Abstract
Purpose
The objective of this study was to extend previous published findings in the authors’ laboratory using a new automated technology to quantitatively characterize nonparticipatory perioral stiffness in healthy male adults.
Method
Quantitative measures of perioral stiffness were sampled during a nonparticipatory task using a computer-controlled linear motor servo programmed to impose a series of tensile displacements over a span of approximately 24 mm at the oral angle in 20 healthy young male adults. Perioral electromyograms were simultaneously sampled to confirm nonparticipation or passive muscle state. Perioral stiffness, derived as a quotient from resultant force (ΔF) and oral span (ΔX), was modeled with regression techniques, and subsequently compared to previously reported perioral stiffness data for female adults.
Results
Multilevel regression analysis revealed a significant quadratic relation between the perioral stiffness and interangle span; however, no significant difference was found between adult males and females.
Conclusion
These normative measures will have application to future studies designed to objectively assess the effects of pathology (i.e., progressive neuromotor disease, traumatic brain insult) and intervention (pharmacologic, neurosurgical, and reconstructive surgery of the face [i.e., cleft lip, trauma, missile injuries]) on facial animation and speech kinematics.
Keywords: upper lip, lower lip, interangle, biomechanics, rigidity
Over the past three decades, objective evaluation of oromotor performance in health and disease has included measures of active force production (Barlow & Abbs, 1984, 1986; Barlow & Burton, 1990; Barlow, Iacono, Paseman, Biswas, & D’Antonio, 1998; Barlow & Muller, 1991; Barlow & Netsell, 1986; Barlow & Rath, 1985; Gentil, Garcia-Ruiz, Pollak, Benabid, 1999; Gentil & Tournier, 1998; McHenry, Minton, Hartley, Calhoun, & Barlow, 1999; Pinto, Gentil, Fraix, Benabid, & Pollak, 2003; Trotman, Barlow, & Faraway, 2007), displacement (see review in Barlow, Finan, Andreatta, & Boliek, 2008; Fogel & Stranc, 1984), and electromyography (Barlow & Muller, 1991; Blair & Smith, 1986; Folkins & Larson, 1978). Relatively little is known, however, about the biomechanics of the lower face, including muscle and labial tissue stiffness (Barlow & Muller, 1991; Muller, Milenkovic, & MacLeod, 1985; Seibel & Barlow, 2007). Muller and his colleagues (1985) provided some of the first experimental observations on the perioral biomechanics in healthy adult subjects, and indicated that precise measurement and modeling of the peripheral motor system is essential to better understand the neural control of speech.
Muscular stiffness is defined as the resistance to imposed stretch and is obtained instrumentally by imposing a known displacement (ΔX) and measuring the resultant force (ΔF) (Ho, Azar, Weinstein & Bowley, 1982). This quantitative measure is closely related to the clinical symptom known as rigidity and is often manifest in individuals with Parkinson’s disease (PD). Rigidity of an extremity is often described by patients as pain, restriction of motion, soreness, or fatigue (Siegler & Beck, 1989). An assessment of rigidity in the patient with PD typically involves the swing test, passive displacement of the upper and lower limbs or face by the clinician. These qualitative assessment techniques can be unreliable among clinicians and rely on an extensive degree of cooperation and task comprehension by the patients (Schwab, 1964). Without instrumentation to quantify these clinical symptoms, clinicians often face difficulties differentiating the central or peripheral (i.e., muscle, joint) causes of these physical signs (Siegler & Beck, 1989). Attempts have been made to quantify rigidity by forcing the limb to a target point and extracting an impedance measure from the imposed force and displacement. However, this method requires decision on how the limb should be forced and the estimate of stiffness is greatly affected by the rate of muscle stretch (Prochazka et al., 1997; Webster, 1966). An instrumental measure of passive joint stiffness at the elbow in PD patients was correlated to the motor subscales of the Unified Parkinson’s Disease Rating System (UPDRS) (Sepheri et al., 2007). Because rigidity is subject to modulation by central and peripheral mechanisms, it has been used clinically to assess neurologic status and document the effects of disease progression, pharmacologic and neurosurgical intervention (Barlow & Hammer, 2008; Prochazka et al., 1997), and most recently to explore the effects of cheiloplasty in children with cleft lip (Trotman et al., 2007).
Muscular stiffness is essential to the regulation of posture and interjoint coordination (Nichols, 2002). The mechanical properties of skeletal muscle arise from the integration of sensory feedback, descending inputs, and the intrinsic mechanics of the muscle (Shiller, Laboissière, & Ostry, 2002; Struppler, 1993). Damage to peripheral nerves and the muscle itself can result in a permanent loss of sensory feedback with consequent alterations in muscular stiffness and interjoint coordination. Likewise, impairments in central regulatory mechanisms of tonic descending inputs can alter muscle stiffness and affect coordination. In pathologic stiffness, there can be a variety of aberrant mechanisms including an increase in centrally-mediated tonic drive on the lower motor neurons (Hunker, Abbs, & Barlow, 1982), increased gamma motor drive to muscle spindles (Andrews, Burke, & Lance, 1972; Dietrichson, 1971), and/or loss of recurrent inhibition (Magladery, 1964). Thus, it would seem that study of stiffness in the human face, given its vulnerability in the presence of pathology (brain injury, neuromotor disease, craniofacial anomaly including cleft lip, or muscle-connective tissue damage) represents an important direction for the investigation and treatment of musculoskeletal disorders affecting kinematics of speech and nonspeech behaviors.
Measurements of jaw and lip stiffness reinforce the important role of muscle biomechanics in speech and non-speech movements. Measurements of jaw stiffness during speech and non–speech tasks have demonstrated the importance of considering both postural and voluntary control of jaw stiffness in the presence of external loads (Shiller, Houle, & Ostry, 2005). For example, up-regulation of jaw stiffness has been shown to decrease kinematic variability during speech (Shiller, Laboissiere, & Ostry, 2002). Similar mechanisms may be operative among other speech articulatory subsystems. Jaw perturbation during speech production indicated that passive properties (stiffness) of the lips and jaw could contribute as a compensatory mechanism for accomplishing speech tasks (Gomi, Honda, Ito & Murano, 2002; Ito, Gomi, & Honda, 2000). A study by Hunker and colleagues (1982) found that labial stiffness was positively correlated with decreases in the range and speed of lip movements during speech in individuals with PD when compared to age-matched healthy adults. Electromyographic recordings from the OOS and OOI muscles indicated a positive correlation between labial stiffness, clinical rigidity, and hypokinesia.
Concerned with perioral biomechanics, Muller et al. (1985) sampled perioral span-tension and force-velocity relations over a displacement of 20 millimeters imposed horizontally and tangential to the oral angle. They advocated a perpendicular orientation of the displacement and load sensitive transducers in order to facilitate sampling and more accurately reflect force vectors and soft tissue properties of this complex muscle system. A follow-up study derived active and passive stiffness coefficients by sampling perioral force over a displacement range spanning 25–70 millimeters using an interangle actuator (Barlow & Muller, 1991). Sex was a significant main effect for active force. In another instrumental adaptation to the perioral system, passive compression force was sampled at midline for the upper and lower lip in 20 subjects. Stiffness values for the lower lip were greater than the upper lip, with higher values obtained from males when compared to their female counterparts (Ho, Azar, Weinstein, & Bowley, 1982). Recently, Seibel and Barlow (2007) elaborated previous findings and sampled perioral stiffness from eight normal healthy female adults using a digitally-controlled linear servo motor in a repeated-measures design to produce a lateral tangential stretch of the oral angle under an electrophysiologically verified nonparticipatory (rest) condition. The positive relation between perioral stiffness and imposed displacement was highly significant using a nonlinear regression technique.
The goal of the current study was to extend the observations of perioral stiffness sampled in females (Seibel & Barlow, 2007) to include a similar measurement of nonparticipatory interangle perioral stiffness in young male adult subjects. It was hypothesized that nonparticipatory stiffness of the male perioral tissue-muscle complex would increase as a function of imposed displacements between the oral angles. A secondary objective was to statistically compare the male and female perioral nonparticipatory stiffness functions. It was hypothesized that the slope of the male stiffness function would be greater than their female counterparts due to differences in perioral muscle and connective tissue properties.
Participants and Method
Twenty males between the ages of 21 to 31 years (Mean=25.1, SD=2.8) with no prior history of neurological disorder and free of orofacial and/or speech impairment participated in this study. Informed consent, approved by the University of Kansas Human Subjects Internal Review Board, was obtained from all subjects after the procedure had been fully explained. All subjects were seated in a comfortable chair with the head positioned firmly between two dense foam mastoid-occipital cups and stabilized circumferentially with a padded Velcro cuff. Subjects were instructed to relax the facial muscles and remain motionless and speechless during testing.
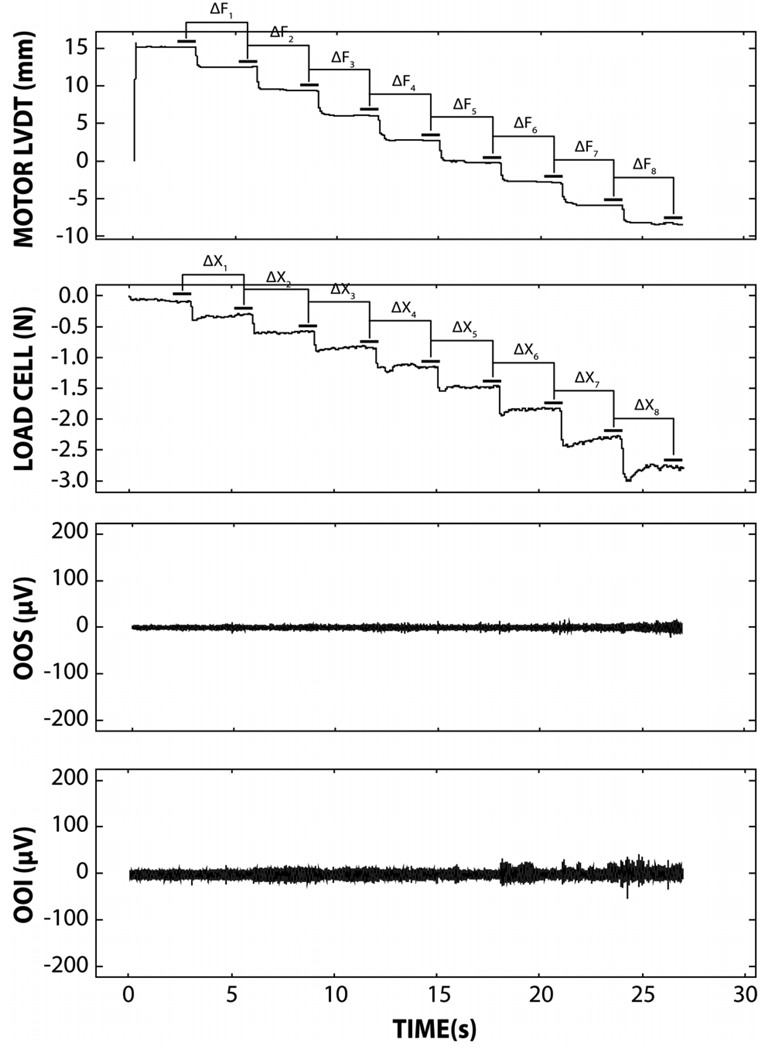
A specially designed linear servo motor was digitally programmed to systematically stretch the perioral tissue while simultaneously digitizing the imposed interangle displacement and resultant force. At the beginning of the experiment, the translator hook was placed inside the subjects’ right oral angle (Figure 1). The servo position offset control was electronically balanced to zero with the subjects’ interangle span (Lø) at rest position. The stator hook, referenced to the housing of the linear motor, was coupled to the contralateral oral angle. Thus, net tensile loads were realized between both oral angles. The servo motor was programmed to generate an automated sequence of 8-step displacements, which increased interangle span. Each step was approximately 3 millimeters and was sustained for exactly 3 seconds. The load cell record shows a systematic increase in force with each successive stretch of the lip angle. The transition time between steps was 100 milliseconds to reduce the possibility of evoking a lip stretch reflex. A single data block was completed in 27 seconds with a total imposed stretch of approximately 24 mm (Figure 2). A total of 5 such data blocks were sampled from each subject. Following instrumentation setup, the total test time per subject was approximately 3 minutes.
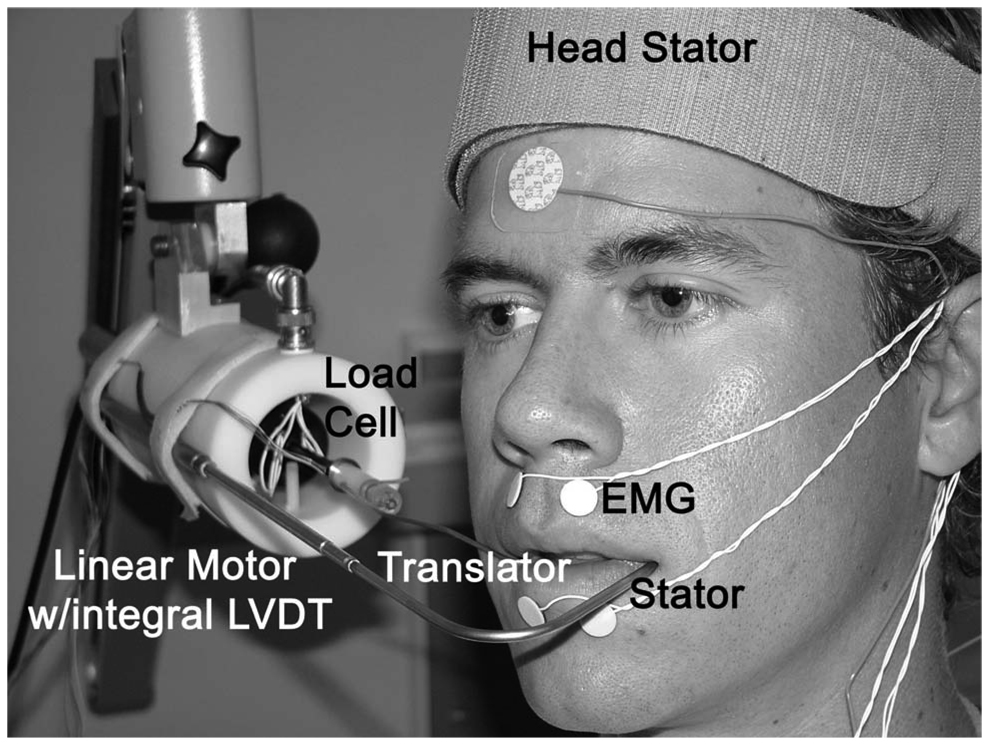
Figure 1.
Instrumentation to assess the force-displacement relation (stiffness) for the perioral system at the lateral tangential oral plane. A linear servo motor was mounted to a Zeiss 3-D articulating arm for orienting the device to the subject’s face. The stator hook was positioned at the left corner of the subject’s mouth for stabilization purposes. The translator was positioned at the right corner of the mouth to impose tensile stretch under position feedback.
Figure 2.
A single data block, including the full sequence of 8 imposed lip span displacements (top panel), resultant interangle force (second panel), OOSm (third panel) and OOIm (fourth panel) electromyograms. The change in resultant force (ΔF) is divided by the change in lip interangle displacement (ΔX) during the final 1 second period at each sequential step in span to derive interangle stiffness coefficients.
Bipolar, electrophysiological sampling of perioral muscle activity was achieved using miniature Ag/AgCl hydrogel surface electrodes (4 mm diameter) placed over the orbicularis oris superior (OOS) and orbicularis oris inferior (OOI) muscles (5 mm interelectrode distance, 1 mm from the lip vermilion). The resulting electromyograms (EMG) were used to document subjects’ nonparticipation during the application of tensile loads to the oral angles. Biopotentials from each electrode pair were conditioned by a Grass P511 bioamplifier (Gain=20k, Bandpass filter @ 30Hz-1kHz). Voltage signals associated with the linear motor (displacement), load cell (lip force), and EMG activity were digitized in real-time at 2000 samples per second over 16-bits of vertical resolution (ML795 Powerlab/16SP ADInstruments, Inc).
Analyses of digitized displacement and lip force signals were completed with a software program known as Servo_Orostiff v1.0 developed in our laboratory using LabVIEW™ v.8.0. This program was used to calculate stiffness, defined as the change in force divided by the change in interangle displacement (ΔFn/ΔXn) between any two consecutive displacement steps (see Figure 2). Stiffness coefficients (N/mm) were plotted over a 24 mm range of interangle lip displacement and fit using multilevel regression techniques.
The root mean square (RMS) of the OOS and OOI EMG signals was measured at each of the 8 stretch levels to determine the presence of reflex and/or voluntary activity. These data were subjected to regression analysis to test for a potential relation between EMG activation and interangle span.
In order to test for a potential sex difference in lip biomechanics, the male perioral stiffness data sampled in the current study were compared to a previously published data set obtained from a group of female subjects sampled with the same instrumentation and test conditions (Seibel & Barlow, 2007). A multilevel regression analysis was conducted to determine whether the nonlinear relation between stiffness and interangle span differed significantly between males and females, using SAS Version 9.1 (SAS Institute, 2004).
Results
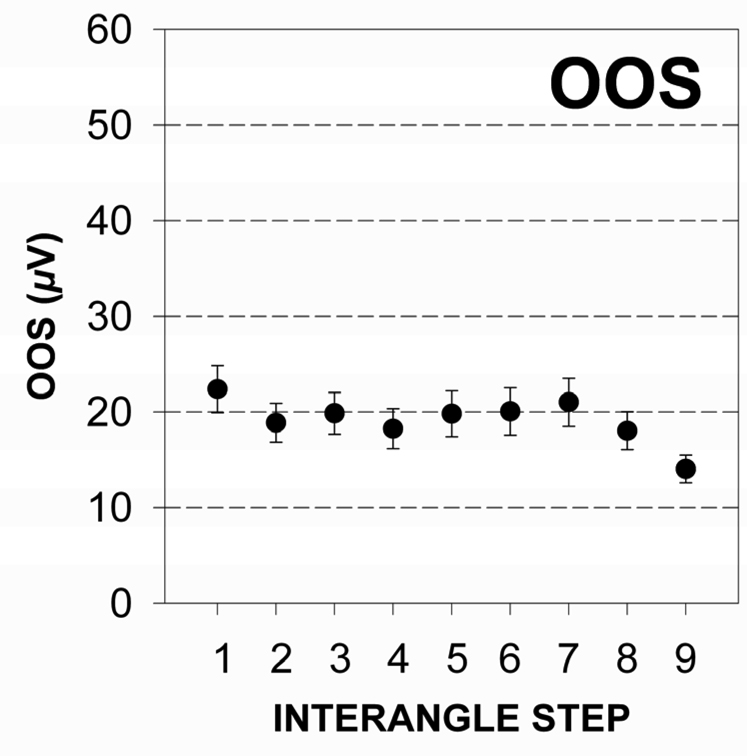
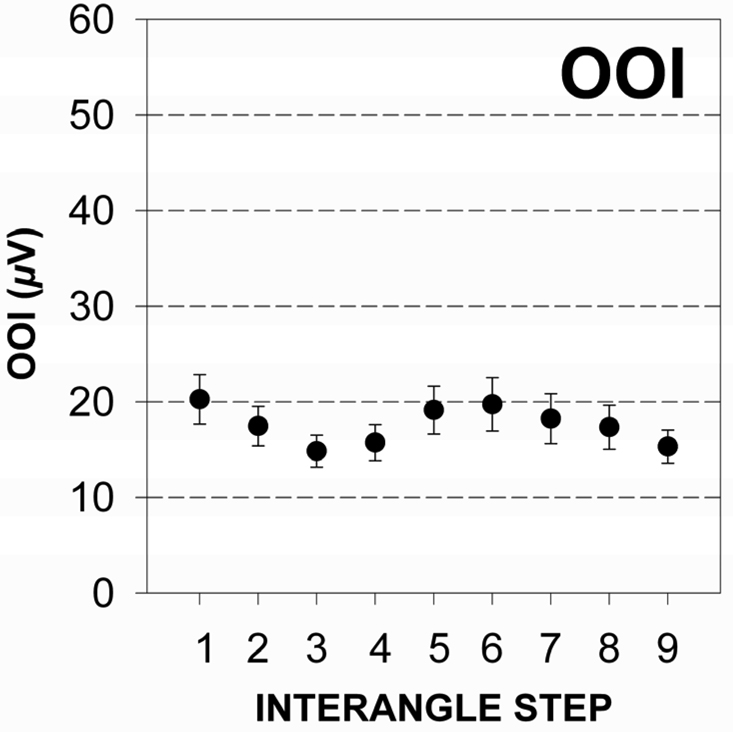
Perioral muscle activity remained remarkably constant as interangle span was increased confirming the nonparticipatory nature of the experimental task. The distribution of EMG RMS values for the OOS and OOI muscle recording sites pooled among subjects is shown in Figure 3a and 3b. The mean RMS level was 19.14 µV (SD = 21.99) and 17.57 µV (SD = 22.67) for the OOS and OOI muscle recording sites, respectively. Simple linear regression analyses for EMG RMS versus interangle span indicated a non-significant slope for the upper lip (t = −1.87, p = .06), and lower lip (t = −.53, p = .59). This finding confirmed the nonparticipatory nature of the stiffness task.
Figure 3.
a & 3b The distribution of mean and standard error of EMG RMS values for upper (3a) and lower lip (3b) recording sites for all male test subjects associated with a nonparticipatory condition.
The results of the biomechanical analysis showed a significant quadratic relation between perioral stiffness and imposed displacement (Figure 4). This translated into progressively higher stiffness values as interangle span increased. In order to examine how much the perioral stiffness values vary between subjects as well as within subjects, a multilevel regression model was fit to the stiffness values separately for each step. The fitted model is given by the expression (1):
| (1) |
where uoj ~ N(0, τ00 ) and rij ~ N(0,σ2) for trial i and subject j. This (unconditional means) model expresses the stiffness values as the sum of an overall subject mean (γ00 ), a series of random deviations from that mean (uoj), and a random error ( rij) associated with the ith trial in the jth subject. The within-subject variances (σ2 ) were significant for all 8 steps (p = .01), suggesting that subjects do differ in their stiffness within each step. A further analysis demonstrated that males yielded greater within-level variance than females for all 8 interangle stretches. Additionally, the minimal Intra-Class Correlations (ICCs), with an average of .04, suggest less variation between subject means than would be expected, given the within-subject variance (Table 1).
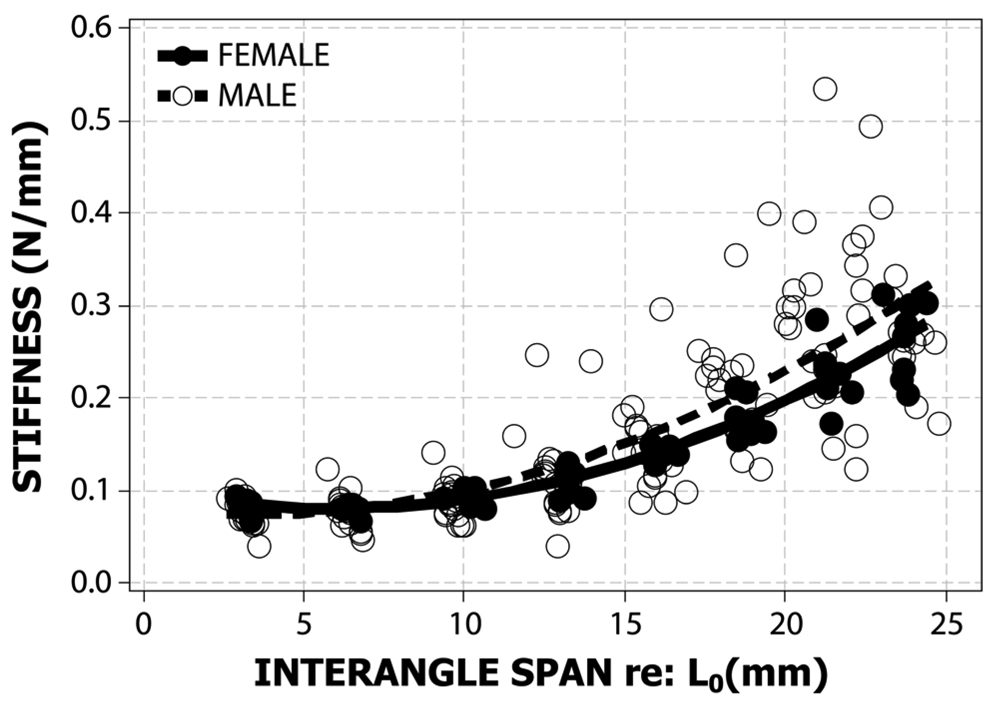
Figure 4.
Regression functions for both male (dotted black line, open circles) and female subjects (solid black line, filled circles). The regression equation for the male group is Interangle_StiffnessMALE = 0.0767− 0.0030*Interangle_Span + 0.0005*Interangle_Span2 (R-Sq=58%). The regression equation for the female group derived from Seibel and Barlow (2007) is Interangle_StiffnessFEMALE = 0.0945−0.0055*Interangle_Span + 0.0005*Interangle_Span2 (R-Sq=77%).
Table 1.
Between- and Within-Level Variances of Perioral Stiffness Scores.
| τ00 | σ2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Step | Estimate | SE | z | Estimate | SE | z | ICC | Estimate | SD | z | |
| 1 | .00032 | .00015 | 2.16* | .00107 | .00014 | 7.48** | .23 | .00067 | .00018 | 3.69** | |
| 2 | 0 | . | . | .00983 | .00118 | 8.34** | 0 | .0039 | .00157 | 2.51* | |
| 3 | .00009 | .00033 | .27 | .00510 | .00068 | 7.48** | .02 | .00243 | .00086 | 2.82** | |
| 4 | 0 | . | . | .00904 | .00109 | 8.34** | 0 | .00349 | .00145 | 2.41* | |
| 5 | 0 | . | . | .01079 | .00129 | 8.34** | 0 | .00771 | .00184 | 4.19** | |
| 6 | .00002 | .00018 | .09 | .00296 | .00040 | 7.48** | .01 | .00213 | .00051 | 4.14** | |
| 7 | 0 | . | . | .01269 | .00152 | 8.34** | 0 | .00857 | .00198 | 4.33** | |
| 8 | .00077 | .00067 | 1.04 | .00809 | .00108 | 7.48** | .08 | .00404 | .00138 | 2.93** | |
Note. The original responses that have not been aggregated (averaged) for each subject were used (N = 28); ICC1 = τ̂00/(τ̂00 + σ̂2);
p < .05
p < .01.
From the polynomial multiple-regression model, the predicted perioral stiffness scores can be obtained by the expressions (2) and (3):
| (2) |
and
| (3) |
The general growth pattern of stiffness as a function of interangle span is consistent with a previous study involving young adult females (Seibel & Barlow, 2007). The current study showed a significant quadratic relationship between the stiffness and interangle displacement (β = .0005, t = 6.65, p < .01). However, the interaction between quadratic relationship and sex was not significant (β = .00, t = .02, p = .98), indicating that a sex difference is absent in this quadratic relation. While there was no age difference among subjects in the two studies (Table 2), body weight was significantly greater in the male subject pool.
Table 2.
Mean age and body weight for male and female subjects.
| Male | Female (Seibel & Barlow, 2007) | |
|---|---|---|
| Number of Subjects | 20 | 8 |
| Age (years) | 25.12 (SD=2.79) | 25.19 (SD=2.54) |
| Weight (kg)* | 74.76 (SD=13.96) | 59.25 (SD=8.26) |
Significant difference at p=.007
Discussion
The results of this study show a predictable quadratic relation between perioral stiffness and imposed displacement during an electrophysiologically verified nonparticipatory condition among twenty healthy male subjects. Intersubject variance in stiffness increased as interangle span progressed from Lø. This is likely due to individual differences in anatomical structure of the lower face which contributes to subtle differences in the slope of individual stiffness growth functions. Because tonic EMG activity was constant over the range of interangle span, the increase in stiffness is due to elasticity of muscle and connective tissue within the perioral complex. The lack of a sex difference in interangle stiffness coefficients contradicts the results of Ho, Azar, Weinstein, & Bowley (1982) who reported that the lips of males were stiffer than female lips. This observed difference is likely due to the fact that Ho et al. (1982) sampled lip forces at midline along a vertical trajectory. They attributed the observed sex difference to perioral muscle and connective tissue mass. Sex is likely to play a role in perioral dynamics since adult males have been shown to produce significantly greater levels of midline compression force compared to a matched cohort of females (Barlow & Rath, 1985).
The present study has also demonstrated that real time data acquisition and analysis of perioral stiffness during a ‘do not contract’ nonparticipatory condition can be completed within 30 seconds in cooperative subjects. Accurate methods for assessing perioral stiffness will make it possible to test the hypothesized relation between perioral stiffness and hypokinesia in PD (Hunker et al., 1982). It will also be possible to assess the effects of anti-PD medications on perioral stiffness and orofacial kinematics during speech. A quantitative analysis of perioral biomechanics is likely to add valuable information for neurologists, neurosurgeons, plastic surgeons and speech pathologists who manage orofacial movement disorders (van Lieshout et al., 2002). The next logical step is to test this application in individuals with facial movement disorders associated with progressive neuromotor disease (i.e., Parkinson’s disease, amyotrophic lateral sclerosis, and cerebellar disease), neuroinflammatory conditions (multiple sclerosis), craniofacial anomalies (cleft lip), or acquired insults to the nervous system (stroke, traumatic brain injury, bomb blast, and missile injuries). In order to expand this biomechanical measurement application to a wider range of clinical subjects, including children, a miniature face-referenced linear actuator is indicated that would preclude the need for head restraint. Such a system has been developed in our laboratory and is being evaluated in a randomized clinical trial involving children undergoing lip revision surgery associated with cleft lip (Trotman, Barlow, & Faraway, 2007).
Acknowledgements
This study was supported in part by the Sutherland Foundation, NIH R01 003311 (SM Barlow), NIH R01 DE13814 (C-A Trotman), and NIH P30 DC005803. Special gratitude is expressed toward Douglas Kieweg, BS, and Lalit Venkatesan, MSCoE for assistance in digital signal processing.
Contributor Information
Shin-Ying Chu, Communication Neuroscience Laboratories, University of Kansas, 1000 Sunnyside Avenue, Room 3001, Lawrence, Kansas 66045-7555, shinying@ku.edu
Steven M. Barlow, Professor, SPLH, Neuroscience, Humn Biology, and Bioengineering, Director, Communication Neuroscience Laboratories, University of Kansas, 1000 Sunnyside Avenue, Room 3001, Lawrence, Kansas 66045-7555, TL 785-864-4447, FX 785-864-4403, smbarlow@ku.edu
Jaehoon Lee, Department of Psychology, Biostatistics Core, Center for Biobehavioral Neuroscience of Communication Disorders, University of Kansas, Lawrence, Kansas 66045-7555, jaehoon@ku.edu
References
- Andrews CJ, Burke D, Lance JW. The response to muscle stretch and shortening in Parkinson rigidity. Brain. 1972;95:795–812. doi: 10.1093/brain/95.4.795. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Abbs JH. Orofacial fine motor control impairments in congenital spasticity: evidence against hypertonus related performance deficits. Journal of Neurology. 1984;34:145–150. doi: 10.1212/wnl.34.2.145. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Abbs JH. Fine force and position control of select orofacial structures in the Upper Motor Neuron Syndrome. Experimental Neurology. 1986;94:699–713. doi: 10.1016/0014-4886(86)90248-7. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Burton MK. Ramp-and-hold force control in the upper and lower lips: developing new neuromotor assessment applications in traumatically brain injured adults. Journal of Speech and Hearing Research. 1990;33:660–675. doi: 10.1044/jshr.3304.660. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Finan DS, Andreatta RD, Boliek C. Kinematic measurement of the human vocal tract. In: McNeil M, editor. Clinical Management of Sensorimotor Speech Disorders. NewYork: Thieme Medical Publishers; 2008. pp. 80–99. [Google Scholar]
- Barlow SM, Hammer M. Pallidotomy and deep brain stimulation in Parkinson’s disease: effects on speech and voice. In: McNeil M, editor. Clinical Management of Sensorimotor Speech Disorders. New York: Thieme Medical Publishers; 2008. pp. 362–364. [Google Scholar]
- Barlow SM, Iacono RP, Paseman LA, Biswas A, D’Antonio LD. The effects of experimental posteroventral pallidotomy on force and speech aerodynamics in Parkinson’s disease. In: Cannito MP, Yorkston KM, Beukelman DR, editors. Speech Motor Control. Baltimore, MD: Paul H. Brookes Publishing Company; 1998. pp. 117–156. [Google Scholar]
- Barlow SM, Muller EM. The relation between interangle span and in vivo resultant force in the perioral musculature. Journal of Speech and Hearing Research. 1991;34:252–259. doi: 10.1044/jshr.3402.252. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Netsell RW. Differential fine force control of the upper and lower lips. Journal of Speech and Hearing Research. 1986;29:163–169. doi: 10.1044/jshr.2902.163. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Rath EM. Maximum voluntary closing forces in the upper and lower lips of humans. Journal of Speech and Hearing Research. 1985;28:373–376. doi: 10.1044/jshr.2803.373. [DOI] [PubMed] [Google Scholar]
- Blair C, Smith A. EMG recording from human lip muscles: can single muscles be isolated? Journal of Speech and Hearing Research. 1986;29:256–266. doi: 10.1044/jshr.2902.256. [DOI] [PubMed] [Google Scholar]
- Dietrichson P. Tonic ankle reflex in Parkinson rigidity and in spasticity: the possible role of the fusimotor system. Acta Neurology Scandinavia. 1971;47:163–182. doi: 10.1111/j.1600-0404.1971.tb07474.x. [DOI] [PubMed] [Google Scholar]
- Fogel ML, Stranc MF. Lip function: a study of normal lip parameters. British Journal of Plastic Surgery. 1984;37:542–549. doi: 10.1016/0007-1226(84)90147-4. [DOI] [PubMed] [Google Scholar]
- Folkins JW, Larson CR. In search of a tonic vibration reflex in the human lip. Brain Research. 1978;151:409–412. doi: 10.1016/0006-8993(78)90898-3. [DOI] [PubMed] [Google Scholar]
- Gentil M, Garcia-Ruiz P, Pollak P, Benabid AL. Effect of stimulation of the subthalamic nucleus on oral control of patients with parkinsonism. Journal of Neurology, Neurosurgery and Psychiatry. 1999;67:329–333. doi: 10.1136/jnnp.67.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentil M, Tournier CL. Differences in fine control of forces generated by the tongue, lips and fingers in humans. Archives of Oral Biology. 1998;43:517–523. doi: 10.1016/s0003-9969(98)00042-9. [DOI] [PubMed] [Google Scholar]
- Gomi H, Honda M, Ito T, Murano EZ. Compensatory articulation during bilabial fricative production by regulating muscle stiffness. Journal of Phonetics. 2002;30(3):261–279. [Google Scholar]
- Ho TP, Azar K, Weinstein S, Bowley WW. Physical properties of human lips: experimental and theoretical analysis. Journal of Biomechanics. 1982;15:859–866. doi: 10.1016/0021-9290(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Hunker CJ, Abbs JH, Barlow SM. The relationship between parkinsonian rigidity and hypokinesia in the orofacial system: a quantitative analysis. Neurology. 1982;32:755–761. doi: 10.1212/wnl.32.7.749. [DOI] [PubMed] [Google Scholar]
- Ito T, Gomi H, Honda M. Task dependent jaw-lip coordination examined by jaw perturbation during bilabial-consonant utterances. Proceedings of the 5th Seminar on Speech Production, Bavaria. 2000:41–44. [Google Scholar]
- Magladery JW. Central facilitating and inhibiting mechanisms in the control of muscle tone. Clinical Pharmacology and Therapeutics. 1964;5:805–811. [Google Scholar]
- McHenry M, Minton J, Hartley L, Calhoun J, Barlow SM. Age-related changes in orofacial force generation in women. Laryngoscope. 1999;109:827–830. doi: 10.1097/00005537-199905000-00027. [DOI] [PubMed] [Google Scholar]
- Muller EM, Milenkovic PH, MacLeod GE. Perioral tissue mechanics during speech production. In: DeLisi C, Eisendfeld J, editors. Proceedings of the Second IMAC International Symposium on Biomedical Systems Modeling; North Holland; Amsterdam. 1985. pp. 363–371. [Google Scholar]
- Nichols TR. The contributions of muscles and reflexes to the regulation of joint and limb mechanics. Clinical Orthopaedics and Related Research. 2002;403:43–50. doi: 10.1097/00003086-200210001-00006. [DOI] [PubMed] [Google Scholar]
- Pinto S, Gentil M, Fraix V, Benabid AL, Pollak P. Bilateral subthalamic stimulation effects on oral force control in Parkinson’s disease. Journal of Neurology. 2003;250:179–187. doi: 10.1007/s00415-003-0966-7. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Bennett DJ, Stephens MJ, Patrick SK, Sears-Duru R, Roberts T, Jhamandas JH. Measurement of rigidity in Parkinson’s disease. Movement Disorders. 1997;12:24–32. doi: 10.1002/mds.870120106. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS 9.1.3 Language Reference: Concepts. Cary, NC: SAS Institute Inc; 2004. [Google Scholar]
- Schwab RS. Problems in clinical estimation of rigidity (hypertonia) Clinical Pharmacology and Therapeutics. 1964;5:942–946. [Google Scholar]
- Seibel LM, Barlow SM. Automatic measurement of non-participatory stiffness in the perioral complex. Journal of Speech, Language, and Hearing Research. 2007;50:1272–1279. doi: 10.1044/1092-4388(2007/089). [DOI] [PubMed] [Google Scholar]
- Sepehri B, Esteki A, Ebrahimi-Takamjani E, Shahidi G-A, Khamseh F, Moinodin M. Quantification of rigidity in Parkinson’s disease. Annals of Biomedical Engineering. 2007;35(12):2196–2203. doi: 10.1007/s10439-007-9379-6. [DOI] [PubMed] [Google Scholar]
- Siegler EL, Beck LH. Stiffness: a pathophysiologic approach to diagnosis and treatment. Journal of General Internal Medicine. 1989;4:533–540. doi: 10.1007/BF02599555. [DOI] [PubMed] [Google Scholar]
- Shiller DM, Houle G, Ostry DJ. Voluntary control of human jaw stiffness. Journal of Neurophysiology. 2005;94:2207–2217. doi: 10.1152/jn.00164.2005. [DOI] [PubMed] [Google Scholar]
- Shiller DM, Laboissière R, Ostry DJ. Relationship between jaw stiffness and kinematic variability in speech. Journal of Neurophysiology. 2002;88:2329–2340. doi: 10.1152/jn.00286.2002. [DOI] [PubMed] [Google Scholar]
- Struppler A. Tremor and Skeletal Muscle Tone. Proceedings of the 2nd International Workshop on Microphysiological Recordings during Stereotactic Neurosurgery: Motor Thalamus. Stereotactic Neurosurgery. 1993;60:152–156. [Google Scholar]
- Trotman C-A, Barlow SM, Faraway JJ. Functional outcomes of cleft lip surgery. Part 3. Measurement of lip forces. The Cleft Palate-Craniofacial Journal. 2007;44:614–623. doi: 10.1597/06-138.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lieshout PHHM, Rutjens CAW, Spauwen PHM. The dynamics of interlip coupling in speakers with a repaired unilateral cleft-lip history. Journal of Speech, Language, and Hearing Research. 2002;45:5–19. doi: 10.1044/1092-4388(2002/001). [DOI] [PubMed] [Google Scholar]
- Webster DD. Rigidity in extrapyramidal disease. Journal of Neurosurgery. 1966;2 Supl II:299–309. [Google Scholar]