. The differences in mean observation periods for PrPSc-positive and PrPSc-negative Sc237-inoculated mice were statistically significant (727 ± 15 and 528 ± 30 days, respectively; P < 0.0001, unpaired t test). Western blotting was performed both with mAb 3F4, which detects hamster but not mouse PrP (23), and polyclonal antibody R073, which detects both hamster and mouse PrP (26) to determine the type of PrPSc present. Mouse PrPSc was readily detectable, but no hamster PrPSc could be detected (Fig. 1).
Figure 1.
Western blot analysis of brain homogenates treated with proteinase K using anti-PrP antibodies R073, which detects both mouse and hamster PrP (lanes 1–3) and 3F4, which detects hamster PrP only (lanes 4–6) (40). Lanes 1 and 4: Sc237-inoculated hamster; lanes 2 and 5: Sc237-inoculated CD-1 mouse positive for murine PrPSc; lanes 3 and 6: Sc237-inoculated mouse negative for PrPSc. Numbers adjacent to horizontal lines indicate positions of molecular mass markers (kDa). Ten microliters of a 10% brain homogenate was loaded in each lane.
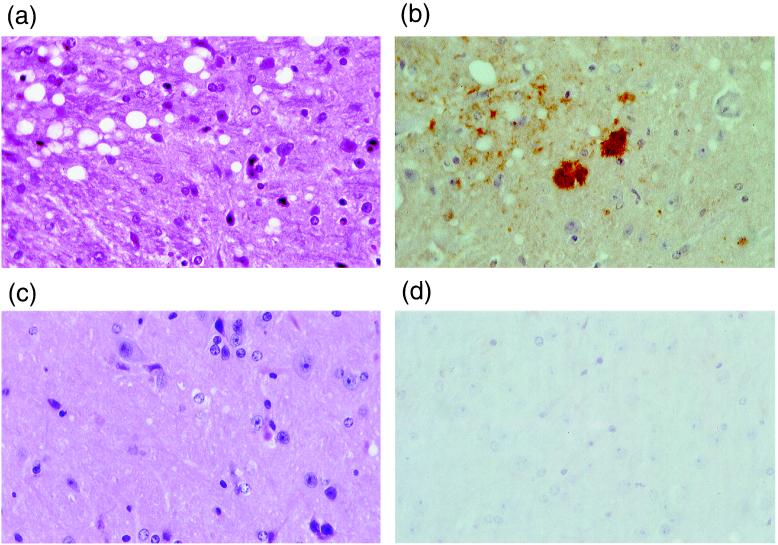
Mice from each group were subjected to full neuropathological examination. Several Sc237-inoculated mice showed the histological features of spongiform encephalopathy with PrP amyloid plaques, consistent with typical prion disease (Fig. 2). Age-matched, PBS-inoculated controls, which died at a similar time postinoculation, all had normal histology and negative PrP immunohistochemistry.
Figure 2.
Neuropathological examination of Sc237-inoculated (a) and (b) and PBS-inoculated (c) and (d) CD-1 mice (41). (a and c) Hematoxylin- and eosin-stained sections showing spongiform neurodegeneration in a. (b and d) PrP immunohistochemistry showing abnormal PrP immunoreactivity including PrP-positive plaques in b. (c and d) Normal appearances. Magnifications: ×150.
Passage of Brain Homogenate from Sc237- or Mock-Inoculated CD-1 Mice in Both Mice and Hamsters.
To investigate whether prion propagation had occurred in Sc237-inoculated mice and to study the characteristics of any infectious prions detected, we performed second-passage transmissions into CD-1 mice, Tg20 mice, which overexpress wild-type mouse PrP and have shortened incubation periods for mouse prions (27), and Syrian hamsters. Two Sc237-inoculated CD-1 mice were chosen for passage, one was PrPSc positive, the other negative. Two PBS-inoculated CD-1 mice were passaged as a negative control.
All animals in all three groups (CD-1 and Tg20 mice and Syrian hamsters) inoculated with the Sc237-inoculated PrPSc-positive CD-1 mouse brain developed typical scrapie signs with incubation periods as shown in Table 2.
Table 2.
Passage of infectivity from Sc237- or PBS-inoculated CD-1 mice into both mice and hamsters (38)
| Inoculum | Host | Affected/inoculated | Incubation period, days ± SEM |
|---|---|---|---|
| 5540 | CD-1 | 9 /9 | 197 ± 0 |
| (Sc237-inoculated CD-1/PrPSc-positive) | Tg20 | 4 /4 | 122 ± 7 |
| SHa | 10 /10 | 127 ± 4 | |
| 7009 | CD-1 | 0 /5 | >400 |
| (Sc237-inoculated CD-1/PrPSc-negative) | Tg20 | 0 /9 | >400 |
| SHa | 10 /10 | 172 ± 9 | |
| 5544 | CD-1 | 0 /8 | >400 |
| (PBS-inoculated CD-1/PrPSc-negative) | Tg20 | 0 /5 | >400 |
| SHa | 0 /10 | >400 |
Inoculation from an Sc237-inoculated PrPSc-negative CD-1 mouse into the three types of animal resulted in transmission only to hamsters with very prolonged and more variable incubation periods (148–238 days) (Table 2). None of the animals inoculated with material from the PBS-inoculated CD-1 mice had shown any scrapie-like symptoms at up to 650 days postinoculation.
All animals from these passage groups were examined by Western blotting and/or neuropathology. All clinically affected animals demonstrated classical neuropathological features of prion disease with widespread spongiform vacuolation and positive PrP immunoreactivity (data not shown). Western blotting revealed the presence of protease-resistant PrPSc (Fig. 3).
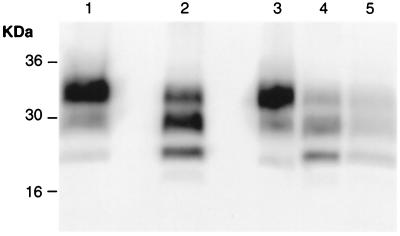
Figure 3.
Western blot analysis of proteinase K-treated brain homogenates using anti-PrP antibody R073 to determine PrPSc types (40). Lane 1: Sc237-inoculated Syrian hamster; lane 2: Sc237-inoculated CD-1 mouse; lanes 3–5: passage of Sc237-inoculated CD-1 mouse into Syrian hamster (lane 3), CD-1 mouse (lane 4), and Tg20 mouse (lane 5). Numbers adjacent to horizontal lines indicate positions of molecular mass markers (kDa).
End-Point Titration of CD-1-Passaged Sc237 Prions.
The prion titer in CD-1 mice inoculated with Sc237 hamster prions was determined by end-point titration, both in Tg20 mice and Syrian hamsters (Table 3). Prion titers in Sc237-inoculated PrPSc-positive brain were estimated at ≈108 LD50/g in hamsters and ≈107 LD50/g in Tg20 mice. Titration of prions from Sc237-inoculated PrPSc-negative CD-1 mouse brain revealed a titer of ≈106 LD50/g in hamsters but there was no transmission to Tg20 mice.
Table 3.
End-point titration of Sc237-inoculated CD-1 mice in Syrian hamsters and Tg20 mice (39)
| Inoculum | Host | LD50/g brain |
|---|---|---|
| 5540 | SHa | 8.9 × 107 |
| (Sc237-inoculated CD-1/PrPSc-positive) | Tg20 | 1.9 × 107 |
| 7009 | SHa | 7.7 × 105 |
| (Sc237-inoculated CD-1/PrPSc-negative) | Tg20 | <3.3 × 103 |
Molecular Analysis of Strain Characteristics of CD-1 Mouse Passaged Sc237 Prions.
Prion strains can be differentiated by differences in PrPSc fragment sizes and glycoform ratios on Western blots after proteinase K cleavage. We compared PrPSc in hamsters inoculated with Sc237 prions with that seen in Sc237-inoculated CD-1 mice and in brains of hamsters and mice inoculated with Sc237-inoculated PrPSc-positive and PrPSc-negative CD-1 mice (Fig. 3). The PrPSc type seen in Sc237-inoculated CD-1 mice differed sharply from that in Sc-237-inoculated hamsters, both with respect to fragment sizes and glycoform ratios after proteinase K digestion. Size of unglycosylated PrP fragment was 21.7 kDa in Sc237-inoculated CD-1 mice, and the most abundant glycoform was monoglycosylated [ratios (mean ± SEM): 34.6 ± 1.6% di-; 45.3 ± 1.6% mono-, and 20.0 ± 0.4% unglycosylated PrP]. In Sc237-inoculated hamsters, diglycosylated PrP predominated (ratios: 83.0% di-; 15.2% mono-, and 1.8% unglycosylated PrP), and the unglycosylated fragment was approximately 20.7 kDa.
On passage of prions from Sc237-inoculated CD-1 mice to additional CD-1 mice, and also to Tg20 mice, the same PrPSc type was generated, with fragment sizes and glycoform ratios indistinguishable from those in the Sc-237-inoculated CD-1 mice. However, on passage in Syrian hamsters, the PrPSc type reverted to that seen in Sc237-inoculated hamsters (Fig. 3).
Discussion
Implication of Demonstration of Subclinical Prion Infection.
In prion diseases, infectious titers in the brain rise progressively throughout prolonged, clinically silent periods that precede the onset of disease. Thus asymptomatic animals may harbor significant infectious titers in brain and other tissues. However, there may be subclinical, as distinct from such preclinical, forms of prion infection, where animals become asymptomatic carriers of infectivity and do not develop clinical disease in their lifetimes (7, 28). Such carrier states are well recognized in other infectious diseases. However, in prion diseases, where incubation periods are extremely prolonged, distinction between subclinical and preclinical states is more difficult. It certainly can be argued that animals dying after a typical lifespan without clinical signs of prion disease but harboring high levels of infectivity represent the late preclinical stage of “transmissions” where the “incubation period” exceeds the normal lifespan (29). The distinction between the terms subclinical and preclinical is essentially a semantic one in this context. Here we use the term subclinical infection operationally to refer to animals in which prion replication is occurring but which have not developed clinical signs of prion disease during a normal lifespan.
We have demonstrated that conventional mice inoculated with Sc237 prions harbor high levels of PrPSc and high prion titers in their brains without developing clinical signs of prion disease within their normal lifespan. These results imply the existence of subclinical prion infections that can be induced by challenge with prions from another species. However, whether or not this infectivity is classified as preclinical or subclinical, it has important public health implications. Iatrogenic transmission could occur from apparently healthy humans who may harbor high prion titers and many animal species (including sheep, pigs, and poultry) were exposed to BSE prions via contaminated feed and could have developed subclinical prion infection. It is known that BSE prions retain their distinctive strain characteristics after passage in a number of other species including humans (4, 13), arguing that such BSE passaged in species other than cattle also may be pathogenic to humans. The possibility that subclinical BSE might be present in other species and thereby present a threat to human health has been raised (30) but not yet rigorously investigated. Furthermore, these data argue in favor of screening apparently healthy cattle after slaughter to investigate whether significant levels of subclinical or preclinical BSE are present.
Secondly, because animals can harbor high levels of infectivity without developing clinical signs of prion disease, these results argue that PrPSc and indeed prions (whether or not they are identical) may not themselves be highly neurotoxic. Such results are in accordance with earlier findings of a lack of correlation between clinical disease and neuropathological features of prion disease (31), prion diseases in which PrPSc is barely or not detectable (32–35), and studies in mice with reduced levels of PrPC expression that have extremely high levels of PrPSc and prions in the brain and yet remain well for several months after their wild-type counterparts succumb (36). Conversely, Tg20 mice, with high levels of PrPC, have short incubation periods and yet produce low levels of PrPSc after inoculation with mouse prions (27). In addition, brain grafts producing high levels of PrPSc do not damage adjacent tissue in PrP knockout (Prnpo/o) mice (37). The cause of neurodegeneration in prion diseases remains unclear. It remains possible that prion neurodegeneration is related, at least in part, to loss of function of PrPC. That Prnpo/o mice (other than those associated with overexpression of the Prnp-like gene Prnd; ref. 38) do not develop neurodegeneration could be caused by compensatory adaptations during neurodevelopment. Complete or near complete ablation of PrP expression in an adult mouse using conditional gene expression methods has not yet been achieved. An alternative hypothesis is that a toxic, possibly infectious, intermediate is produced in the process of conversion of PrPC to PrPSc, with PrPSc, present as highly aggregated material, being a relatively inert end-product. The steady-state level of such a toxic monomeric or oligomeric PrP intermediate then could determine rate of neurodegeneration. One possibility is that Sc237-inoculated CD-1 mice propagate prions very slowly and that such a toxic intermediate is generated at extremely low levels that are tolerated by the mouse. The fact that the PrPSc-negative Sc237-inoculated CD-1 mice were the ones culled earlier than those that were PrPSc positive, allows the assumption that they may have become PrPSc positive had they lived longer. A more detailed study of the time course of accumulation of infectivity will be necessary to investigate this further.
Transmission of Infectivity from Subclinical Animals.
The transmission properties of prions from the subclinical Sc237-inoculated CD-1 mice were remarkable. With respect to transmissions to additional CD-1 or Tg20 mice, the 100% attack rate and highly consistent incubation periods suggest transmission in the absence of a barrier. However, the incubation periods, notably in the Tg20 mice, which succumb to RML mouse prions in around 60 days (27), are very prolonged. The 100% attack rate argues against this being a consequence of low prion titer in the inoculum. Incubation period at end point dilution in Tg20 mice of RML mouse prions is around 109 days (37). Remarkably, passage in hamsters of this isolate also showed a 100% attack rate and consistent incubation periods suggestive of transmission in the absence of a barrier. Again, incubation periods were extremely prolonged and differed markedly from the transmission properties of Sc237/263K prions in hamsters (8, 10, 39). Indeed, the incubation period seen would correspond to an Sc237 titer in Syrian hamsters of <103 LD50/g brain, which is completely inconsistent with the titers measured; Sc237 incubation periods at end point dilution in Syrian hamsters are around 130 days (40). That a 100% attack rate was seen at a 127-day incubation period argues against persistent Sc237 inoculum, rather than newly formed prions, being responsible for the pathogenicity to hamsters. Together, these data suggested production of novel infectivity, pathogenic for both mice and hamsters on passage of Sc237 to CD-1 mice.
A recent report has suggested that hamster scrapie (263K) may persist in the brains of inoculated C57BL/10 mice for prolonged periods without replication (41). Our data are not consistent with infectivity in the PrPSc-positive Sc237-inoculated CD-1 mice being the result of persistence of residual Sc237 hamster scrapie inoculum. High levels of mouse PrPSc (and no hamster PrPSc) are detectable on Western blot, and prions pathogenic for mice are generated. Intracerebral inoculation is known to result in wide distribution of the inoculum outside the brain via the circulation and, presumably as a result of other clearance mechanisms, brain titers fall to undetectable levels within a few days (42). Prion titers present in the brains of these mice (≈108 LD50/g mouse brain assayed in hamsters) considerably exceed those inoculated (≈8.5 × 106). Together, these data argue strongly for prion replication in these mice. It is possible that the prions detected in the brains of the C57BL/10 mice in the earlier study were not caused by persistence of inoculated 263K, but by propagation of prions with the properties we describe. The species origin of PrPSc (hamster or mouse) in the 263K-inoculated C57BL/10 mice was not reported. The observation periods postinoculation were generally much shorter than those we report here. That those mice with the longest survival postinoculation produced the shortest incubation periods on passage of infectivity into hamsters is consistent with propagation, rather than simply persistence, of prions in this earlier study (41).
Re-Evaluation of Species Barriers.
Importantly, these data seriously question our current understanding of species barriers. The assessment of species barriers has relied on the development of a clinical disease in inoculated animals. On this basis there is a highly efficient barrier limiting transmission of Sc237 prions to mice. However, although not developing a clinical disease, and indeed living as long as mock-inoculated mice, Sc237-inoculated mice may accumulate high levels of prions in their brains. Previous studies on the species barrier between hamsters and mice (using the Sc237 or 263K strain) did not report whether PrPSc and/or infectivity were present in clinically unaffected animals (8, 12) or have attempted passage from mice only up to 280 days postinoculation (10). The barrier to primary passage appears in this case to be to the development of rapid neurodegeneration and the resulting clinical syndrome rather than a barrier to prion propagation itself.
The transmission characteristics of prions generated in the brains of Sc237-inoculated CD-1 mice argue that one or more distinct prion strains have been generated. The finding that Sc237-inoculated CD-1 mice in which PrPSc could not be detected on Western blot were the ones that had been culled after shorter periods than mice with detectable PrPSc argues that prion propagation is occurring in all of these mice, but is detectable only after prolonged incubation periods. That high levels of hamster infectivity were present in the PrPSc-negative Sc237-inoculated CD-1 mouse (examined at 463 days postinoculation) in the absence of detectable mouse infectivity, whereas very high and relatively comparable titers of both mouse and hamster infectivity were present in the PrPSc-positive Sc237-inoculated CD-1 mouse (examined at 730 days postinoculation) suggests that more than one strain may be propagating in these mice, with preferential replication of a strain with higher pathogenicity for hamsters early in the incubation period. One possibility is that early replication of a prion strain pathogenic only for hamsters is induced in Sc237-inoculated CD-1 mice, then later followed by the generation of a second strain that is pathogenic for mice. More extensive passage studies, including cloning of strains at end-point dilution in both mice and hamsters, will be required to investigate this further and to characterize the strain(s) of prions generated in the brains of Sc237-inoculated CD-1 mice.
Acknowledgments
We thank C. Weissmann for Tg20 mice and L. Doey, L. Hudson, and R. Bond for technical assistance. This work was funded by the Medical Research Council and Wellcome Trust.
Abbreviations
- CJD
Creutzfeldt–Jakob disease
- BSE
bovine spongiform encephalopathy
- PrP
prion protein
- PrPSc
disease-related isoform of PrP
- PrPC
cellular PrP
References
- 1.Griffith J S. Nature (London) 1967;215:1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner S B. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 3.Pattison I H. In: Slow, Latent and Temperate Virus Infections, NINDB Monograph 2. Gajdusek C J, Gibbs C J, Alpers M P, editors. Washington DC: U.S. Government Printing; 1965. pp. 249–257. [Google Scholar]
- 4.Collinge J, Sidle K C L, Meads J, Ironside J, Hill A F. Nature (London) 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 5.Hill A F, Desbruslais M, Joiner S, Sidle K C L, Gowland I, Collinge J. Nature (London) 1997;389:448–450. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 6.Bruce M E, Will R G, Ironside J W, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, et al. Nature (London) 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 7.Collinge J. Lancet. 1999;354:317–323. doi: 10.1016/S0140-6736(99)05128-4. [DOI] [PubMed] [Google Scholar]
- 8.Scott M, Foster D, Mirenda C, Serban D, Coufal F, Wälchli M, Torchia M, Groth D, Carlson G, DeArmond S J, et al. Cell. 1989;59:847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- 9.Kimberlin R H, Walker C A. J Gen Virol. 1977;34:295–304. doi: 10.1099/0022-1317-34-2-295. [DOI] [PubMed] [Google Scholar]
- 10.Kimberlin R H, Walker C A. J Gen Virol. 1978;39:487–496. doi: 10.1099/0022-1317-39-3-487. [DOI] [PubMed] [Google Scholar]
- 11.Kimberlin R H, Walker C A. J Gen Virol. 1979;42:107–117. doi: 10.1099/0022-1317-42-1-107. [DOI] [PubMed] [Google Scholar]
- 12.Prusiner S B, Scott M, Foster D, Pan K M, Groth D, Mirenda C, Torchia M, Yang S L, Serban D, Carlson G A, et al. Cell. 1990;63:673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- 13.Bruce M, Chree A, McConnell I, Foster J, Pearson G, Fraser H. Philos Trans R Soc London B. 1994;343:405–411. doi: 10.1098/rstb.1994.0036. [DOI] [PubMed] [Google Scholar]
- 14.Bessen R A, Marsh R F. J Virol. 1992;66:2096–2101. doi: 10.1128/jvi.66.4.2096-2101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bessen R A, Marsh R F. J Virol. 1994;68:7859–7868. doi: 10.1128/jvi.68.12.7859-7868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telling G C, Parchi P, DeArmond S J, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner S B. Science. 1996;274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 17.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen F E, Prusiner S B. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 18.Wadsworth J D F, Hill A F, Joiner S, Jackson G S, Clarke A R, Collinge J. Nat Cell Biol. 1999;1:55–59. doi: 10.1038/9030. [DOI] [PubMed] [Google Scholar]
- 19.Collinge J, Palmer M S, Sidle K C L, Hill A F, Gowland I, Meads J, Asante E, Bradley R, Doey L J, Lantos P L. Nature (London) 1995;378:779–783. doi: 10.1038/378779a0. [DOI] [PubMed] [Google Scholar]
- 20.Carlson G A, Kingsbury D T, Goodman P A, Coleman S, Marshall S T, DeArmond S J, Westaway D, Prusiner S B. Cell. 1986;46:503–511. doi: 10.1016/0092-8674(86)90875-5. [DOI] [PubMed] [Google Scholar]
- 21.Bolton D C, Meyer R K, Prusiner S B. J Virol. 1985;53:596–606. doi: 10.1128/jvi.53.2.596-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed L J, Muench H. J Am Hyg. 1938;27:493–497. [Google Scholar]
- 23.Kascsak R J, Rubenstein R, Merz P A, Tonna DeMasi M, Fersko R, Carp R I, Wisniewski H M, Diringer H. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson G S, Hill A F, Joseph C, Hosszu L, Power A, Waltho J P, Clarke A R, Collinge J. Biochim Biophys Acta Protein Struct Mol Enzymol. 1999;1431:1–13. doi: 10.1016/s0167-4838(99)00038-2. [DOI] [PubMed] [Google Scholar]
- 25.Bell J E, Gentleman S M, Ironside J W, McCardle L, Lantos P L, Doey L, Lowe J, Fergusson J, Luthert P, McQuaid S, et al. Neuropathol Appl Neurobiol. 1997;23:26–35. [PubMed] [Google Scholar]
- 26.Serban D, Taraboulos A, DeArmond S J, Prusiner S B. Neurology. 1990;40:110–117. doi: 10.1212/wnl.40.1.110. [DOI] [PubMed] [Google Scholar]
- 27.Fischer M, Rülicke T, Raeber A, Sailer A, Oesch B, Brandner S, Aguzzi A, Weissmann C. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 28.Frigg R, Klein M A, Hegyi I, Zinkernagel R M, Aguzzi A. J Virol. 1999;73:9584–9588. doi: 10.1128/jvi.73.11.9584-9588.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickinson A G, Fraser H, Outram G W. Nature (London) 1975;256:732–733. doi: 10.1038/256732a0. [DOI] [PubMed] [Google Scholar]
- 30.Collinge J, Rossor M. Lancet. 1996;347:916–917. doi: 10.1016/s0140-6736(96)91407-5. [DOI] [PubMed] [Google Scholar]
- 31.Collinge J, Owen F, Poulter M, Leach M, Crow T J, Rossor M N, Hardy J, Mullan M J, Janota I, Lantos P L. Lancet. 1990;336:7–9. doi: 10.1016/0140-6736(90)91518-f. [DOI] [PubMed] [Google Scholar]
- 32.Medori R, Montagna P, Tritschler H J, LeBlanc A, Cortelli P, Tinuper P, Lugaresi E, Gambetti P. Neurology. 1992;42:669–670. doi: 10.1212/wnl.42.3.669. [DOI] [PubMed] [Google Scholar]
- 33.Collinge J, Palmer M S, Sidle K C L, Gowland I, Medori R, Ironside J, Lantos P L. Lancet. 1995;346:569–570. doi: 10.1016/s0140-6736(95)91405-6. [DOI] [PubMed] [Google Scholar]
- 34.Hsiao K K, Scott M, Foster D, Groth D F, DeArmond S J, Prusiner S B. Science. 1990;250:1587–1590. doi: 10.1126/science.1980379. [DOI] [PubMed] [Google Scholar]
- 35.Lasmezas C I, Deslys J-P, Robain O, Jaegly A, Beringue V, Peyrin J-M, Fournier J-G, Hauw J-J, Rossier J, Dormant D. Science. 1997;275:402–405. doi: 10.1126/science.275.5298.402. [DOI] [PubMed] [Google Scholar]
- 36.Bueler H, Raeber A, Sailer A, Fischer M, Aguzzi A, Weissmann C. Mol Med. 1994;1:19–30. [PMC free article] [PubMed] [Google Scholar]
- 37.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Nature (London) 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 38.Moore R C, Lee I Y, Silverman G L, Harrison P M, Strome R, Heinrich C, Karunaratne A, Pasternak S H, Chishti M A, Liang Y, et al. J Mol Biol. 1999;292:797–817. doi: 10.1006/jmbi.1999.3108. [DOI] [PubMed] [Google Scholar]
- 39.Marsh R F, Kimberlin R H. J Infect Dis. 1975;131:104–110. doi: 10.1093/infdis/131.2.104. [DOI] [PubMed] [Google Scholar]
- 40.Prusiner S B, Groth D F, Cochran S P, Masiarz F R, McKinley M P, Martinez H M. Biochemistry. 1980;19:4883–4891. doi: 10.1021/bi00562a028. [DOI] [PubMed] [Google Scholar]
- 41.Race R, Chesebro B. Nature (London) 1998;392:770. doi: 10.1038/33834. [DOI] [PubMed] [Google Scholar]
- 42.Sailer A, Büeler H, Fischer M, Aguzzi A, Weissmann C. Cell. 1994;77:967–968. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]