Abstract
Small-molecule Smac mimetics are being developed as a novel class of anticancer drugs. Recent studies have shown that Smac mimetics target cellular inhibitor of apoptosis protein (cIAP)-1/2for degradation and induce tumor necrosis factor-α (TNFα)–dependent apoptosis in tumor cells. In this study, we have investigated the mechanism of action and therapeutic potential of two different types of novel Smac mimetics, monovalent SM-122 and bivalent SM-164. Our data showed that removal of cIAP-1/2 by Smac mimetics or small interfering RNA is not sufficient for robust TNFα-dependent apoptosis induction, and X-linked inhibitor of apoptosis protein (XIAP) plays a critical role in inhibiting apoptosis induction. Although SM-164 is modestly more effective than SM-122 in induction of cIAP-1/2 degradation, SM-164 is 1,000 times more potent than SM-122 as an inducer of apoptosis in tumor cells, which is attributed to its much higher potency in binding to and antagonizing XIAP. SM-164 induces rapid cIAP-1 degradation and strong apoptosis in the MDA-MB-231 xenograft tumor tissues and achieves tumor regression, but has no toxicity in normal mouse tissues. Our study provides further insights into the mechanism of action for Smac mimetics and regulation of apoptosis by inhibitor of apoptosis proteins. Furthermore, our data provide evidence that SM-164 is a promising new anticancer drug for further evaluation and development.
Introduction
Defects in the apoptosis machinery provide a survival advantage to cancer cells and confer resistance of cancer cells to current anticancer therapies (1, 2). Targeting critical apoptosis inhibitors is an attractive new cancer therapeutic strategy (2–4). Inhibitor of apoptosis proteins (IAP) are a class of key apoptosis regulators characterized by the presence of one to three domains known as baculoviral IAP repeat (BIR) domains (5–7). Among these IAP proteins, cellular IAP-1 (cIAP-1) and cIAP-2 play a critical role in regulation of tumor necrosis factor (TNF) receptor–mediated apoptosis (8–10), and X-linked IAP (XIAP) is a central regulator of both death receptor–mediated and mitochondria-mediated apoptosis pathways. XIAP inhibits apoptosis by suppressing caspase activity (7, 11–14). Whereas the third BIRdomain (BIR3) of XIAP selectively targets an initiator caspase-9 (7), the BIR2 domain, together with the linker immediately preceding it, inhibits effector caspase-3/caspase-7 (12–14). Consistent with their role in inhibition of apoptosis, XIAP and cIAP-1 were found to be highly expressed in cancers of diverse tumor types (15–18) and are considered as attractive new cancer therapeutic targets (19, 20).
Second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low isoelectric point (Smac/DIABLO) is an endogenous antagonist of IAP proteins (21, 22). In its dimeric form, Smac via its AVPI tetrapeptide binding motif interacts with both BIR2 and BIR3 domains in XIAP and abrogates the inhibition of caspase-3/caspase-7 and caspase-9 by XIAP (23, 24). Smac also binds to cIAP-1/2 and can cause the degradation of cIAP-1 (25, 26). Intense research efforts have been devoted to the design and development of small-molecule Smac mimetics as a new class of anticancer drugs (4, 27–32). Two types of Smac mimetics have been reported (i.e., monovalent and bivalent Smac mimetics). The monovalent compounds are designed to mimic the binding of a single AVPI binding motif to IAP proteins (27–29), whereas the bivalent compounds contain two AVPI binding motif mimetics tethered together through a linker (4, 30–32). Both types of Smac mimetics have been shown to induce apoptosis in solid tumor and leukemia cells as single agents (4, 27, 28, 30, 33).
Several recent independent studies have shown that Smac mimetics induce rapid degradation of cIAP-1/2, which leads to nuclear factor-κB activation, production and secretion of TNFα, and TNFα-dependent induction of apoptosis (31, 32, 34, 35). These studies established that induction of cIAP-1/2 degradation is a key early event in apoptosis induction by Smac mimetics, and cIAP-1/2 are critical cellular targets for Smac mimetics. Interestingly, although Smac mimetics were designed based on the interaction of XIAP and Smac (4, 27, 28), the role of XIAP in apoptosis induction by Smac mimetics has not been well defined.
In this study, we investigated the mechanism of action and therapeutic potential of two different types of Smac mimetics: monovalent SM-122 and bivalent SM-164 (ref. 30; Fig. 1A). We showed that removal of cIAP-1/2 by Smac mimetics or small interfering RNA (siRNA) is not sufficient for TNFα-dependent apoptosis induction. Using complementary approaches, we showed that XIAP plays a critical role in inhibiting apoptosis induction by Smac mimetics. By concurrently targeting cIAP-1/2 for degradation and efficiently antagonizing XIAP, bivalent SM-164 is an extremely potent inducer of apoptosis in tumor cells and in xenograft tumor tissues as a single agent. SM-164 effectively inhibits tumor growth and is capable of achieving tumor regression, but causes no toxicity in normal mouse tissues. This study provides further insights into the mechanism of action for Smac mimetics and regulation of apoptosis by IAPs. Furthermore, our data provide evidence that SM-164 is a promising new anticancer drug for further evaluation and development.
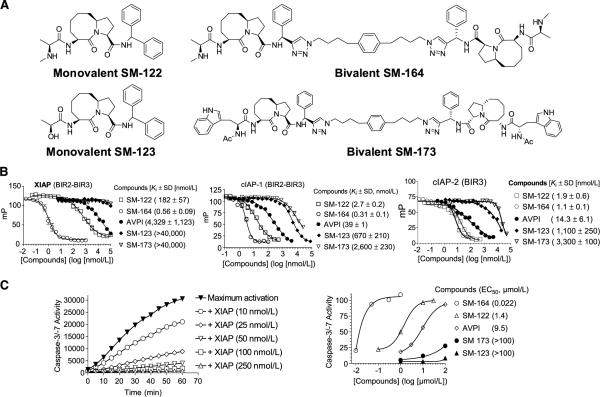
Figure 1.
Nonpeptidic, monovalent, and bivalent Smac mimetics bind to cIAP-1/2 and XIAP proteins with high affinities and antagonize XIAP. A, chemical structures of monovalent SM-122 and bivalent SM-164. B, competitive binding of Smac mimetics and their control compounds to recombinant XIAP and cIAP-1 proteins containing both BIR2 and BIR3 domains and to cIAP-2 protein containing only the BIR3 domain, as determined using fluorescence polarization assays. C, dose-dependent inhibition of caspase-3/caspase-7 activity by recombinant XIAP containing both BIR2 and BIR3 domains and antagonism of Smac mimetics against XIAP to restore the activity of caspase-3/caspase-7.
Materials and Methods
Drugs, reagents, and antibodies
SM-122, SM-164, SM-123, and SM-173 were synthesized using the methods described previously (30) and were determined by high-performance liquid chromatography to have >95% purity. Taxotere was purchased from the University of Michigan Hospital pharmacy. MG-132, Z-IETD-FMK, and Z-DEVD-FMK were purchased from Calbiochem. The following primary antibodies were used in the study: anti–cleaved caspase-8, anti-XIAP, and anti-PARP (Cell Signaling Technology); anti–cIAP-1 (a kind gift from John Silke, La Trobe University, Victoria, Australia); anti–cIAP-2 (R&D Systems); and anti–caspase-3, anti–caspase-9, and anti–procaspase-8 (Stressgen Biotechnologies) for Western blot analysis.
Binding assays
Binding affinities of Smac mimetics to different XIAP, cIAP-1, and cIAP-2 proteins were determined using fluorescence polarization–based assays and the details are provided in Supplementary data.
Cell lines and cell culture
MDA-MB-231 breast cancer, SK-OV-3 ovarian cancer, and MALME-3M melanoma cell lines were obtained from the American Type Culture Collection and maintained in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin-streptomycin at 37°C in 5% CO2. Isogenic XIAP wild-type (XIAP+/+) and XIAP knockout (XIAP−/−) HCT-116 colon cancer cell lines were a kind gift from Dr. Fred Bunz (Johns Hopkins University, Baltimore, MD). Jurkat cell lines transfected with vector control or XIAP plasmid were a kind gift from Dr. Colin Duckett (University of Michigan, Ann Arbor, MI).
Cell growth, cell death, and apoptosis
Cell growth was evaluated by a WST-8 assay (Dojindo) as described previously (30). Cell viability was quantitated by microscopic examination in a trypan blue exclusion assay. Apoptosis analysis was done using an Annexin V/propidium iodide apoptosis detection kit (Roche) according to the manufacturer's instructions.
Western blot analysis
Cells and xenograft tumor tissues were lysed using radioimmunoprecipitation assay lysis buffer (PBS containing 1% NP40, 0.5% Na-deoxycholate, and 0.1% SDS) supplemented with 1 μmol/L phenylmethylsulfonyl fluoride and 1 protease inhibitor cocktail tablet per 10 mL on ice for 20 min, and lysates were then cleared by centrifugation before protein concentration determination using the Bio-Rad protein assay kit according to the manufacturer's instructions. Proteins were electrophoresed onto 4–20% SDS-PAGE gels (Invitrogen) and transferred onto polyvinylidene difluoride membranes. Following blocking in 5% milk, membranes were incubated with a specific primary antibody, washed, and incubated with horseradish peroxidase–linked secondary antibody (Amer-sham). The signals were visualized with the chemiluminescent horseradish peroxidase antibody detection reagent (Denville Scientific). When indicated, the blots were stripped and reprobed with a different antibody.
RNA interference
SiRNA was used to knock down caspase-3, caspase-8, caspase-9, XIAP, cIAP-1 (Dhamarcon Research, Inc.), and cIAP-2 (BD Biosciences). Nontargeting control siRNA was purchased from Ambion. Transfections were done using Lipofectamine RNAiMAX (Invitrogen) in the reverse manner according to the manufacturer's instructions. Briefly, 5 to 10 nmol siRNA and 5 μL Lipofectamine RNAiMAX were mixed in each well of six-well plates for 20 min, followed by growing 3 × 105 cells in the siRNA mix for 24 to 48 h; knockdown efficacy was examined with Western blotting. Transfected cells were treated as indicated.
Measurement of TNFα concentration
Secreted TNFα concentrations were determined in 200 μL of cell culture medium using the Quantikine HS Human TNFα ELISA kit (R&D Systems). The assay was done according to the manufacturer's instructions.
In vivo pharmacodynamic characterization
For in vivo pharmacodynamic studies, the MDA-MB-231 xenograft tumor model was used. To develop xenograft tumors, 5 × 106 MDA-MB-231 cancer cells with Matrigel were injected s.c. on the dorsal side of severe combined immunodeficient (SCID) mice from Charles River, one tumor per mouse. Mice bearing MDA-MB-231 xenograft tumors were treated with a single i.v. dose of SM-164 at 5 mg/kg, Taxotere at 7.5 mg/kg, or vehicle control. Tumor tissues and normal mouse tissues were harvested at indicated time points. Tumor tissues were analyzed by Western blotting to examine levels of cIAP-1 and XIAP, caspase processing, and poly(ADP-ribose) polymerase (PARP) cleavage in tumor tissues. Tumor and normal mouse tissues were examined by terminal deoxyribonucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) staining, as described in Supplementary Methods, to detect apoptosis induction and by H&E staining for histopathologic change. All animal experiments were done under the guidelines of the University of Michigan Committee for Use and Care of Animals.
In vivo antitumor efficacy study
SCID mice (8–10 per group) bearing MDA-MB-231 xenograft tumors were treated i.v. with 1 and 5 mg/kg of SM-164 or 7.5 mg/kg of Taxotere or vehicle control daily, 5 d/wk for 2 wk. Tumor sizes and animal weights were measured thrice a week. Data are represented as mean tumor volumes ± SE.
Statistical analysis
Statistical analyses were done by two-way ANOVA and unpaired two-tailed t test, using Prism (version 4.0, GraphPad). P < 0.05 was considered statistically significant.
Results
Smac mimetics bind to multiple IAP proteins with high affinities
SM-122 was designed as a nonpeptidic mimetic of the Smac AVPI peptide, and SM-164 as a bivalent Smac mimetic containing two SM-122 analogues tethered together by a chemical linker (Fig. 1A; ref. 30). SM-123 and SM-173 were designed as much less potent monovalent and bivalent control compounds, respectively (Fig. 1A; ref. 30).
The binding affinities of SM-164 and SM-122 to XIAP, cIAP-1, and cIAP-2 proteins were determined using fluorescence-polarization based assays (Fig. 1B; Supplementary Figs. S1–S3). SM-164 and SM-122 have Ki values of 0.56 and 182 nmol/L, respectively, to XIAP protein containing both BIR2 and BIR3 domains (Fig. 1B). SM-164 and SM-122 have Ki values of 0.31 and 2.7 nmol/L, respectively, to cIAP-1 protein containing both BIR2 and BIR3 domains (Fig. 1B). Because we were unable to obtain soluble cIAP-2 protein containing both BIR2 and BIR3 domains, we evaluated the binding affinities of our Smac mimetics to cIAP-2 BIR3-only protein. SM-164 and SM-122 bind to cIAP-2 BIR3 protein with Ki values of 1.1 and 1.9 nmol/L, respectively (Fig. 1B). Hence, bivalent SM-164 is 300 times more potent than the monovalent SM-122 for binding to XIAP and is 9 times more potent than SM-122 for binding to cIAP-1. SM-122 and SM-164 have similar high binding affinities to cIAP-2. The monovalent control compound, SM-123, is 100 to 1,000 times less potent than SM-122, and SM-173 is 1,000 times less potent than SM-164, for binding to these IAP proteins (Fig. 1B).
Because XIAP functions as a potent antagonist of caspase-3/caspase-7 (12–14), we evaluated SM-164 and SM-122 for their ability to antagonize XIAP in cell-free functional assays. Recombinant XIAP containing linker-BIR2-BIR3 (residues 120–356) effectively inhibited the activity of caspase-3/caspase-7 in a dose-dependent manner and achieved complete inhibition at 50 nmol/L (Fig. 1C). Both SM-164 and SM-122 dose-dependently antagonized XIAP and promoted caspase activity, but SM-164 was 60 times more potent than SM-122 (Fig. 1C). SM-123 and SM-173 had minimal effect in this functional assay up to 100 μmol/L (Fig. 1C). Thus, these biochemical data show that the major difference between bivalent SM-164 and monovalent SM-122 is their potency in targeting XIAP. This is consistent with our previous study that SM-164 is >100 times more potent than SM-122 in competing off a biotinylated Smac mimetic to cellular XIAP in an immunoprecipitation pull-down assay (30).
Smac mimetics induce caspase-8– and caspase-3–dependent apoptosis in cancer cells
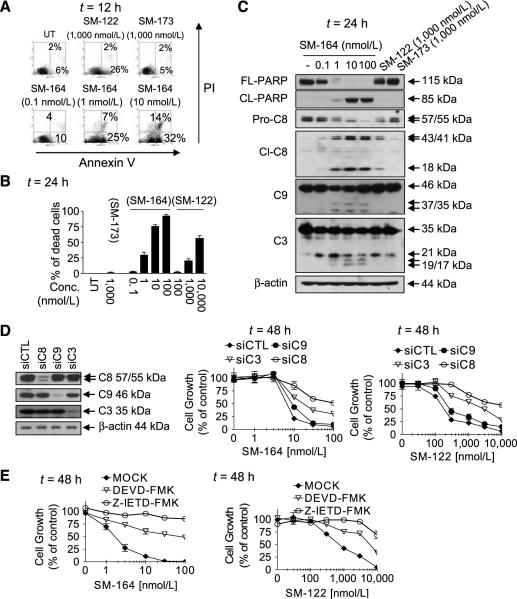
Smac mimetics were found to be effective in induction of apoptosis as single agents in a number of tumor cell lines (data not shown). We selected the MDA-MB-231 breast cancer, SK-OV-3 ovarian cancer, and MALME-3M melanoma cell lines as representative sensitive models for detailed studies.
Both SM-164 and SM-122 induced apoptosis and cell death in each of these three cancer cell lines in a dose-dependent manner, but SM-164 was much more potent than SM-122 (Fig. 2A and B; Supplementary Figs. S5 and S6). For example, treatment with SM-164 at 1 nmol/L for 12 hours induced 32%, 33%, and 37% of the MDA-MB-231, SK-OV-3 and MALME-3M cells to undergo apoptosis, respectively (Fig. 2A; Supplementary Figs. S5A and S6A). Western blot analysis further showed that SM-164 markedly decreased the levels of procaspase-3, caspase-8, caspase-9, and pro-PARP and increased the levels of processed caspase-8, caspase-9, and caspase-3 and cleaved PARP in all the three cell lines at 1 to 10 nmol/L (Fig. 2C; Supplementary Figs. S5C and S6B). SM-164 at 1 nmol/L was as effective as SM-122 at 1,000 nmol/L in induction of apoptosis, cell death, caspase processing, and PARP cleavage. Furthermore, SM-164 induced caspase processing and PARP cleavage in a time-dependent manner (Supplementary Fig. S4).
Figure 2.
Monovalent and bivalent Smac mimetics induce apoptosis in the MDA-MB-231 cancer cell line in a caspase-3– and caspase-8–dependent manner. MDA-MB-231 cells were treated as indicated. Apoptosis was analyzed by propidium iodide (PI)/Annexin V double staining using flow cytometry (A); cell viability was determined by trypan blue dye exclusion assay (B); and cleavage of caspases and PARP was analyzed by Western blotting (C). D, cells were transfected with siRNA against caspase-3, caspase-8, and caspase-9 for 48 h, followed by treatment with SM-164 (middle) or SM-122 (right) for 48 h. Knockdown efficacy was examined by Western blotting and cell growth inhibitory activity by a WST assay. E, cells were treated with Z-DEVD-FMK (25 μmol/L) or Z-IETD-FMK (25 μmol/L) for 1 h, followed by treatment with a Smac mimetic for 48 h. Cell growth inhibitory activity was examined by a WST assay. Points, mean of triplicates; bars, SD.
To examine the role of caspase-8, caspase-3, and caspase-9 in apoptosis induction by Smac mimetics, we used siRNA against these caspases in MDA-MB-231 and SK-OV-3 cancer cell lines. Knockdown of caspase-3 and caspase-8 markedly attenuated the activity of both SM-164 and SM-122, whereas knockdown of caspase-9 had a minimal effect (Fig. 2D; Supplementary Fig. S5D). In addition, Z-IETD-FMK, a selective caspase-8 inhibitor, and Z-DEVD-FMK, a selective caspase-3/caspase-7 inhibitor, markedly inhibited the activity of both Smac mimetics (Fig. 2E; Supplementary Fig. S5E). Hence, although these three caspases are all processed, only caspase-8 and caspase-3 play a crucial role in apoptosis induction by Smac mimetics and caspase-9 seems to have a minimal role, consistent with a previous study (34).
Smac mimetics induce TNFα-dependent apoptosis
Recent studies have shown that Smac mimetics induce apoptosis in tumor cells through a TNFα-dependent pathway (31, 32, 34, 35). Indeed, these sensitive cancer cell lines secreted detectable levels of TNFα in the cell culture medium without Smac mimetic treatment, and the secretion of TNFα was further enhanced by SM-164 and SM-122 (Supplementary Fig. S7A and B). Furthermore, cell death induction by SM-164 and SM-122 was effectively blocked by a TNFα, but not by a TNF-related apoptosis-inducing ligand neutralizing antibody (Fig. 3A; Supplementary Fig. S7C and D), indicating that apoptosis induction by both SM-164 and SM-122 is TNFα dependent.
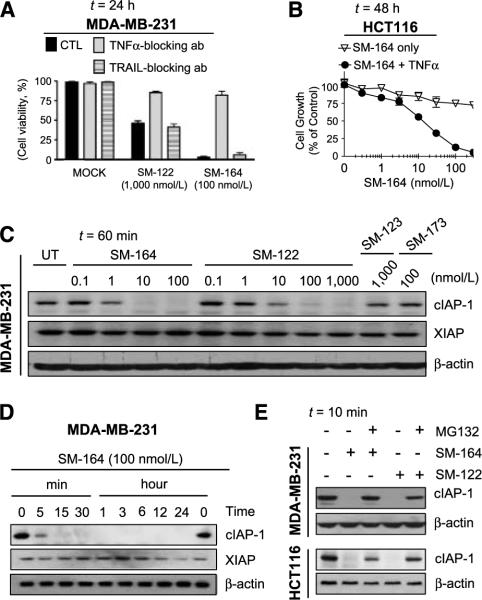
Figure 3.
Smac mimetics induce TNFα-dependent apoptosis and cIAP-1 degradation. A, MDA-MB-231 cells were pretreated with or without TNFα-or TRAIL- blocking antibody, followed by treatment with Smac mimetics. Cell viability was determined with trypan blue assay. B, HCT116 colon cancer cells were treated with SM-164 alone, TNFα alone, or the combination for 48 h. Cell growth inhibition was determined by a WST assay. C and D, MDA-MB-231 cells were treated with Smac mimetics as indicated and the levels of cIAP-1 and XIAP were examined by Western blotting. E, MDA-MB-231 or HCT116 cells treated with or without MG-132, followed by the treatment with 100 nmol/L of SM-122 or SM-164. The levels of cIAP-1 were examined by Western blotting.
To explore whether the resistance of cancer cells to Smac mimetics is due to the lack of secreted TNFα, we examined the levels of secreted TNFα. All resistant cancer cell lines tested, such as the MDA-MB-453 breast, DU-145 prostate, and MDA-MB-435 melanoma cancer cell lines, had undetectable levels of TNFα in the cell culture medium (Supplementary Fig. S7A). Both SM-164 and SM-122 also failed to induce detectable levels of TNFα in these resistant cancer cell lines (Supplementary Fig. S7B and data not shown). However, addition of exogenous TNFα can significantly enhance the activity of these Smac mimetics, especially for SM-164, in resistant cancer cell lines such as HCT116 and MDA-MB-453 (Fig. 3B; Supplementary Fig. S7E). These data indicated that the secreted TNFα is essential for the activity of Smac mimetics as single agents.
Both SM-164 and SM-122 induce rapid cIAP-1 degradation in cancer cells
Consistent with previous studies (31, 32, 34), both SM-122 and SM-164 effectively and potently induced cIAP-1 degradation in sensitive cancer cell lines (Fig. 3C–E; Supplementary Fig. S8). SM-164 at 1 nmol/L for 60 minutes reduced cIAP-1 markedly and at 10 to 100 nmol/L to undetectable levels, and SM-122 effectively induced degradation of cIAP-1 at 10 to 100 nmol/L (Figs. 3C and E and 4A and B; Supplementary Fig. S8A). Induction of cIAP-1 degradation by both compounds was very rapid, occurring within 5 to 10 minutes of drug treatment (Fig. 3D and E; Supplementary Fig. S8B and C). SM-164 and SM-122 also induced rapid cIAP-1 degradation in resistant cancer cell lines (Fig. 3E; Supplementary Fig. S9A and B). MG-132, a proteasome inhibitor, effectively blocked cIAP-1 degradation by SM-122 and SM-164 in both sensitive and resistant cancer cell lines (Fig. 3E; Supplementary Fig. S8C), indicating that cIAP-1 degradation by SM-122 and SM-164 is proteasome mediated, consistent with the observation for other Smac mimetics (31, 32). The levels of XIAP protein in MDA-MB-231 and SK-OV-3 cancer cell lines were also reduced on treatment with SM-164 (Fig. 3D; Supplementary Fig. S8B). However, the reduction of XIAP took place at the 12- to 24-hour time points, several hours after robust apoptosis induction, suggesting that degradation of XIAP is not required for apoptosis induction by Smac mimetics.
Figure 4.
Removal of cIAP-1/2 alone is not sufficient to induce robust TNFα-dependent apoptosis. MDA-MB-231 (A) and SK-OV-3 (B) cell lines were treated with SM-122 alone, TNFα alone, or the combination for 24 h. The levels of cIAP-1 and cIAP-2 were examined by Western blotting and cell viability was determined by trypan blue assay. MDA-MB-231 (C) and SK-OV-3 (D) cell lines were transfected with siRNA against cIAP-1, cIAP-2, both cIAP-1 and cIAP-2, or control siRNA for 48 h, followed by treatment with TNFα for 24 h. The levels of cIAP-1 and cIAP-2 were examined by Western blotting and cell viability was determined by trypan blue assay.
Removal of cIAP-1/2alone is not sufficient to induce TNFα-dependent apoptosis
We observed that SM-122 at 100 nmol/L markedly decreased the levels of cIAP-1 (Figs. 3C and E and 4A and B; Supplementary Fig. S8A and C) and cIAP-2 (Fig. 4A and B), but induced minimal cell death in each sensitive cell line at this concentration (Figs. 2B and 4A and B; Supplementary Figs. S5B and S6B), suggesting that removal of cIAP-1/2 alone by Smac mimetics may not be sufficient for apoptosis induction.
To investigate whether the lack of apoptosis induction by SM-122 at 100 nmol/L is due to insufficient levels of secreted TNFα, we treated cells with an excess amount of exogenous TNFα in combination with SM-122. Addition of exogenous TNFα only had minimal effect on cell killing by SM-122 (Fig. 4A and B), indicating that the lack of robust apoptosis induction by SM-122 at 100 nmol/L is not simply due to insufficient amount of secreted TNFα.
Furthermore, efficient knockdown of cIAP-1 or cIAP-2 by siRNA, individually or together, had little effect on cell death in the MDAMB-231 and SK-OV-3 cell lines, with or without exogenous TNFα (Fig. 4C; Supplementary Fig. S10). Taken together, our data show that removal of cIAP-1/2 is not sufficient to achieve robust TNFα-dependent cell killing.
XIAP is a critical cellular target for Smac mimetics
Caspase-3 plays a crucial role in apoptosis induction by Smac mimetics (Fig. 2D and E; Supplementary Fig. S5D and E; ref. 34). Because XIAP binds to and inhibits caspase-3, we reasoned that the inhibition of caspase-3 by XIAP must be relieved for efficient apoptosis induction by Smac mimetics and XIAP is a critical cellular target for Smac mimetics.
To investigate the role of XIAP in apoptosis induction by Smac mimetics, we knocked down XIAP in sensitive cancer cell lines. Because XIAP knockdown can attenuate the production and secretion of TNFα (Supplementary Fig. S11A; ref. 36), exogenous TNFα was added in these XIAP knockdown experiments. The results showed that in all three cancer cell lines, efficient knockdown of XIAP by siRNA dramatically sensitized cancer cells to SM-122 in the presence of exogenous TNFα (Fig. 5A; Supplementary Fig. S11B and C). For example, whereas SM-122 at 100 nmol/L in combination with TNFα killed 25% of the MDA-MB-231 cells when control siRNA was used, the same combination killed 75% of the cells when XIAP was knocked down. These data showed that degradation of cIAP-1/2 by SM-122 and down-regulation of XIAP by siRNA are highly effective in induction of TNFα-dependent apoptosis. These data further suggested that SM-122 at 100 nmol/L effectively induced cIAP-1/2 degradation, but is ineffective in antagonizing cellular XIAP in these cancer cells, consistent with its relatively weak functional antagonism against XIAP (Fig. 1C). However, SM-122 at higher concentrations was still capable of inducing robust cell death in these sensitive cancer cell lines (Fig. 2B; Supplementary Figs. S5B and S6B), indicating that SM-122 can still effectively antagonize cellular XIAP but higher concentrations are needed, consistent with the functional data (Fig. 1C).
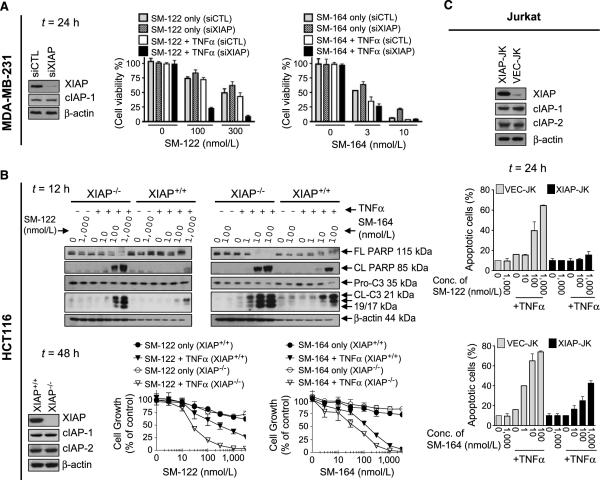
Figure 5.
XIAP plays an important role in mediating TNFα-dependent apoptosis induced by Smac mimetics. A, MDA-MB-231 cell line was transfected with XIAP siRNA for 48 h, followed by the treatment of SM-122 or SM-164 in combination with TNFα (10 ng/mL) for 48 h. Cell viability was determined by trypan blue dye exclusion. B, HCT 116 XIAP+/+ and XIAP−/− cell lines were treated with SM-122 or SM-164 alone or in combination with TNFα (0.1 ng/mL) as indicated. PARP cleavage and activation of caspase-3 were examined by Western blotting and cell growth inhibition by a WST assay. C, Jurkat cell lines were treated with SM-122, SM-164, TNFα (300 ng/mL) alone, or the combination of SM-122 or SM-164 with TNFα for 24 h. Apoptosis was analyzed by propidium iodide staining/Annexin V double staining using flow cytometry.
In sharp contrast, SM-164 at 3 to 10 nmol/L effectively induced cell death with or without TNFα in all these sensitive cancer cell lines (Fig. 5A; Supplementary Fig. S11B and C). Furthermore, knockdown of XIAP only modestly sensitized the cancer cells to SM-164 in the presence of exogenous TNFα in these sensitive cancer cell lines. These data suggested that SM-164 at these concentrations not only efficiently induces marked cIAP-1 degradation but also effectively antagonizes cellular XIAP. This is consistent with our binding and functional data that SM-164 binds to XIAP with a very high affinity and is an ultrapotent antagonist of XIAP (Fig. 1B and C).
Knockout of XIAP sensitizes cancer cells to Smac mimetics for TNFα-dependent apoptosis induction
To complement the siRNA experiments and to more precisely define the role of XIAP in TNFα-dependent apoptosis induction by Smac mimetics, we used HCT116 XIAP+/+ and XIAP−/− isogenic cell lines (37). Because both these cell lines were resistant to SM-122 and SM-164 as single agents (Supplementary Fig. S9A), we investigated the role of XIAP using Smac mimetics in combination with TNFα. SM-122 at 100 to 1,000 nmol/L effectively induced cIAP-1 degradation in both cell lines (Supplementary Fig. S9A, bottom). The combination of SM-122 at 100 to 1,000 nmol/L with exogenous TNFα had a modest effect in induction of caspase-3 activation, PARP cleavage, and cell growth inhibition in the HCT-116 XIAP+/+ cell line (Fig. 5B). In contrast, the same combination was highly effective in the isogenic XIAP−/− cell line in induction of robust caspase-3 activation and strong PARP cleavage and in inhibition of cell growth (Fig. 5B). These data clearly showed that XIAP strongly attenuated TNFα-dependent apoptosis induction by SM-122 in the HCT116 cells and knockout of XIAP dramatically sensitized the cells to SM-122 in combination with TNFα.
SM-164 effectively induced cIAP-1 degradation at 10 to 100 nmol/L in both cell lines (Supplementary Fig. S9A). The combination of SM-164 at these concentrations with TNFα was effective in induction of caspase-3 activation and PARP cleavage and in inhibition of cell growth in the XIAP+/+ cell line (Fig. 5B). Although knockout of XIAP sensitized the HCT116 cells to SM-164 in combination with TNFα, the effect was much less than that observed with the combination of SM-122 with TNFα (Fig. 5B).
Overexpression of XIAP renders cancer cells resistant to TNFα-dependent apoptosis induction by Smac mimetics
We next investigated the effect of XIAP overexpression in cancer cells on TNFα-dependent apoptosis induction by Smac mimetics. Because stable transfection of XIAP in those sensitive cancer cell lines was unsuccessful, we used the previously well-characterized XIAP stably transfected Jurkat leukemia cell line (XIAP-JK) and its vector control cell line (VEC-JK; ref. 38). The VEC-JK cell line with low expression of XIAP was sensitive to SM-122 or SM-164 in combination with TNFα(Fig. 5C). In striking contrast, the XIAP-JK cell line with high levels of XIAP expression became extremely resistant to the combination of SM-122 with TNFα. For instance, whereas SM-122 at 100 and 1,000 nmol/L efficiently and rapidly degraded cIAP-1 in XIAP-JK cells (Supplementary Fig. S9B), it was ineffective in induction of apoptosis in combination with TNFα (Fig. 5C). Moreover, although the combination of SM-164 with TNFα was capable of inducing apoptosis in the XIAP-JK cell line, it was much less effective than in the VEC-JK cell line. These data show that overexpression of XIAP effectively attenuates TNFα-dependent apoptosis induction by both SM-164 and SM-122 but with a stronger effect on SM-122.
Collectively, the data obtained using complementary approaches provide strong evidence that XIAP plays a key role in attenuating apoptosis induction by Smac mimetics. Because bivalent SM-164 is a more potent antagonist of XIAP than monovalent SM-122, it exhibits much stronger potency than SM-122 in TNFα-dependent apoptosis induction.
SM-164 rapidly degrades cIAP-1 and displays strong in vivo antitumor activity
To further investigate its therapeutic potential and in vivo mechanism, we evaluated SM-164 in the MDA-MB-231 xenograft model in mice.
SM-164 induced rapid cIAP-1 degradation and robust apoptosis in the MDA-MB-231 xenograft tumor tissues (Fig. 6A). A single dose of SM-164 at 5 mg/kg markedly decreased the level of cIAP-1 protein within 1 hour and the effect lasted for at least 24 hours. Robust activation of caspase-8, caspase-9, and caspase-3 and cleavage of PARP were observed at the 3-hour time point and persisted for 24 hours. A TUNEL assay further showed that SM-164 induced strong apoptosis in tumor tissues as early as the 3-hour time point, and more than 50% of tumor cells were TUNEL positive at the 6-hour time point (Fig. 6C), consistent with the strong caspase processing and PARP cleavage at this time point (Fig. 6A). The strong apoptosis induction by SM-164 was still evident at the 24-hour time point. H&E staining showed that SM-164 caused profound damage to tumor tissues (Fig. 6D). In contrast to the strong apoptosis induction and damage in xenograft tumor tissues, SM-164 had no effect on all the normal mouse tissues examined, including highly proliferative tissues such as the small intestine, stomach, liver, and spleen (Fig. 6D; Supplementary Fig. S12A and B).
Figure 6.
SM-164 induces rapid cIAP-1 degradation and robust apoptosis in tumor tissues and achieves tumor regression, but causes minimal toxicity to mouse tissues. A, SCID mice (two mice per group) bearing established MDA-MB-231 xenograft tumors were treated with a single i.v. dose of SM-164, Taxotere (TXT), or vehicle (VEH). Tumor tissues were harvested at the indicated time points. Degradation of cIAP-1 and XIAP, activation of caspases, and PARP cleavage in tumor tissues were analyzed by Western blotting. B, SCID mice (two mice per group) bearing established MDA-MB-231 xenograft tumors were treated with a single i.v. dose of SM-122 or vehicle. Tumor tissues were harvested at the indicated time points. cIAP-1 degradation and PARP cleavage in tumor tissues were analyzed by Western blotting. Tumor tissues harvested from mice treated with SM-164 were included as controls. C, apoptosis by TUNEL staining. To score apoptosis, at least 1,000 cells were counted under a microscope. D, SCID mice (two to three mice per group) bearing established MDA-MB-231 xenograft tumors were treated with a single dose of SM-164 (5 mg/kg, i.v.) or vehicle. Tumor and mouse tissues were harvested at the 24-h time point and examined by H&E staining. Xenograft tumor tissues treated with SM-164 were shown with cell shrinkage, nuclear pyknosis, and chromatin condensation. E, SCID mice (8–10 per group) bearing established MDA-MB-231 xenograft tumors were treated i.v. with SM-164, Taxotere, or vehicle. Points, mean tumor volume; bars, SE.
Using the MDA-MB-231 xenograft model, we also examined SM-122 for its ability to induce cIAP-1 degradation and apoptosis in tumor tissues in vivo. Administration of a single dose of SM-122 at 100 mg/kg i.v., a near maximum tolerated dose, effectively induced cIAP-1 degradation in tumor tissues but had minimal effect on PARP cleavage (Fig. 6B). Hence, our in vivo data further indicated that cIAP-1 degradation is not sufficient for apoptosis induction.
We next evaluated SM-164 for its ability to inhibit tumor growth. Consistent with its potent activity in apoptosis induction in xenograft tumor tissues, SM-164 was highly effective in inhibition of tumor growth and capable of achieving tumor regression in the MDA-MB-231 xenograft model (Fig. 6E). Treatment with SM-164 at 1 mg/kg completely inhibited tumor growth during the treatment. Treatment with SM-164 at 5 mg/kg reduced the tumor volume from 147 ± 54 mm3 at the beginning of the treatment (day 25) to 54 ± 32 mm3 at the end of the treatment (day 36), a reduction of 65%. The strong antitumor activity by SM-164 was long lasting and not transient. Importantly, no significant weight loss or other sign of toxicity was observed for mice treated with SM-164 at 1 or 5 mg/kg (Supplementary Fig. S13). Treatment with Taxotere at 7.5 mg/kg was effective in inhibition of tumor growth, as compared with the control treatment, but failed to achieve tumor regression. SM-164 at 5 mg/kg is statistically more effective than Taxotere at the end of the treatment (P < 0.01) or when the tumor size in the control group reached 750 mm3 (P < 0.02). Taken together, our data showed that SM-164 has a very strong in vivo antitumor activity at nontoxic dose schedules.
Discussion
Several recent studies showed that Smac mimetics induce rapid degradation of cIAP-1/2, and this leads to activation of nuclear factor κB and production and secretion of TNFα, resulting in TNFα-dependent apoptosis induction (31, 32, 34). These studies establish that degradation of cIAP-1/2 by Smac mimetics is essential for apoptosis induction. Our study, however, clearly showed that removal of cIAP-1/2 alone either by a Smac mimetic or by siRNA is not sufficient for induction of TNFα-dependent apoptosis in both sensitive and resistant cancer cell lines (Fig. 4; Supplementary Fig. S14).
Using several complementary approaches, we showed that in addition to cIAP-1/2, XIAP plays a major role in regulating TNFα-dependent apoptosis induction by Smac mimetics. Down-regulation of XIAP by siRNA or XIAP knockout significantly sensitizes tumor cells to apoptosis induction by Smac mimetics (Fig. 5A and B; Supplementary Fig. S11B and C). Conversely, overexpression of XIAP markedly attenuates the activity (Fig. 5C). The important role of XIAP in regulating apoptosis induction by Smac mimetics is consistent with the notion that XIAP potently inhibits caspase-3 (12, 14) and caspase-3 plays a crucial role in TNFα-dependent apoptosis induction by Smac mimetics (Fig. 2D and E; Supplementary Fig. S5D and E; ref. 34). Hence, cIAP-1/2 and XIAP are the major and independent blockades in TNFα-dependent apoptosis by Smac mimetics, and concurrent removal of these blockades is needed to achieve efficient apoptosis induction in tumor cells.
The requirement that both cIAP-1/2 and XIAP be removed for efficient apoptosis induction by Smac mimetics also explains the differences in the cellular activities of bivalent SM-164 and monovalent SM-122. Because bivalent SM-164 contains two “AVPI” binding motifs and simultaneously interacts with both the BIR2 and BIR3 domains in XIAP (30), it is an extremely potent antagonist of XIAP (Fig. 1C). Our data strongly suggest that bivalent SM-164 at concentrations of 1 to 10 nmol/L not only efficiently induces cIAP-1 degradation but also effectively antagonizes cellular XIAP, thus leading to robust TNFα-dependent apoptosis induction.
In comparison, monovalent SM-122 binds to XIAP BIR3 with a high affinity but to BIR2 with a low affinity (30). Because the BIR2 domain, together with the immediate preceding linker, binds to and inhibits caspase-3 (12, 14), SM-122 is a much weaker antagonist of XIAP than SM-164 in relieving caspase-3 inhibition (Fig. 1C). However, SM-164 is only nine times more potent than SM-122 for binding to cIAP-1 protein containing both BIR2 and BIR3 domains. Furthermore, SM-164 and SM-122 bind to cIAP-1 protein containing only the BIR3 domain with essentially the same high affinities (Supplementary Fig. S3; Fig. 1B). Both compounds bind to cIAP-1 BIR2-containing protein with only millimolar affinities (data not shown). These data indicate that unlike with XIAP, both Smac mimetics interact with cIAP-1 primarily through the BIR3 domain. Consistently, SM-164 is only modestly more potent than SM-122 in induction of cIAP-1 degradation in cells. Therefore, our present study suggests that the much higher potency of SM-164 in antagonizing XIAP than SM-122 primarily accounts for the 1,000-fold difference in their ability to induce TNFα-dependent apoptosis in tumor cells.
Our study indicated that for robust induction of TNFα-dependent apoptosis, a Smac mimetic must be capable of not only targeting cIAP-1/2 for degradation but also effectively antagonizing XIAP to remove inhibition of caspase-3. Because most of monovalent Smac mimetics have been designed to target the BIR3 domain of XIAP (27–29), our data suggest that for the design of more effective monovalent Smac mimetics, their potency against XIAP BIR2 domain needs to be improved.
To investigate if SM-164 is selectively toxic against tumor cells, we evaluated SM-164 alone or in combination with TNFα against a panel of normal human cells (Supplementary Fig. S15). These include normal human fibroblasts, epithelial cells, and endothelial cells. Our data showed that SM-164 has no or minimal toxicity, either alone or in combination with TNFα against these normal human cells. Of interest, all the normal cells examined have very low expression of cIAP-1, as compared with tumor cell lines, suggesting that normal cells do not depend on cIAP-1 for blocking apoptotic signals.
Our in vivo data clearly show that a single dose of SM-164 is effective in induction of rapid cIAP-1 degradation, caspase activation, and strong apoptosis in the MDA-MB-231 tumor tissues, while having no toxicity to any of the normal mouse tissues examined. Hence, SM-164 is selectively toxic to tumor tissues versus normal tissues. SM-164 is effective in inhibition of tumor growth and is capable of achieving partial tumor regression.
In summary, our present study provides clear evidence that cIAP-1/2 and XIAP are crucial and independent blockades that need to be concurrently removed by Smac mimetics for efficient TNFα-dependent apoptosis to proceed. By concurrently targeting cIAP-1/2 and XIAP, SM-164 is a highly effective inducer of apoptosis in tumor cells in vitro and in vivo. Furthermore, SM-164 is selectively toxic to tumor cells in vitro and to tumor tissues in vivo. SM-164 warrants extensive testing as a new anticancer drug.
Supplementary Material
Acknowledgments
Grant support: Breast Cancer Research Foundation (S. Wang), Prostate Cancer Foundation (S. Wang), National Cancer Institute/NIH grant R01CA109025 (S. Wang), Ascenta Therapeutics, Inc. (S. Wang), and University of Michigan Cancer Center grant P30CA046592.
We thank Dr. John Silke for providing us with cIAP-1 antibody, Dr. Fred Bunz for isogenic XIAP knockout HCT-116 colon cancer cell lines, and Dr. Colin Duckett for Jurkat cell lines stably transfected with XIAP.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest S. Wang: commercial research grant, ownership interest, and consultant/advisory board, Ascenta Therapeutics. The other authors disclosed no potential conflicts of interest.
References
- 1.Ponder BA. Cancer genetics. Nature. 2001;411:336–41. doi: 10.1038/35077207. [DOI] [PubMed] [Google Scholar]
- 2.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–95. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 3.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFα-mediated cell death. Science. 2004;305:1471–4. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 5.Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999;18:5242–51. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang YL, Li XM. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 2000;10:169–77. doi: 10.1038/sj.cr.7290046. [DOI] [PubMed] [Google Scholar]
- 7.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–10. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 8.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–52. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 9.Fotin-Mleczek M, Henkler F, Samel D, et al. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J Cell Sci. 2002;115:2757–70. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]
- 10.Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFα-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 11.Sun C, Cai M, Gunasekera AH, et al. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature. 1999;401:818–22. doi: 10.1038/44617. [DOI] [PubMed] [Google Scholar]
- 12.Riedl SJ, Renatus M, Schwarzenbacher R, et al. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 13.Chai J, Shiozaki E, Srinivasula SM, et al. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104:769–80. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R. X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes. J Biol Chem. 2001;276:27058–63. doi: 10.1074/jbc.M102415200. [DOI] [PubMed] [Google Scholar]
- 15.Jaffer S, Orta L, Sunkara S, Sabo E, Burstein DE. Immunohistochemical detection of antiapoptotic protein X-linked inhibitor of apoptosis in mammary carcinoma. Hum Pathol. 2007;38:864–70. doi: 10.1016/j.humpath.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Imoto I, Tsuda H, Hirasawa A, et al. Expression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapy. Cancer Res. 2002;62:4860–6. [PubMed] [Google Scholar]
- 17.Nakagawa Y, Abe S, Kurata M, et al. IAP family protein expression correlates with poor outcome of multiple myeloma patients in association with chemotherapy-induced overexpression of multidrug resistance genes. Am J Hematol. 2006;81:824–31. doi: 10.1002/ajh.20656. [DOI] [PubMed] [Google Scholar]
- 18.Kluger HM, McCarthy MM, Alvero AB, et al. The X-linked inhibitor of apoptosis protein (XIAP) is up-regulated in metastatic melanoma, and XIAP cleavage by phenoxodiol is associated with carboplatin sensitization. J Transl Med. 2007;5:6. doi: 10.1186/1479-5876-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–59. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 20.Fulda S. Inhibitor of apoptosis proteins as targets for anticancer therapy. Expert Rev Anticancer Ther. 2007;7:1255–64. doi: 10.1586/14737140.7.9.1255. [DOI] [PubMed] [Google Scholar]
- 21.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c -dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 22.Verhagen AM, Ekert PG, Pakusch M, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 23.Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–62. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Rich RL, Myszka DG, Wu H. Requirement of both the second and third BIRdomains for the relief of X-linked inhibitor of apoptosis protein (XIAP)-mediated caspase inhibition by Smac. J Biol Chem. 2003;278:49517–22. doi: 10.1074/jbc.M310061200. [DOI] [PubMed] [Google Scholar]
- 25.Samuel T, Welsh K, Lober T, Togo SH, Zapata JM, Reed JC. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J Biol Chem. 2006;281:1080–90. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]
- 26.Yang QH, Du C. Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J Biol Chem. 2004;279:16963–70. doi: 10.1074/jbc.M401253200. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, Nikolovska-Coleska Z, Yang CY, et al. Structure-based design, synthesis, and evaluation of conformationally constrained mimetics of the second mitochondria-derived activator of caspase that target the X-linked inhibitor of apoptosis protein/caspase-9 interaction site. J Med Chem. 2004;47:4147–50. doi: 10.1021/jm0499108. [DOI] [PubMed] [Google Scholar]
- 28.Zobel K, Wang L, Varfolomeev E, et al. Design, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACS Chem Biol. 2006;1:525–33. doi: 10.1021/cb600276q. [DOI] [PubMed] [Google Scholar]
- 29.Sun H, Nikolovska-Coleska Z, Lu J, et al. Design, synthesis, and evaluation of a potent, cell-permeable, conformationally constrained second mitochondria derived activator of caspase (Smac) mimetic. J Med Chem. 2006;49:7916–20. doi: 10.1021/jm061108d. [DOI] [PubMed] [Google Scholar]
- 30.Sun H, Nikolovska-Coleska Z, Lu J, et al. Design, synthesis, and characterization of a potent, nonpeptide, cell-permeable, bivalent Smac mimetic that concurrently targets both the BIR2 and BIR3 domains in XIAP. J Am Chem Soc. 2007;129:15279–94. doi: 10.1021/ja074725f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varfolomeev E, Blankenship JW, Wayson SM, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell. 2007;131:669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Vince JE, Wong WW, Khan N, et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell. 2007;131:682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 33.Chauhan D, Neri P, Velankar M, et al. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM) Blood. 2007;109:1220–7. doi: 10.1182/blood-2006-04-015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen SL, Wang L, Yalcin-Chin A, et al. Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–56. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 36.Gaither A, Porter D, Yao Y, et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-α signaling. Cancer Res. 2007;67:11493–8. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 37.Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 2004;64:3006–8. doi: 10.1158/0008-5472.can-04-0046. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson JC, Cepero E, Boise LH, Duckett CS. Upstream regulatory role for XIAP in receptor-mediated apoptosis. Mol Cell Biol. 2004;24:7003–14. doi: 10.1128/MCB.24.16.7003-7014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.