Abstract
Objective
To determine whether first trimester maternal serum Placental Protein 13 (PP13) concentrations can be used in the risk assessment for preeclampsia.
Study Design
This case-control study included 50 patients with preeclampsia and 250 patients with normal pregnancies. Samples were collected between 8-13 weeks of gestation. Serum PP13 concentrations were measured by ELISA and expressed as medians and multiples of the median (MoM) for gestational age. Sensitivity and specificity were derived from receiver operating characteristic curve analysis.
Results
1) Serum PP13 concentration in the first trimester was significantly lower in patients who developed preterm and early-onset preeclampsia than in those with normal pregnancies; and 2) At 80% specificity, a cutoff of 0.39 MoM had a sensitivity of 100% for early-onset preeclampsia and 85% for preterm preeclampsia.
Conclusion
Maternal serum first trimester PP13 appears to be a reasonable marker for risk assessment, but a weak marker for severe preeclampsia at term, and ineffective for identifying mild preeclampsia at term.
Keywords: Risk assessment, screening, maternal serum biochemistry, high-risk pregnancy, prenatal care
INTRODUCTION
Preeclampsia complicates approximately 5% of all pregnancies and remains a leading cause of maternal and perinatal morbidity and mortality.1–6 It is increasingly recognized that patients presenting with preeclampsia at early gestational ages have a worse form of the disease with a higher frequency of multi-systemic involvement and small for gestational age (SGA) fetuses than those presenting at term.4;7–16 Early-onset preeclampsia (<34 weeks of gestation) is characterized by uteroplacental vascular insufficiency and damage to the placental villous tissues.2;13;17–22 Indeed, patients with early-onset disease are more likely to have abnormal uterine and umbilical artery Doppler velocimetry studies23–31 and lesions recognized by placental histological examination.20;32–44 Moreover, the perinatal morbidity and mortality is higher in early-onset disease,4;8;15 as are the frequencies of HELLP syndrome10;45–48 and placental abruption.49–52
Risk assessment for preeclampsia remains a major challenge in prenatal care. A wide range of markers have been the subject of investigation, ranging from uterine artery Doppler velocimetry23-31 to analytes such as soluble vascular endothelial growth factor receptor-1,16;53-76 placental growth factor,16;53;61;70;72-83 soluble endoglin70;74;84-87 and others.88
Placental Protein 13 (PP13)89-92 is a member of the galectin family,93;94 predominantly expressed by the placenta, specifically by the syncytiotrophoblast, where it is localized on the brush-border membrane at the maternal-fetal interface.94;95 Recently, maternal serum PP13 concentrations were found to be significantly reduced during the first trimester among women who subsequently developed preeclampsia.96;97
The purpose of this study was to determine whether PP13 serum concentrations in the first trimester of pregnancy can be used in the risk assessment for preeclampsia. We have conducted a nested case-control study in a Hispanic population, which has been reported to have an increased relative risk for preeclampsia than that of non-Hispanic Caucasian women.98
MATERIALS AND METHODS
A nested case-control study was designed using data from a prospective, longitudinal study conducted by the Perinatology Research Branch of the National Institute of Child Health and Human Development (NICHD). This cohort included pregnant patients seeking care at the prenatal clinics of the Sotero del Rio Hospital in Santiago, Chile. First trimester blood samples were obtained upon enrollment, beginning at 7 weeks of gestation.54 All women provided written informed consent prior to the collection of samples. The collection and utilization of the samples was approved by the Institutional Review Boards of both the Sotero del Rio Hospital, Santiago, Chile, and the National Institute of Child Health and Human Development (NICHD/NIH/DHHS) Bethesda, Maryland.
Women aged 18-45 years with a singleton gestation who delivered after 26 weeks were eligible for inclusion. Patients were classified into the following study groups: 1) preeclampsia (n=50), and 2) normal pregnancy (n=250). Women in the preeclampsia group were further classified as: 1) preterm preeclampsia (n=13); 2) severe preeclampsia at term (n=21); and 3) mild preeclampsia at term (n=16). From the group of patients with preterm preeclampsia, six women with early-onset preeclampsia (preeclampsia requiring delivery before 34 weeks of gestation) were examined as a separate group. Each patient with preeclampsia was matched to five women with normal pregnancies. The cases were matched by gestational age at venipuncture (±1 week) and duration of storage of the specimen (±2 weeks).
Baseline demographics, blood pressure measurements, and urinalyses from the first prenatal visit through the postpartum period as well as subsequent outcome of pregnancy were collected prospectively by practitioners throughout prenatal care. Serum samples were collected at the time of the first prenatal visit and at regular intervals thereafter.
Definitions
Gestational age (GA) was determined by the last menstrual period (LMP) and verified by fetal biometry in the first or second trimester of pregnancy in all patients. Preeclampsia was defined as hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on at least two occasions, 4 hours to 1 week apart) associated with proteinuria (>300 mg in a 24-hour urine collection or one dipstick measurement of ≥2+).99 Severe preeclampsia was defined as systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥110 mmHg and/or proteinuria greater than 5 grams in a 24-hour collection or ≥3+ on dipstick.5 Patients with preeclampsia were sub-classified as either early-onset (<34 weeks) or preterm (<37 weeks) preeclampsia according to the gestational age at which delivery was required. Small for gestational age (SGA) was defined as a birthweight below the 10th percentile for the gestational age at birth, according to the national birthweight distribution of a Hispanic population.100 Normal pregnancy was defined as one that resulted in the delivery of an appropriate-for-gestational age neonate at term without complications.
PP13 immunoassay
Samples of peripheral blood from pregnant women were obtained by venipuncture, centrifuged and stored at -80°C. Maternal serum concentration of PP13 was measured using a solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) with a pair of PP13-specific monoclonal antibodies, marked with amplified biotin-extravidin-horseradish-peroxidase complex, and developed with tetramethylbenzidene substrate, as previously described.92 Optical density was measured at 450 nm against a 650 nm background. Concentrations were determined by extrapolation from a standard curve constructed using recombinant PP13 standards (0-500 pg/mL). The sensitivity of the assay was 5 pg/ml. The intra-and inter-assay coefficients of variation (CV) for this study were 7.3% and 19.5%, respectively. The laboratory staff performing the assays was blinded to pregnancy outcome.
Statistical analysis
Baseline demographics, clinical and delivery characteristics were compared using Fisher’s exact test for categorical variables and Wilcoxon Rank-sum test for continuous variables. The first trimester concentration of PP13 for each subject was converted into a multiple of the gestational age-specific median (MoM) following the method described by Cuckle and Wald.101 In brief, this was done by computing the median PP13 concentration of pregnancies with normal outcomes. Medians were calculated for each completed week of gestation at venipuncture and adjustment was then made using weighted (by number of patients) regression to model the relationship between PP13 concentration and gestational age. Because of the wide window range of gestational age the cubic weighted regression model showed superior fit over the linear weighted regression model for this population.
MoM was then computed for all subjects (cases and controls) with the following formula:
Where, i = subject, j = GA, Median is the regressed value.
The gestational age adjusted PP13 MoM was then further adjusted to body mass index (BMI) because of the significant correlation between PP13 MoM and BMI (p=0.048). In the normal pregnancy group, there was no association between PP13 MoM and maternal age (p=0.34) or parity (p=0.15), thus, PP13 MoM was initially adjusted only to GA and BMI. However, due to the correlation of PP13 MoM to parity and maternal age in the preeclampsia group, PP13 MoM was subsequently adjusted to four parameters: GA, BMI, maternal age and parity. No adjustment was made to ethnicity considering the common Hispanic origin.
PP13 MoM was first compared between women with normal pregnancy and those with preeclampsia. Subsequently, a subgroup analysis was performed based on the clinical subtypes of preeclampsia. The comparison of PP13 MoM between groups was performed with the Wilcoxon Rank-sum test.
The sensitivity and specificity for different thresholds of PP13 MoM were derived from receiver operating characteristic (ROC) curves, which included all cases with preeclampsia and then a set of curves generated for the different clinical subtypes of the syndrome (mild preeclampsia at term, severe preeclampsia at term, preterm and early-onset preeclampsia). The area under the curve (AUC), along with the 95% CI and p-values were derived. Diagnostic indices (sensitivity and specificity) for first trimester maternal serum PP13 MoMs were calculated. Data were analyzed using SAS®.
RESULTS
Demographic and clinical characteristics
Table I displays the demographic characteristics of the study population. Women with severe preeclampsia at term were younger than those with normal pregnancies. Patients with other clinical subtypes of preeclampsia did not differ in maternal age from the group of normal pregnancy. The mean BMIs at enrollment for patients with mild and severe preeclampsia at term, and for patients with preterm preeclampsia were significantly higher than those of patients with normal pregnancies. The proportion of nulliparous women was significantly higher in the group of severe preeclampsia at term than in all other study groups. The median gestational age at venipuncture did not differ among the study groups.
Table I.
Demographic characteristics of the study groups.
| Study groups | |||||
|---|---|---|---|---|---|
| Normal pregnancy (n=250) | Mild preeclampsia at term (n=16) | Severe Preeclampsia at term (n=21) | Preterm preeclampsia (n=13) | Early-onset preeclampsia (n=6) | |
| Maternal age (years) | 24.5 ± 5.6 | 27.2 ± 7.6 | 21.5 ± 4.7† | 27.2 ± 7.1 | 28.3 ± 7.7 |
| Maternal body mass index (kg/m2) | 22.1 ± 2.0 | 24.3 ± 2.6‡ | 25.7 ± 5.1‡ | 25.9 ± 5.3† | 26.5 ± 5.7 |
| Nulliparity (%) | 49.2 | 56.3 | 81.0† | 46.2 | 33.3 |
| Gestational age at blood draw (weeks) | 11.1 (8.0-13.9) | 11.5 (8.6-13.4) | 11.9 (9.3-13.9) | 11.6 (8.4-13.7) | 10.6 (9.0-13.3) |
Values are expressed as mean ± standard deviation or median (range)
p<0.01, compared to the normal pregnancy group
p<0.001 compared to the normal pregnancy group
Early-onset preeclampsia cases were included in the preterm preeclampsia group
Table II displays the clinical characteristics of the study population. Cesarean delivery was more frequent in the groups with preeclampsia than among women with normal pregnancies. The mean birthweight of neonates born to women with severe preeclampsia at term and to those with preterm preeclampsia was significantly lower than for those born to women with normal pregnancies. In the group of patients with preterm preeclampsia, 9 of the 13 patients delivered an SGA neonate. In the group of patients with term preeclampsia there were 7 SGA neonates (5 with severe preeclampsia).
Table II.
Clinical characteristics of the study groups.
| Study groups | |||||
|---|---|---|---|---|---|
| Normal pregnancy (n=250) | Mild preeclampsia at term (n=16) | Severe preeclampsia at term (n=21) | Preterm preeclampsia (n=13) | Early-onset Preeclampsia (n=6) | |
| GA at delivery (weeks) | 39.7 ± 1.1 | 39.1 ± 1.2 | 39.0 ± 1.0† | 34.0 ± 3.0‡ | 31.5 ± 2.5‡ |
| Cesarean delivery (%) | 8.4 | 18.8 ‡ | 33.3 ‡ | 61.5 ‡ | 83.3 ‡ |
| Birthweight (grams) | 3448.0±278.2 | 3386.3±545.0 | 3143.8±472.9† | 1872.3±722.7‡ | 1226.7±383.3‡ |
| Female neonates (%) | 44.4 | 50.0 | 33.3 | 46.2 | 33.3 |
| Highest systolic BP (mmHg) | 122.5 ± 6.9 | 144.1 ± 5.8‡ | 158.9 ± 17.0‡ | 163.1 ± 22.5‡ | 165.0 ± 18.7‡ |
| Highest diastolic BP (mmHg) | 77.2 ± 5.0 | 95.1 ± 5.8‡ | 101.8 ± 12.5‡ | 106.2 ± 13.2‡ | 108.3 ± 14.7‡ |
| Highest Proteinuria (by dipstick) | 0 (0-1) | 2 (1-2) | 3 (2-4) | 3 (2-4) | 3 (2-4) |
Values are expressed as percent, mean ± standard deviation, or median (range)
p<0.01, compared to the normal pregnancy group
p<0.001 compared to the normal pregnancy group
Early-onset preeclampsia cases were included in the preterm preeclampsia group
P < 0 .01 compared with the normal pregnancy group
BP, blood pressure
PP13 concentrations and MoMs across the first trimester
The week-specific PP13 concentration medians between eight to thirteen weeks of gestation (for each completed week of gestation) in women with a normal pregnancy were 60.25 pg/mL (n=23), 67.59 pg/mL (n=46), 167.41 pg/mL (n=33), 114.33 pg/mL (n=40), 88.62 pg/mL (n=49), and 135.00 pg/mL (n=59), respectively. This pattern fitted a cubic weighted regression model compared to linear or other weighted regression models.
First trimester PP13 MoMs in patients with preeclampsia
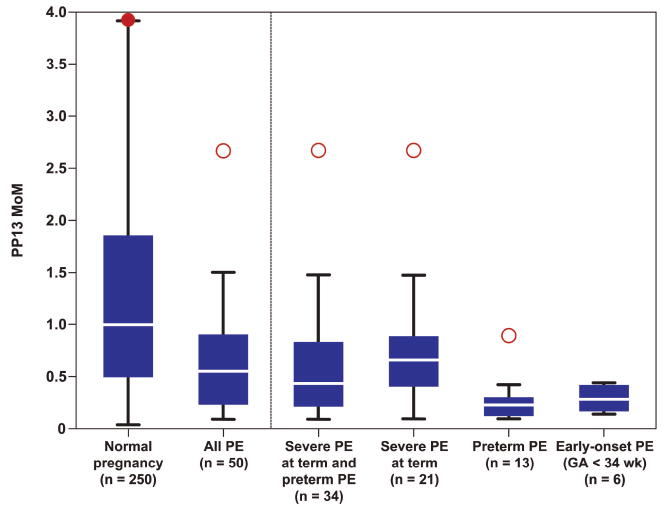
Patients with preeclampsia were first studied as one group in both models; they had a significantly lower median PP13 MoM than women who had a normal pregnancy (Figure 1). The comparison of the sub-groups of preeclampsia to the normal pregnancy group demonstrated the following: 1) In the first model (Table IIIA), the median PP13 MoM was significantly lower in patients who subsequently developed preterm preeclampsia, early-onset preeclampsia, and severe preeclampsia at term than in women with a normal pregnancy.2) In the second model (Table IIIB), only patients who subsequently developed preterm preeclampsia and early-onset preeclampsia had a significantly lower PP13 MoM than in women with a normal pregnancy.
Figure 1. PP13 multiples of the median (MoMs) according to the study groups.
The results are based on the first model where PP13 MoM was adjusted to GA and BMI. The boxplot represents the medians (as a horizontal line within the box), the 25th and 75th quartiles and the maximum and minimum for each group. Red dots refer to outliers in the right position, the filled red dot refers to a clipped outlier outside the range of the figure. PE: preeclampsia.
Table IIIA.
Median first trimester maternal serum PP13 MoMs according to the study groups adjusted to gestational age and body mass index
| Study groups | Median MoMs | 95% CI for the median MoMs | p-values† |
|---|---|---|---|
| Normal pregnancy (n=250) | 1.00 | 0.83 - 1.10 | Reference value |
| All preeclampsia (n=50) | 0.59 | 0.41 - 0.83 | <0.001 |
| Early-onset preeclampsia (n=6) | 0.26 | 0.10 - 0.40 | 0.002 |
| Preterm preeclampsia (n=13) | 0.24 | 0.11 - 0.40 | <0.001 |
| Severe preeclampsia at term (n=21) | 0.65 | 0.43 - 0.85 | 0.022 |
| Mild preeclampsia at term (n=16) | 0.89 | 0.66 - 1.24 | 0.491 |
p-values for the comparison of each study group with the normal pregnancy group. Median PP13 MoMs were significantly lower (p<0.05) than in the normal pregnancy group for each of the following groups: all preeclampsia, severe preeclampsia at term, preterm preeclampsia, and early-onset preeclampsia. Early-onset preeclampsia cases were included in the preterm preeclampsia group.
Table IIIB.
Median first trimester maternal serum PP13 MoMs according to the study groups adjusted to gestational age, body mass index, parity, and maternal age.
| Study groups | Median MoMs | 95% CI for the median MoMs | p-values† |
|---|---|---|---|
| Normal pregnancy (n=250) | 1.00 | 0.87 - 1.11 | Reference value |
| All preeclampsia (n=50) | 0.59 | 0.41 - 0.80 | <0.001 |
| Early-onset preeclampsia (n=6) | 0.25 | 0.10 - 0.38 | 0.001 |
| Preterm preeclampsia (n=13) | 0.22 | 0.13 - 0.38 | <0.001 |
| Severe preeclampsia at term (n=21) | 0.73 | 0.41 - 1.03 | 0.073 |
| Mild preeclampsia at term (n=16) | 0.84 | 0.57 - 1.26 | 0.390 |
p-values for the comparison of each study group with the normal pregnancy group. Median PP13 MoMs were significantly lower (p<0.05) than in the normal pregnancy group for each of the following groups: all preeclampsia, preterm preeclampsia, and early-onset preeclampsia. Early-onset preeclampsia cases were included in the preterm preeclampsia group.
Preeclampsia risk assessment by median PP13 MoMs
ROC curves for PP13 MoMs were generated for all cases of preeclampsia (Figure 2) and for the clinical subtypes of preeclampsia (Figures 3A and 3B) according to both adjustment models. The diagnostic indices of each model that were generated according to the ROC curves are presented in Tables IVA and IVB.
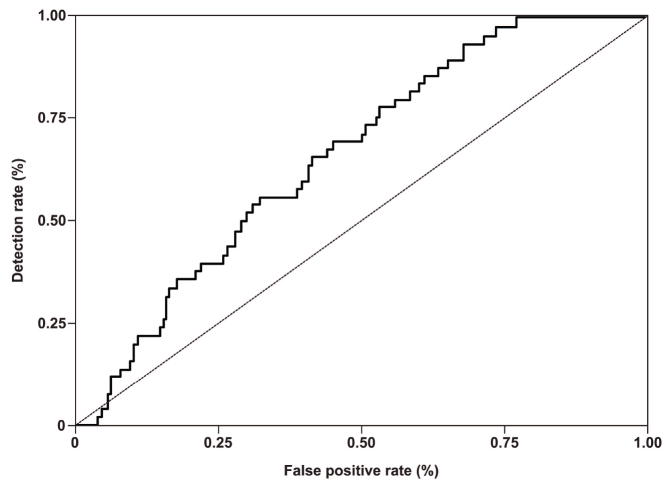
Figure 2. Receiver operating characteristic (ROC) curve depicting the sensitivity and specificity of PP13 MoM in maternal serum for the identification of all cases of preeclampsia.
The results are based on the second model where PP13 MoM was adjusted to GA, BMI, maternal age and parity.
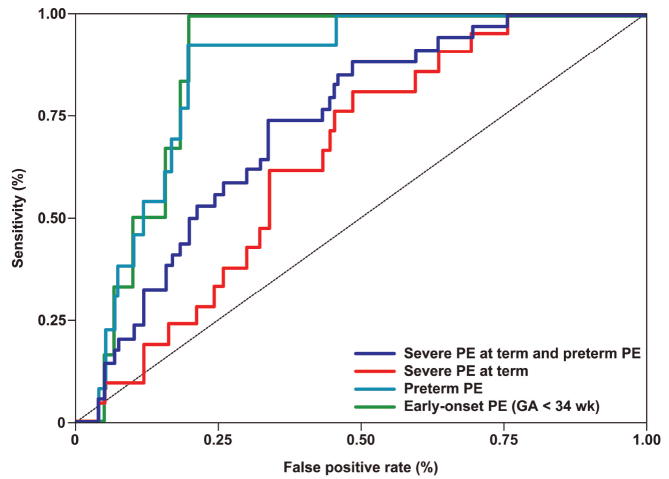
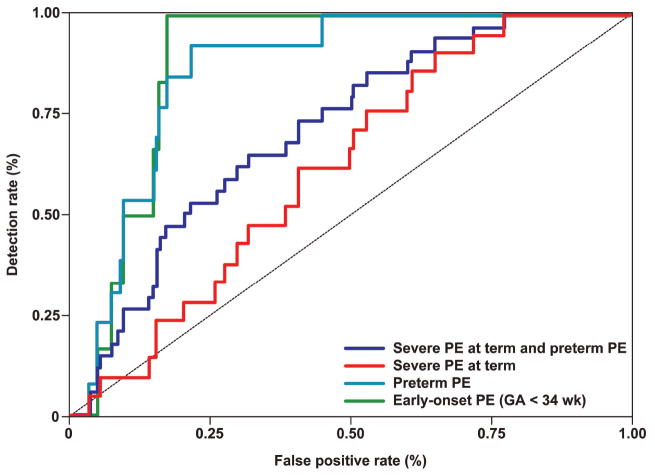
Figure 3. Receiver operating characteristic (ROC) curves depicting the sensitivity and specificity of first trimester maternal serum PP13 MoM for the identification of the different clinical subtypes of preeclampsia.
A: ROC curves were generated based on the first model where PP13 MoM was adjusted to GA and BMI. PE = preeclampsia. The diagnostic indices that were generated from these curves are presented in Table IVA.
B: ROC curves were generated based on the second model where PP13 MoM was adjusted to GA,BMI, maternal age, and parity. PE = preeclampsia. The diagnostic indices that were generated from these curves are presented in Table IVB.
Table IVA.
Diagnostic indices and area under the curve (AUC) of first trimester maternal serum PP13 MoMs for the subsequent development of preeclampsia after adjustment for gestational age and body mass index.
| Study groups | AUC (95% CI) | p-value† | Sensitivity at 80% specificity (MoM cutoff) | Specificity at 80% sensitivity (MoM cutoff) |
|---|---|---|---|---|
| All preeclampsia (n=50) | 0.67 (0.60-0.74) | <0.001 | 34% (0.40) | 43% (1.12) |
| Early-onset preeclampsia (n=6) | 0.87 (0.81-0.93) | 0.002 | 83% (0.40) | 82% (0.33) |
| Preterm preeclampsia (n=13) | 0.86 (0.79-0.92) | <0.001 | 77% (0.40) | 80% (0.40) |
| Severe preeclampsia at term (n=21) | 0.65 (0.55-0.75) | 0.022 | 24% (0.40) | 52% (0.98) |
| Preterm and term severe preeclampsia combined (n=34) | 0.73 (0.65-0.81) | <0.001 | 44% (0.40) | 55% (0.85) |
| Mild preeclampsia at term (n=16) | 0.55 (0.45-0.66) | 0.490 | 13% (0.40) | 39% (1.24) |
p-values of the AUC was calculated compared to AUC = 0.5, which is a random prediction.
Early-onset preeclampsia cases were included in the preterm preeclampsia group.
CI, Confidence Interval
Table IVB.
Diagnostic indices and area under the curve (AUC) of first trimester maternal serum PP13 MoMs for the subsequent development of preeclampsia after adjustment for gestational age, body mass index, parity and maternal age.
| Study groups | AUC (95% CI) | p-value† | Sensitivity at 80% specificity (MoM cutoff) | Specificity at 80% sensitivity (MoM cutoff) |
|---|---|---|---|---|
| All preeclampsia (n=50) | 0.66 (0.5-0.74) | <0.001 | 36% (0.39) | 44% (1.10) |
| Early-onset preeclampsia (n=6) | 0.88 (0.83-0.93) | 0.001 | 100% (0.39) | 84% (0.31) |
| Preterm preeclampsia (n=13) | 0.86 (0.79-0.93) | <0.001 | 85% (0.39) | 82% (0.38) |
| Severe preeclampsia at term (n=21) | 0.62 (0.52-0.72) | 0.073 | 24% (0.39) | 40% (1.19) |
| Preterm and term severe preeclampsia combined (n=34) | 0.71 (0.63-0.79) | <0.001 | 47% (0.39) | 49% (1.01) |
| Mild preeclampsia at term (n=16) | 0.56 (0.45-0.68) | 0.389 | 13% (0.39) | 36% (1.26) |
p-values of the AUC was calculated compared to AUC = 0.5, which is a random prediction.
Early-onset preeclampsia cases were included in the preterm preeclampsia group.
CI, Confidence Interval
Using the first model, the following results were found: 1) When the specificity was fixed at 80% (20% false positive rate), the sensitivity was 83% for early-onset preeclampsia, 77% for preterm preeclampsia, 24% for severe preeclampsia at term and 44% for preterm and term severe preeclampsia combined. 2) When the sensitivity was fixed at 80%, the specificity was 82% for early-onset preeclampsia, 80% for preterm preeclampsia, 52% for severe preeclampsia at term, and 55% for the latter two combined (Table IVA).
Using the second model, the following results were found: 1) PP13 MoMs had a better sensitivity for early-onset preeclampsia (100%) and preterm preeclampsia (85%) when the specificity was fixed at 80%; however, the changes in other subgroups of preeclampsia were less prominent. 2) The specificity (at 80% sensitivity) of PP13 MoMs were lower in the subgroups of severe preeclampsia at term, preterm and term severe preeclampsia combined, and mild preeclampsia at term (Table IVB), indicating that the adjustment to a larger repertoire of confounders improve the accuracy only for preeclampsia that develops preterm.
DISCUSSION
Principal findings of the study
1) The maternal serum concentration of PP13 in the first trimester was significantly lower in patients who subsequently developed early-onset and preterm preeclampsia than in those who had a normal pregnancy outcome; 2) Maternal serum concentrations of PP13 may be of use in the risk assessment for preterm preeclampsia; and 3) The first trimester serum concentration of PP13 did not identify women who will develop mild preeclampsia at term.
Placental Protein 13 (galectin-13) – structure, function, and localization
PP13 was first isolated and cloned from human term placenta.89;90 The protein was mainly found as a homodimer of 16 kDa subunits linked by disulfide bonds.89-91;94 PP13 has been designated as galectin-13 because of its conserved structural homology and carbohydrate-recognition domain, as well as its ability to bind sugars resembling to members of the galectin family.93;94 The protein demonstrated endogenous lysophospholipase activity91;94 and elicited, through influx of calcium ions, depolarization of trophoblasts, as well as liberation of linoleic and arachidonic acids from the trophoblast membrane.92 PP13 is a soluble protein which can be externalized to the cell surface by non-classical pathways, though it lacks a transmembrane domain and a transport signal.94 It is predominantly expressed by the placenta, specifically, the syncytiotrophoblast, where it is localized on the brush-border membrane at the maternal-fetal interface.94;95 In addition to its detection in maternal serum, PP13 has been isolated from fetal serum and amniotic fluid.92
Maternal serum PP13 and adverse pregnancy outcome
Two previous studies have examined the potential value of maternal serum PP13 in the risk assessment for preeclampsia in the first trimester.96;97 Nicolaides et al. reported a case-control study indicating that patients who developed preeclampsia requiring delivery before 34 weeks of gestation had a lower median PP13 serum concentration expressed in MoMs than those who had a normal delivery at term (MoM: 0.07; p<0.001).96 Moreover, the information derived from maternal serum PP13 concentrations could be combined with the results of uterine artery Doppler velocimetry in the first trimester to estimate the risk for the subsequent development of preeclampsia requiring delivery before 34 weeks. The combination could accomplish a detection rate of 90% with a false-positive rate of 6%.96 Spencer et al. reported the results of a nested case-control study which examined the value of PP13 combined with second-trimester Doppler velocimetry of the uterine arteries in the prediction of early-onset preeclampsia (delivery prior to 35 weeks).97 The median PP13 concentrations as well as MoMs were significantly lower in patients who subsequently developed early-onset preeclampsia than in those in the control group. However, second-trimester Doppler velocimetry did not add significant information to that provided by PP13.97 Therefore, there are now three studies indicating that patients who subsequently develop early-onset preeclampsia have lower maternal serum concentrations of PP13 in early pregnancy. The sensitivity and specificity reported by Spencer et al.97 were lower than those reported by Nicolaides et al.96 Our results are in keeping with those reported by Nicolaides et al.
The prevalence of preterm preeclampsia in the general population is very low;16;102 resulting in a low positive predictive value for first trimester PP13 concentrations. Therefore, the combination of PP13 with other biomarkers or first and/or second trimester ultrasound measurements would increase its positive predictive value. In fact, this approach has not only been used for PP13,96;97 but also for the combination of maternal plasma PlGF with abnormal uterine artery Doppler velocimetry in the second trimester.16
Previous investigations have studied patients from other ethnic groups. For example, 30% of patients with preeclampsia in one study were Caucasian,96 whereas in another study, this proportion was 86%.97 Our findings suggest that PP13 performs well in a Hispanic population.
Potential mechanisms for a reduction in maternal serum PP13 concentration in patients destined to develop preeclampsia
The expression of PP13 is down-regulated in the placentas of patients with preterm preeclampsia.95 However, the mechanisms responsible for this have not been determined. Because the syncytiotrophoblast is in direct contact with the maternal blood, it is tempting to speculate that a deficient production of PP13 may account for the lower maternal serum concentration of this protein in patients destined to develop preeclampsia. Since the lower concentration of PP13 is observed in the first trimester of pregnancy,96;97 months before the development of clinical disease, this suggests that the decreased concentration in maternal blood is not the consequence of the disease.
Strengths and limitations
The strength of this study is that it provides evidence that a low concentration of PP13 in the first trimester is a risk factor for preterm preeclampsia and severe preeclampsia at term. These data have been generated in a different ethnic group from those studied in the past.96;97 The limitations of this study are those inherent to any case-control study, namely the potential for biases and the inability to calculate predictive values.
The relatively high inter-assay CV for PP13 (19.5%) could be attributed to the very low maternal serum concentrations of PP13 (at the pg range). This is challenging for the detection accuracy of the ELISA method. To improve the test accuracy, a new amplification method for the PP13 ELISA is currently being developed, to replace the use of the biotin-avidin-HRP amplification system by other amplification methods, such as with the use of lanthanides.
A considerable source of variability for serum markers, such as PP13, whose concentrations change with gestation is the inaccuracy in dating the pregnancy. When gestational age is estimated by ultrasound biometry, this source of variability is reduced, and as a consequence, there is less overlap in the distribution of marker concentrations in MoMs between affected and unaffected pregnancies. In the present study, gestational age was largely based on menstrual dates. Thus, the observed discriminatory power of PP13 in the detection of preeclampsia is likely to have underestimated the true performance. However, in both this study and previous studies where gestation was based on an ultrasound scan,96;97 the overlap in serum PP13 concentrations between preeclampsia and unaffected pregnancies was small. Therefore, any improvement brought about by more precise dating is probably marginal.
Future investigation
A large cohort study is required to determine whether the observations reported from nested case-control studies can be replicated in a large population and whether the likelihood ratios are such that PP13 determinations in early pregnancy can contribute to the risk assessment for preeclampsia. The identification of an analyte which changes in the first trimester of pregnancy in patients destined to develop preeclampsia is attractive because it offers the maximal opportunity for intervention. Future studies are required to determine the factors responsible for deficient production of PP13 in the placentas of patients who subsequently develop preeclampsia and the consequences of reduced PP13 bioavailability. Moreover, Nicolaides et al.96 and Spencer et al.97 reported the value of maternal serum PP13 measurements combined with Doppler sonography for the risk assessment for preeclampsia. Furthermore, a recent study103 has indicated benefit for sequential testing with PP13, suggesting that a prospective study that will combine first trimester PP13 with other analytes and sonographic findings may yield an effective assessment tool for the risk of subsequently developed preeclampsia in later stages of pregnancy.
Acknowledgments
The authors are grateful to the patients and the nursing staff of the Sotero del Rio Hospital for their valuable involvement in this study.
This research was supported, in part, by the Intramural Research Program of the National Institute of Child Health and Human Development, Eunice Kennedy Shriver National Institutes of Health, Department of Health and Human Services, and Grants 31851, 42872 from Israel Chief Scientist (HM). This paper was presented as a poster (No: 422) at the 27th Annual Meeting of the Society for Maternal-Fetal Medicine in San Francisco, CA (9th February, 2007).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003–10. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 2.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179:1359–75. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 3.Confidential Enquiries in Maternal Deaths. Why Mothers Die 1997-1999. London: RCOG Press; 2002. [Google Scholar]
- 4.Sibai BM, Caritis S, Hauth J. What we have learned about preeclampsia. Semin Perinatol. 2003;27:239–46. doi: 10.1016/s0146-0005(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Clinical Excellence. Routine antenatal care for healthy pregnant woman. London: National Institute for Clinical Excellence; 2003. [Google Scholar]
- 6.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 7.Dekker GA, de Vries JI, Doelitzsch PM, Huijgens PC, von Blomberg BM, Jakobs C, et al. Underlying disorders associated with severe early-onset preeclampsia. Am J Obstet Gynecol. 1995;173:1042–48. doi: 10.1016/0002-9378(95)91324-6. [DOI] [PubMed] [Google Scholar]
- 8.Myatt L, Miodovnik M. Prediction of preeclampsia. Semin Perinatol. 1999;23:45–57. doi: 10.1016/s0146-0005(99)80059-7. [DOI] [PubMed] [Google Scholar]
- 9.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96:950–55. [PubMed] [Google Scholar]
- 10.Murphy DJ, Stirrat GM. Mortality and morbidity associated with early-onset preeclampsia. Hypertens Pregnancy. 2000;19:221–31. doi: 10.1081/prg-100100138. [DOI] [PubMed] [Google Scholar]
- 11.Xiong X, Demianczuk NN, Saunders LD, Wang FL, Fraser WD. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol. 2002;155:203–09. doi: 10.1093/aje/155.3.203. [DOI] [PubMed] [Google Scholar]
- 12.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143–48. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 13.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–94. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharya S, Campbell DM. The incidence of severe complications of preeclampsia. Hypertens Pregnancy. 2005;24:181–90. doi: 10.1081/PRG-200059873. [DOI] [PubMed] [Google Scholar]
- 15.Gaugler-Senden IP, Huijssoon AG, Visser W, Steegers EA, de Groot CJ. Maternal and perinatal outcome of preeclampsia with an onset before 24 weeks’ gestation. Audit in a tertiary referral center. Eur J Obstet Gynecol Reprod Biol. 2006;128:216–21. doi: 10.1016/j.ejogrb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326–13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–16. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–40. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 20.Myatt L. Role of placenta in preeclampsia. Endocrine. 2002;19:103–11. doi: 10.1385/ENDO:19:1:103. [DOI] [PubMed] [Google Scholar]
- 21.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 22.Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil. 2001;29:518–22. doi: 10.1016/s1297-9589(01)00180-1. [DOI] [PubMed] [Google Scholar]
- 23.Campbell S, Diaz-Recasens J, Griffin DR, Cohen-Overbeek TE, Pearce JM, Willson K, et al. New doppler technique for assessing uteroplacental blood flow. Lancet. 1983;1:675–77. doi: 10.1016/s0140-6736(83)91970-0. [DOI] [PubMed] [Google Scholar]
- 24.Harrington KF, Campbell S, Bewley S, Bower S. Doppler velocimetry studies of the uterine artery in the early prediction of pre-eclampsia and intra-uterine growth retardation. Eur J Obstet Gynecol Reprod Biol. 1991;42(Suppl):S14–S20. [PubMed] [Google Scholar]
- 25.Bower S, Schuchter K, Campbell S. Doppler ultrasound screening as part of routine antenatal scanning: prediction of pre-eclampsia and intrauterine growth retardation. Br J Obstet Gynaecol. 1993;100:989–94. doi: 10.1111/j.1471-0528.1993.tb15139.x. [DOI] [PubMed] [Google Scholar]
- 26.Harrington K, Cooper D, Lees C, Hecher K, Campbell S. Doppler ultrasound of the uterine arteries: the importance of bilateral notching in the prediction of pre-eclampsia, placental abruption or delivery of a small-for-gestational-age baby. Ultrasound Obstet Gynecol. 1996;7:182–88. doi: 10.1046/j.1469-0705.1996.07030182.x. [DOI] [PubMed] [Google Scholar]
- 27.Albaiges G, Missfelder-Lobos H, Lees C, Parra M, Nicolaides KH. One-stage screening for pregnancy complications by color Doppler assessment of the uterine arteries at 23 weeks’ gestation. Obstet Gynecol. 2000;96:559–64. doi: 10.1016/s0029-7844(00)00946-7. [DOI] [PubMed] [Google Scholar]
- 28.Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol. 2001;18:441–49. doi: 10.1046/j.0960-7692.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- 29.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–60. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 30.Papageorghiou AT, Yu CK, Nicolaides KH. The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2004;18:383–96. doi: 10.1016/j.bpobgyn.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Papageorghiou AT, Yu CK, Cicero S, Bower S, Nicolaides KH. Second-trimester uterine artery Doppler screening in unselected populations: a review. J Matern Fetal Neonatal Med. 2002;12:78–88. doi: 10.1080/jmf.12.2.78.88. [DOI] [PubMed] [Google Scholar]
- 32.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–91. [PubMed] [Google Scholar]
- 33.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia. J Pathol. 1970;101:vi. [PubMed] [Google Scholar]
- 34.Khong TY, De WF, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 35.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–55. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 36.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van AA. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–74. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 37.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–23. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 38.Fisher SJ. The placental problem: linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod Biol Endocrinol. 2004;2:53. doi: 10.1186/1477-7827-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyall F. Priming and remodelling of human placental bed spiral arteries during pregnancy--a review. Placenta. 2005;26(Suppl A):S31–S36. doi: 10.1016/j.placenta.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Sargent IL, Borzychowski AM, Redman CW. Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online. 2006;13:680–86. doi: 10.1016/s1472-6483(10)60659-1. [DOI] [PubMed] [Google Scholar]
- 41.Kadyrov M, Kingdom JC, Huppertz B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am J Obstet Gynecol. 2006;194:557–63. doi: 10.1016/j.ajog.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 42.Espinoza J, Romero R, Mee KY, Kusanovic JP, Hassan S, Erez O, et al. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med. 2006;34:447–58. doi: 10.1515/JPM.2006.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson WB, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens. 1976;5:115–27. [PubMed] [Google Scholar]
- 44.Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol. 1977;4:573–93. [PubMed] [Google Scholar]
- 45.Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004;103:981–91. doi: 10.1097/01.AOG.0000126245.35811.2a. [DOI] [PubMed] [Google Scholar]
- 46.Barton JR, Sibai BM. Diagnosis and management of hemolysis, elevated liver enzymes, and low platelets syndrome. Clin Perinatol. 2004;31:807–33. vii. doi: 10.1016/j.clp.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Egerman RS, Sibai BM. HELLP syndrome. Clin Obstet Gynecol. 1999;42:381–89. doi: 10.1097/00003081-199906000-00022. [DOI] [PubMed] [Google Scholar]
- 48.Abramovici D, Friedman SA, Mercer BM, Audibert F, Kao L, Sibai BM. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation: does the HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome matter? Am J Obstet Gynecol. 1999;180:221–25. doi: 10.1016/s0002-9378(99)70178-x. [DOI] [PubMed] [Google Scholar]
- 49.Tikkanen M, Nuutila M, Hiilesmaa V, Paavonen J, Ylikorkala O. Clinical presentation and risk factors of placental abruption. Acta Obstet Gynecol Scand. 2006;85:700–05. doi: 10.1080/00016340500449915. [DOI] [PubMed] [Google Scholar]
- 50.Lindqvist PG, Happach C. Risk and risk estimation of placental abruption. Eur J Obstet Gynecol Reprod Biol. 2006;126:160–64. doi: 10.1016/j.ejogrb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Toivonen S, Heinonen S, Anttila M, Kosma VM, Saarikoski S. Reproductive risk factors, Doppler findings, and outcome of affected births in placental abruption: a population-based analysis. Am J Perinatol. 2002;19:451–60. doi: 10.1055/s-2002-36868. [DOI] [PubMed] [Google Scholar]
- 52.Ananth CV, Smulian JC, Vintzileos AM. Incidence of placental abruption in relation to cigarette smoking and hypertensive disorders during pregnancy: a meta-analysis of observational studies. Obstet Gynecol. 1999;93:622–28. doi: 10.1016/s0029-7844(98)00408-6. [DOI] [PubMed] [Google Scholar]
- 53.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–75. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 54.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 55.Bujold E, Romero R, Chaiworapongsa T, Kim YM, Kim GJ, Kim MR, et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 2005;18:9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 56.Lyall F, Greer IA, Boswell F, Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br J Obstet Gynaecol. 1997;104:223–28. doi: 10.1111/j.1471-0528.1997.tb11050.x. [DOI] [PubMed] [Google Scholar]
- 57.Kupferminc MJ, Daniel Y, Englender T, Baram A, Many A, Jaffa AJ, et al. Vascular endothelial growth factor is increased in patients with preeclampsia. Am J Reprod Immunol. 1997;38:302–06. doi: 10.1111/j.1600-0897.1997.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 58.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–63. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 59.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–51. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 61.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 62.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191:1240–46. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–47. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 64.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;122:33–39. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 65.Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, et al. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90:4895–903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- 66.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, et al. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–73. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 68.Stepan H, Faber R. Elevated sFlt1 level and preeclampsia with parvovirus-induced hydrops. N Engl J Med. 2006;354:1857–58. doi: 10.1056/NEJMc052721. [DOI] [PubMed] [Google Scholar]
- 69.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–75. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 71.Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ’mirror syndrome’ (Ballantyne’s syndrome) J Matern Fetal Neonatal Med. 2006;19:607–13. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–07. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 73.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol. 2006;195:255–59. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 74.Tjoa ML, Levine RJ, Karumanchi SA. Angiogenic factors and preeclampsia. Front Biosci. 2007;12:2395–402. doi: 10.2741/2241. [DOI] [PubMed] [Google Scholar]
- 75.Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Arai F, et al. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens Res. 2007;30:151–59. doi: 10.1291/hypres.30.151. [DOI] [PubMed] [Google Scholar]
- 76.Muller PR, James AH, Murtha AP, Yonish B, Jamison MG, Dekker G. Circulating angiogenic factors and abnormal uterine artery Doppler velocimetry in the second trimester. Hypertens Pregnancy. 2006;25:183–92. doi: 10.1080/10641950600912968. [DOI] [PubMed] [Google Scholar]
- 77.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–44. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 78.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–72. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 79.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregnancy. 2004;23:101–11. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 80.Levine RJ, Thadhani R, Qian C, Lam C, Lim KH, Yu KF, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 81.Aggarwal PK, Jain V, Sakhuja V, Karumanchi SA, Jha V. Low urinary placental growth factor is a marker of pre-eclampsia. Kidney Int. 2006;69:621–24. doi: 10.1038/sj.ki.5000075. [DOI] [PubMed] [Google Scholar]
- 82.Bersinger NA, Odegard RA. Second-and third-trimester serum levels of placental proteins in preeclampsia and small-for-gestational age pregnancies. Acta Obstet Gynecol Scand. 2004;83:37–45. [PubMed] [Google Scholar]
- 83.Zwahlen M, Gerber S, Bersinger NA. First trimester markers for pre-eclampsia: placental vs. non-placental protein serum levels. Gynecol Obstet Invest. 2007;63:15–21. doi: 10.1159/000094672. [DOI] [PubMed] [Google Scholar]
- 84.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–49. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 85.Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Correlation between soluble endoglin, vascular endothelial growth factor receptor-1 and adipocytokines in preeclampsia. J Clin Endocrinol Metab. 2007;92:2672–9. doi: 10.1210/jc.2006-2349. [DOI] [PubMed] [Google Scholar]
- 86.Lopez-Novoa JM. Soluble endoglin is an accurate predictor and a pathogenic molecule in pre-eclampsia. Nephrol Dial Transplant. 2007;22:712–14. doi: 10.1093/ndt/gfl768. [DOI] [PubMed] [Google Scholar]
- 87.Luft FC. Soluble endoglin (sEng) joins the soluble fms-like tyrosine kinase (sFlt) receptor as a pre-eclampsia molecule. Nephrol Dial Transplant. 2006;21:3052–54. doi: 10.1093/ndt/gfl439. [DOI] [PubMed] [Google Scholar]
- 88.Slaghekke F, Dekker G, Jeffries B. Endogenous inhibitors of nitric oxide and preeclampsia: a review. J Matern Fetal Neonatal Med. 2006;19:447–52. doi: 10.1080/14767050600852171. [DOI] [PubMed] [Google Scholar]
- 89.Bohn H, Kraus W, Winckler W. Purification and characterization of two new soluble placental tissue proteins (PP13 and PP17) Oncodev Biol Med. 1983;4:343–50. [PubMed] [Google Scholar]
- 90.Than GN, Bohn H, Szabo DG. Advances in Pregnancy Related Protein Research. Boca Raton, FL: CRC press; 1993. pp. 1–333. [Google Scholar]
- 91.Than NG, Sumegi B, Than GN, Berente Z, Bohn H. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new lysophospholipase, homologue of human eosinophil Charcot-Leyden Crystal protein. Placenta. 1999;20:703–10. doi: 10.1053/plac.1999.0436. [DOI] [PubMed] [Google Scholar]
- 92.Burger O, Pick E, Zwickel J, Klayman M, Meiri H, Slotky R, et al. Placental protein 13 (PP-13): effects on cultured trophoblasts, and its detection in human body fluids in normal and pathological pregnancies. Placenta. 2004;25:608–22. doi: 10.1016/j.placenta.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 93.Visegrady B, Than NG, Kilar F, Sumegi B, Than GN, Bohn H. Homology modelling and molecular dynamics studies of human placental tissue protein 13 (galectin-13) Protein Eng. 2001;14:875–80. doi: 10.1093/protein/14.11.875. [DOI] [PubMed] [Google Scholar]
- 94.Than NG, Pick E, Bellyei S, Szigeti A, Burger O, Berente Z, et al. Functional analyses of placental protein 13/galectin-13. Eur J Biochem. 2004;271:1065–78. doi: 10.1111/j.1432-1033.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 95.Than NG, Rahman OA, Kovats T, Nagy B, Magenheim R, Dienes J, Hupuczi P, Rigo J, Sziller I, Meiri H, Bohn H, Papp Z. PP13 expression is altered in preeclampsia. J Soc Gynecol Invest. 2006;13(Suppl 2):128A. Ref Type: Abstract. [Google Scholar]
- 96.Nicolaides KH, Bindra R, Turan OM, Chefetz I, Sammar M, Meiri H, et al. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet Gynecol. 2006;27:13–17. doi: 10.1002/uog.2686. [DOI] [PubMed] [Google Scholar]
- 97.Spencer K, Cowans NJ, Chefetz I, Tal J, Meiri H. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;29:128–34. doi: 10.1002/uog.3876. [DOI] [PubMed] [Google Scholar]
- 98.Wolf M, Shah A, Jimenez-Kimble R, Sauk J, Ecker JL, Thadhani R. Differential risk of hypertensive disorders of pregnancy among Hispanic women. J Am Soc Nephrol. 2004;15:1330–38. doi: 10.1097/01.asn.0000125615.35046.59. [DOI] [PubMed] [Google Scholar]
- 99.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 100.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, et al. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil. 2004;132:1155–65. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 101.Cuckle HS, Wald N. Testing using single markers. In: Wald N, Leck I, editors. Antenatal and Neonatal Screening. 2. Oxford: Oxford University Press; 2000. pp. 1–22. [Google Scholar]
- 102.Rasmussen S, Irgens LM. Fetal growth and body proportion in preeclampsia. Obstet Gynecol. 2003;101:575–83. doi: 10.1016/s0029-7844(02)03071-5. [DOI] [PubMed] [Google Scholar]
- 103.Gonen R. PP13 as an early marker for preeclampsia. J Prenatal Med. 2007;35(S2):S24–S25. [Google Scholar]