Abstract
The emergence of multicellular animals could only take place once evolution had produced molecular mechanisms for cell adhesion and communication. Today, all metazoans express integrin-type adhesion receptors and receptors for growth factors. Integrins recognize extracellular matrix proteins and respective receptors on other cells and, following ligand binding, can activate the same cellular signaling pathways that are regulated by growth factor receptors. Recent reports have indicated that the two receptor systems also collaborate in many other ways. Here, we review the present information concerning the role of integrins as assisting growth factor receptors and the interplay between the receptors in cell signaling and in the orchestration of receptor recycling.
Keywords: Integrin, Cell adhesion, Growth factor, Signaling, Endocytosis
Integrin family of adhesion receptors
The integrin family of cell adhesion receptors mediates the anchoring of cell to the protein fibrils and networks that compose the extracellular matrix (ECM). In addition, certain members of the integrin family can bind to plasma proteins or respective receptors on other cells. Based on their structure and phylogeny, human integrin α subunits can be divided into four categories (Johnson et al. 2009). Basically, all metazoans seem to have integrins that recognize arginine-glycine-aspartic acid (RGD) motifs in their ligands. In vertebrates, they act as receptors for ECM glycoproteins, such as fibronectin, vitronectin, and fibrinogen. The classical fibronectin receptor, α5β1 integrin, plays an important role during development, since mice lacking α5β1 die early during embryonic development (Yang et al. 1993). Integrin α8β1 is another fibronectin receptor and is important for the development of the kidney (Muller et al. 1997) and inner ear (Littlewood Evans and Muller 2000). Platelet fibrinogen receptor, αIIbβ3 integrin, is important for hemostasis, whereas the physiological role of the five αV-containing heterodimers (αVβ1, αVβ3, αVβ5, αVβ6, αVβ8) is less obvious. Deficiency of all five receptors in αV-null animals causes a surprisingly mild phenotype (Bader et al. 1998). The development of most organs is close to normal, and some of the animals may be born alive, although they die soon after birth because of defects in their blood vessels (Bader et al. 1998). The deficiency of αVβ8 integrin has been show to affect brain vessel formation (Zhu et al. 2002). However, in general, the role of αV integrins in angiogenesis has been under debate since inhibitors of αV function can block angiogenesis in animal models (Brooks et al. 1994), whereas the genetic deletion of αVβ3 and αVβ5 integrins actually promotes angiogenesis (Reynolds et al. 2002). Furthermore, small concentrations of RDG-mimetic integrin inhibitors may also promote angiogenesis (Reynolds et al. 2009).
Laminin receptor integrins are also widespread in the animal kingdom suggesting their early evolution. Integrin α3β1 appears to be important for the normal development of the lung, kidney (Kreidberg et al. 1996), and skin (DiPersio et al. 1997), whereas α6β4 integrin is an essential component of hemidesmosomes (Dowling et al. 1996). Integrin β4 is exceptional when compared with all other integrins because of its large intracellular part. In addition to β4, α6 can have another β partner, namely β1 integrin. α7β1 is specific to muscles and, when genetically defective, causes a type of muscular dystrophy in mice (Mayer et al. 1997), whereas α7 mutations may lead to congenital myopathy in man (Hayashi et al. 1998).
Integrins α4 and α9 can both form a heterodimer with β1 integrin. In addition, α4 can have another β partner, namely β7. Based on their structural features, the two α subunits are considered to form a separate subgroup of integrins (Johnson et al. 2009). Integrin α4 can bind to fibronectin in ECM in an RGD-independent manner. It can also mediate cell-cell interaction and act as a receptor for VCAM (vascular cell adhesion molecule), which is a transmembrane protein belonging to the immunoglobulin superfamily. Integrin α4 is important for early steps of embryonic development, and its deficiency causes embryonic lethality (Yang et al. 1995). Integrin α9β1 has several ECM ligands, including tenascin C. Functionally, α9β1 integrin has been associated with the regulation of lymphangiogenesis (Huang et al. 2000).
The fourth phylogenic subgroup of integrin α subunits is composed of members that differ from all other integrin subunits based on the finding that they contain an extra domain in their structure. After the cloning of the integrin α subunits, the extra domain was named an I domain, i.e., an inserted domain. Later, the αI domain was realized to be the ligand-binding domain in this subset of integrins. The integrin αI domain takes a Rossmann fold and, therefore, is similar to the von Willebrand factor A domain (Michishita et al. 1993). Based on this finding, integrin I domains are also called integrin A domains. The αI domain integrins are only found in chordates (Johnson et al. 2009). In vertebrates, the αI domain integrins can be further divided into two functionally different subgroups, namely the leukocyte integrins (αDβ2, αEβ7, αLβ2, αMβ2, and αXβ2; for a review, see Evans et al. 2009) and the collagen receptor integrins (α1β1, α2β1, α10β1, and α11β1; for a review, see Heino 2007). These integrins seem to have specialized functions related to, amongst others, native and acquired immunity, thrombosis, and the development of connective tissues.
Integrins anchor cells to the ECM and have an important role in the maintenance of tissue integrity. However, integrins are also signaling receptors (for a review, see Legate et al. 2009). Unlike many growth factor receptors, integrins have no enzymatic activity, and their signaling function is dependent on their binding to intracellular proteins. Integrin heterodimers may take alternative conformations in which the integrin cytoplasmic domains are either together or separated from each other (for a review, see Arnaout et al. 2007; Luo et al. 2007). Recent studies have stressed the flexibility of the integrin structure and suggest that integrins can have many different conformations and activation states (Zhu et al. 2008). The cytoplasmic domains of the integrin β subunits alone have been reported to bind to tens of different cytoskeletal and signaling proteins (for a review, see Legate and Fassler 2009). The interactions of integrin α subunits with intracellular proteins are much less studied, but α subunits are nevertheless thought to play an important part in heterodimer-specific signaling events. Given the finding that the intracellular domains are relatively short, only a limited number of proteins can bind at any one time. Therefore, integrins can probably gather various types of protein complexes.
Talin is an actin-binding protein that can also recognize a specific motif in the cytoplasmic domains of integrin β subunits. Talin has been found to be an important activator of integrin-ligand-binding function (Tadokoro et al. 2003). Its binding to the tail of β subunit breaks a salt bridge between α and β subunits and induces a conformational change leading to the extension of the integrin ectodomain, whereas the inactive integrin is considered to be bent. Kindlins 1–3 bind to a distinct motif in β cytoplasmic domain when compared with talin (Moser et al. 2009). Kindlins act together with talin in the regulation of integrin activity (Moser et al. 2009). Other proteins binding to β cytoplasmic domains include the protein tyrosine kinases, such as focal adhesion kinase, Src, and integrin-linked kinase. These kinases and numerous other kinases, phosphatases, GTP-ases, and adaptor proteins can link the integrins to downstream signaling pathways (Legate et al. 2009).
Because of its large cytoplasmic domain, β4 integrin is considered to behave in a different manner from all other integrin β subunits. Tyrosine kinases, such as Src family kinases, can phosphorylate the cytoplasmic domain β4 and create a binding site for Shc that further activates Ras and its downstream signaling pathways (Mainiero et al. 1997; Shaw et al. 1997).
Direct binding of integrins to growth factors
Integrins are known to regulate the same signaling pathways as growth factor receptors (Fig. 1). A classic example of this is the ability of a subset of integrins to engage ligands and subsequently activate the Shc-Ras-Mitogen-activated protein kinase pathway in a manner similar to growth factor receptors (Wary et al. 1996, 1998). More recently, we have realized that some integrins may also bind directly to growth factors (Fig. 1). The best-known example is αVβ6 integrin, which plays a crucial role in the activation of transforming growth factor-β (TGF-β). The latency-associated peptide (LAP) in latent TGF-β (LTGF-β) has an RGD motif that can be recognized by αVβ6 integrin. The binding of LTGF-β to αVβ6 integrin leads to the activation of the growth factor (Munger et al. 1999). In addition, LTGF-β can be activated by plasmin (Sato and Rifkin 1989), by metalloproteinases (Yu and Stamenkovic 2000), or by binding to thrombospondin (Crawford et al. 1998). Despite the finding that the αVβ6-mediated activation of LTGF-β represents only one of many alternative activation mechanisms, αVβ6 seems to be critical for TGF-β action in many animal models for fibrotic diseases. The lack of β6 integrin makes mice resistant to pulmonary fibrosis induced by bleomysin (Munger et al. 1999), and to kidney (Ma et al. 2003) and biliary (Wang et al. 2007) fibrosis. On the other hand, the overexpression of β6 integrin in mouse skin induces the spontaneous formation of chronic wounds (Hakkinen et al. 2004). Integrin αVβ8 can also bind to LTGF-β and activate the growth factor. The activation mechanism is reported to involve a membrane-type matrix metalloproteinase, MT1-MMP (Mu et al. 2002). Integrin αVβ8 is expressed on brain astrocytes and is considered to mediate cross-talk with αVβ8-negative endothelial cells in a TGF-β-dependent manner (Cambier et al. 2005). The finding that αVβ8 integrin-deficient mice have lethal defects in the development of the blood vessels in the brain (Zhu et al. 2002) further stresses the physiological importance of this mechanism. LTGF-β might also be recognized by αVβ1, αVβ5 (Munger et al. 1998), αVβ3 (Asano et al. 2005), and α8β1 (Lu et al. 2002). However, the physiological relevance of these interactions is not clear. For example, integrin αVβ1 is abundantly expressed in various cell lines, but its existence in tissues is uncertain.
Fig. 1.
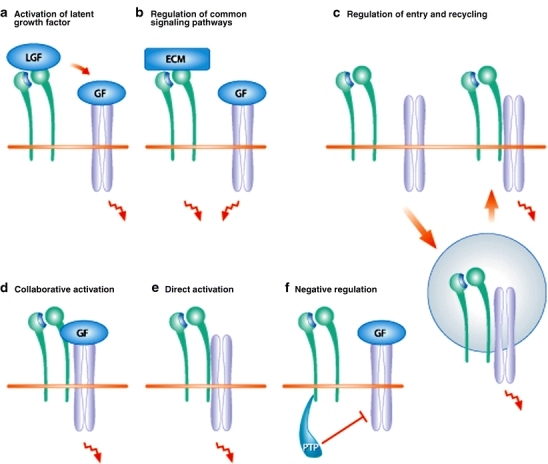
Various forms of collaboration between integrins (green) and growth factor receptors (blue). a Integrins may directly bind to latent growth factors (LGF) and activate them. Subsequently, activation growth factors (GF) can bind to their signaling receptors. b The binding of integrins to extracellular matrix (ECM) proteins often activates the same signaling pathways as GF binding to GF receptors. c Receptors can signal when located either on the cell surface or in endosomes. Co-endocytosis of integrins and GF receptors regulates cellular signaling at multiple levels. d, e Integrins can activate GF receptors in a collaborative or direct manner. In both cases, integrins create an environment in which the GF receptors can properly interact with the downstream signaling molecules. In the collaborative mechanism, the binding of GFs to their receptors precedes receptor activation (d), whereas in the direct mechanism, the GF receptors are activated without ligand binding (e). f Integrins can also activate protein tyrosine phosphatases (PTP) and suppress signaling by GF receptors
In addition to LTGF-β, integrins can also bind to other growth factors that regulate angiogenesis and lymphangiogenesis. Integrin α9β1 can bind to vascular endothelial growth factors A, C, and D (VEGF-A, -C, -D; Vlahakis et al. 2005, 2007). The role of α9β1 seems to be critical especially during lymphangiogenesis, since the lack of α9β1 in the mouse prevents lymphangiogenesis (Huang et al. 2000). VEGF-A can also be recognized by α3β1 and αVβ3 integrins (Hutchings et al. 2003). However, these integrins can only bind to the 165-kDa splicing varant of VEGF-A, whereas α9β1 can also recognize the 121-kDa variant (Vlahakis et al. 2005). Integrin binding to VEGF-A seems to regulate endothelial cell migration, and results from in vivo assays, such as the chick chorioallantoic membrane assay, indicate that the interaction is relevant for angiogenesis (Vlahakis et al. 2007). In addition to VEGFs, angiopoietins, such as angiopoietin-1 and -2, form a further group of growth factors required for angiogenesis. Integrin α5β1 has been shown to recognize angiopoietin-1 and -2 proteins (Carlson et al. 2001), but the in vivo role of this interaction is unclear.
Growth factors that can act as direct ligands for integrins also include fibroblast growth factor-1 (FGF-1), nerve growth factor (NGF), and two other neurotrophins. Integrin αVβ3 has been shown to be a receptor for FGF-1 and to modulate FGF-1 signaling through its tyrosine kinase receptor (Mori et al. 2008). NGF, brain-derived neurotrophic factor, and NT3 are ligands for α9β1 integrin. Integrin-NGF interaction stimulates cell proliferation and migration. In addition to modulating the signaling by NGF receptor TrkA/NTRK1, NGF binding to α9β1 may activate integrin signaling (Staniszewska et al. 2008).
Semaphorin 7A (Sema7A, CD108) is known to regulate axon outgrowth through β1-integrin receptors and to contribute to the formation of the lateral olfactory tract. Sema7A is also expressed on activated T cells and stimulates cytokine production in monocytes and macrophages through α1β1 integrin (Suzuki et al. 2007). This function of Sema7A has been reported to be based on the ability of Sema7A to act as a direct ligand for α1β1 integrin (Suzuki et al. 2007).
The biological consequences of growth factor binding to an integrin seem to be variable (Table 1). In the case of LTGF-β, the obvious role of the integrin is to activate the latent growth factor by inducing a conformational change or presenting the latent growth factor to a protease. Integrins may also assist the binding of a growth factor to its receptor, or the growth factor may even activate integrin signaling. In many experiments, cells have been allowed to attach to cell culture wells that have been precoated with growth factors. Under these conditions, the cells can adhere and migrate. However, whether integrin can take advantage of growth factors in the same manner under in vivo conditions remains unclear.
Table 1.
Direct binding of integrins to growth factors (VEGF vascular endothelial growth factor, NGF nerve growth factor, FGF fibroblast growth factor, NT neurotrophin)
| Integrin | Growth factor | Physiological role | Reference |
|---|---|---|---|
| α1β1 | Semaphorin 7A | Inflammation | Suzuki et al. 2007 |
| α3β1 | VEGF-A (165) | Angiogenesis | Hutchings et al. 2003 |
| α5β1 | Angiopoietin-1 | Angiogenesis | Carlson et al. 2001 |
| Angiopoietin-2 | Angiogenesis | Carlson et al. 2001 | |
| α9β1 | VEGF-A (121, 165) | Angiogenesis | Vlahakis et al. 2007 |
| VEGF-C, VEGF-D | Lymphangiogenesis | Vlahakis et al. 2005 | |
| NGF | Cell proliferation | Staniszewska et al. 2008 | |
| Brain-derived neurotrophic factor | Staniszewska et al. 2008 | ||
| NT3 | Staniszewska et al. 2008 | ||
| αVβ3 | FGF-1 | FGF signaling | Mori et al. 2008 |
| VEGF-A (165) | Angiogenesis | Hutchings et al. 2003 | |
| αVβ6 | LTGF-β | TGF-β activation. | Munger et al. 1999 |
| Fibrosis and inflammation | |||
| αVβ8 | LTGF-β | TGF-β activation. | Cambier et al. 2005 |
| Formation of brain blood vessels. |
Integrins and growth factor receptors
Integrins and growth factor receptors are known to regulate the same signaling pathways, and together they regulate cell differentiation, proliferation, and survival. The balance of the various activating and inhibiting signals is considered to determine cell fate. However, the interplay between integrins and growth factor receptors is more complex than the simple independent activation of the same signaling pathways (Fig. 1). The binding of integrins to their ligands can cause the formation of large integrin clusters (Kim et al. 2004; Miyamoto et al. 1995). In some experimental models, inside-out integrin activation has also induced the lateral movement of ligand-free integrins on the cell surface and the formation of similar clusters (Cambi et al. 2006; Connors et al. 2007). Clustered integrins also seem to bunch together other surface receptors, including growth factor receptors (Yamada and Even-Ram 2002). Moreover, integrins seem to have a regulatory role in the activation of growth factor receptors. Integrin-dependent activation of growth factor receptors in these clusters can be either direct or collaborative (Yamada and Even-Ram 2002). In the collaborative mechanism, the binding of growth factors to the growth factor receptors precedes receptor activation, whereas the role of the integrins is to create an environment in which the growth factor receptors can properly interact with the downstream signaling molecules (Fig. 1). In the direct mechanism, the integrin-mediated aggregation of growth factor receptors induces the formation of clusters in which the growth factor receptors are able, for example, to phosphorylate each other. Thus, the presence of the growth factors is not needed in the direct mechanism (Fig. 1).
One of the physiologically most relevant examples of collaboration between integrins and growth factors is adhesion-dependent survival. Adherent cells are widely known to depend on integrin-mediated adhesion for responsiveness to growth factors. Loss of adhesion in normal cells results in growth arrest or anoikis (detachment induced apoptosis) attributable to impaired signaling induced by growth factors and cytokines (Danen and Yamada 2001; Frisch and Francis 1994; Schwartz and Assoian 2001). The mechanisms supporting permissive signals from integrins in normal cells seem to vary depending on the cell type and are not fully understood. However, the described mechanisms include the loss of PI3K-Akt pathway signaling, focal adhesion kinase inactivation, the activation of Jun N-terminal kinase, and the phosphatase-induced inactivation of protein kinase Cε (Frisch and Ruoslahti 1997; Ivaska et al. 2003; Ruoslahti 1999). Further insight into the co-operation between adhesion and survival has come from studies in cancer cells. Resistance to anoikis is emerging as a hallmark of metastatic malignancies (Liotta and Kohn 2004), and many different mechanisms have been described to regulate the escape from anoikis. These mechanisms are similar to those seen in drug resistance and include the upregulation of kinases (Douma et al. 2004) and scaffolding molecules (Parsons et al. 2009), the suppression of apoptotic pathways (Simpson et al. 2008), and, recently, the hypoxia-induced inhibition of receptor tyrosine kinase (RTK) endocytosis (Wang et al. 2009) and the downregulation of α5β1 (Rohwer et al. 2008), a critical anoikis regulator in normal epithelial cells. Ligation of integrins results in the activation of many RTKs in the absence of a ligand including epidermal growth factor receptor (EGFR), insulin receptor, platelet-derived growth factor receptor (PDGFR), VEGFR2, and Met (for a review, see Streuli and Akhtar 2009). In many cases, this seems to involve the induction of a direct interaction between integrins and growth factor receptors following integrin ligation. However, this is not the case with the collaboration of α6β4 and Met receptor. Cells expressing only Met in the absence of α6β4 integrin are unable to respond to human growth factor (HGF), the ligand for Met. Interestingly, the interaction between Met and a truncated form of β4 integrin that is unable to bind laminin is sufficient to restore HGF responses (Trusolino et al. 2001). This demonstrates that integrins can also collaborate with RTKs in an adhesion-independent manner.
Indeed, in most cases, integrins seem to function as positive regulators of growth factor receptor signaling. However, integrin α1β1 has a unique negative role in regulating EGFR and VEGFR2 signaling (Fig. 1). In response to adhesion to collagen, the integrin α1-subunit cytoplasmic tail interacts with a non-receptor protein tyrosine phosphatase TCPTP. This leads to the activation of TCPTP spatially at the membrane and results in the dephosphorylation of EGFR and VEGFR2 and thus attenuation of their signaling (Mattila et al. 2005, 2008).
Studies of transgenic animals lacking expression of β4 integrin in the breast epithelium have highlighted the importance of the integrin-RTK co-operation in cancer initiation in vivo. Initiation and progression of breast cancer can be studied by using a mouse model of ErbB2-induced mammary carcinoma. When β4-integrin is deleted from the mammary epithelium of these mice, the onset of tumors and their invasive growth is suppressed. This is attributable to the requirement for β4-integrin to amplify the signaling of ErbB2 in vivo to promoter tumor progression. Even though ErbB2 receptor does not bind growth factors, it readily heterodimerizes with members of the ErbB-family of RTKs that are activated by growth factors (Bublil and Yarden 2007). Therefore, integrins probably function also to amplify growth-factor-induced signals in vivo. Interestingly, β4-integrin is necessary for ErbB2-mediated tumorigenesis, as mice lacking expression of β4-integrin in the mammary epithelium show suppressed tumor onset and invasive growth (Guo et al. 2006).
Endocytosis and recycling
Integrin endo-exocytic traffic is now widely accepted to contribute to cell migration by supporting adhesion site dynamics and the localized targeting of the adhesion receptors. Integrins are constantly endocytosed from the cell surface and are then rapidly recycled to the plasma membrane either by the short-loop small GTPase Rab4 recycling route (in the case of αvβ3) or by the long-loop recycling via a Rab11-positive compartment (in the case of most β1-integrins). Integrin endocytosis has been described via several entry routes including clathrin-dependent and -independent pathways. These principles of integrin endo/excocytic traffic have been review extensively and are not discussed here in detail (Jones et al. 2006; Pellinen and Ivaska 2006). However, recent work on integrin traffic is revealing the complexity of the way that integrin traffic contributes to cell motility. As is becoming obvious, the link between migration and integrin traffic is highly cell-context-dependent, and processes supporting cell motility in the widely used, traditional, in vitro assays may indeed be totally insignificant in three-dimensional and other models that are more relevant in vivo. Small GTPases have been shown to regulate integrin traffic in part based on their distinct expression patterns. Rab21 small GTPase regulates integrin endo/exocytosis by interacting with the α-subunit of most integrins in several different cell lines (Pellinen et al. 2006). This is important for cell migration, at least in breast and prostate cancer cells in two dimensions. In addition, Rab21 regulates cell anchorage and cytokinetic furrow formation during cell division in two dimensions (Pellinen et al. 2008). In contrast, Caswell et al. (2007) have reported that, in epithelial ovarian carcinoma cells, Rab25 interacts directly with the integrin β-subunit. Interestingly, Rab25 regulates α5β1 integrin traffic spatially in extending pseudopods, resulting in increased migration on cell-derived matrix and increased invasion in three-dimensional matrices, but has no effect on cell migration in two dimensions.
Endocytosis was initially considered as a mechanism for the downregulation of signaling by growth factor receptors following ligand engagement. Today, spatially regulated signaling of receptors from the endosome is well established (Mosesson et al. 2008). Recently, growth factor receptors and some other adhesion receptors have been shown to regulate integrin endocytosis and vice versa (Fig. 1). Neuropilin-1 is a transmembrane glycoprotein that functions as an adhesion receptor and a co-receptor for both pro- and anti-angiogenic factors (Olsson et al. 2006; Shimizu et al. 2000). Interestingly, neuropilin-1 seems to regulate the internalization of active α5β1 integrin in endothelial cells and to regulate their ability to assemble fibronectin (Valdembri et al. 2009). Recently, the expression of T-cadherin, a glycophosphatidylinositol-anchored cadherin molecule, has also been shown to suppress the internalization of β1-integrins, whereas the stimulation of EGFR with EGF induces the internalization of β1-integrin (Mukoyama et al. 2007). These studies demonstrate that integrin endocytosis can be governed by other cell-surface receptors. Conversely, the internalization of the junctional adhesion molecule JAM-A has recently been shown to be dependent on integrin internalization, and the chemotactic migration of neutrophils requires the co-endocytosis of both receptors (Cera et al. 2009). These examples are interesting and suggest that the cross-talk between the various receptors is also governed at the levels of endocytosis. Even though integrins and growth factor receptors have been shown to co-cluster and form complexes, whether they can also be co-endocytosed together in a ligand-dependent or ligand-independent way remains unclear.
More progress has been made in understanding the recycling of integrins and growth factor receptors and the cross-talk between the various integrins. As is becoming apparent, the trafficking of diverse cargo along distinct endocytic pathways results in functional interdependence whereby one pathway influences the other. Integrin αvβ3 traffics through the Rab4-positive rapid-recycling pathway. Interestingly, this regulates the recycling of another RGD-binding receptor α5β1 integrin. When αvβ3 is functional and rapidly trafficking, α5β1 integrin is recycled less, and cells migrate persistently in two dimensions (Caswell et al. 2008; White et al. 2007). However, the inhibition of αvβ3 with the small molecule antagonist cilengitide results in an increased recycling of α5β1. This results in random motility in two dimensions but an increased invasiveness in three dimensions (White et al. 2007). Interestingly, α5β1 forms a complex with EGFR and Rab GTPase effector Rab-coupling protein in the recycling endosomal compartment. Inhibition of αvβ3 also results in increased recycling of EGFR to the plasma membrane resulting in increased invasion and activation of the Akt pathway (Caswell et al. 2008). EGFR has been shown to recycle when engaged with lower affinity ligands such as TGFα (Ebner and Derynck 1991; Waterman and Yarden 2001). However, recent data suggest that alterations in other cellular signaling pathways result in the recycling of other growth factor receptors. PDFGRβ does not recycle normally. However, it has been shown to recycle rapidly if it becomes hyperphosphorylated upon loss of its negative regulator TCPTP protein tyrosine phosphatase (Hellberg et al. 2009; Karlsson et al. 2006). This has not yet been linked to integrin traffic, but since integrin adhesion regulates TCPTP activity (Mattila et al. 2005), and since PDGFRβ can co-cluster and be activated without a ligand by the integrins (Schneller et al. 1997), integrin traffic might also regulate PDGFRβ function. In endothelial cells, intimate cross-talk seems to occur between the trafficking of αvβ3 integrin and VEGFR1 and -2 receptors. VEGFR1 activates the recycling of αvβ3 integrin from the Rab4-positive compartment and is involved in regulating fibronectin polymerization (Jones et al. 2009). Conversely, inhibition of αvβ3 with cilengitide alters the recycling of VEGFR2, thus promoting angiogenesis (Reynolds et al. 2009). This is unexpected as integrin inhibitors are currently undergoing clinical trials as anti-angiogenic agents. Thus, a new platform for integrin and growth factors receptor co-operation and cross-talk is emerging at the endosomal compartment (summarized in Table 2), and future findings in this area are likely to provide important new insights into invasion, transformation, and the regulation of angiogenesis.
Table 2.
Integrin cross-talk with other receptor systems in endosomes (VEGFR vascular endothelial growth factor receptor, EGFR epidermal growth factor receptor, JAM-A junctional adhesion molecule-A)
| Integrin | Other receptors | Physiological role | Reference |
|---|---|---|---|
| αvβ3 | Integrin α5β1 | Integrin αvβ3 inhibits recycling of α5β1; reduced invasion | White et al. 2007 |
| αvβ3 | EGFR | Integrin αvβ3 inhibits recycling of EGFR; reduced invasion | Caswell et al. 2008 |
| α5β1 | Neuroplilin-1 | Neuropilin-1 induces α5β1 endocytosis | Valdembri et al. 2009 |
| β1 | T-cadherin | T-cadherin inhibits β1-integrin endocytosis | Mukoyama et al. 2007 |
| β1 | JAM-A | JAM-A internalization depends on β1-integrin endocytosis; neutrophil chemotaxis | Cera et al. 2009 |
| αVβ3 | VEGFR1 | VEGFR1 stimulates recycling of αvβ3; fibronectin assembly | Jones et al. 2009 |
| αVβ3, αVβ5 | VEGFR2 | Integrin inhibition results in increased VEGFR2 recycling and angiogenesis | Reynolds et al. 2009 |
Concluding remarks
The emerging information about growth factor and cell adhesion receptors has unveiled a multiplicity of mechanisms regarding the way that the receptor systems work together. Integrin binding to ECM proteins and growth factor binding to their own receptors regulate many common signaling pathways. Integrins may directly bind to some growth factors and, in the case of TGF-β, activate them (Munger et al. 1999). Furthermore, activated integrins form receptor clusters that also contain growth factor receptors. In these protein complexes, integrins are required for the formation of a platform in which growth factor receptors can properly interact with downstream signaling partners (Yamada and Even-Ram 2002). Interestingly, within the integrin-organized complexes, growth factor receptors can be activated even without ligands. The most recent observations have revealed a new level in the interplay between integrins and growth factor receptors. Previously, both receptor types were known to be internalized and recycled back to the cell surface. However, current reports indicate that integrins orchestrate the intracellular trafficking of growth factor receptors (Caswell and Norman 2008; Caswell et al. 2008).
This new information might help to explain the contradictory results that have generated debate during the past few years with regard to integrin function. Especially in the cases of αV integrins and α2β1 integrin, results obtained in the knockout phenotypes have been in conflict with those from experiments involving the use of integrin inhibitors (Hynes 2002; Zhang et al. 2008). Recent observations indicate that the lack of integrins affects the levels of growth factor receptors on the cell surface (Zhang et al. 2008). Furthermore, integrin inhibitors not only might block integrin binding to ligands, but may also affect signaling by growth factor receptors (Reynolds et al. 2009). Thus, the new data stress the role of integrins as integral parts of the cellular signaling machinery. Furthermore, the direct role of specific integrins must be reconsidered in certain biological processes, such as angiogenesis.
Acknowledgements
The authors thank Mr. Timo Kattelus for the illustrations.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol. 2005;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha V integrins. Cell. 1998;95:507–519. doi: 10.1016/S0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha V beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol. 2007;19:124–134. doi: 10.1016/j.ceb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Cambi A, Joosten B, Koopman M, de Lange F, Beeren I, Torensma R, Fransen JA, Garcia-Parajo M, van Leeuwen FN, Figdor CG. Organization of the integrin LFA-1 in nanoclusters regulates its activity. Mol Biol Cell. 2006;17:4270–4281. doi: 10.1091/mbc.E05-12-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(V)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–26525. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–263. doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, Norman JC. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cera MR, Fabbri M, Molendini C, Corada M, Orsenigo F, Rehberg M, Reichel CA, Krombach F, Pardi R, Dejana E. JAM-A promotes neutrophil chemotaxis by controlling integrin internalization and recycling. J Cell Sci. 2009;122:268–277. doi: 10.1242/jcs.037127. [DOI] [PubMed] [Google Scholar]
- Connors WL, Jokinen J, White DJ, Puranen JS, Kankaanpaa P, Upla P, Tulla M, Johnson MS, Heino J. Two synergistic activation mechanisms of alpha2beta1 integrin-mediated collagen binding. J Biol Chem. 2007;282:14675–14683. doi: 10.1074/jbc.M700759200. [DOI] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/S0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. Alpha3beta1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner R, Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991;2:599–612. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009;122:215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/S0955-0674(97)80124-X. [DOI] [PubMed] [Google Scholar]
- Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Hakkinen L, Koivisto L, Gardner H, Saarialho-Kere U, Carroll JM, Lakso M, Rauvala H, Laato M, Heino J, Larjava H. Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol. 2004;164:229–242. doi: 10.1016/s0002-9440(10)63113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ, Ozawa E, Goto Y, Nonaka I, Tsukahara T, Wang JZ, Hoffman EP, Arahata K. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet. 1998;19:94–97. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- Heino J. The collagen family members as cell adhesion proteins. Bioessays. 2007;29:1001–1010. doi: 10.1002/bies.20636. [DOI] [PubMed] [Google Scholar]
- Hellberg C, Schmees C, Karlsson S, Ahgren A, Heldin CH. Activation of protein kinase C {alpha} is necessary for sorting the PDGF {beta}-receptor to Rab4a-dependent recycling. Mol Biol Cell. 2009;20:2856–2863. doi: 10.1091/mbc.E08-12-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/MCB.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings H, Ortega N, Plouet J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J. 2003;17:1520–1522. doi: 10.1096/fj.02-0691fje. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ivaska J, Bosca L, Parker PJ. PKCepsilon is a permissive link in integrin-dependent IFN-gamma signalling that facilitates JAK phosphorylation of STAT1. Nat Cell Biol. 2003;5:363–369. doi: 10.1038/ncb957. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Lu N, Denessiouk K, Heino J, Gullberg D. Integrins during evolution: evolutionary trees and model organisms. Biochim Biophys Acta. 2009;1788:779–789. doi: 10.1016/j.bbamem.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–557. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Jones MC, Caswell PT, Moran-Jones K, Roberts M, Barry ST, Gampel A, Mellor H, Norman JC. VEGFR1 (Flt1) regulates Rab4 recycling to control fibronectin polymerization and endothelial vessel branching. Traffic. 2009;10:754–766. doi: 10.1111/j.1600-0854.2009.00898.x. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Kowanetz K, Sandin A, Persson C, Ostman A, Heldin CH, Hellberg C. Loss of T-cell protein tyrosine phosphatase induces recycling of the platelet-derived growth factor (PDGF) beta-receptor but not the PDGF alpha-receptor. Mol Biol Cell. 2006;17:4846–4855. doi: 10.1091/mbc.E06-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin {alpha}L{beta}2. J Cell Biol. 2004;167:1241–1253. doi: 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Kohn E. Anoikis: cancer and the homeless cell. Nature. 2004;430:973–974. doi: 10.1038/430973a. [DOI] [PubMed] [Google Scholar]
- Littlewood Evans A, Muller U. Stereocilia defects in the sensory hair cells of the inner ear in mice deficient in integrin alpha8beta1. Nat Genet. 2000;24:424–428. doi: 10.1038/74286. [DOI] [PubMed] [Google Scholar]
- Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM. Integrin alpha8beta1 mediates adhesion to LAP-TGFbeta1. J Cell Sci. 2002;115:4641–4648. doi: 10.1242/jcs.00145. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Murgia C, Wary KK, Curatola AM, Pepe A, Blumemberg M, Westwick JK, Der CJ, Giancotti FG. The coupling of alpha6beta4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila E, Pellinen T, Nevo J, Vuoriluoto K, Arjonen A, Ivaska J. Negative regulation of EGFR signalling through integrin-alpha1beta1-mediated activation of protein tyrosine phosphatase TCPTP. Nat Cell Biol. 2005;7:78–85. doi: 10.1038/ncb1209. [DOI] [PubMed] [Google Scholar]
- Mattila E, Auvinen K, Salmi M, Ivaska J. The protein tyrosine phosphatase TCPTP controls VEGFR2 signalling. J Cell Sci. 2008;121:3570–3580. doi: 10.1242/jcs.031898. [DOI] [PubMed] [Google Scholar]
- Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- Michishita M, Videm V, Arnaout MA. A novel divalent cation-binding site in the A domain of the beta 2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell. 1993;72:857–867. doi: 10.1016/0092-8674(93)90575-B. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wu CY, Yamaji S, Saegusa J, Shi B, Ma Z, Kuwabara Y, Lam KS, Isseroff RR, Takada YK, Takada Y. Direct binding of integrin alphaVbeta3 to FGF1 plays a role in FGF1 signaling. J Biol Chem. 2008;283:18066–18075. doi: 10.1074/jbc.M801213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(V)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukoyama Y, Utani A, Matsui S, Zhou S, Miyachi Y, Matsuyoshi N. T-cadherin enhances cell-matrix adhesiveness by regulating beta1 integrin trafficking in cutaneous squamous carcinoma cells. Genes Cells. 2007;12:787–796. doi: 10.1111/j.1365-2443.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Muller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–613. doi: 10.1016/S0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphaVbeta1. Mol Biol Cell. 1998;9:2627–2638. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha V beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/S0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Patel P, Brat DJ, Colbert L, Vertino PM. Silencing of TMS1/ASC promotes resistance to anoikis in breast epithelial cells. Cancer Res. 2009;69:1706–1711. doi: 10.1158/0008-5472.CAN-08-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen T, Ivaska J. Integrin traffic. J Cell Sci. 2006;119:3723–3731. doi: 10.1242/jcs.03216. [DOI] [PubMed] [Google Scholar]
- Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol. 2006;173:767–780. doi: 10.1083/jcb.200509019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen T, Tuomi S, Arjonen A, Wolf M, Edgren H, Meyer H, Grosse R, Kitzing T, Rantala JK, Kallioniemi O, Fassler R, Kallio M, Ivaska J. Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev Cell. 2008;15:371–385. doi: 10.1016/j.devcel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- Rohwer N, Welzel M, Daskalow K, Pfander D, Wiedenmann B, Detjen K, Cramer T. Hypoxia-inducible factor 1alpha mediates anoikis resistance via suppression of alpha5 integrin. Cancer Res. 2008;68:10113–10120. doi: 10.1158/0008-5472.CAN-08-1839. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/S0065-230X(08)60772-1. [DOI] [PubMed] [Google Scholar]
- Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller M, Vuori K, Ruoslahti E. AlphaVbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/S0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Murakami Y, Suto F, Fujisawa H. Determination of cell adhesion sites of neuropilin-1. J Cell Biol. 2000;148:1283–1293. doi: 10.1083/jcb.148.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Staniszewska I, Sariyer IK, Lecht S, Brown MC, Walsh EM, Tuszynski GP, Safak M, Lazarovici P, Marcinkiewicz C. Integrin alpha9 beta1 is a receptor for nerve growth factor and other neurotrophins. J Cell Sci. 2008;121:504–513. doi: 10.1242/jcs.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T, Takagi J, Rennert PD, Kolodkin AL, Kumanogoh A, Kikutani H. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell. 2001;107:643–654. doi: 10.1016/S0092-8674(01)00567-0. [DOI] [PubMed] [Google Scholar]
- Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, Serini G. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:e25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphaVbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Roche O, Yan MS, Finak G, Evans AJ, Metcalf JL, Hast BE, Hanna SC, Wondergem B, Furge KA, Irwin MS, Kim WY, Teh BT, Grinstein S, Park M, Marsden PA, Ohh M. Regulation of endocytosis via the oxygen-sensing pathway. Nat Med. 2009;15:319–324. doi: 10.1038/nm.1922. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/S0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/S0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- Waterman H, Yarden Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–152. doi: 10.1016/S0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- White DP, Caswell PT, Norman JC. AlphaVbeta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol. 2007;177:515–525. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, Sheppard D. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J Biol Chem. 2007;282:15187–15196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nat Cell Biol. 2002;4:E75–E76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ramirez NE, Yankeelov TE, Li Z, Ford LE, Qi Y, Pozzi A, Zutter MM. Alpha2beta1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell-specific manner. Blood. 2008;111:1980–1988. doi: 10.1182/blood-2007-06-094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. Beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


