Abstract
Although it has been known for decades that the tight junctions of fluid-transporting epithelia are leaky to ions, it has not been possible to determine directly whether significant transjunctional water movement also occurs. An optical microscopic technique was developed for the direct visualization of the flow velocity profiles within the lateral intercellular spaces of a fluid-absorptive, cultured renal epithelium (MDCK) and used to determine the velocity of the fluid flow across the tight junction. The flow velocity within the lateral intercellular spaces fell to near zero adjacent to the tight junction, showing that significant transjunctional flow did not occur, even when transepithelial fluid movement was augmented by imposition of osmotic gradients.
More than 30 years ago, the tight junctions (TJs) of fluid-absorbing epithelia were shown to be leaky to ions and, thus, to constitute a paracellular pathway with a selectively permeable seal between the apical and basal compartments. Despite extensive studies of the solute permeability of the paracellular pathways of a wide variety of epithelia, the junctional permeability to water has remained undetermined because of the lack of an experimentally feasible method for the direct measurement of the rate of fluid flow across the TJ as well as within the lateral intercellular spaces (LIS) separating the cells. An understanding of the mechanism of transepithelial fluid transport requires knowledge of the fraction of transepithelial fluid flow that is paracellular; however, current estimates for transjunctional fluid flow still range from 0 to 100% (1–3). Indirect arguments have been advanced for important transjunctional flows in gallbladder (1), small intestine (4), and renal proximal tubule (5), but there has not been a definitive demonstration of transjunctional water flow in these native epithelia despite decades of experimental efforts with a variety of techniques (3). Convective flux across the TJ is not only a potentially important mechanism for solute and water reabsorption, but also complicates the determination of the driving forces for solute transport. In particular, the presence of convective Na and Cl fluxes across the TJ can give rise to an apparent coupling of the two fluxes in the overall transport equations (6, 7).
MATERIALS AND METHODS
Flow Visualization.
Fluid flow within the LIS can be visualized by introducing a marker (e.g., a fluorescent dye) that is trapped in the LIS and observing the concentration profile of the marker along the LIS. Within the small dimensions of the LIS, the concentration profile of a marker is determined by the balance of convection tending to build the concentration and diffusion tending to dissipate the flow-induced concentration gradients. Thus, the flow velocity at every location within the LIS can be determined from knowledge of the local marker concentration, local diffusion coefficient of the marker, and cross-sectional area of the region. The convection-induced concentration profile of a high molecular weight marker, which diffuses slowly within the LIS, should be more pronounced than that of a fast-diffusing, low molecular weight one.
The volume flow, Fv, at any location within the LIS may be calculated from the local marker concentration, C, by application of the diffusion equation to the marker flux, F, along the LIS as follows:
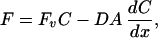 |
1 |
where Fv is volume flow, C is marker concentration, D is the diffusion coefficient, A is the LIS cross-sectional area, and x is the distance along the LIS. For fluorescent solutes that are trapped within the LIS, the steady-state marker flux is zero and Eq. 1 may be reduced to:
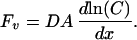 |
2 |
In the present study, the marker concentration, C, was determined from the intensity of the fluorescence at focal planes along the LIS, normalized relative to the measured value nearest the TJ (0.32 μm beneath the TJ). The logarithm of the normalized intensity was multiplied by the previously determined marker diffusion coefficient, D, and plotted as a function of x. The cross-sectional area of LIS, A, was measured as a function of x and fitted by a fourth-order polynomial. To calculate Fv, the logarithm of the normalized intensity values along the LIS was taken, and the data were fitted by a fourth-order polynomial by using the method of least squares. The first term of the resultant polynomial, multiplied by the LIS cross-sectional area function, defines the volume flow rate at x = 0, the level of the TJ.
Specimen Preparation.
Low-resistance MDCK cells (transepithelial resistance: 65 ± 9.6 Ω⋅cm2; ref. 14) were grown to confluence on Anocell permeable supports (Whatman) that had been treated to reduce pore size sufficiently to be impermeable to the fluorescent dextran marker (8). A sharpened glass micropipette (≈1 μm diameter), filled with Ringer’s solution containing 3 mM fluorescein dextran (70,000 Mr, Molecular Probes), was used to puncture through the epithelial layer at several locations and introduce 5- to 10-pl droplets of the fluorescently labeled solution between the epithelium and the permeable support. Over the next several hours, the fluorescein dextran remained trapped between the epithelium and the permeable support and diffused from the puncture sites into the LIS of adjacent undisturbed regions of the cell layer. In some cases, small regions of the epithelial layer at the puncture site lifted up slightly from the permeable support during the injection, but all measurements were made in nearby regions in which the LIS geometry was undisturbed. The support with its adherent cell layer was perfused with bicarbonate-buffered Ringer’s solution at 37°C in a bilateral chamber mounted on the stage of a confocal microscope as previously described (8, 14). Transepithelial osmotic gradients were generated with 3,000 Mr polyethylene glycol (PEG, Sigma). Preliminary experiments showed that 3,000 Mr PEG could not enter the LIS by penetrating through either the TJ or permeable support. Protein kinase A was stimulated by the addition of 10 μM of (Sp)-adenosine cyclic 3′,5′-phosphorothioate (Sp-cAMPS, Biolog, La Jolla, CA).
When the LIS were loaded with 2′,7′-bis(2-carboxethyl)-5(and 6)-carboxyfluorescein (BCECF, Molecular Probes), a 2-mM solution was introduced into the apical bathing solution, perfusion of the apical bath was stopped for 1 or 2 min to allow the dye to diffuse across the TJ and into the LIS and then perfusion of Ringer’s solution was restarted. The basolateral bath was perfused continuously during BCECF loading.
Microscopy and Image Processing.
The marker fluorescence was visualized with a 100×/1.32 objective lens (Nikon) on an inverted microscope (Diaphot, Nikon) to which a confocal scanner (Odyssey, Noran Instruments, Middleton, WI) was attached. The optical performance of the confocal scanner had been thoroughly characterized previously (9) both before and after the instrument was modified for the low-light-level observation of living cells. The preparation was illuminated with 488-nm laser light, and optical sections of the fluorescence and corresponding differential interference contrast (DIC) images were obtained as the focus was displaced by a stepping motor in 0.32-μm increments from a region slightly above the apical surface of the cells to a location just beyond the cell’s basal surface. Both the fluorescence and DIC images were digitized by using 0.11-μm pixel size in the image plane, and the fluorescence images were analyzed by using digital image processing (Image-1, Universal Imaging, West Chester, PA). Although the LIS of MDCK cells often have a scalloped appearance in cross-section, regions of uniform width extending over several microns could be found. The concentration profile of the fluorescent marker was determined from the average gray level in a small rectangular measuring box positioned inside such a straight region in the digitized image of the LIS.
The width of the LIS at each focal plane in the uniform regions was determined from the digitized fluorescence images after digital deblurring by the method of iterative deconvolution (Cell-Scan, Scanalytics, Vienna, VA). To enhance lateral resolution, these images were acquired with 1.5× additional magnification by reduction of the scanned area so that pixel size in the image plane was effectively 0.077 μm. The requisite point-spread function was generated from optical sections of fluorescently labeled, subresolution latex beads (Molecular Probes). Typically, 20 optical sections were obtained in 0.32-μm focus displacement increments from the level of the TJ to the base of the LIS.
RESULTS
LIS Dimensions.
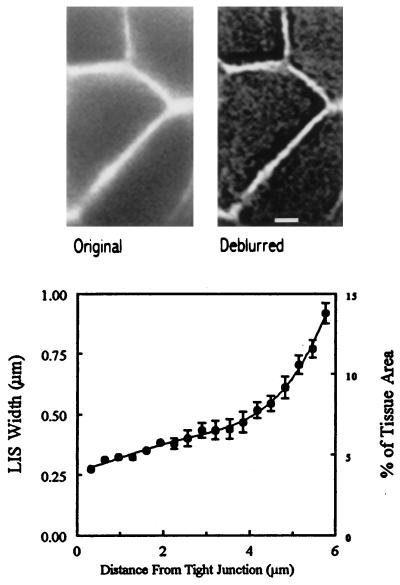
Original and deblurred images of an optical section of fluorescently labeled LIS of MDCK cells are shown in the upper portion of Fig. 1. The lower portion of Fig. 1 shows the width in μm (mean ± SEM) of seven LIS from the deconvolved images as well as the fourth-order polynomial fitted to the data. Because the LIS is shared between neighboring cells, the cross-sectional area at each focal plane also can be expressed as a percentage of the epithelial apical surface area (Fig. 1, Lower). The calculated volume of the LIS for a single cell is 46 fl, or about 5.5% of the average cell volume (800 fl). LIS cross-sectional area was unaffected by the imposition of transepithelial osmotic gradients (data not shown).
Figure 1.
(Upper) A confocal optical section approximately halfway through the MDCK cell epithelium showing the LIS filled with 70,000 Mr fluorescein dextran before (Left) and after (Right) digital deconvolution. Scale bar indicates 2 μm. (Lower) Mean ± SEM width of uniform regions of the LIS of seven MDCK cells are shown as a function of the focal distance from the TJ. Optical sections, obtained at 0.32-μm focus intervals, were digitally deblurred (Cell-Scan) by using an experimentally determined point-spread function as described in Materials and Methods.
Marker Fluorescence Profiles.
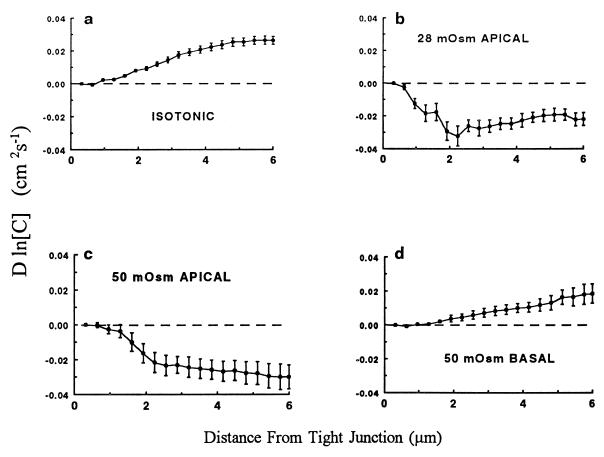
Fig. 2 shows the logarithm of the concentration profiles for the 70,000 Mr fluorescein dextran after normalization and multiplication by the dextran diffusion coefficient as described in Materials and Methods. The diffusion coefficient for 70,000 Mr dextran was calculated from the value recently obtained for 10,000 Mr caged fluorescein dextran within the LIS of MDCK cells grown on permeable supports (O.K., P. Bungay, and K.R.S., unpublished work). Those experiments showed that molecular hindrance within the LIS of MDCK cells grown on permeable supports reduced the diffusivity of 10,000 Mr dextran to about 25% of its free solution value. The diffusion coefficient for the 70,000 Mr dextran at 37°C used in the present calculations was 1 × 10−7 cm2/s, one-fourth of its free solution diffusivity.
Figure 2.
Intensity (mean ± SEM) of the fluorescence in digitized confocal images of 70,000 Mr fluorescein dextran along the length of the LIS of MDCK cells is expressed as the logarithm of concentration multiplied by the dextran diffusion coefficient in the LIS. All intensities were corrected for differences in gain of the photomultiplier tube in the confocal microscope as well as for variations in the intensity of the laser light used for excitation. (a) The profile obtained under control conditions (n = 51) during perfusion of bicarbonate-buffered Ringer’s solution at 37°C. (b) The effect of a 28 mOsm osmotic gradient (n = 7) created by adding 28 mM PEG (3,000 Mr) to the apical perfusate. (c) The intensity profile obtained when 50 mM PEG (n = 8) was added to the apical perfusate. (d) The effect of 50 mM PEG (n = 8) in the basal perfusate.
The curves in Fig. 2 depict the mean ± SEM normalized marker concentration profiles: (i) under isotonic conditions, (ii) when transepithelial fluid absorption was stopped by the addition of 28 mM PEG to the apical bathing solution, (iii) when the transepithelial fluid flux was reversed to secretion by the introduction of 50 mM PEG to the apical bath, or (iv) when transepithelial fluid absorption was accelerated by the addition of 50 mM PEG to the basolateral bathing solution. The normalized concentration curves along the LIS during cessation or reversal of flow (Fig. 2 b and c) differ markedly from those during spontaneous fluid absorption (Fig. 2a) or during enhanced absorption (Fig. 2d). Interpretation of these curves in terms of localized fluid volume flow rate requires incorporation of the cross-sectional area function from Fig. 1.
Volume Flows.
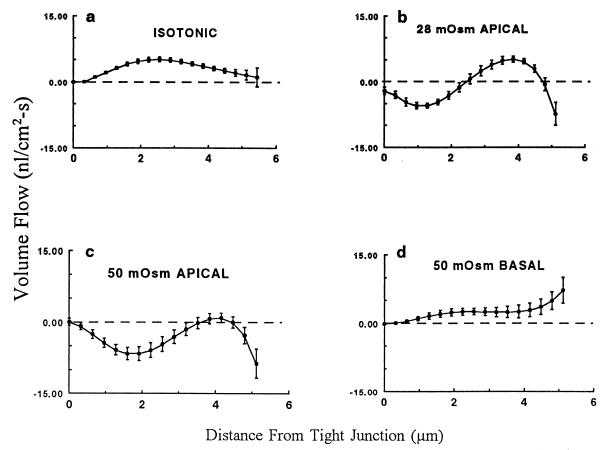
Fig. 3a shows the mean ± SEM volume flow in nl/cm2⋅s along the LIS under control (isotonic) conditions, calculated from the data in Figs. 1 and 2 according to Eq. 2. Table 1 lists the volume flow rates (mean ± SEM) at the level of the TJ calculated from the first term in the fitted polynomial curves as described in Materials and Methods. As indicated in Table 1, volume flow across the TJ is insignificant under isotonic conditions. The computed volume flow at the basolateral mouth of the LIS under control conditions (0.72 ± 3.94 nl/cm2⋅s) is subject to large error because of variability in the geometry of the 0.5 μm of the LIS closest to the basal surface. The mean volume flow exiting the LIS is reasonably close to the estimated transepithelial absorptive fluid flux of 0.3 nl/cm2⋅s predicted from the experimentally measured cell membrane water permeabilities and osmotic gradient (11). The shape of the volume flow curve along the LIS and the magnitude of the volume flow at the mouth of the LIS are consistent with the conclusion that the bulk of the transported fluid exits the cell across the lateral rather than the basal membrane. Acceleration of fluid absorption by the imposition of 50 mOsm transepithelial osmotic gradient (Fig. 3d) failed to induce a significant volume flow across the TJ although it substantially increased the flow at the basal end of the LIS. Again, variability in LIS geometry in the last 0.5 μm of the LIS precluded an accurate estimate of the volume flow rate at the mouth of the interspace.
Figure 3.
The rate of volume flow along the LIS (mean ± SEM) calculated from Figs. 1 and 2 is shown. Absorptive flows are denoted as positive and secretory flows as negative. (a) The isotonic condition. (b) The flow with 28 mM PEG in the apical perfusate. (c) The flow with 50 mM PEG in the apical perfusate. (d) The flow with 50 mM PEG in the basal perfusate. The predicted values of volume flow at the TJ (mean ± SEM from Table 1) are indicated as the data point at zero on the x axis.
Table 1.
Volume flows at the TJ
| Condition | Volume flow, nl/cm2/s | P vs. 0 | N |
|---|---|---|---|
| Control | −0.036 ± 0.13 | ns | 51 |
| 28 mOsm apical | −2.089 ± 0.82 | ns | 7 |
| 50 mOsm apical | +0.058 ± 0.72 | ns | 8 |
| 50 mOsm basolateral | −0.135 ± 0.10 | ns | 8 |
| BCECF control | +0.639 ± 1.13 | ns | 20 |
| Sp-cAMPS 50 mOsm apical | −0.688 ± 0.31 | <0.05 | 14 |
| Sp-cAMPS 100 mOsm basolateral | −0.114 ± 0.158 | ns | 11 |
N is the number of preparations. Volume flow rates (mean ± SEM) were derived from the first term of the fourth-order polynomial fit to the data as described in Eq. 2. ns, not significant.
Cessation of osmotic flow, which should occur during perfusion of a 28-mOsm hypertonic solution in the apical bath (Fig. 3b), or probable transepithelial fluid flow reversal with a 50-mOsm hypertonic perfusate (Fig. 3c) resulted both in reversed (negative) and positive flows along the LIS, without any significant transjunctional fluid flux (Table 1). Substantial reversed fluid flow proceeded through the 1.0–1.5 μm at the basal end of the LIS; however, large errors in the measurement precluded an accurate estimation of these flows.
BCECF Profile.
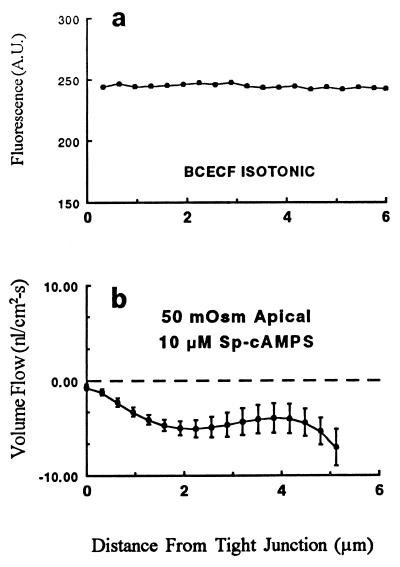
As predicted from the diffusion equation, the convection-induced concentration profile of a rapidly diffusing solute should be less pronounced than that of the slowly diffusing 70,000 Mr dextran. In addition, introduction of a tracer into the LIS with a substantially higher diffusion coefficient should help to rule out any artifacts in the intensity of the fluorescence related to LIS geometry. As shown in Fig. 4a, BCECF, a fluorescein derivative of molecular weight 520 with a measured diffusion coefficient in the LIS 9.8 times that of the 70,000 Mr dextran (10, 12), does not show any significant gradient in fluorescence intensity along the LIS under isotonic conditions. This observation is in good agreement with a previous study from our laboratory using wide-field fluorescence microscopy showing that gradients in BCECF concentration along the LIS did not occur (8). We conclude that the intensity gradients seen with the 70,000 Mr dextran are caused by convective flows sweeping this slowly diffusing solute.
Figure 4.
(a) The fluorescence intensity profile for BCECF in the LIS in the absence of an imposed osmotic gradient (n = 20). (b) The mean ± SEM volume flow rate in the LIS when the apical perfusate was 50 mOsm hyperosmotic and the epithelium had been treated with 10 μM Sp-cAMPS to increase the permeability of the TJ (n = 14). Note that the ordinate scale differs from that in Fig. 3.
Volume Flow Across a Leaky TJ.
To ascertain whether our method could detect transjunctional fluid flow when the TJ is sufficiently leaky, we added 10 μM Sp-cAMPS to stimulate protein kinase A and thereby increase TJ permeability. We recently showed that 10 μM Sp-cAMPS increased the Na permeability of the TJ by 33% (13) and reduced transepithelial resistance to one-half of the control value (14). Although transjunctional fluid flow could not be detected after treatment of the monolayers with 10 μM Sp-cAMPS under isotonic conditions or during accelerated absorption produced by perfusion of a 100 mOsm hyperosmotic solution in the basolateral bath, a small, but statistically significant, reversed fluid flux from the LIS across the TJ was observed (Fig. 4b and Table 1) when the apical bath was made hyperosmotic by 50 mOsm.
DISCUSSION
The present results provide determinations of the velocity of fluid movement within the LIS as well as direct measurements of the magnitude of transjunctional fluid flow. We conclude that there is no significant fluid flow through the TJ of MDCK cells, even when fluid absorption is accelerated by the imposition of an osmotic gradient.
Methodological Considerations.
Our approach exploited the balance between convection and diffusion in the confines of the LIS. Use of a relatively large fluorescently labeled probe, 70,000 Mr fluorescein dextran, ensured that dye diffusion was sufficiently slow such that the effects of convection on probe distribution could be visualized. Artifacts in our measurement technique were ruled out with two approaches: the measurement of the intensity profile of a small, fast-diffusing solute (BCECF), and the demonstration that a modest transjunctional fluid flow could be detected when the TJs were made sufficiently leaky by treatment with 10 μM Sp-cAMPS.
Interpretation of the fluorescence intensity data in terms of volume flow rates required knowledge both of the diffusion coefficient for the fluorescent probe within the LIS and of the cross-sectional area of the LIS in living cells. Although the need for such measurements was established decades ago (3), the experiments only recently became feasible as they depend on three newly developed techniques: high-resolution confocal microscopy of living epithelial cells at low light levels (9), digital deblurring of confocal microscopic image stacks (14), and the determination of solute diffusion coefficients within the LIS (10).
We recently measured the diffusion coefficients of two solutes by using photo-activated fluorescence and high-speed photometry in the LIS of MDCK cells grown on glass coverslips (10). The diffusivity within the LIS of a small fluorescent solute, hydroxypyrene-1,3,6-trisulfonic acid (524 Mr), was indistinguishable from that in free solution, an observation in agreement with an earlier study of BCECF diffusivity by fluorescence recovery after photobleaching (12). Although the rate of diffusion of 70,000 Mr fluorescein dextran could not be measured because of limited solubility of the caged dye, the diffusivity of 10,000 Mr fluorescein dextran within the LIS was determined. This study showed that the diffusivity of the 10,000 Mr dextran was 60% of its free solution value in the LIS of cells grown on glass coverslips, a reduction probably caused by molecular hindrance of this large molecule. More recently, we have shown that diffusion of 10,000 Mr fluorescein dextran was further reduced to about 25% of its free solution rate in the LIS of cells grown on permeable supports, whereas the diffusivity of several small solutes remained identical to that in free solution (O.K., P. Bungay, and K.R.S., unpublished work).
LIS cross-sectional area was determined as accurately as is presently feasible by deconvolving optical sections obtained with a confocal microscope. Reduction of the scanned area was used to magnify the image with a resulting reduction of the effective pixel size to 77 nm in the image plane. The operating characteristics of the confocal had been previously determined (9) when the instrument was modified. It was shown to function near the theoretical resolution limit expected for a point-scanning, slit-detection system. Measurements of a subresolution fluorescently labeled latex bead revealed an axial full-width, half-maximum resolution of 0.55 μm and a lateral resolution of 0.4 μm (9). As expected, considerable improvement in both the axial and lateral resolution was achieved by deconvolution of a stack of optical sections. Thus, the LIS cross-sectional area measurements represent a best present-day effort for the determination of this critical parameter in living cells.
Volume flow at the TJ was not detectable under isotonic (Fig. 3a) conditions or when fluid absorption was accelerated by an increase of the basolateral bath osmolality by 50 mOsm (Fig. 3d). Uncertainty in the transjunctional volume flow estimates was very small for these cases, but a somewhat larger error was seen with apical hyperosmotic perfusates (Table 1 and Fig. 3). Such larger errors may have obscured modest reversed flows across the TJ under these rather unphysiologic conditions. However, only when Sp-cAMPS was added could a small, but significant, transjunctional volume flow be detected (Table 1 and Fig. 4b). In all cases, volume flows were less accurately predicted at the basal end of the LIS because of the wide variability seen in the geometry of this part of the intercellular space.
Implications for Fluid Transport Models.
The absence of transjunctional fluid flow in a leaky, fluid-transporting epithelium removes this complication from the model of the mechanism of fluid transport by MDCK cells. Furthermore, the flow velocities along the LIS under isotonic conditions are consistent with water extraction from the adjacent cells along the length of the cell lateral membrane as predicted in the prevailing mathematical models of fluid absorption (3, 6, 7). The high diffusivity in the LIS of small molecules such as BCECF in conjunction with the relatively low flow velocities in the LIS makes it virtually impossible to generate a “standing osmotic gradient” in MDCK cell epithelium. Thus, the model for transepithelial fluid absorption by MDCK epithelium is simplified considerably to one in which solute transport into the LIS creates a region of relatively uniform hypertonicity that draws water from the adjacent cells by simple osmosis. As water enters the LIS, the hydrostatic pressure within the LIS rises. Because of the low distensibility of this compartment (11) fluid is forced across the basement membrane and underlying permeable support.
Although the picture of the MDCK epithelium as a fluid-transporting tissue is virtually complete (3), this state is not the case in native epithelia where transjunctional flows have been postulated, such as gallbladder (1), small intestine (4), and renal proximal tubule (5). Nothing is known about the LIS ionic composition, fluid flow velocity, or diffusivity within the LIS, as well as tight junctional water permeability in these tissues. Until the direct measurements are made, speculation about the route and mechanism of transepithelial fluid flow in these epithelia will continue.
ABBREVIATIONS
- LIS
lateral intercellular spaces
- TJ
tight junction
- PEG
polyethylene glycol
- Sp-cAMPS
(Sp)-adenosine cyclic 3′,5′-phosphorothioate
- BCECF
2′,7′-bis(2-carboxethyl)-5(and 6)-carboxyfluorescein
References
- 1.Shachar-Hill B, Hill A E. J Physiol. 1993;468:463–486. doi: 10.1113/jphysiol.1993.sp019782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeuthen T. Int Rev Cytol. 1995;160:99–161. doi: 10.1016/s0074-7696(08)61554-5. [DOI] [PubMed] [Google Scholar]
- 3.Spring K R. Annu Rev Physiol. 1998;60:105–119. doi: 10.1146/annurev.physiol.60.1.105. [DOI] [PubMed] [Google Scholar]
- 4.Madara J L, Pappenheimer J R. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 5.Whittembury G, Malnic G, Mello-Aires M, Amorena C. Pflügers Arch. 1988;412:541–547. doi: 10.1007/BF00582545. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein A M. J Gen Physiol. 1987;89:501–518. doi: 10.1085/jgp.89.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein A M. Am J Physiol. 1988;254:F297–F305. doi: 10.1152/ajprenal.1988.254.3.F297. [DOI] [PubMed] [Google Scholar]
- 8.Chatton J-Y, Spring K R. J Membr Biol. 1994;140:89–99. doi: 10.1007/BF00232897. [DOI] [PubMed] [Google Scholar]
- 9.Nitschke R, Spring K R. J Microsc Soc Am. 1995;1:1–11. [Google Scholar]
- 10.Xia, P., Bungay, P. M., Gibson, C. C., Kovbasnjuk, O. N. & Spring, K. R. (1998) Biophys. J., in press. [DOI] [PMC free article] [PubMed]
- 11.Timbs M M, Spring K R. J Membr Biol. 1996;153:1–11. doi: 10.1007/s002329900104. [DOI] [PubMed] [Google Scholar]
- 12.Harris P J, Chatton J-Y, Tran P H, Bungay P M, Spring K R. Am J Physiol. 1994;266:C73–C80. doi: 10.1152/ajpcell.1994.266.1.C73. [DOI] [PubMed] [Google Scholar]
- 13.Kovbasnjuk O, Chatton J-Y, Friauf W S, Spring K R. J Membr Biol. 1995;148:223–232. doi: 10.1007/BF00235040. [DOI] [PubMed] [Google Scholar]
- 14.Kovbasnjuk O N, Szmulowicz U, Spring K R. J Membr Biol. 1998;161:93–104. doi: 10.1007/s002329900317. [DOI] [PubMed] [Google Scholar]