Abstract
High altitude and the decreased environmental oxygen pressure have both immediate and chronic effects on the carotid body. An immediate effect is to limit the oxygen available for mitochondrial oxidative phosphorylation, and this leads to increased activity on the afferent nerves leading to the brain. In the isolated carotid body preparation, the afferent nerve activity depends on the ratio of carbon monoxide (CO), an inhibitor of respiratory chain function, to oxygen. The CO-induced increase in afferent neural activity is reversed by light, and the wavelength dependence of this reversal shows that the site of CO (and therefore oxygen) interaction is cytochrome a3 of the mitochondrial respiratory chain. Thus, primary sensing of ambient oxygen pressure is through the oxygen dependence of mitochondrial oxidative phosphorylation. The conductance of ion channels in the cellular membranes may also be sensitive to oxygen pressure and, through this, modulate the sensitivity to oxygen pressure. Longer-term exposure to high altitude results in progressive changes in the carotid body that involve several mechanisms, including cellular energy metabolism and hypoxia inducible factor-1α (HIF-1α). These changes begin within minutes of exposure, but progress such that chronic exposure results in morphological and biochemical alterations in the carotid body, including enlarged cells, increased catecholamine levels, altered cellular appearance, and others. In the chronically adapted carotid body, responses to acute changes in oxygen pressure are enhanced. The adaptive changes due to chronic hypoxia are largely reversed upon return to lower altitudes.
Keywords: carotid body, hypoxia, mitochondria, cytochrome oxidase, oxygen sensor, adaptation to hypoxia
INTRODUCTION
Carotid body is a tiny organ (about 1 mg in a cat) situated bilaterally at the bifurcation of carotid arteries, close to the heart, and drawing its blood supply from the carotid artery. This allows it to sense the PO2 in the arterial blood on the way to the brain. The information on the arterial PO2 is sent to the brain via the carotid sinus nerve (CSN). The brain then uses this input to regulate the efferent neural activity that controls breathing and cardiovascular function. Decreased oxygen pressures in the arterial blood affect the carotid body both acutely (seconds) and chronically (hours and months). The acute responses occur very rapidly and are fully reversible; they play a critical role in maintaining the appropriate oxygen delivery to tissue. The chronic effects, however, not only occur over much longer periods of time, but also involve a variety of adaptive mechanisms that may or may not result in improved oxygen delivery to tissue.
The carotid body contains type I cells (also known as glomus cells), and these are the only cells with synapses linking them to the afferent nerves in the CSN. The glomus cells are believed to be responsible for oxygen sensing under normal conditions and therefore for the rapid reflex responses to alterations in PO2. Over the years, studies have resulted in a wide range of proposals for the identity of the oxygen sensor. These proposals included that the sensor is mitochondrial cytochrome oxidase (Mulligan et al., 1981; Wilson et al., 1994), an oxygen-sensitive K+ channel (López-Barneo et al., 1998; 2001), NADPH oxidase (Acker, 1994), or other entity (Eyzaguirre and Koyano, 1965a, b, c; Streller et al., 2002). It is not the purpose of the present paper to comprehensively review the extensive literature on the subject. Rather, we will provide what is, in our view, evidence on the identity of primary (acute) oxygen sensor and describe some of the adaptive mechanisms that determine the effect of chronic oxygen deficiency on carotid body and respiratory physiology.
IMMEDIATE RESPONSES TO ALTERATIONS IN PO2
Relationship of energy metabolism to function in the carotid body
The carotid body is composed of highly aerobic tissue that depends primarily on mitochondrial oxidative phosphorylation for energy (ATP). Synthesis of ATP by mitochondria is tightly coupled to oxygen consumption; that is, the mitochondria respire only fast enough to replenish ATP as it is used. Decreased availability of oxygen first affects the ability of the mitochondria to maintain the [ATP]/[ADP][Pi] ratio, a measure of the energy available when the ATP is hydrolized to ADP and inorganic phosphate (Pi). Respiration is stimulated by decreasing the [ATP]/[ADP][Pi] ratio and, as a result, the rate of respiration does not decrease until the oxygen deprivation is so severe that the capacity of the control system is exhausted. For simplicity, we will discuss in the following only two limiting cases: (1) when the supply of oxygen is completely shut off and then (2) when the level of oxygen continues, but at a decreased level.
Acute, complete loss of oxygen supply
The level of ATP in neural cells is buffered by creatine phosphate through the creatine phosphokinase reaction. Cells use ATP at rates that are sufficient to consume most of the ATP in the cell in a few seconds. In the isolated, beating rat heart, for example, the total content of ATP and creatine phosphate is 14 µmol/g wet weight, whereas oxygen consumption is 11 to 15 µmol O2/min/g wet weight (e.g., see Nuutinen et al., 1983). Assuming 6 ATP are synthesized per O2, the rate of ATP consumption is 66 to 90 µmol/min/g wet weight. Thus, approximately 10% of the ATP + creatine phosphate in the cardiac myocytes is used and resynthesized each second. In highly aerobic organs, such as the brain and heart, complete and instantaneous loss of oxygen would cause substantial alteration of metabolism within a second. In brain, loss of function would begin within 3 to 5 sec and complete loss of function, accompanied by massive release of ions and neurotransmitters, would occur within 10 to 15 sec (note that, for most physiological conditions, oxyhemoglobin in the blood would buffer oxygen depletion).
Oxygen is available but at a below normal pressure (high altitude)
In the brain, the ATP is about 3 mmol/L and is buffered by creatine phosphate (approximately 5 mmol/L) and creatine (approximately 5 mmol/L), whereas the concentrations of free ADP and Pi are less than 0.05 mmol/L and 0.8 mmol/L, respectively. Decrease in oxygen pressure causes a decrease in the energy available for synthesis of ATP and, as a result, the rate of ATP synthesis is decreased. The reactions using ATP, however, do not depend on oxygen pressure, and ATP utilization continues unchanged. Initially, the rate of synthesis falls below the rate of utilization and the ATP concentration falls, increasing the concentrations of ADP and Pi (decreasing the [ATP]/[ADP][Pi]). This process continues, but the decrease in the energy state stimulates the rate of ATP synthesis (rate of respiration) until it again equals the rate of utilization. A new steady state, but at a lower [ATP]/[ADP][Pi], is established. The decrease in ATP levels is typically small, because a decrease of 5% in the ATP level (from 3 to 2.85 mmol/L) would increase the concentration of ADP by at least fourfold, from 0.05 to 0.20 mmol/L. Equally importantly, the creatine phosphokinase reaction is near equilibrium:
and the equilibrium constant K is approximately 40. Thus the creatine phosphate would decrease from 5 to 2.5 mmol/L and the creatine increase from 5 to 7.5 mmol/L. More importantly, inorganic phosphate would rise from 0.8 to 3.3 mmol/L. Thus a decrease of only 5% in ATP would result in the ([ATP]/ [ADP] [Pi]) decreasing by a factor of 17. This is sufficient to increase the respiratory rate of the mitochondria at constant high oxygen pressure by 10- to 20-fold, even if the intramitochondrial [NADH]/[NAD+] ratio remained unchanged. Most available data indicate the intracellular Ca2+ in the glomus cells increases with decreasing oxygen pressure. Many mitochondrial dehydrogenases are activated by Ca2+, and this would likely increase the [NADH]/[NAD+] and further enhance the potential increase in respiratory capacity. Thus, the mitochondrial respiratory capacity can be fully turned on before there is a substantial decrease in cellular [ATP]. There would also be large changes in other metabolic regulators, such as adenosine monophosphate (AMP). Through equilibration of the adenylate kinase reaction (2 ADP = ATP + AMP), the 5% decrease in [ATP] described above would result in a much larger (fourfold) increase in [ADP], while the increase in [AMP] would be 16-fold. In the brain, cellular and metabolic heterogeneity, combined with the difficulty in obtaining sufficiently accurate analysis, has made correlation of neuronal function and metabolite levels highly problematic.
Inhibitors of mitochondrial function have been known for decades to transiently stimulate afferent activity of the carotid body (Anichkov and Belen’kii, 1963), and many studies have examined their effect on both isolated carotid body preparations and glomus cells. Among the compounds causing transient increase in afferent activity are respiratory chain inhibitors such as rotenone, myxothiazol, antimycin A, and cyanide; inhibitors of energy coupling such as oligomycin; and uncouplers of oxidative phosphorylation such as dinitrophenol (DNP) and trifluoromethyl carbonylcyanide of phenylhydrazone (FCCP). Each of these agents can cause transient increase in afferent activity in carotid body preparations and significant increase in intra-cellular [Ca2+] in glomus cells. In thin slices of the carotid body, catecholamine release increases with decreasing glucose concentration (López-Barneo and Pardal, 2003). Thus, deficiency in substrate for the citric acid cycle, and thereby for oxidative phosphorylation, also mimics hypoxia. High levels of mitochondrial inhibitors abolish the oxygen chemosensory response in the carotid body (Lahiri, 2004; Mulligan et al., 1981). It should be noted, however, that the carotid body afferent activity can still, at least transiently, be stimulated by changes in CO2/H+ (Lahiri, 2004; Mulligan et al., 1981). Inhibitors and uncouplers can have effects in addition to those on oxidative phosphorylation, however, and inhibiting oxidative phosphorylation causes extensive alterations in cell metabolism and biology. Taken together, the inhibitor-uncoupler studies infer that oxygen sensing is through oxidative phosphorylation. Variability among the observed responses and uncertainty about the mechanism of action, however, make these studies less than conclusive.
Ion channels and oxygen sensing in the carotid body
Historically, there have been two main hypotheses concerning the oxygen sensor responsible for the rapid response. One is that the oxygen sensor is mitochondrial cytochrome oxidase, and this, through its effects on oxidative phosphorylation, transmits the information to the rest of the cell (discussed above). The other was that glomus cells have an oxygen-sensitive K+ channel for which conductance is lowered with decreasing oxygen pressure. The calcium-sensitive K+ channels (BK) of type I cells have been reported to be suppressed by hypoxia (PO2 < 30 torr) (Peers, 1990; Tang et al., 2003). This suppression is followed by cell depolarization, influx of Ca2+ through voltage-gated channels, and cellular Ca2+ rise, triggering neurotransmitters release. A similar effect can be induced by iron chelation in normoxia (PO2 > 100 torr) (Roy et al., 2004). The mechanism by which BK+ channel conductance is suppressed by hypoxia is still being investigated. It has been suggested that BK+ channels interact with heme-related compounds that are attached to the membrane (Tang et al., 2003; Williams et al., 2004). Williams et al. (2004) has suggested BK+ channel inhibition to heme oxygenase (HO-2). HO is a membrane-bound enzyme that oxidatively breaks down heme to CO, biliverdin, and iron. CO was previously shown to stimulate opening of BK channels (Wang and Wu, 1997), and it has been suggested that, in the presence of O2, HO-2 breaks down heme, generating CO and keeping the BK channel open. Lowering the oxygen pressure would then suppress the CO levels and thereby channel conductance. Hoshi and Lahiri (2004) concurred with Williams et al. (2004), but there remain many uncertainties (see Fig. 1 of Hoshi and Lahiri, 2004). There is also controversy with respect to K+ channel properties. For example, TEAS and 4-AP have been reported to mimic hypoxia by some workers (Lopez-Barneo et al., 1998), but not by others (Doyle and Donnelley, 1994; Lahiri et al., 1998). Although mitochondrial cytochrome oxidase is the primary oxygen sensor (see below), it is possible that oxygen-dependent ion channels help to modulate the sensitivity to changes in oxygen pressure.
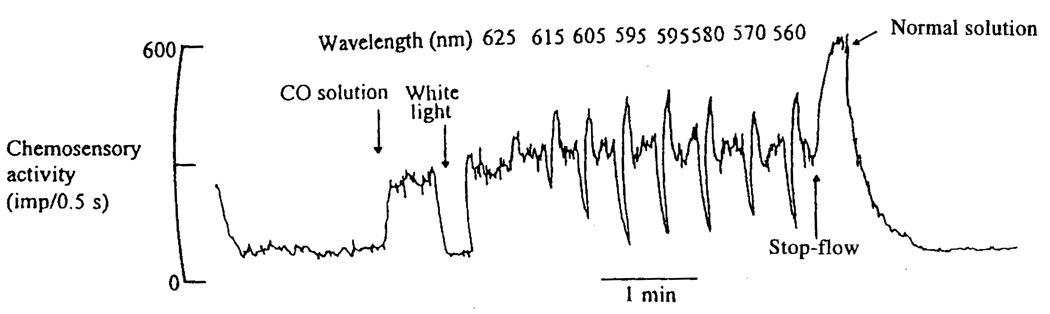
FIG. 1.
Reversal of the CO-induced increase in the oxygen-dependent sinus nerve activity of the isolated perfused-superfused carotid body of the cat by light. The carotid body is placed in a small chamber in a temperature-controlled water bath and superfused with Tyrode solution equilibrated with a gas phase containing the nitrogen (estimated O2, 10 to 20 mmHg). The carotid artery is perfused with Tyrode solution equilibrated with either a gas mixture containing % argon or one containing the same oxygen pressure, but consisting of air and CO. The two electrodes measure afferent electrical pulses by the differential amplification of the measured potential, resulting in an electrical spike as each impulse passes down the fiber. After an initial period of perfusion (38°C) with medium equilibrated with air, the perfusate was changed to one equilibrated to gas mixture containing O2: CO at 130 and 560 mmHg, respectively (CO solution). The CO-induced increase in neural activity was completely eliminated by illuminating the carotid body with a bright white light, but only while the light was on. The carotid body was then illuminated for 6-sec intervals with monochromatic light separated by a recovery period. The wavelength of the monochromatic light was varied from 625 to 560 nm to show the wavelength dependence of the reversal (taken with modification from Wilson et al., 1994).
Carbon monoxide and oxygen sensing by the carotid body
The kind of experiments that can be carried out on the carotid body in vivo are limited by systemic responses and the presence of blood. In vivo, CO binds to hemoglobin and suppresses oxygen delivery, and this obscures other effects on the sensory mechanism. An important development was an isolated preparation that included the afferent neurons and maintained its chemosensory responses when perfused–superfused with hemoglobin-free media (Iturriaga et al., 1991). When such a hemoglobin-free preparation was developed, it became possible to study the effects of agents such as CO.
When the isolated perfused–superfused carotid body preparation is treated with media containing O2/CO mixtures, the afferent activity depends on the O2/CO ratio. This indicates that CO and O2 compete for the oxygen binding site, and the affinity of the sensor for oxygen progressively decreases as the CO is increased. The measured sensory function (PO2 sensed) is a function of the actual oxygen pressure (PO2), the inhibitor concentration [CO], and the inhibitory constant (PCO):
The CO-induced increase in afferent activity can be fully reversed by illuminating the carotid body with a bright light (Fig. 1), indicating that absorption of light by the CO complex results in the CO being dissociated from its binding site.
The light-induced reversal of CO binding makes it possible to change the effective CO concentration in a highly controlled and rapid manner by just switching on or off a light source of an appropriate wavelength and intensity. The transition in afferent activity when a bright light (sufficient to effectively fully dissociate the CO) is turned on or off occurs with a half-time of 2 to 3 sec, consistent with the very rapid carotid body response in vivo. This half-response time is also consistent with the time required for oxidative phosphorylation to substantially change the cellular energy level.
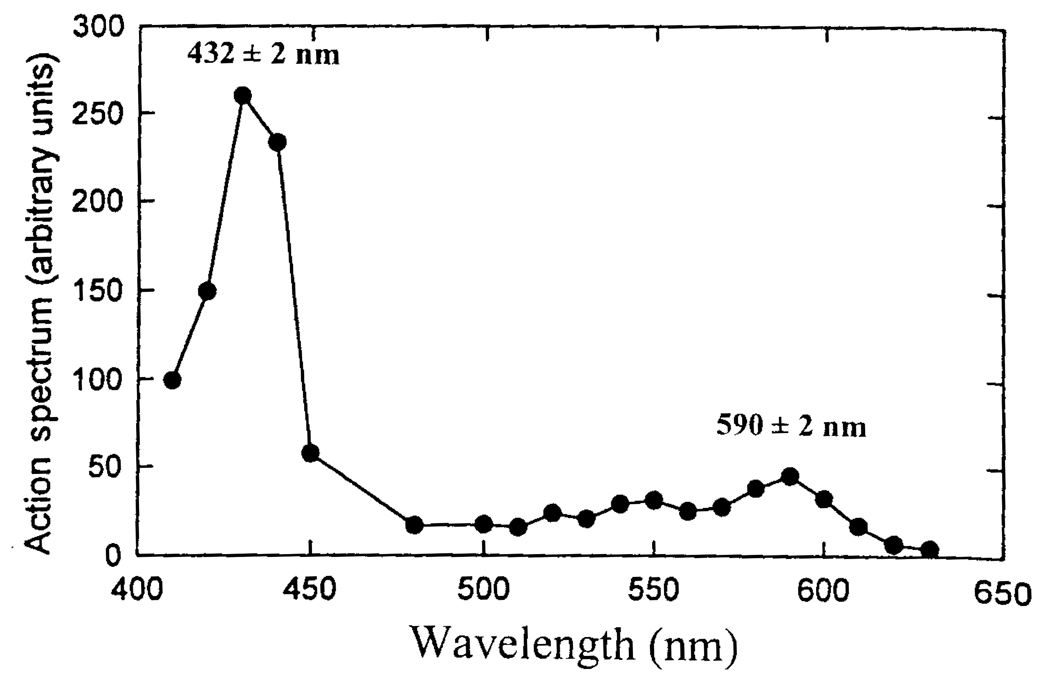
Reversible, light-induced photodissociation has been observed for many CO complexes of reduced hemes. Historically, this phenomenon has been used to identify the mitochondrial oxidase (cytochrome a3) (Keilin and Hartree, 1939; Kubowitz and Haas, 1932; Melnick, 1941, 1942; Warburg, 1926, 1927; Warburg and Neglein, 1928a, b, 1929), other oxidases in a wide range of microbes and plants (see Castor and Chance, 1955, 1959; Keilin, 1966), and mixed function oxidases (Cooper et al., 1970; Estabrook, 1963). When monochromatic light is used to reverse CO binding, the efficiency of the reversal is a direct measure of the absorption of the CO complex at that wavelength (see Fig. 1). This is because only photons specifically absorbed by the heme-CO complex can provide the energy for breaking the CO-iron bond and cause dissociation of the CO. The absorption spectrum of the heme-CO complex can be obtained by determining the light-induced change as a function of wavelength, using light intensities that give only partial saturating effect (Wilson et al., 1994). When normalized to the same light intensity for all wavelengths, a plot of the light-induced reversal against wavelength (action spectrum; see Fig. 2) is the absorption spectrum of the heme-CO complex. Light that is absorbed by other pigments or scattered away does not introduce energy into the heme-CO complex and therefore cannot cause dissociation of the CO or contribute to the action spectrum. In the carotid body preparation, the action spectrum was measured by the changes in afferent neural activity. The action spectrum for the light-induced reversal of the CO-induced increase in afferent nerve activity of the carotid body is that of the CO complex of reduced cytochrome a3, the terminal oxidase of the mitochondrial respiratory chain (Wilson et al., 1994). Thus, cytochrome a3 of the mitochondrial respiratory chain is the oxygen sensor responsible for the rapid alteration in afferent neural activity when the oxygen pressure changes.
FIG. 2.
The wavelength dependence of the light-induced reversal of the CO effect after normalizing the responses to that for the same light intensity at all the different wavelengths (taken from Wilson et al., 1994, with modification).
Relationship between the photochemical action spectrum and absorption spectra of the cytochromes in vivo
Lahiri and Acker (1999) reported that, although light reversed the effect of CO on the afferent activity, the absorption spectra did not show dissociation of a substantial fraction of the cytochrome a3-CO complex. This is consistent with the properties of cytochrome a3 of the respiratory chain. The action spectrum reported by Warburg for "atmungsferment" (see Fig. 3) was early recognized by Keilin and Hartree to be that of the reduced cytochrome a3-CO complex (Keilin, 1966; Keilin and Hartree, 1939). They noted, however, that even light intensities much higher than necessary to fully reverse inhibition of oxygen consumption by CO did not significantly diminish the absorption band of the cytochrome a3-CO complex. This apparent discrepancy arises because the unliganded, reduced, cytochrome a3 produced by dissociation of the CO by light can react with either O2 or CO. The "on" rate constant for the reaction with O2 is 4 × 108 mol−1 sec−1, while that for CO is 8 × 104 mole−1 sec−1 (Erecinska and Wilson, 1980), and most of the cytochrome a3 from which CO is photodissociated reacts with O2 and not with CO. In addition, the maximal activity of the cytochrome oxidase (turnover number of more than 5000 sec−1) is also much higher than the overall respiratory chain activity (about 5 sec−1). As a result, mitochondrial respiration can be restored by photodissociation of only a very small fraction of the total cytochrome a3—CO complex.
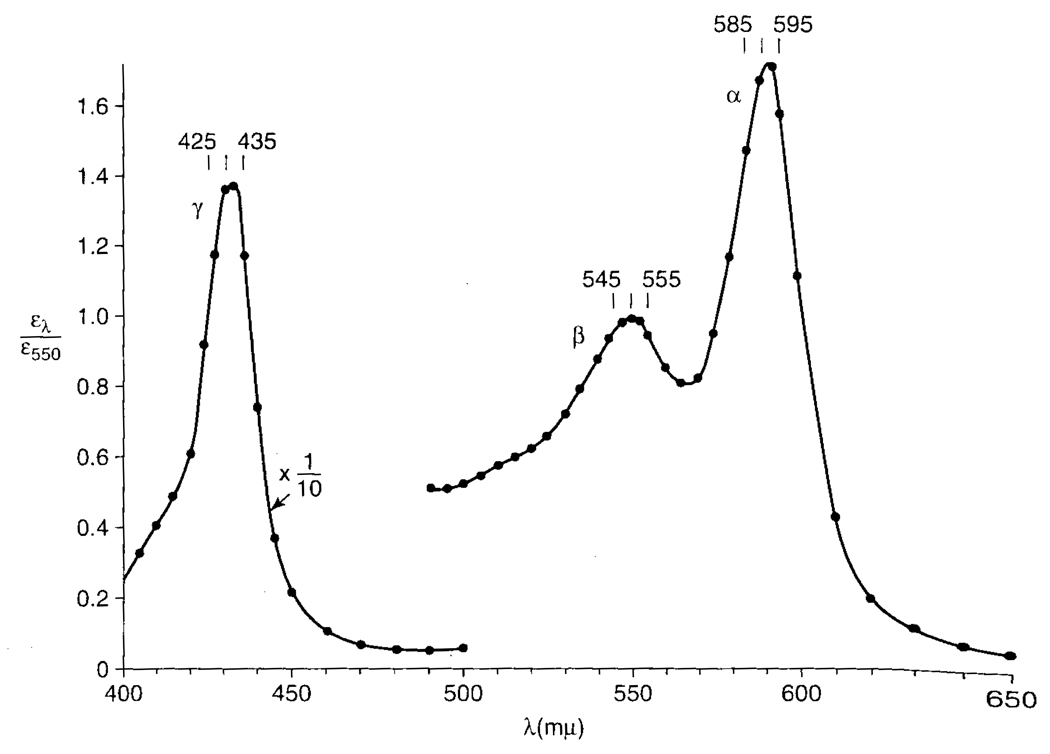
FIG. 3.
The wavelength dependence of the light-induced reversal of the inhibition of yeast respiration by carbon monoxide as measured by the rate of oxygen consumption. This photochemical action spectrum was taken from Castor and Chance (1955), with the light response presented relative to that at 550 nm. This spectrum is similar to those reported by Kubowitz and Haas (1932) and others.
It is instructive to note that the CO concentrations used are about 1 mmol/L, about 400 times the Kd. Thus, in the dark there are about 400 reduced cytochrome a3-CO complexes for every free reduced cytochrome a3 available to react with oxygen. At each oxygen pressure, respiration occurs at the rate necessary to make the ATP as fast as it is used. The energy state (relative to no CO at the same oxygen pressure) is decreased as necessary to provide the appropriate concentration of reduced cytochrome a3. When the light is turned on, each reduced cytochrome a3 produced by photodissociation of the reduced cytochrome a3–CO complex reacts with oxygen, is oxidized, and then re-reduced by the respiratory chain. Light intense sufficiently to photodissociate 0.25% of the cytochrome a3-CO complex instantaneously doubles the concentration of reduced cytochrome a3 and thereby doubles the rate of respiration (and of ATP synthesis). Since the rate of ATP synthesis in the light initially exceeds its rate of utilization, the cellular energy state rises, progressively inhibiting respiration until the rates of ATP synthesis and utilization are again equal and a new steady state is achieved. When the light is turned off, the process is reversed.
Oxygen dependence of mitochondrial oxidative phosphorylation in vivo
For cytochrome a3 to act as the oxygen sensor for rapid detection of altered oxygen pressure, mitochondrial oxidative phosphorylation must be sensitive to oxygen pressures available to the cells in vivo. Blood flowing through the carotid body comes from the carotid artery, where the PO2 is typically 80 to 100 mmHg. The carotid body afferent activity rises significantly if the arterial oxygen pressure falls to 60 mmHg. The oxygen pressure at the mitochondria is much lower, however, because as the blood enters the carotid body the oxygen pressure falls rapidly due to extraction by the cells. By the time it reaches the microvessels, the average oxygen pressures are about 50 mmHg (Lahiri et al., 1993a, b; Rumsey et al., 1991), and the minimal values should be near 20 mmHg, assuming the distributions are similar to those for other tissues (see Ziemer et al., 2005). Diffusion from the blood in the microvessels to the mitochondria is expected to further decrease the oxygen pressure, but it is reasonable to expect the average oxygen pressure at the mitochondria to be about 40 mmHg, with minimum values of about 15 mmHg. Thus a significant portion of the mitochondria of the glomus cells is exposed to oxygen pressures of less than 20 mmHg when the arterial oxygen pressures are 80 mmHg.
Mitochondrial respiration has been reported to continue with an essentially unchanged rate to oxygen pressures of less than 1 mmHg, with P50 values generally reported to be <0.1 mmHg (e.g., see Wilson et al., 1988). The P50 is dependent on the metabolic energy state, however, (Wilson et al., 1979a, b; 1988), and when isolated mitochondria are maintained in media with a high [ATP]/[ADP][Pi], the measured P50 for oxygen consumption is higher (0.4 to 0.5 mmHg). When the oxygen dependence of respiration was measured in cell suspensions in which the oxygen depletion (from 10 to <1 mmHg) required only a few seconds, the P50 was significantly higher (1 to 2 mmHg in cardiac myocytes; Rumsey et al., 1990). These data show significant dependence in respiratory rate on oxygen pressures at 10 mmHg and below. In such experiments, a significant amount of time, minimally several seconds, is required for the oxygen pressure to decrease from 10 mmHg to essentially zero. The cellular energy state ([ATP]/[ADP][Pi]) and therefore the regulation of mitochondrial respiration begin to change within 1 sec of the change in oxygen pressure and counter the effect of decreased oxygen pressure. As a result, the oxygen dependence of oxidative phosphorylation on oxygen pressure can be better obtained by measuring the reduction of internal electron carriers of the respiratory chain, particularly cytochrome c. Cytochrome c is the electron donor for the mitochondrial oxidase, and its level of reduction reflects changes in the internal electron transfer reactions of the oxidase. For mitochondria suspended in media with a high [ATP]/[ADP][Pi] and or in suspensions of intact cells, significant cytochrome c reduction was observed as the oxygen pressure decreased to below 30 mmHg (Wilson et al., 1979a, b; Wilson and Erecinska, 1985). This shows that electron transfer and energy coupling in the cytochrome oxidase depend on oxygen pressure of up to at least 30 mmHg. This range of sensitivity is consistent with oxidative phosphorylation functioning as the primary oxygen sensor of the carotid body.
Comparison of oxygen sensing by the carotid body with glucose sensing by the pancreatic beta cells
There are many sensory systems in the body, and it is instructive to compare the chemosensory activity of the carotid body with glucose sensing by the pancreas. The pancreatic beta cells sense the concentration of glucose in the blood and use this signal to control secretion of insulin. The two sensory systems share many properties, although the sensors per se are different:
The sensor uses the sensed molecule as a metabolic substrate (glucokinase or cytochrome a3) and is an integral part of energy metabolism in the cell.
Increased levels of the sensed molecule (glucose or oxygen) increase production of ATP (through glycolysis and/or oxidative phosphorylation and the cellular energy level).
Ion channels in the cell membrane are controlled by metabolism through the change in energy level (ATP-dependent K+ channel in beta cells; not yet established in glomus cells, but could involve Ca2+-activated K+ channel).
Membrane potentials decrease and intracellular Ca2+ levels increase.
Exocytotic release of the appropriate response element (insulin or neurotransmitter) increases.
For the beta cells the released insulin passes into the blood, but in the carotid body, increased neurotransmitters at the neural synapses cause increased activity on the afferent neurons.
Identification of the primary chemosensor has required extensive investigation in both systems. Glucokinase was definitively identified as the glucose sensor when subclasses of diabetes were found that were caused by genetic alterations in the Km or/and Vmax of glucokinase (for reviews see Henquin et al., 2003; Zelent et al., 2005). This allowed quantitative correlation between specific changes in the kinetic properties of glucokinase and the alterations of blood glucose levels characteristic of the disease. Carbon monoxide provides a similar experimental tool for the carotid body. The affinity of the sensor for oxygen could be quantitatively changed, and these changes directly correlated with the resulting alterations in afferent neural activity. The light-induced reversal of the effect of CO and ability to determine the absorption spectrum of the CO complex has a specificity equivalent to the genetic information on the glucose sensor. The light-induced reversal of the CO effect can arise from only one molecular species, and this species has an absorption spectrum the same as the measured photochemical action spectrum.
Property 3 has significant differences in the mechanism by which the cellular membrane responds to the change in energy metabolism. In the pancreatic beta cells, the increase in vesicular release is in response to an increase in energy levels, whereas in the carotid body, it is in response to a decrease in energy level. Beta cells have an ATP-sensitive K+ channel that closes with increasing energy level, thereby decreasing K+ permeability and the membrane potential. In the carotid body, vesicular release of neurotransmitters increases with decreasing energy level, and this may be a normal response of neurons. In the striatum of the brain, for example, the levels of extracellular dopamine are reported to increase with decreasing oxygen pressure throughout the physiological range of oxygen pressures (Huang et al., 1994; Pastuszko et al., 1993). This suggests that neurotransmitter release-reuptake systems are intrinsically sensitive to the metabolic energy level within neurons.
CHRONIC HYPOXIA IN ADULT ANIMALS ENHANCES RESPONSES TO ACUTE HYPOXIA
Oxygen sensing and maturation in the carotid body
The carotid body chemoreceptors have minimal sensitivity to hypoxia at birth and become more sensitive to hypoxia over the first few days or weeks of life (Carroll et al., 1993). It is therefore surprising that some investigators have used glomus cells from neonatal rats (e.g., Buckler, 1997). It should also be noted that the observation that chronic hypoxia increases oxygen sensitivity is for the carotid body of adult animals (Lahiri et al., 1987; Nielson et al., 1988; Vizek et al., 1987).
Sensory response in adult animals
Decreasing oxygen pressure results in decreasing cellular energy state ([ATP]/[ADP][Pi]) and a wide range of other metabolic alterations in systems coupled to energy metabolism: It has been suggested that there is a decrease in the activity of large-conductance voltage-gated potassium (BK) channels (Peers, 1990), and this results in depolarization of the glomus cell membrane and opening of the voltage-gated Ca2+ channels. Influx of Ca2+ then results in an increase in intracellular [Ca2+]. Neurotransmitters are released and act on the afferent nerve endings that synapse with the glomus cells, generating the afferent neural discharge. The mechanism(s) responsible for membrane depolarization and the possible role of the reported oxygen sensitivity of the K+ channels in this process are not known. These oxygen-sensitive K+ channels are reported to be closed when the PO2 falls below 80 torr, whereas the oxygen-dependent increase in CSN discharge rates occurs at arterial PO2 <~ 60 torr (Lahiri, 1994). The enhanced sensory response suggests that neurotransmitter release is increased (Barnard et al., 1987; He et al., 2004; Neilson et al., 1988; Vizek et al., 1987).
Hypoxia-inducible factor
In the chronic state (more than 2 min) of hypoxia, the hypoxia-inducible factor-lα (HIF-1α) becomes elevated (Fig. 4). HIF-1α is continuously formed and degraded in the cytoplasm in normoxia. The HIF-1α combines with constitutively expressed HIF-1β to form HIF-1, which moves into the nucleus to induce gene transcription (Bunn and Poyton, 1996). HIF-1α is hydroxylated on proline residue by the enzyme prolyl hydroxylases prior to degradation. The prolyl hydroxylases have a relatively high Km for O2, and the hydroxylation is rate limiting for degradation under physiologic condition (Semenza, 2004). Decreased oxygen pressure can result in significant accumulation of HIF-1α in less than 2 min, and it is rapidly degraded again on reoxygenation (<1 min). In addition to O2, 2-oxogluterate, Fe2+, and ascorbic acid are required for hydroxylation of HIF-1α. Roy et al. (2004) reported that addition of Fe2+ chelators resulted in rapid accumulation of HIF-1α. Oxygen deficiency is reported to cause accumulation of HIF-1α, an increase that can be inhibited by respiratory chain inhibitors (Agani et al., 2000; Baby et al., 2005).
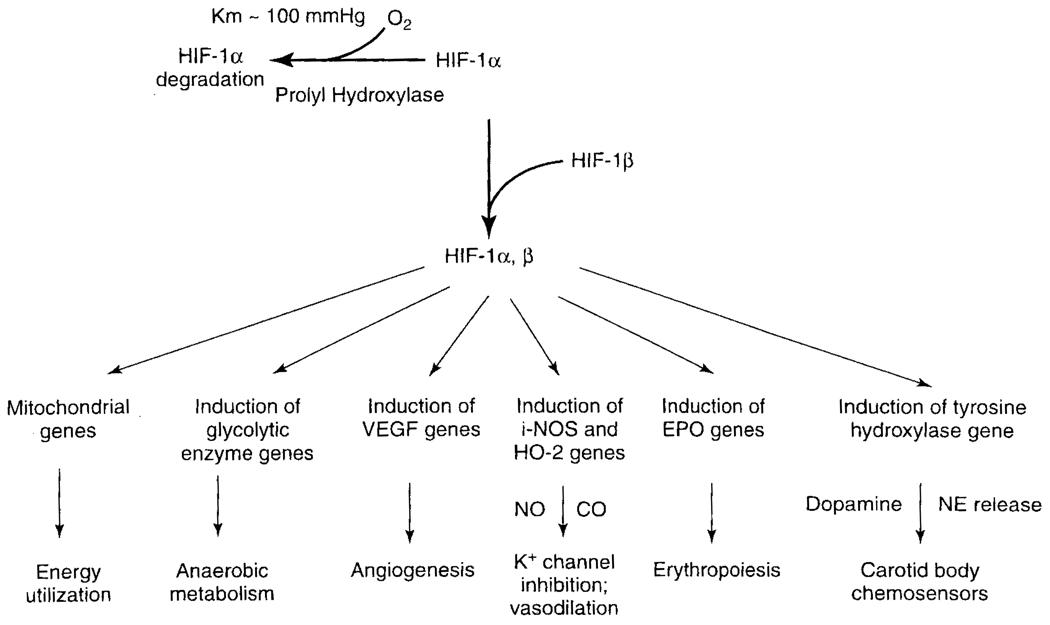
FIG. 4.
Schematic representation of the HIF-1 hypoxia response pathway (modified from Guillemin and Krasnow, 1997). When oxygen decreases, the rate of proline hydroxylation decreases, leading to accumulation of HIF-1α. This binds to HIF-1β to form an α, β heterodimer. The HIF-1 1α/β heterodimer binds to and activates expression of various genes, including those encoding glycolytic enzymes (for anaerobic metabolism), VEGF (for angiogenesis), inducible nitric oxide synthase and heme-oxygenase-2 (for NO and CO production and vasodilatation), EPO (for erythropoiesis), and possibly tyrosine hydroxylase (for dopamine production to influence carotid body chemoreception). The products of these genes help the cell survive at low oxygen and/or act to restore normal oxygen levels. Some gene targets of HIF-1 are induced in most hypoxic cells, while others, like EPO, are only induced in specific tissues and hence also require tissue-specific regulators. Mitochondrial inhibitors suppressed HIF-1α and show HIF-1α signaling is coupled to mitochondrial respiratory chain function. Coupling is present through the energy requirements for synthesis of HIF-1α, but it is likely there are other links as well.
Recently, Kline et al. (2002) reported that mice partially deficient in HIF-1α had a marked decrease in carotid body response to acute hypoxia, but that the in vivo ventilatory response was not compromised. Furthermore, wild-type mice exposed to hypoxia for 3 days manifested an augmented ventilatory response to a subsequent acute hypoxic challenge. In contrast, chronic hypoxia resulted in a diminished ventilatory response to acute hypoxia in the partially HIF-1α deficient mice. Thus partial HIF-1α deficiency has a significant effect on carotid body neural activity and ventilatory adaptation to chronic hypoxia.
Subjects born and raised at high altitude
Lahiri and Milledge (1965), working in the Himalayan high altitude, and Severinghaus et al. (1966), working in the South American altiplano, found that the adult natives showed a blunted ventilatory response to acute alteration of inspired PO2 both at rest and during exercise. The age at which this pulmonary adaptation occurred was not known; neither was it known whether it was acquired or genetic. This investigation was later extended (Lahiri et al., 1976). Recent work by Moore et al. (2000) has shown that Tibetans, compared to other high-altitude residents, demonstrate higher ventilation and hypoxic ventilatory response. The conclusion was that the responses to chronic hypoxia were determined by environmental rather than genetic factors. There remain conflicting reports as to the extent to which the response returns to normal during the years after high altitude dwellers move to sea level (Leon-Velarde et al., 2003).
Subsequently, the ventilatory response to acute hypoxia has been found to be blunted in animals reared in a hypoxic environment (Eden and Hanson, 1987) and for neonatal rats (9 to 14 days old) born and raised in a normobaric hypoxic chamber (10% O2) (Wyatt et al., 1995). The latter investigators also measured Ca2+ sensitive K+ currents in glomus cells from carotid bodies from the hypoxic and normoxic rats. The responses to hypoxia (PO2 12 to 20 mmHg) reported were (1) K+ current density was significantly lower in cells from hypoxic rats; (2) hypoxia caused similar, reversible inhibitions in cells from the two groups; that is, resting membrane potentials were similar; (3) charybdotoxin (20 nmol/L) inhibited the K+ current as expected in normal cells, but had no effect on that from hypoxic rats; and (4) acute hypoxia depolarized the normoxic cells, but was without effect on cells from hypoxic rats. It was concluded that the O2 sensing mechanism survived in the chronically hypoxic rats but there was a failure to depolarize in response to hypoxia. The failure to increase chemosensory discharge would explain the absence of an increase in rate of respiration. A similar conclusion was reached by Sterni et al. (1999) from measurements of the peak [Ca2+] response in glomus cells to acute hypoxia in rats maintained chronically hypoxic from birth to 11 days. The response, however, began to be restored upon return to normoxia. That is, the set point for the response returned to the normal (higher) level of PO2 In all these studies, the voltage-gated Ca2+ current density remained normal or increased (Hempelman, 1996; Sterni et al., 1999). More importantly, there was a dissociation between inhibition of O2-sensitive BK+ current and membrane depolarization, and this is surprising. The glomus cells from the rats that were hypoxic from birth are also insensitive to charybdotoxin. The lack of depolarization should have been accompanied by a lack of O2-sensitive inhibition of BK current.
Intermittent apnea at high altitude
Sleep at high altitude gives rise to central apnea and periodic breathing in newcomers. Briefly, at times during sleep breathing stops, resulting in a decrease in alveolar and arterial PO2 and increased CO2. Arterial chemoreceptor activity then stimulates the respiratory system and restarts breathing, restoring the arterial PO2 and removing the respiratory stimulus (e.g., Lahiri et al., 1983). This results in a pattern of periodic cessation of breathing, or apnea. Studies have been made of the effect of intermittent hypoxia on arterial chemoreceptor activity in animals. The results depend on the method used to produce intermittent hypoxia (Greenberg et al., 1999; Hui et al., 2003; Prabhakar, 2001). Peng and Prabhakar (2004) reported that arterial chemoreceptor activity was augmented after 3 days of intermittent hypoxia, while sustained hypoxia of similar duration depressed the arterial chemoreceptor activity. In contrast, Hui et al. (2003) found that the effects of sustained hypoxia were always greater than for intermittent hypoxia (see also Di Giulio et al., 2003; Lahiri et al., 2003).
Structural correlates of function in chronic hypoxia
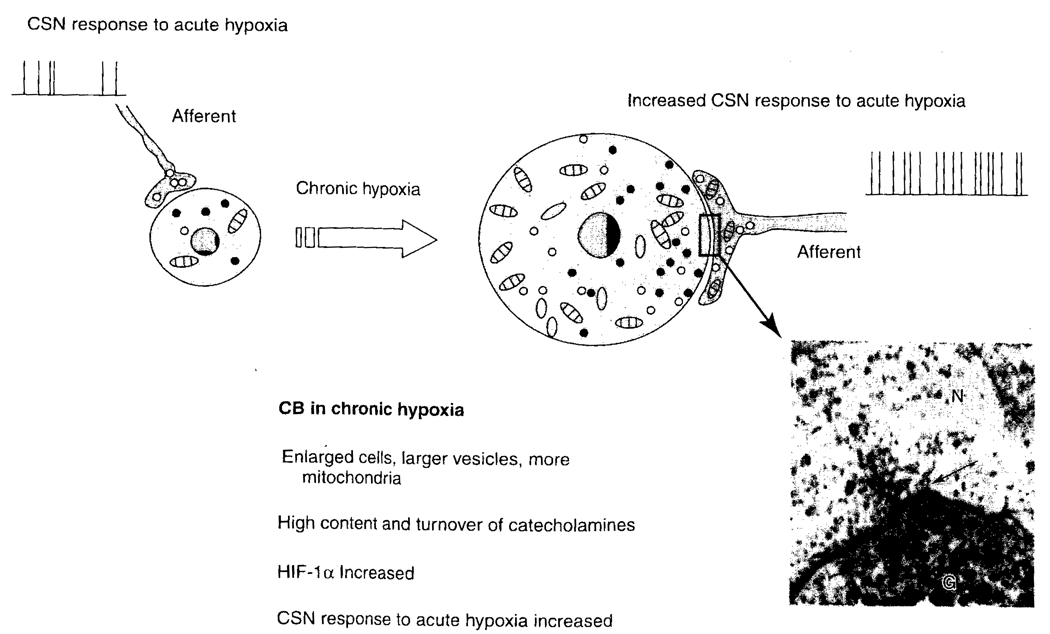
Chronically hypoxic rats have enlarged type I cells, depending on the degree of hypoxia (altitude). For example, these cells undergo 4× hypertrophy at 4880 m in 3 weeks time (McGregor et al., 1984). The vesicles become more numerous and the mitochondria increase in number, without increasing in size. Along the length of the synaptic junction, the clear-core vesicles in the nerve endings are arranged in a concentric ring. These vesicles migrate toward the synapse (see Fig. 5). The catecholamine content and turnover are increased in chronic hypoxia, as are HIF-1α and the sensory response to acute hypoxia.
FIG. 5.
Schematic drawing of the changes in the CB during chronic hypoxia (modified after Joseph and Pequignot, 2003). Carotid bodies from animals subjected to chronic hypoxia have an increased response to acute hypoxia. N, nerve ending; G, glomus cell; the arrow indicates a synaptic connection.
Neurotransmitters in chronic hypoxia
Dopamine and norepinephrine
Tyrosine hydroxylase activity is markedly increased in the carotid body. The levels increase by six- to sevenfold, and dopamine and norepinephrine levels increased 15- and 10-fold, respectively (Hanbauer et al., 1981; Hui et al., 2003).
Acetylcholine
Acetylcholine is known to be excitatory (Fitzgerald, 2000) in the carotid body. He et al. (2004) showed that carotid sinus nerve activity evoked by acetylcholine was significantly larger after chronic hypoxia, suggesting increased expression of cholinergic receptors. But their investigation suggested that cholinergic mechanism was not responsible for the enhanced response after chronic hypoxia.
5-Hydroxytryptamine (5-HT)
5-HT has been found in the CB and it has been reported to be stimulatory. The levels do not change in chronic hypoxia (Hui et al., 2003).
Substance-P (SP)
Neuropeptide SP is present in the carotid body and has been reported to be stimulatory (Shirahata et al., 1991). The effect of chronic hypoxia is not known.
Endothelin-1 (ET-1)
Although ET-1 is not detectable under normoxia, it increases markedly in chronic hypoxia (He et al., 1996). It is reported to stimulate carotid chemoreceptor afferents (Chen et al., 2000), and this is potentiated by hypoxia.
Enkephalins (ENK)
Hypoxia stimulates release of ENK (Hanson et al., 1986). Naloxane (ENK antagonist) has been reported to inhibit carotid body chemoreception and whole-animal ventilation in response to acute hypoxia (Pokorski and Lahiri, 1981). But little is known about the mechanism of action of ENK or if it has a role in the response to chronic hypoxia.
Adenosine and purinergic receptors
Adenosine has been reported to stimulate CSN activities of the rat CB (McQueen and Ribeiro, 1986; Spergel and Lahiri, 1993). The effect of ATP is mediated via purinergic receptor activation, as well as direct activation of ion channels. Recent studies suggest that Ach and ATP act as neurotransmitters and are released in CB excitation. ATP has been reported to be released from CB glomus cells in hypoxia, and this was blocked by L-type Ca2+ channel blockers (Buttigieg and Nurse, 2004), but its role in the response to chronic hypoxia is not known.
Angiotensin
Leung et al. (2000) demonstrated that chronic hypoxia resulted in upregulation of expression of angiotensin II receptors. This might enhance CSN activity to angiotensin II, which regulates the salt and water homeostasis.
Signaling gases
Carbon monoxide
Heme oxygenase 1 and 2 (HO-1, HO-2) catalyze formation of CO and HO-1 is constitutively expressed in CB cells, suggesting that CB is capable of CO synthesis. Inhibition of HO-2 by zinc protoporphyrin-IX augments the chemosensory activity, suggesting that endogenous CO is inhibitory (Prabhakar et al., 1995). Inhibition (PCO ~ 60 to 80 torr) and stimulation (PCO ~ 500 torr) of carotid sinus nerve activities suggest a dual nature for CO (Lahiri et al., 1993b). CO is also known to hyperpolarize glomus cells, possibly via BK channels, and inhibition of this effect by hypoxia has been reported (Williams et al., 2004).
Nitric oxide
Nitric oxide synthase (NOS) is localized in nerve fibers and vascular endothelium of the CB, and acute hypoxia inhibits NOS activity. The inhibition of CSN activity by NO is partly mediated via Ca2+ channels in glomus cells, suggesting that NO may be a second messenger in O2 sensing of the CB (Chugh et al., 1994; Grimes et al, 1995; Prabhakar et al., 1995; Wang et al., 1993). Although acute hypoxia depresses NO production, the effect of chronic hypoxia is not known. NO is an inhibitor of mitochondrial cytochrome a3 and is competitive with respect to oxygen. It has been proposed that NO effectively decreases the oxygen affinity of mitochondrial respiration, making it more dependent on oxygen pressure (Brown and Cooper, 1994; Buerk and Lahiri, 2000). This is unlikely since the NO concentrations used were well above physiological levels, and such an inhibition would be counterproductive. The intrinsic oxygen sensitivity of the mitochondria (absence of NO) is consistent with their role in oxygen sensing.
ACKNOWLEDGMENT
This work was supported in part by R37-HL-43413 to SL and NS-31465 to DFW from the U.S. National Institutes of Health.
REFERENCES
- Acker H. Mechanisms and meanings of cellular oxygen sensing in the organism. Respir. Physiol. 1994;95:1–10. doi: 10.1016/0034-5687(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Agani FH, Pichiule P, Chavez JC, LaManna JC. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J. Biol. Chem. 2000;275:35863–35867. doi: 10.1074/jbc.M005643200. [DOI] [PubMed] [Google Scholar]
- Anichov SV, Belen’kii ML. Pharmacology of the Carotid Body Chemoreceptors. New York: Macmillan; 1963. [Google Scholar]
- Baby SM, Roy A, Mokashi A, Lahiri S. Role of mitochondria in the regulation of hypoxia-inducible factor-1α in the rat carotid body glomus cells (under revision) 2005 doi: 10.1007/s00418-005-0028-6. In press. [DOI] [PubMed] [Google Scholar]
- Barnard P, Andronikou S, Pokorski M, Smatresk N, Mokashi A, Lahiri S. Time-dependent effect of hypoxia on carotid body chemosensory function. J. Appl. Physiol. 1987;63:685–691. doi: 10.1152/jappl.1987.63.2.685. [DOI] [PubMed] [Google Scholar]
- Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBs Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J. Physiol. 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerk DG, Lahiri S. Evidence that nitric oxide plays a role in O2 sensing from tissue NO and PO2 measurements in cat carotid body. In: Lahiri S, Prabhakar NR, Forster RE II, editors. Oxygen Sensing, Molecule to Man. Kluwer Academic/Plenum Publishers; 2000. pp. 337–347. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem. Biophys. Res. Commun. 2004;322:82–87. doi: 10.1016/j.bbrc.2004.07.081. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Bamford OS, Fitzgerald RS. Postnatal maturation of carotid chemoreceptor responses to O2 and CO2 in the cat. J. Appl. Physiol. 1993;75:2383–2391. doi: 10.1152/jappl.1993.75.6.2383. [DOI] [PubMed] [Google Scholar]
- Castor LN, Chance B. Photochemical action spectra of carbon monoxide-inhibited respiration. J. Biol. Chem. 1955;217:453–465. [PubMed] [Google Scholar]
- Castor LN, Chance B. Photochemical determinations of the oxidases of bacteria. J. Biol. Chem. 1959;234:1587–1592. [PubMed] [Google Scholar]
- Chen J, He L, Dinger B, Fidone S. Cellular mechanisms involved in rabbit carotid body excitation elicited by endothelin peptides. Respir. Physiol. 2000;121:13–23. doi: 10.1016/s0034-5687(00)00113-4. [DOI] [PubMed] [Google Scholar]
- Chugh DK, Katayama M, Mokashi A, Bebout DE, Ray DK, Lahiri S. Nitric oxide-related inhibition of carotid chemosensory nerve activity in the cat. Respir. Physiol. 1994;97:147–156. doi: 10.1016/0034-5687(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Cooper DY, Schleyer H, Rosenthal O. Some chemical properties of cytochrome P-450 and its carbon monoxide compound (P-450-CO) Ann. N.Y. Acad. Sci. 1970;174:205–217. doi: 10.1111/j.1749-6632.1970.tb49787.x. [DOI] [PubMed] [Google Scholar]
- Di Giulio C, Huang WX, Mokashi A, Roy A, Cacchio M, Macri MA, Lahiri S. Sustained hypoxia promotes hyperactive response of carotid body in the cat. Respir. Physiol. Neurobiol. 2003;134:69–74. doi: 10.1016/s1569-9048(02)00203-3. [DOI] [PubMed] [Google Scholar]
- Doyle TP, Donnelly DF. Effect of Na+ and K+ channel blockade on baseline and anoxia-induced catecholamine release from rat carotid body. J. Appl. Physiol. 1994;77:2606–2611. doi: 10.1152/jappl.1994.77.6.2606. [DOI] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J. Physiol. 1987;392:11–19. doi: 10.1113/jphysiol.1987.sp016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Wilson DF. Inhibitors of cytochrome c oxidase. Pharmacol. Ther. 1980;8:1–20. [Google Scholar]
- Estabrook R. The light reversible carbon monoxide inhibition of the steroid C21-hydroxylase system of the adrenal cortex. Biochem. Z. 1963;338:741–755. [PubMed] [Google Scholar]
- Eyzaguirre C, Koyano H. Effecs of hypoxia, hypercapnia, and pH on the chemoreceptor activity of the carotid body in vitro. J. Physiol. 1965a;178(3):385–409. doi: 10.1113/jphysiol.1965.sp007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C, Koyano H. Effects of some pharmacological agents on chemoreceptor discharges. J. Physiol. 1965b;178(3):410–437. doi: 10.1113/jphysiol.1965.sp007635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre CL, Koyano H. Effects of electrical stimulation on the frequency of chemoreceptor discharges. J. Physiol. 1965c;178:485–462. doi: 10.1113/jphysiol.1965.sp007636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald RS. Oxygen and carotid body chemo-transduction: the cholinergic hypothesis—a brief history and new evaluation. Respir. Physiol. 2000;120:89–104. doi: 10.1016/s0034-5687(00)00091-8. [DOI] [PubMed] [Google Scholar]
- Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J. Appl. Physiol. 1999;86:298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- Grimes PA, Mokashi A, Stone RA, Lahiri S. Nitric oxide synthase in autonomic innervation of the cat carotid body. J. Auton. Nerv. Syst. 1995;54:80–86. doi: 10.1016/0165-1838(95)00006-j. [DOI] [PubMed] [Google Scholar]
- Guillemin K, Krasnow MA. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- Hanbauer I, Karoum F, Hellstrom S, Lahiri S. Effects of hypoxia lasting up to one month on the catecholamine content in rat carotid body. Neuroscience. 6:81–86. doi: 10.1016/0306-4522(81)90245-1. [DOI] [PubMed] [Google Scholar]
- Hanson G, Jones L, Fidone S. Physiological chemoreceptor stimulation decreases enkephalin and substance P in the carotid body. Peptides. 1986;7:767–769. doi: 10.1016/0196-9781(86)90093-8. [DOI] [PubMed] [Google Scholar]
- He L, Chen J, Dinger B, Stensaas L, Fidone S. Endothelin modulates chemoreceptor cell function in mammalian carotid body. Adv. Exp. Med. Biol. 1996;410:305–311. doi: 10.1007/978-1-4615-5891-0_46. [DOI] [PubMed] [Google Scholar]
- He L, Dinger B, Fidone S. Effect of chronic hypoxia on cholinergic chemotransmission in rat carotid body. J. Appl. Physiol. 2004;98:614–619. doi: 10.1152/japplphysiol.00714.2004. [DOI] [PubMed] [Google Scholar]
- Hempleman SC. Increased calcium current in carotid body glomus cells following in vivo acclimatization to chronic hypoxia. J. Neurophysiol. 1996;76:1880–1886. doi: 10.1152/jn.1996.76.3.1880. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P. Hierarchy of the β-cell signals controlling insulin secretion. Eur. J. Clin. Invest. 2003;33:742–750. doi: 10.1046/j.1365-2362.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Lahiri S. Cell biology. Oxygen sensing: oxygen sensing it’s a gas! Science. 2004;306:2050–2051. doi: 10.1126/science.1107069. [DOI] [PubMed] [Google Scholar]
- Huang C-C, Lejevardi N, Tammela O, Pastuszko A, Delivoria-Papadopoulos M, Wilson DF. Relationship of extracellular dopamine in striatum of newborn piglets to cortical oxygen pressure. Neurochem. Res. 1994;19:649–655. doi: 10.1007/BF00967702. [DOI] [PubMed] [Google Scholar]
- Hui AS, Striet JB, Gudelsky G, Soukhova GK, Gozal E, Beitner-Johnson D, Guo SZ, Sachleben LR, Jr, Haycock JW, Gozal D, Czyzyk-Krzeska MF. Regulation of catecholamines by sustained and intermittent hypoxia in neuroendocrine cells and sympathetic neurons. Hypertension. 2003;42:1130–1136. doi: 10.1161/01.HYP.0000101691.12358.26. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Rumsey WL, Mokashi A, Spergel D, Wilson DF, Lahiri S. In vitro perfused–superfused cat carotid body for physiological and pharmacological studies. J. Appl. Physiol. 1991;70(3):1393–1400. doi: 10.1152/jappl.1991.70.3.1393. [DOI] [PubMed] [Google Scholar]
- Joseph V, Pequignot J-E. Neurochemical process involved in acclimatization to long-term hypoxia. In: Lahiri S, Semenza GL, Prabhakar NR, editors. Oxygen Sensing: Responses and Adaptation to Hypoxia. New York: Marcel Dekker; 2003. pp. 467–487. [Google Scholar]
- Keilin D. The History of Cell Respiration and Cytochrome. New York: Cambridge University Press; 1966. pp. 117–139. [Google Scholar]
- Keilin D, Hartree EF. Cytochrome and cytochrome oxidase. Proc. Roy. Soc. B. 1939;127:167–191. [Google Scholar]
- Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defecive carotid body function and impaired ventilatory responses to chronc hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc. Natl. Acad. Sci. U. S. A. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubowitz F, Haas E. Ausbau der photochemische Methoden zur Untersuchung des sauerstoffübertragenden Ferments. Biochem. Z. 1932;255:247–277. [Google Scholar]
- Lahiri S. Chromophores in O2 chemoreception: the carotid body model. News in Physiological Science. 1994;9:161–165. [Google Scholar]
- Lahiri S. Oxygen sensing in health and disease. J. Appl. Physiol. 2004;96:1170–1172. [Google Scholar]
- Lahiri S, Acker H. Redox-dependent binding of CO to heme protein controls PO2-sensitive chemoreceptor discharge of the rat carotid body. Respir. Physiol. 1999;115:169–177. doi: 10.1016/s0034-5687(99)00014-6. [DOI] [PubMed] [Google Scholar]
- Lahiri S, DeLaney RG, Brody JS, Simpser M, Velasquez T, Motoyama EK, Polgar C. Relative role of environmental and genetic factors in respiratory adaptation to high altitude. Nature. 1976;261:133–135. doi: 10.1038/261133a0. [DOI] [PubMed] [Google Scholar]
- Lahiri S, DiGuilio C, Roy A. Lessons from chronic intermittent and sustained hypoxia at high altitudes. Respir. Physiol. Neurobiol. 2002;130:223–233. doi: 10.1016/s0034-5687(01)00343-7. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Iturriaga R, Mokashi A, Ray DK, Chugh D. CO reveals dual mechanisms of O2 chemoreception in the cat carotid body. Respir. Physiol. 1993b;94:227–240. doi: 10.1016/0034-5687(93)90050-k. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Milledge JS. Sherpa physiology. Nature. 1965;207:610–612. doi: 10.1038/207610a0. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Mulligan E, Andronikou S, Shirahata M, Mokashi A. Carotid body chemosensory function in prolonged normobaric hyperoxia in the cat. J. Appl. Physiol. 1987;62:1924–1931. doi: 10.1152/jappl.1987.62.5.1924. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Roy A, Rozanov C, Mokashi A. K+-current modulated by PO2 in type I cells in rat carotid body is not a chemosensor. Brain Res. 1998;794:162–165. doi: 10.1016/s0006-8993(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Rumsey WL, Wilson DF, Iturriaga R. Contribution of in vivo microvascular PO2 in the cat carotid body chemotransduction. J. Appl. Physiol. 1993a;75(3):1035–1043. doi: 10.1152/jappl.1993.75.3.1035. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Smatresk N, Pokorski M, Barnard P, Mokashi M. Efferent inhibition of carotid body chemoreception in chronically hypoxic cats. Am. J. Physiol. 1983;245:R678–R683. doi: 10.1152/ajpregu.1983.245.5.R678. [DOI] [PubMed] [Google Scholar]
- Leon-Velarde F, Gamboa A, Rivera-Ch M, Palacios J-A, Robbins PA. Peripheral chemoreflex function in high-altitude natives and patients with chronic mountain sickness. J. Appl. Physiol. 2003;94:1269–1278. doi: 10.1152/japplphysiol.00858.2002. [DOI] [PubMed] [Google Scholar]
- Leung PS, Lam SY, Fung ML. Chronic hypoxia upregulates the expression and function of AT1 receptor in rat carotid body. J. Endocrinol. 2000;167:517–524. doi: 10.1677/joe.0.1670517. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, Montoro R, Ortega-Sáaenz P, Ureña J. Oxygen regulated ion channels: functional roles and mechanisms. In: Lopez-Barneo J, Weir EK, editors. Oxygen Regulation of Ion Channels and Gene Expression. Amonk, NY: Future; 1998. pp. 127–144. [Google Scholar]
- López-Barneo J, Pardal R. Carotid body thin slices: new answers to old questions. In: Lahiri S, Semenza GL, Prabhakar NR, editors. Oxygen Sensing: Responses and Adaptation to Hypoxia. New York: Marcel Dekker; 2003. pp. 315–330. [Google Scholar]
- López-Barneo J, Pardal R, Ortega-Sáenz P. Cellular mechanisms of oxygen sensing. Annu. Rev. Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- McGregor KH, Gil J, Lahiri S. A morphometric study of the carotid body in chronically hypoxic rats. J. Appl. Physiol. 1984;57:1430–1438. doi: 10.1152/jappl.1984.57.5.1430. [DOI] [PubMed] [Google Scholar]
- McQueen S, Ribeiro JA. Pharmacological characterization of the receptor involved in chemoexcitation induced by adenosine. Br. J. Pharmacol. 1986;88:615–620. doi: 10.1111/j.1476-5381.1986.tb10242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick JL. The photochemical action spectra of the Pasteur enzyme and the respiratory ferment in yeast. J. Biol. Chem. 1941;141:269–281. [Google Scholar]
- Melnick JL. The photochemical spectrum of cytochrome oxidase. J. Biol. Chem. 1942;146:385–390. [Google Scholar]
- Moore LG, Armaza F, Villena M, Vargas E. Comparative aspects of high altitude adaptation in human populations. Adv. Exp. Med. Biol. 2000;475:45–62. doi: 10.1007/0-306-46825-5_6. [DOI] [PubMed] [Google Scholar]
- Mulligan E, Lahiri S, Storey BT. Carotid body O2 chemoreception and mitochondrial oxidative phosphorylation. J. Appl. Physiol. 1981;51(2):438–446. doi: 10.1152/jappl.1981.51.2.438. [DOI] [PubMed] [Google Scholar]
- Nielson AM, Bisgard GB, Vidruk EH. Carotid body chemoreceptor activity during acute and sustained hypoxia in goats. J. Appl. Physiol. 1988;53:247–252. doi: 10.1152/jappl.1988.65.4.1796. [DOI] [PubMed] [Google Scholar]
- Nuutinen EM, Nelson D, Wilson DF, Erecinska M. Regulation of Coronary Blood Flow: Effects of 2,4-dinitrophenol and Theophylline. Amer. J. Physiol. 1983;244:H396–H405. doi: 10.1152/ajpheart.1983.244.3.H396. [DOI] [PubMed] [Google Scholar]
- Pastuszko A, Saadat-Lajevardi N, Chen J, Tammela O, Wilson DF, Delivoria-Papadopoulos M. Effects of graded levels of tissue oxygen pressure on dopamine metabolism in the striatum of newborn piglets. J. Neurochem. 1993;60:161–166. doi: 10.1111/j.1471-4159.1993.tb05834.x. [DOI] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells—selective effect on the Ca2+-activated K+ current. Neurosci. Lett. 1990;119:253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J. Appl. Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- Pokorski M, Lahiri S. Effects of naloxone on carotid body chemoreception and ventilation in the cat. J. Appl. Physiol. 1981;51:1533–1538. doi: 10.1152/jappl.1981.51.6.1533. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J. Appl. Physiol. 2000;88:2289–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J. Appl. Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Dinerman JL, Agani FH, Snyder SH. Carbon monoxide: a role in carotid body chemoreception. Proc. Natl. Acad. Sci. 1995;92:1994–1997. doi: 10.1073/pnas.92.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK, Chang CH, Agani FH, Haxhiu MA. Nitric oxide in the sensory function of the carotid body. Brain Res. 1993;625:16–22. doi: 10.1016/0006-8993(93)90132-7. [DOI] [PubMed] [Google Scholar]
- Roy A, Li J, Baby SM, Mokashi A, Buerk DG, Lahiri S. Effects of iron-chelators on ion-channels and HIF-1α in the carotid body. Respir. Physiol. Neurobiol. 2004;141:115–123. doi: 10.1016/j.resp.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Rumsey WL, Iturriagga R, Spergel D, Lahiri S, Wilson DF. Optical measurements of the dependence of chemoreception on oxygen pressure in the cat carotid body. Am. J. Physiol. 1991;261:C614–C622. doi: 10.1152/ajpcell.1991.261.4.C614. [DOI] [PubMed] [Google Scholar]
- Rumsey WL, Schlosser C, Nuutinen M, Robiolio M, Wilson DF. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J. Biol. Chem. 1990;265:15392–15399. [PubMed] [Google Scholar]
- Semenza GL. Hydroxylation of HIF-1β: oxygen sensing at the molecular level. Physiologist. 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW, Bainton CR, Carcelen A. Respiratory insensitivity to hypoxia in chronically hypoxic man. Respir. Physiol. 1966;1:308–334. doi: 10.1016/0034-5687(66)90049-1. [DOI] [PubMed] [Google Scholar]
- Shirahata M, Fitzgerald RS, Lahiri S. Sympathetic influence on carotid chemoreceptor response to substance P in the cat. J. Auton. Nerv. Syst. 1991;35:143–152. doi: 10.1016/0165-1838(91)90057-a. [DOI] [PubMed] [Google Scholar]
- Spergel D, Lahiri S. Differential modulation by extracellular ATP of carotid chemosensory responses. J. Appl. Physiol. 1993;74:3052–3056. doi: 10.1152/jappl.1993.74.6.3052. [DOI] [PubMed] [Google Scholar]
- Sterni LM, Bamford OS, Wasicko MJ, Carroll JL. Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. Am. J. Physiol. 1999;277:L645–L652. doi: 10.1152/ajplung.1999.277.3.L645. [DOI] [PubMed] [Google Scholar]
- Streller T, Huckstorf C, Pfeiffer C, Acker H. Unusual cytochrome a592 with low PO2 affinity correlates as putative oxygen sensor with rat carotid body hemoreceptor discharge. FASEB J. 2002;16:1277–1279. doi: 10.1096/fj.02-0166fje. [DOI] [PubMed] [Google Scholar]
- Tang XD, Xu R, Reynolds MF, Garcia ML, Heinemann SH, Hoshi T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 2003;425:531–535. doi: 10.1038/nature02003. [DOI] [PubMed] [Google Scholar]
- Vizek M, Pickett CK, Weil JV. Increased carotid body hypoxic sensitivity during acclimatization to hypobaric hypoxia. J. Appl. Physiol. 1987;63:2403–2410. doi: 10.1152/jappl.1987.63.6.2403. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Bredt DS, Fidone SJ, Stensaas LJ. Neurons synthesizing nitric oxide innervate the mammalian carotid body. J. Comp. Neurol. 1993;336:419–432. doi: 10.1002/cne.903360308. [DOI] [PubMed] [Google Scholar]
- Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J. Biol. Chem. 1997;272:8222–82226. doi: 10.1074/jbc.272.13.8222. [DOI] [PubMed] [Google Scholar]
- Warburg O. Über die Wirkung des Kolenoxyds auf den Stoffwechsel der Hefe. Biochem. Z. 1926;177:471–486. [Google Scholar]
- Warburg O. Über die Wirkung des Kolenoxyd und Stickoxyd auf Atmung und Gärung. Biochem. Z. 1927;189:354–380. [Google Scholar]
- Warburg O, Negelein E. Über die Einfluss der Wellenlänge auf die Verteilung des Atmungsferments (Absorptionsspektrum des Atmungsferments) Biochem. Z. 1928a;193:339–346. [Google Scholar]
- Warburg O, Negelein E. Über die photochemische Dissoziation bei intermittierender Bleichuterung und das absolute Absorptionssperktrum des Atmungsferments. Biochem. J. 1928b;202:202–228. [Google Scholar]
- Warburg O, Negelein E. Über das Absorptionsspektrum des Atmungsferments. Biochem. Z. 1929;214:64–1000. [Google Scholar]
- Williams SEJ, Wootton P, Mason HS, Bould L, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Sci. Express. 2004 Nov;4:1–3. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Erecinska M. Effect of oxygen concentration on cellular metabolism. Chest. 1985;88s:229s–232s. doi: 10.1378/chest.88.4_supplement.229s. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Erecinska M, Drown C, Silver LA. The oxygen dependence of cellular energy metabolism. Arch. Biochem. Biophys. 1979a;195:485–493. doi: 10.1016/0003-9861(79)90375-8. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Mokashi A, Chugh D, Vinogradov S, Osanai S, Lahiri S. The primary oxgyen sensor of the cat carotid body is cytochrome a3 of the mitochondrial respiratory chain. FEBS Lett. 1994;351:370–374. doi: 10.1016/0014-5793(94)00887-6. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Owen CS, Erecinska M. Quantitative dependence of mitochondrial oxidative phosphorylation on oxygen concentration. Arch. Biochem. Biophys. 1979b;195:494–504. doi: 10.1016/0003-9861(79)90376-x. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Rumsey WL, Green TJ, Vanderkooi JM. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen. J. Biol. Chem. 1988;263:2712–2718. [PubMed] [Google Scholar]
- Wyatt CN, Wright C, Bee D, Peers C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc. Natl. Acad. Sci. 1995;92:295–299. doi: 10.1073/pnas.92.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelent D, Najafi H, Odili S, Buettger C, Weik-Collins H, Li C, Doliba N, Grimsby J, Matshinsky FM. Glucokinase and glucose homeostasis: proven concepts and new ideas. Biochem. Soc. Trans. 2005;33:306–310. doi: 10.1042/BST0330306. [DOI] [PubMed] [Google Scholar]
- Ziemer LS, Lee WMF, Vinogradov SA, Sehgal C, Wilson DF. Oxygen distribution in murine tumors: characterization using oxygen-dependent quenching of phosphorescence. J. Appl. Physiol. 2005;98 doi: 10.1152/japplphysiol.01140.2004. in press. [DOI] [PubMed] [Google Scholar]