Abstract
Adhesion and migration are integrated cell functions that build, maintain and remodel the multicellular organism. In migrating cells, integrins are the main transmembrane receptors that provide dynamic interactions between extracellular ligands and actin cytoskeleton and signalling machineries. In parallel to integrins, other adhesion systems mediate adhesion and cytoskeletal coupling to the extracellular matrix (ECM). These include multifunctional cell surface receptors (syndecans and CD44) and discoidin domain receptors, which together coordinate ligand binding with direct or indirect cytoskeletal coupling and intracellular signalling. We review the way that the different adhesion systems for ECM components impact cell migration in two- and three-dimensional migration models. We further discuss the hierarchy of these concurrent adhesion systems, their specific tasks in cell migration and their contribution to migration in three-dimensional multi-ligand tissue environments.
Keywords: Cell adhesion, Cell migration, Integrins, Extracellular matrix, Cytoskeleton
Introduction
Cell adhesion and migration are fundamental to the formation and maintenance of multicellular organisms. Cell adhesion is provided by adhesion molecules, which are expressed at the cell surface of all nucleated cells, mediate extracellular binding to cell and tissue substrates and transmit mechanical docking to the intracellular actomyosin or intermediate filament cytoskeleton. Cell adhesion underlies many important physiological processes, including cell spreading, polarity, anchoring and differentiation. When cell-tissue interactions undergo dynamic turn-over, adhesion molecules further mediate cell migration and positioning during dynamic phases of the body, including morphogenesis, wound healing and, in a de-regulated form, during cancer invasion and metastasis (Hood and Cheresh 2002; Hynes 2002). Likewise, cell adhesion and migration underlie many functions of the immune system, including leukocyte recirculation, pathogen recognition and effector function (Friedl and Weigelin 2008).
To fulfil these various tasks in the different cell types and tissue contexts, diverse sets of adhesion receptors contribute to cell interaction with tissue components and to migration. Adhesion receptor ligands are another cell, a multimeric particle or macromolecules that are immobilized in the tissue, such as extracellular matrix (ECM) ligands. Important ECM proteins recognized by adhesion receptors are collagens, fibronectin, vitronectin, fibrinogen and laminin (van der Flier and Sonnenberg 2001). Non-protein ECM ligands comprise proteoglycan polysaccharides, such as heparan sulphate, chondroitin sulphate and keratin sulphate, and the non-proteoglycan polysaccharide hyaluronan (Iozzo 1998; Heino and Kapyla 2009).
Several classes of cell surface receptors fulfil adhesion and cytoskeletal coupling functions and provide a range of adhesion strength, specificity and turn-over rates during cell migration (Fig. 1). High affinity adhesion to ECM ligands is predominantly provided by receptors of the integrin family (Hynes 2002) and CD44 (Goodison et al. 1999). Whereas integrins predominantly recognize extracellular protein scaffolds, such as interstitial collagen (Takada et al. 2007), CD44 preferentially binds to extracellular carbohydrate polymers, such as glycoproteins, glycosaminoglycans and hyaluronic acid (HA; Ponta et al. 2003). In addition to these “classical” adhesion receptors, other surface receptors can bind ECM components and induce signalling, including the syndecans (Bernfield et al. 1999) and the receptor tyrosine kinases discoidin domain receptors (DDRs; Yoshimura et al. 2005). Whereas integrin- and CD44-mediated adhesions have previously received substantial attention (Humphries et al. 2003), alternative adhesion mechanisms and their contribution to cell anchoring and migration have been less well studied. We summarize here the way that integrin- and non-integrin-mediated cell-substrate interactions contribute to different types of cell migration.
Fig. 1.
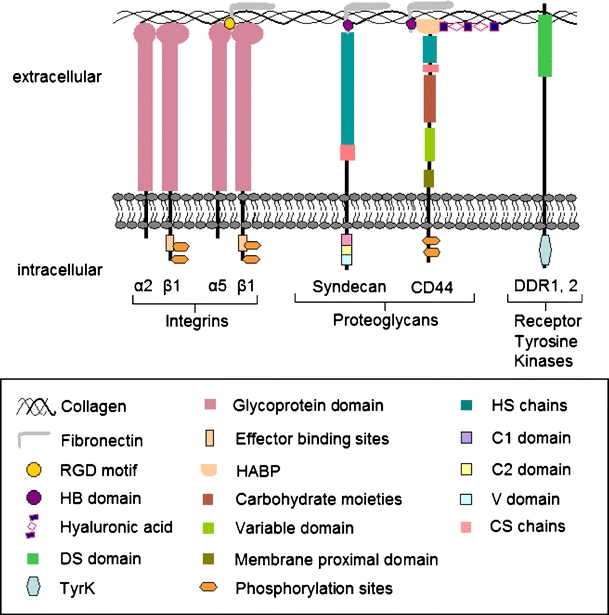
Classes of adhesion receptors involved in cell adhesion and migration. Important domains of adhesion receptors and interstitial ECM ligands collagen, fibronectin and hyaluronan are shown
Modes of cell migration and adhesion requirements
Cell migration is not a uniform event but comprises distinct modes of cell migration that are executed by different cell types and contexts. These migration types vary in cell shape, adhesion strength and migration speed and also in whether cell-cell junctions are retained (Fig. 2).
Fig. 2.
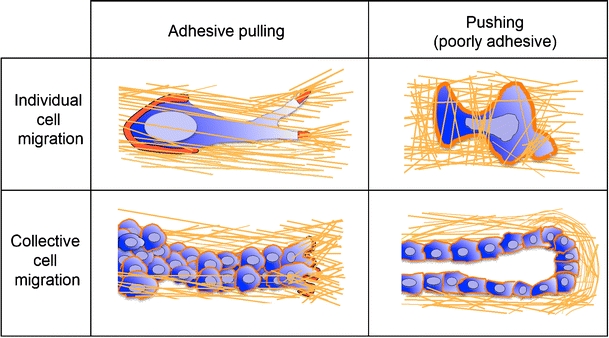
Various migration strategies. Dependent on cell-matrix adhesion strengths and cell contrctility, force generation occurs either via attachment to the ECM substrate and pulling or by cell propulsion. Dependent on the stability of cell-cell adhesion, cells migrate either individually or within multicellular strands (red actin cytoskeleton)
Arguably, the most “simple” migration mode is amoeboid migration (Friedl et al. 2001). Cells that move in an amoeboid manner include primordial germ cells, lymphocytes, dendritic cells, lymphoma cells and neutrophils, all of which exhibit low or no integrin-mediated traction force generation (Entschladen et al. 1997; Friedl et al. 1998; Blaser et al. 2006; Lammermann et al. 2008). These cells form relatively instable adhesion sites to a substrate; such sites rapidly turn-over and, depending on extracellular context and signalling, allow for rapidly adaptive migration (Friedl et al. 2001). Amoeboid movement is driven by a roundish to ellipsoid cell shape, non-focalized but rather diffusely organized adhesion sites to the substrate and a strictly cortical actin cytoskeleton that lacks stress fibres (Friedl and Wolf 2003). Two types of force generation contribute to amoeboid movement. Based on relatively weak attachment and speading to the substrate, a fast “gliding” type of movement is enabled that supports a weakly adhesive pulling-type migration across two-dimensional (2D) and in three-dimensional (3D) environments (Friedl and Wolf 2009). Alternatively, in non-adhesive cells, polarized dendrite or bleb formation coupled to rear-retraction fail to attach to 2D substrate and lack adhesion sites and, thus, provide an intercalating propulsive-type of migration in 3D tissues (Fackler and Grosse 2008). The biophysical basis of this blebbing-type migration is incompletely understood as it shows signs of adhesion-independent migration. Instead of focalized adhesion complexes, a predominantly cortical actin cytoskeleton drives cell polarization and physical translocation by shape change and cytoskeletal stiffness in complex tissue environments (Paluch et al. 2006; Friedl and Wolf 2009). In cells with increased adhesion and cytoskeletal contractility, cell-matrix interactions become focalized and the cell adopts an elongated spindle-shaped morphology; this mode of migration is termed mesenchymal migration and is used by fibroblasts, myoblasts and many cancer cells (Friedl et al. 1998; Friedl 2004), generates substantial adhesion and traction force towards the surrounding tissue and further mediates proteolytic tissue remodelling by the function of cell-produced proteases (Wolf and Friedl 2009). Both ligand binding and intracellular coupling of cell adhesions are turned over in the range of minutes to hours thereby regulating both local adhesion strength and the dynamics of the cell (Zamir and Geiger 2001; Ballestrem et al. 2001). During morphogenesis, regeneration and cancer invasion, cells that retain cell-cell junctions by means of cadherins and other cell-cell adhesion mechanisms migrate collectively either as monolayer sheets or as 3D strands, sprouts or isolated clusters (Friedl et al. 2004). Two types of force generation in collective migration are under discussion at present, depending on the amount of anterior adhesion and traction force generation. If one or several front- and mid-row cells generate adhesion and traction force towards the substrate, collective migration results from adhesive “pulling” mediated by adhesion-complexes that connect to the actin cytoskeleton, similar to mesenchymal movement (Hegerfeldt et al. 2002; Trepat and Wasserman 2009). This type of migration is commonly detected in sprouting vessels, epithelial sheets during wound healing and cancer invasion (Hegerfeldt et al. 2002; Friedl and Gilmour 2009). A second, less well understood mode of collective force generation is multicellular pushing, which is produced by a stable stick-like stalk and a relatively roundish terminal bud that protrudes into soft tissue in the absence of apparent force-generating adhesion complexes (Ewald et al. 2008). Thus, different cellular and molecular requirements for adhesion and force generation govern the different types of cell migration. Whereas, until recently, integrins have received ample attention and are considered as the main mediators of adhesion force generation during cell migration, the promigratory functions of non-integrin adhesion systems remain to be integrated into these concepts of cell movement.
Integrins
Integrins are heterophilic cell adhesion molecules consisting of non-covalently connected α and β chains that together determine ligand-binding specificity and intracellular coupling (Humphries 2000; Hynes 2002). Based on ECM/ligand recognition, integrins mediate binding to laminins (α2β1, α3β1, α6β1, α7β1), fibrillar collagens (α1β1, α2β1, α3β1, α10β1, α11β1) and Arg-Gly-Asp (RGD) motifs contained in many ECM proteins (α5β1, αvβ3, αvβ5, αIIbβ3; Takada et al. 2007). Thereby, integrins are the most important cell surface receptors for cell interactions with ECM structures.
Integrins control the strength and turn-over of cell interactions with ECM scaffolds (Hynes 1992). After ligand binding, integrins multimerize in the plasma membrane in a focalized manner and connect, via intracellular signalling and adaptor proteins, to the actin cytoskeleton, termed focal contact or focal adhesion (Burridge and Chrzanowska-Wodnicka 1996; Zaidel-Bar et al. 2007; Moser et al. 2009). Alternatively, diffuse integrin distribution devoid of microscopically detectable clustering and focalization of adhesion site mediates substrate binding and intracellular signalling (Friedl et al. 1998).
To mediate cell movement, the integrin-mediated cell-substrate interactions and linkages to the actin cytoskeleton form and turn-over, the dynamics and polarity of which determine cell speed and directional persistence. The way that integrins govern migration type and rates depends upon net adhesion strength per cell, type of cell-matrix interactions and type of substrate. On 2D ECM substrates, including collagen, fibronection or vitronectin, integrin-mediated adhesions are preferentially mediated by α5β1, αvβ3 and α2β1, respectively (Takada et al. 2007). In integrin-dependent migration models, the highest velocities result from an intermediate level of net adhesion strength allowing both rapid focal contact formation and the generation of traction forces, which are regulated by the small GTPase Rho (DiMilla et al. 1991; Beningo et al. 2006) . Accordingly, increasing adhesion to the substrate slows cells down and favours cell immobilization and anchoring attributable to delayed rear-retraction. Likewise, at low net adhesion, migration rates are impaired because of reduced binding strength and force generation at the leading edge, resulting in partial or complete loss of migration (Palecek et al. 1997). The impact of adhesion- and traction force-dependent integrin functions are commonly established in haptokinetic migration across a 2D ligand-coated substrate, so that some degree of attachment is indispensible for migration (Huttenlocher et al. 1995). This principle of adhesion-driven migration applies to many, if not all, actin-associated integrins, notably β1, β2, β3, and β4 integrins (Rabinovitz and Mercurio 1997; Maaser et al. 1999; Vicente-Manzanares et al. 2009). Consequently, both cell adhesion and migration are impaired by adhesion-perturbing anti-integrin antibodies or genetic deletion of integrins so that the cells either round up and partly detach or completely lose contact to the substrate (Friedl and Wolf 2003). If, however, cells move through a 3D ECM, distinct physical principles apply because cells are entirely surrounded by ECM and, even after loss of adhesion, continue to interact passively with the substrate (Friedl and Brocker 2000).
For cells of high integrin availability moving through a 3D ECM, including fibroblasts and certain cancer cells, integrins mediate adhesive pulling at the leading edge so that traction force towards the substrate is continually being generated (Fig. 2; Maaser et al. 1999; Petrie et al. 2009). Consequently, cell elongation and mesenchymal migration are dependent on integrin-mediated adhesion and focalization of the actin cytoskeleton to matrix contacts. The integrin engagement leads to the activation of focal adhesion kinase, Rho/Rac guanine nucleotide exchange factors and Rho kinase (ROCK), which together drive the formation and turn-over of adhesions (Iwanicki et al. 2008). Following interference with integrin-mediated adhesion, mesenchymal migration is abrogated and no alternative adhesion receptors compensate for ablated integrin-dependent force generation and mesenchymal migration (Maaser et al. 1999; Grinnell 2008, 2009). In mesenchymal migration, integrin β1 or αvβ3 integrins cooperate with cell surface proteases, notably matrix metalloproteinases (MMPs) leading to the generation of small microtracks bordered by remodelled collagen fibres (Deryugina et al. 1998; Wolf et al. 2007). Secondary to traction force generation, mesenchymal migration leads to contraction and remodelling of the ECM in vitro and to wound healing (Cooke et al. 2000; Larsen et al. 2006).
If integrin-mediated traction force is low or negligible, moving cells utilize the amoeboid migration mode (Fig. 2; Friedl et al. 2001). Whereas, on 2D surfaces, amoeboid migration is still dependent on low adhesion mediated by integrins (Lammermann et al. 2008), amoeboid migration in 3D fibrillar collagen or interstitial tissue in vivo persists either partly or fully after integrins are blocked by antibody or are genetically ablated (Friedl et al. 1998; Lammermann et al. 2008). In integrin-independent amoeboid migration, the leading edge produces pseudopodia, dendites or roundish-shaped blebs, all of which are dependent on cortical actin networks but do not form focalized adhesion complexes with the ECM substrate (Friedl et al. 1998; Lammermann and Sixt 2009). The mechanisms underlying integrin-independent migration are incompletely unterstood but are best explained by actin forward flow followed by cell intercalation between matrix pores and gaps and cell contraction mediated by myosin II to move both the cell rear and the nucleus forward (Lammermann et al. 2008). Thus, grossly different magnitudes of integrin-mediated adhesion and force generation are associated with the diverse migration modes.
Accordingly, in 3D ECM-based models, cells can switch between high and low integrin-dependence of migration. The lowering of integrin-mediated adhesion by blocking with antibodies or by interfering with the integrin-effector c-src does not abrogate migration of cancer cells in 3D ECM-based models but is followed by persistent robust cell movement (Carragher et al. 2006; Zaman et al. 2006). Whether such plasticity is restricted to cancer cells and which alternative adhesion mechanisms compensate for impaired integrin function remain unclear.
Because of their multifunctional nature and ubiquitous expression, integrins contribute to most cell-tissue interaction models substantially and impact other adhesion pathways in response to ECM. Thus, as we will discuss below, the study of adhesion mechanisms other than integrins is often compromised by an overlap with integrin-mediated substrate recognition and function.
Syndecans
Syndecans are a familiy of transmembrane cell surface heparan sulphate proteoglycans (HSPGs) with four members, syndecan 1–4. All vertebrate cells express at least one syndecan family member in a cell-type and tissue-specific manner, which is further modified during cell activation (Tkachenko et al. 2005). Syndecans bind to ECM ligands and further cooperate with other cell surface receptors for ligand-binding and signalling. Via their heparin-binding ectodomain, syndecans bind to extracellular glycosaminoglycans, including heparan sulphate and chondroitin sulphate, and to other ECM molecules, including collagen types I, III and IV, fibronectin and vitronectin (Beauvais et al. 2004). By distinct mechanisms, syndecans further bind via residues within the HSPG side chains to cytokines and growth factors, including fibroblast growth factor-2 and epidermal growth factor and “present” them laterally to their specific receptors (Wu et al. 2003; Tkachenko et al. 2005).
Syndecans mediate both cell adhesion and co-signalling, often in the same context, which renders the distinction between adhesion and co-signalling function difficult, if not impossible. In most cell models, overexpression of syndecans enhances cell adhesion and haptokinetic migration in normal and neoplastic cells, whereas interference with syndecan function decreases cell migration (Table 1). As an example, in endothelial cells, binding of syndecan-4 to fibronectin-cated substrate results in syndecan-4 co-clustering with integrin α5β1 and the activation of Rac-1 through the intracellular scaffold protein synectin (Tkachenko et al. 2006; Morgan et al. 2007). Consequently, syndecans enhance cell spreading, polarization and migration in vitro and cell migration and tissue remodelling during wound healing and angiogenesis in vivo (Table 1; Echtermeyer et al. 2001; Stepp et al. 2002; Morgan et al. 2007).
Table 1.
Regulation of cell migration by syndecans (2D two-dimensional, 3D three dimensional)
| Syndecan type | Cell type | Experimental model | Function | Reference |
|---|---|---|---|---|
| Syndecan-1 | Breast cancer cells | 2D spreading and migration assay; migration through 3D matrigel after overexpression | Increased spreading, adhesion and migration on 2D collagen I; increased cell invasion in 3D Matrigel | Burbach et al. 2004 |
| Myeloma cells | In vivo model of experimental metastasis in mice | Increased metastasis and tumour growth | Khotskaya et al. 2009 | |
| Syndecan-2 | Intestinal epithelial cells | 2D adhesion and migration after overexpression | Increase of adhesion, spreading on collagen type I (in cooperation with α2β1 integrin) | Choi et al. 2009 |
| Colon carcinoma cells | Migration across type-I-collagen-coated surface after overexpression | Increased migration speed | Park et al. 2002 | |
| Melanoma cells | Migration through polycarbonate filter (transwell) after overexpression | Increased migration and invasion | Lee et al. 2009 | |
| Syndecan-3 | Neuronal cells | Migration through polycarbonate filter (transwell) | Increased migration | Hienola et al. 2006 |
| Syndecan-4 | Melanoma cells | Adhesion and migration across fibronectin-coated 2D surface | Increased adhesion and migration | Chalkiadaki et al. 2009 |
| Fibroblasts | Adhesion and migration in 3D fibrin-fibronectin-matrix | Increased adhesion and migration | Midwood et al. 2004, 2006 |
Syndecans interact with a large number of ligands, which in parallel also bind to other cell surface receptors. Thus, a functional synergy between syndecans and integrins leads to an overlap in adhesion-dependent signalling pathways (Morgan et al. 2007). Given that syndecans functionally synergize with integrins, the extent to which syndecans can be understood as true adhesion receptors awaits further clarification by using models that isolate the direct contribution of syndecans to adhesion from its co-receptor and signalling activities.
Discoidin domain receptors (DDR)
Discoidin domain receptors (DDRs) belong to the discoidin-like domain-containing subfamily of receptor tyrosine kinases, with two members in mammalian cells: DDR1, expressed as five isoforms (DDR1a-e), and DDR2 with no known isoforms (Alves et al. 2001; Vogel et al. 1997). As main ligands, DDRs bind to all triple-helical fibrillar collagens, and DDR1 additionally binds to collagens type IV, VI and VIII (Curat et al. 2001). DDR1 is expressed in many epithelia (Vogel et al. 1997), and in tissue-infiltrating leukocytes (Kamohara et al. 2001), whereas DDR2 is expressed in muscle cells, kidney, lung, brain and connective tissue (Vogel 1999); both DDRs are upregulated in many cancer cell types (Vogel et al. 2006). After ligand binding, DDRs induce various intracellular signalling pathways, including the activation of Wiskott-Aldrich syndrome protein and Pyk-2s, the SH2-domain-containing-transforming protein and SH2-domain-containing phosphatase 2, all of which indirectly promote actin dynamics (Buday et al. 2002; Koo et al. 2006; Vogel et al. 2006). Accordingly, DDRs enhance cell migration, proliferation, and survival (Vogel 1999). In contrast to other receptor tyrosine kinases, initial signalling through DDRs occurs within minutes but peaks only several hours later, implicating DDRs in sustained and slow rather than acute responses to the ECM (Vogel et al. 1997, 2006).
Despite their collagen-binding capability, whether DDRs can be considered as classical adhesion receptors is unclear. DDR signalling typically co-engages with integrins (Shintani et al. 2008). DDRs enhance integrin-mediated cell adhesion to collagen (Kamohara et al. 2001) and enhance integrin-mediated signalling (Shintani et al. 2008). In addition, in some models, DDR1 overexpression enhances cell attachment to collagen that cannot be directly attributed to integrin function, suggesting either a direct adhesion function of DDR1 or the coengegement of yet another adhesion system (Kamohara et al. 2001).
DDRs regulate cell migration in an isoform-specific manner. DDR1a-overexpressing leukemia and glioma cells show enhanced migration into 3D collagen lattices, whereas overexpression of DDR1b reduces migration in both cell types (Kamohara et al. 2001; Ram et al. 2006). On the basis that all DDRs bind to collagen by a similar mechanism, the signalling pathways that underlie such different fine-tuning of migration are unknown.
DDRs not only enhance cytoskeletal dynamics but further induce a more complex “invasion program”. DDR signalling upregulates pro-invasive MMPs 2 and 9, which leads to enhanced proteolytic degradation of ECM (Hou et al. 2002) and invasion and metastasis of tumour cells in vivo (Vogel et al. 1997). Although experimental overexpression of DDR1 acts in a promigratory manner (Ram et al. 2006), whether lower endogenous levels mediate the same effect remains unclear. To improve the discrimination between adhesive and signalling functions of DDRs, future studies should address DDR adhesion and other functions in integrin-independent models.
CD44
CD44 is a highly glycosylated member of the hyaladherin or link protein superfamily of adhesion molecules and is either expressed as the standard form (CD44s) or as one of 12 distinct isoforms (CD44v1-v12; Ponta et al. 2003; Naor et al. 2008). CD44 binds its main ligand, HA, via the hyaluronan-binding domain (Banerji et al. 2007). Other ligands, which instead interact with variable membrane-proximal domains of CD44, include heparan sulphate (exon v3), chondroitin sulphate (exon 5) and, via unmapped sites with probably weaker binding strength, collagen types I and VI, fibronectin and laminin and cell surface receptors, such as E- and L-selectin (Ponta et al. 2003; Bendall et al. 2004; Naor et al. 2007). The cytoplasmic domain of CD44 recruits the actin-binding proteins ezrin, radixin and moesin (ERM; Legg et al. 2002) and ankyrin and thereby physically bridges extracellular ligands to the actin cytoskeleton and induces intracellular signalling (Bourguignon 2008; Singleton et al. 2004). In addition to its adhesion function, CD44 serves as a co-receptor for other signalling receptors, such as the receptor tyrosine kinase mesenchymal-epithelial transition factor (c-Met), epidermal growth factor receptor and tumour growth factor-β (Orian-Rousseau et al. 2002).
CD44s is expressed by all nucleated vertebrate cells, including most cancer cells (Naor et al. 1997; Tanabe et al. 1993). Activated cells and many cancer cells additionally express CD44 variants, such as CD44v3-v10 on keratinocytes and CD44v6 on many transformed cells ((Brown et al. 1991); Wang et al. 2009). CD44-mediated signalling occurs via various pathways. HA binding leads to the activation of several effectors, including c-Src (Ouhtit et al. 2007), Rac1 and RhoA (Bourguignon et al. 2000; Bourguignon et al. 2001). In turn, active Rho and its effector ROCK promote the recruitment of the cytoskeleton-protein ankyrin-1 and engage with the conserved cytoplasmic domain of CD44 and thereby provide a second link between CD44 and the actin cytoskeleton (Singleton and Bourguignon 2004).
In addition to binding to tissue-anchored HA, CD44 immobilizes HA at the cell surface (Rilla et al. 2008). In early cell adhesion, cell-surface-tethered HA captures ECM substrata prior to integrin-mediated focal contact formation (Zimmerman et al. 2002). Cell-surface HA is constitutively present at the tips of cell protrusions, such as pseudopodia and lamellipodia, and engages with extracellular ECM substrate before integrin clustering and the formation of focal adhesions are detectable (Zimmerman et al. 2002; Rilla et al. 2008).
In most 2D haptokinetic migration models, CD44 enhances migration, either directly by mediating attachment to the substrate or by enhancing promigratory signalling. In normal and neoplastic cells, CD44 supports adhesion and migration across HA-coated surfaces (Zhu et al. 2006). Hereby, in contrast to integrins and syndecans, CD44 does not cluster at contact sites but rather seems to form uniform interaction zones to the substrate with a trend for redistribution to the cell rear (Jacobson et al. 1984; Goebeler et al. 1996; Friedl et al. 1997). In migrating leukocytes, CD44 is virtually excluded from the leading edge and accumulates together with ERM proteins in the posterior uropod, albeit that its function here is unclear (Sanchez-Madrid and del Pozo 1999; Wagner et al. 2008). Many cells, irrespective of adhesion strength and the substrate across which they migrate, release substantial amounts of CD44, the function of which however is unclear, from the rear of the cell (Bazil and Horejsi 1992; Friedl et al. 1997). In addition to an adhesion function, the CD44-HA interaction results in the rapid activation of surface proteolysis via endopeptidases, including MT1-MMP and ADAMs (a disintegrin and metalloproteinase family), which cleave the CD44 ectodomain and thereby limit CD44-mediated cell attachment (Nagano et al. 2004). Thus, the dual role of CD44 comprises cell attachment and the secondary release of CD44-mediated adhesion bonds.
In contrast to in vitro findings with respect to the contribution of CD44 to cell migration on an HA substrate, CD44-deficient mice lack obvious defects of development, regeneration and immune function, suggesting intact interstitial cell migration (Protin et al. 1999; Naor et al. 2008). To what extent other adhesion receptors, including β1 integrins, the receptor for hyaluronan-mediated motility (RHAMM) or layilin as alternative receptors for HA compensate for the loss of CD44 remains to be addressed (Protin et al. 1999; Naor et al. 2007; Chen et al. 2008).
In cancer models, the interaction of CD44 variants with HA supports lymphoma and melanoma progression and metastasis, which is prevented by blocking antibodies targeting CD44v4-v10 or by expressing CD44 with an inactive HA-binding domain (Wallach-Dayan et al. 2001). Conversely, over-expression of soluble CD44 ectodomain in malignant melanoma and mammary carcinoma cells inhibits growth, local invasion and metastasis in vivo, suggesting a role for the membrane-anchoring of CD44 (Ahrens et al. 2001; Peterson et al. 2000). However, given the lack of a phenotype in CD44-deficient mice, the in vivo relevance of CD44-mediated adhesion and migration detected in vitro remains unclear. Thus, like syndecans, CD44 and its variants provide multiple adhesion and co-signalling functions, the mechanistic contribution of which to cell migation remains incompletely understood.
Concluding remarks
The various adhesion systems expressed by vertebrate cells serve overlapping functions for cell positioning, anchoring and signalling but simultaneously retain additional independent and unique properties for each receptor. Whereas the molecular structure and associated signalling machinery of each receptor system have been examined in detail, only the role of integrins in cell migration has been conclusively established. Conversely, the functions of syndecans, DDRs and CD44 in the different types of cell migration, their integration into distinct adhesion and de-adhesion functions of the cell, their functional overlap and their spatiotemporal coordination during cell-matrix interaction and migration within multi-ligand enviroments remain unknown. Syndecans and DDRs cooperate with integrins synergistically in mediating cell adhesion and migration; this compromises defined experimental control of their functions. In order to experimentally overcome the governance of integrins and to gain better conceptual insights into each adhesion system independently, future strategies will require models of limited integrin availablility or integrin deficiency. Integrin-deficient cell models that still retain their cytoskeletal and polarization machinery will be instrumental in addressing specific migration modes and mechanisms and their response to physical tissue properties. To this end, the discrimination of the bona fide adhesion function of DDRs, syndecans and CD44 from their other co-receptor functions and from intracellular docking to cytoskeletal and signalling scaffolds, which contribute to cell adhesion and migration by indirect routes, will be of importance. Such approaches will clarify the way that distinct adhesion systems spatiotemorally contribute to the complex process of cell dynamics and of anchoring in multifaceted tissue environments.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
This work was supported by a grant from the Dutch Cancer Foundation (KWF 2008–4031).
Contributor Information
Samuel Schmidt, Phone: +31-243-610907, FAX: +31-243-615317.
Peter Friedl, Phone: +31-243-610907, FAX: +31-243-615317, Email: P.Friedl@ncmls.ru.nl.
References
- Abdulhussein R, McFadden C, Fuentes-Prior P, Vogel WF. Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor. J Biol Chem. 2004;279:31462–31470. doi: 10.1074/jbc.M400651200. [DOI] [PubMed] [Google Scholar]
- Ahrens T, Sleeman JP, Schempp CM, Howells N, Hofmann M, Ponta H, Herrlich P, Simon JC. Soluble CD44 inhibits melanoma tumor growth by blocking cell surface CD44 binding to hyaluronic acid. Oncogene. 2001;20:3399–3408. doi: 10.1038/sj.onc.1204435. [DOI] [PubMed] [Google Scholar]
- Alves F, Saupe S, Ledwon M, Schaub F, Hiddemann W, Vogel WF. Identification of two novel, kinase-deficient variants of discoidin domain receptor 1: differential expression in human colon cancer cell lines. FASEB J. 2001;15:1321–1323. doi: 10.1096/fj.00-0626fje. [DOI] [PubMed] [Google Scholar]
- Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol. 2001;155:1319–1332. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Wright AJ, Noble M, Mahoney DJ, Campbell ID, Day AJ, Jackson DG. Structures of the Cd44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat Struct Mol Biol. 2007;14:234–239. doi: 10.1038/nsmb1201. [DOI] [PubMed] [Google Scholar]
- Bazil V, Horejsi V. Shedding of the CD44 adhesion molecule from leukocytes induced by anti-CD44 monoclonal antibody simulating the effect of a natural receptor ligand. J Immunol. 1992;149:747–753. [PubMed] [Google Scholar]
- Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates alphavbeta3 integrin activity in human mammary carcinoma cells. J Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall LJ, Nilsson SK, Khan NI, James A, Bonnet C, Lock RB, Papa R, Bradstock KF, Gottlieb DJ. Role of CD44 variant exon 6 in acute lymphoblastic leukaemia: association with altered bone marrow localisation and increased tumour burden. Leukemia. 2004;18:1308–1311. doi: 10.1038/sj.leu.2403393. [DOI] [PubMed] [Google Scholar]
- Beningo KA, Hamao K, Dembo M, Wang YL, Hosoya H. Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Arch Biochem Biophys. 2006;456:224–231. doi: 10.1016/j.abb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, Solnica-Krezel L, Heisenberg CP, Raz E. Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell. 2006;11:613–627. doi: 10.1016/j.devcel.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18:251–259. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Shao L, Chen YW. CD44 interaction with Tiam1 promotes Rac1 signaling and hyaluronic acid-mediated breast tumor cell migration. J Biol Chem. 2000;275:1829–1838. doi: 10.1074/jbc.275.3.1829. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Zhou B, Diedrich F, Singleton PA, Hung MC. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2–p185(HER2) and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem. 2001;276:48679–48692. doi: 10.1074/jbc.M106759200. [DOI] [PubMed] [Google Scholar]
- Brown TA, Bouchard T, St John T, Wayner E, Carter WG. Human keratinocytes express a new CD44 core protein (CD44E) as a heparan-sulfate intrinsic membrane proteoglycan with additional exons. J Cell Biol. 1991;113:207–221. doi: 10.1083/jcb.113.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday L, Wunderlich L, Tamas P. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal. 2002;14:723–731. doi: 10.1016/s0898-6568(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Burbach BJ, Ji Y, Rapraeger AC. Syndecan-1 ectodomain regulates matrix-dependent signaling in human breast carcinoma cells. Exp Cell Res. 2004;300:234–247. doi: 10.1016/j.yexcr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Carragher NO, Walker SM, Scott Carragher LA, Harris F, Sawyer TK, Brunton VG, Ozanne BW, Frame MC. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene. 2006;25:5726–5740. doi: 10.1038/sj.onc.1209582. [DOI] [PubMed] [Google Scholar]
- Chalkiadaki G, Nikitovic D, Berdiaki A, Sifaki M, Krasagakis K, Katonis P, Karamanos NK, Tzanakakis GN. Fibroblast growth factor-2 modulates melanoma adhesion and migration through a syndecan-4-dependent mechanism. Int J Biochem Cell Biol. 2009;41:1323–1331. doi: 10.1016/j.biocel.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhuo W, Wang Y, Ao X, An J. Down-regulation of layilin, a novel hyaluronan receptor, via RNA interference, inhibits invasion and lymphatic metastasis of human lung A549 cells. Biotechnol Appl Biochem. 2008;50:89–96. doi: 10.1042/BA20070138. [DOI] [PubMed] [Google Scholar]
- Choi S, Kim Y, Park H, Han IO, Chung E, Lee SY, Kim YB, Lee JW, Oh ES, Yi JY. Syndecan-2 overexpression regulates adhesion and migration through cooperation with integrin alpha2. Biochem Biophys Res Commun. 2009;384:231–235. doi: 10.1016/j.bbrc.2009.04.093. [DOI] [PubMed] [Google Scholar]
- Cooke ME, Sakai T, Mosher DF. Contraction of collagen matrices mediated by alpha2beta1A and alpha(v)beta3 integrins. J Cell Sci. 2000;113:2375–2383. doi: 10.1242/jcs.113.13.2375. [DOI] [PubMed] [Google Scholar]
- Curat CA, Eck M, Dervillez X, Vogel WF. Mapping of epitopes in discoidin domain receptor 1 critical for collagen binding. J Biol Chem. 2001;276:45952–45958. doi: 10.1074/jbc.M104360200. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Bourdon MA, Reisfeld RA, Strongin A. Remodeling of collagen matrix by human tumor cells requires activation and cell surface association of matrix metalloproteinase-2. Cancer Res. 1998;58:3743–3750. [PubMed] [Google Scholar]
- DiMilla PA, Barbee K, Lauffenburger DA. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991;60:15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entschladen F, Niggemann B, Zanker KS, Friedl P. Differential requirement of protein tyrosine kinases and protein kinase C in the regulation of T cell locomotion in three-dimensional collagen matrices. J Immunol. 1997;159:3203–3210. [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Friedl P, Brocker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K (2009) Plasticity of cell migration modes—a multi-scale tuning model. J Cell Biol (in press) [DOI] [PMC free article] [PubMed]
- Friedl P, Maaser K, Klein CE, Niggemann B, Krohne G, Zanker KS. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res. 1997;57:2061–2070. [PubMed] [Google Scholar]
- Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Friedl P, Borgmann S, Brocker EB. Amoeboid leukocyte crawling through extracellular matrix: lessons from the Dictyostelium paradigm of cell movement. J Leukoc Biol. 2001;70:491–509. [PubMed] [Google Scholar]
- Friedl P, Hegerfeldt Y, Tusch M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol. 2004;48:441–449. doi: 10.1387/ijdb.041821pf. [DOI] [PubMed] [Google Scholar]
- Goebeler M, Kaufmann D, Brocker EB, Klein CE. Migration of highly aggressive melanoma cells on hyaluronic acid is associated with functional changes, increased turnover and shedding of CD44 receptors. J Cell Sci. 1996;109:1957–1964. doi: 10.1242/jcs.109.7.1957. [DOI] [PubMed] [Google Scholar]
- Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast mechanics in three-dimensional collagen matrices. J Bodyw Mov Ther. 2008;12:191–193. doi: 10.1016/j.jbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F (2009) Cell migration and signaling in three-dimensional matrix. Annu Rev Cell Dev Biol (in press)
- Hegerfeldt Y, Tusch M, Brocker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–2130. [PubMed] [Google Scholar]
- Heino J, Kapyla J. Cellular receptors of extracellular matrix molecules. Curr Pharm Des. 2009;15:1309–1317. doi: 10.2174/138161209787846720. [DOI] [PubMed] [Google Scholar]
- Hienola A, Tumova S, Kulesskiy E, Rauvala H. N-syndecan deficiency impairs neural migration in brain. J Cell Biol. 2006;174:569–580. doi: 10.1083/jcb.200602043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90:1147–1149. doi: 10.1161/01.res.0000022166.74073.f8. [DOI] [PubMed] [Google Scholar]
- Humphries MJ. Integrin structure. Biochem Soc Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- Humphries MJ, McEwan PA, Barton SJ, Buckley PA, Bella J, Mould AP. Integrin structure: heady advances in ligand binding, but activation still makes the knees wobble. Trends Biochem Sci. 2003;28:313–320. doi: 10.1016/s0968-0004(03)00112-9. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner PB, Parsons JT. FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J Cell Sci. 2008;121:895–905. doi: 10.1242/jcs.020941. [DOI] [PubMed] [Google Scholar]
- Jacobson K, O'Dell D, Holifield B, Murphy TL, August JT. Redistribution of a major cell surface glycoprotein during cell movement. J Cell Biol. 1984;99:1613–1623. doi: 10.1083/jcb.99.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamohara H, Yamashiro S, Galligan C, Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. FASEB J. 2001;15:2724–2726. doi: 10.1096/fj.01-0359fje. [DOI] [PubMed] [Google Scholar]
- Khotskaya YB, Dai Y, Ritchie JP, Macleod V, Yang Y, Zinn K, Sanderson RD. Syndecan-1 is required for robust growth, vascularization and metastasis of myeloma tumors in vivo. J Biol Chem. 2009;284:26085–26095. doi: 10.1074/jbc.M109.018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo DH, McFadden C, Huang Y, Abdulhussein R, Friese-Hamim M, Vogel WF. Pinpointing phosphotyrosine-dependent interactions downstream of the collagen receptor DDR1. FEBS Lett. 2006;580:15–22. doi: 10.1016/j.febslet.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Sixt M. Mechanical modes of “amoeboid” cell migration. Curr Opin Cell Biol. 2009;21:636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park H, Chung H, Choi S, Kim Y, Yoo H, Kim TY, Hann HJ, Seong I, Kim J, Kang KG, Han IO, Oh ES. Syndecan-2 regulates the migratory potential of melanoma cells. J Biol Chem. 2009;284:27167–27175. doi: 10.1074/jbc.M109.034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg JW, Lewis CA, Parsons M, Ng T, Isacke CM. A novel PKC- regulated mechanism controls CD44 ezrin association and directional cell motility. Nat Cell Biol. 2002;4:399–407. doi: 10.1038/ncb797. [DOI] [PubMed] [Google Scholar]
- Maaser K, Wolf K, Klein CE, Niggemann B, Zanker KS, Brocker EB, Friedl P. Functional hierarchy of simultaneously expressed adhesion receptors: integrin alpha2beta1 but not CD44 mediates MV3 melanoma cell migration and matrix reorganization within three-dimensional hyaluronan-containing collagen matrices. Mol Biol Cell. 1999;10:3067–3079. doi: 10.1091/mbc.10.10.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood KS, Valenick LV, Hsia HC, Schwarzbauer JE. Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol Biol Cell. 2004;15:5670–5677. doi: 10.1091/mbc.E04-08-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood KS, Mao Y, Hsia HC, Valenick LV, Schwarzbauer JE. Modulation of cell-fibronectin matrix interactions during tissue repair. J Invest Dermatol Symp Proc. 2006;11:73–78. doi: 10.1038/sj.jidsymp.5650005. [DOI] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- Nagano O, Murakami D, Hartmann D, De Strooper B, Saftig P, Iwatsubo T, Nakajima M, Shinohara M, Saya H. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol. 2004;165:893–902. doi: 10.1083/jcb.200310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997;71:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- Naor D, Nedvetzki S, Walmsley M, Yayon A, Turley EA, Golan I, Caspi D, Sebban LE, Zick Y, Garin T, Karussis D, Assayag-Asherie N, Raz I, Weiss L, Slavin S, Golan I. CD44 involvement in autoimmune inflammations: the lesson to be learned from CD44-targeting by antibody or from knockout mice. Ann N Y Acad Sci. 2007;1110:233–247. doi: 10.1196/annals.1423.025. [DOI] [PubMed] [Google Scholar]
- Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouhtit A, Abd Elmageed ZY, Abdraboh ME, Lioe TF, Raj MH. In vivo evidence for the role of CD44s in promoting breast cancer metastasis to the liver. Am J Pathol. 2007;171:2033–2039. doi: 10.2353/ajpath.2007.070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Paluch E, van der Gucht J, Sykes C. Cracking up: symmetry breaking in cellular systems. J Cell Biol. 2006;175:687–692. doi: 10.1083/jcb.200607159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kim Y, Lim Y, Han I, Oh ES. Syndecan-2 mediates adhesion and proliferation of colon carcinoma cells. J Biol Chem. 2002;277:29730–29736. doi: 10.1074/jbc.M202435200. [DOI] [PubMed] [Google Scholar]
- Peterson RM, Yu Q, Stamenkovic I, Toole BP. Perturbation of hyaluronan interactions by soluble CD44 inhibits growth of murine mammary carcinoma cells in ascites. Am J Pathol. 2000;156:2159–2167. doi: 10.1016/S0002-9440(10)65086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163:4917–4923. [PubMed] [Google Scholar]
- Rabinovitz I, Mercurio AM. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neurooncol. 2006;76:239–248. doi: 10.1007/s11060-005-6874-1. [DOI] [PubMed] [Google Scholar]
- Rilla K, Tiihonen R, Kultti A, Tammi M, Tammi R. Pericellular hyaluronan coat visualized in live cells with a fluorescent probe is scaffolded by plasma membrane protrusions. J Histochem Cytochem. 2008;56:901–910. doi: 10.1369/jhc.2008.951665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180:1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton PA, Bourguignon LY. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp Cell Res. 2004;295:102–118. doi: 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe KK, Ellis LM, Saya H. Expression of CD44R1 adhesion molecule in colon carcinomas and metastases. Lancet. 1993;341:725–726. doi: 10.1016/0140-6736(93)90490-8. [DOI] [PubMed] [Google Scholar]
- Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- Tkachenko E, Elfenbein A, Tirziu D, Simons M. Syndecan-4 clustering induces cell migration in a PDZ-dependent manner. Circ Res. 2006;98:1398–1404. doi: 10.1161/01.RES.0000225283.71490.5a. [DOI] [PubMed] [Google Scholar]
- Trepat X, Wasserman MR. Physical forces during collective cell migration. Nat Physics. 2009;5:426–430. [Google Scholar]
- van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. J Cell Sci. 2009;18:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB J. 1999;13(Suppl):S77–S82. doi: 10.1096/fasebj.13.9001.s77. [DOI] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal. 2006;18:1108–1116. doi: 10.1016/j.cellsig.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Roderburg C, Saffrich R, Diehlmann A, Eckstein V, Ho AD. Adhesion of human hematopoietic progenitor cells to mesenchymal stromal cells involves CD44. Cells Tissues Organs. 2008;188:160–169. doi: 10.1159/000112821. [DOI] [PubMed] [Google Scholar]
- Wallach-Dayan SB, Grabovsky V, Moll J, Sleeman J, Herrlich P, Alon R, Naor D. CD44-dependent lymphoma cell dissemination: a cell surface CD44 variant, rather than standard CD44, supports in vitro lymphoma cell rolling on hyaluronic acid substrate and its in vivo accumulation in the peripheral lymph nodes. J Cell Sci. 2001;114:3463–3477. doi: 10.1242/jcs.114.19.3463. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Wong G, de Heer AM, Xia W, Bourguignon LY. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope. 2009;119:1518–1530. doi: 10.1002/lary.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin Exp Metastasis. 2009;26:289–298. doi: 10.1007/s10585-008-9190-2. [DOI] [PubMed] [Google Scholar]
- Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Zhang L, Yabe T, Kuberan B, Beeler DL, Love A, Rosenberg RD. The involvement of heparan sulfate (HS) in FGF1/HS/FGFR1 signaling complex. J Biol Chem. 2003;278:17121–17129. doi: 10.1074/jbc.M212590200. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Matsuyama W, Kamohara H. Discoidin domain receptor 1: a new class of receptor regulating leukocyte-collagen interaction. Immunol Res. 2005;31:219–230. doi: 10.1385/IR:31:3:219. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci USA. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- Zimmerman E, Geiger B, Addadi L. Initial stages of cell-matrix adhesion can be mediated and modulated by cell-surface hyaluronan. Biophys J. 2002;82:1848–1857. doi: 10.1016/S0006-3495(02)75535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


