Abstract
Here we describe clinical and pathologic evidence of Chagas disease caused in dogs by circulating Trypanosoma cruzi from a newly recognized endemic area in Mexico. We show that the Zumpahuacan isolate, although less virulent than the Sylvio-X10 reference strain that caused acute myocarditis and death, was pathogenic in dogs. Dogs infected with the Zumpahuacan isolate exhibited electrocardiographic alterations, left- and right-ventricle dilation, and hydropericardium. Histologically, diffused perimysial and endomysial lymphoplasmacytic cell infiltration, cardiomyocyte necrosis, and amastigote nests were noted in Zumpahuacan-infected dogs. These findings suggest that the risk of T. cruzi infection and Chagas disease is present in the State of Mexico, and further research is needed to identify the T. cruzi bio-types circulating in southern State of Mexico.
INTRODUCTION
With an estimated 18 million people infected with Trypanosoma cruzi, and 25% of the population at risk, Chagas disease is endemic in Latin America.1–3 In Mexico, ~5.8 million people might be infected with T. cruzi.4,5 The State of Mexico, located in the central highlands of the country, was considered free of T. cruzi until 1998. However, we documented T. cruzi–specific antibodies in 7.1% of humans and 21% of dogs from rural areas of the State of Mexico6 and noted that detailed epidemiologic information is needed to understand the true impact of Chagas disease in these areas.
In this study, we isolated T. cruzi from triatomines collected from Zumpahuacan municipality of the State of Mexico and aimed to evaluate its virulence in dogs. We examined physical, clinical, and histopathologic aspects in dogs that were naturally infected or experimentally infected with the Zumpahuacan isolate of T. cruzi. We chose dog as an experimental model because they are an important domestic reservoir host7,8 and should be an excellent model to study Chagas disease.9–15
MATERIALS AND METHODS
Animals
Twelve of 57 mongrel dogs collected in Zumpahuacan municipality tested positive for anti-T. cruzi antibodies by indirect hemagglutination (IHA) test and enzyme-linked immunosorbent assay (ELISA) and were considered naturally infected (21% prevalence). Apart from cardiomyopathy, no signs of infections related to other cardiomyopathic diseases (e.g., ringworm) were noticed during clinical evaluation, necropsy, and histologic studies, and these dogs were considered affected by Chagas disease only.
For experimental infection, dogs (2 months old) were acquired locally and kept at the research center until used for challenge infection at 8 months of age. Dogs were confirmed serologically negative for T. cruzi–specific antibodies by IHA and ELISA and treated with anti-helminthics and vaccines against regional infectious diseases (canine distemper, parvovirus, canine hepatitis, leptospirosis, and rabies). All dogs received water ad libitum and commercial dog food according to age and development requirements. Experimental protocols were conducted according to Norma-Official-Mexicana (NOM-0062-ZOO-1999) technical specifications for the care and use of laboratory animals.
Parasites
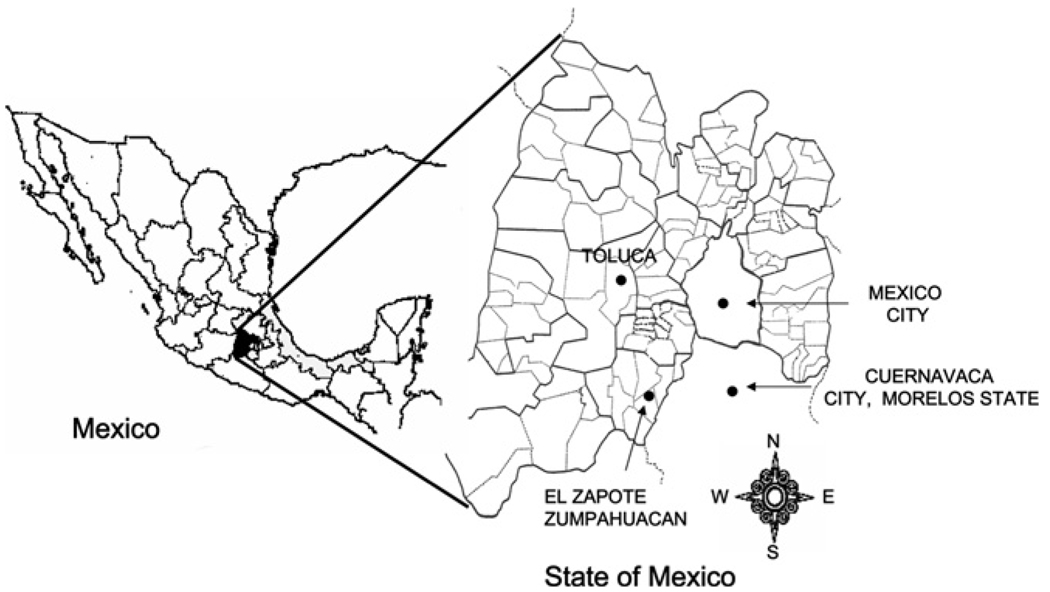
Trypanosoma cruzi (Sylvio-X10) was purchased from American Tissue Culture Collection. A native T. cruzi isolate was obtained from Triatoma pallidipennis collected from El Zapote village of Zumpahuacan municipality, located in the southern State of Mexico (Figure 1). Triatomines feces were examined for epimastigotes by light microscopy,16 and positive samples were diluted in PBS and intraperitoneally injected in Balb/C mice. Blood trypomastigotes were collected by cardiac puncture at Day 14 post-infection (pi). Parasites were purified by Ficoll gradient, seeded on a monolayer of NIH3T3 cells, and cultured and propagated at 37°C, 5% CO2 in DMEM media (pH 7.2) supplemented with 2% FBS, 8 µg/mL ampicillin, and 0.1 mg/mL pyruvate (Gibco/Invitrogen, Grand Island, NY). The native isolate was named “Zumpahuacan.”
FIGURE 1.
Study site in Mexico. The Zumpahuacan isolate was obtained from infected triatomines collected from El Zapote village in the Zumpahuacan municipality of the State of Mexico. Dogs naturally infected with T. cruzi were also obtained from same region.
Experimental infection
Seronegative dogs (N = 10) were blindly distributed. Four dogs were infected with Zumpahuacan and four with Sylvio-X10 (3,500 trypomastigotes/kg body weight). Two dogs served as controls. Dogs were observed daily for general physical condition and every week for serology, clinical condition, and cardiac function.
Serology
Blood samples (7 mL) were obtained by venepuncture (once per week, until 10 weeks pi) and immediately processed, and sera were stored at −20°C. Sera samples were analyzed for anti-T. cruzi antibodies by IHA (Polychaco, Laboratorio-Lemos SRL, Buenos Aires, Argentina) and ELISA (Laboratorio-Lemos SRL, Buenos Aires, Argentina) Chagas diagnostic kits, following the manufacturer’s instructions. The horseradish peroxidase (HRP)-labeled anti-human-IgG in ELISA kit was replaced with anti-dog-IgG (Koma Biotech, Seoul, Korea). The cut-off value for IHA (positive titer at ≥ 1:8 serum dilution) and ELISA (OD450nm ± 2 SD) tests were set using sera from four healthy dogs.17,18 The seropositive and seronegative status of the control dogs was confirmed by InDre (national reference center for diagnosis of T. cruzi infection) by Serodia-Chagas (Fujirebio, Tokyo, Japan) and Chagas STAT-PAK (Chembio, Medford, NY).
Electrocardiography
Changes in cardiac rhythm and conduction were monitored each week (for 10 weeks pi). The EK-8 electrocardiographic machine (Burdick Stylus, Milton, WI) was set at 120 V, 60 H, 20 amps, and 25 W, and six leads were considered at 25 mm/s at 1 mV, standardized to 1 cm for this study.
Necropsy and histologic studies
Necropsy was performed when dogs died because of infection or after humanitarian sacrifice, and macroscopic and microscopic analysis of affected organs was performed. Animals were euthanized according to the Norma Official Mexicana guidelines (NOM-033-ZOO-1995). Tissue samples were fixed in 10% formalin, dehydrated, and embedded in paraffin. Tissue sections (5 µm) were stained with hematoxylin and eosin and evaluated by light microscopy.
RESULTS
Clinical evaluation
The Zumpahuacan- and Sylvio-X10–infected experimental dogs developed a brief febrile episode during 5–16 dpi. No other signs of clinical illness were apparent in experimentally infected and healthy control dogs during the daily physical exam. The naturally infected dogs showed no fever during the observation period. Dogs infected with Sylvio-X10 died at day 29 and 30 pi.
Serology
Trypanosoma cruzi–specific antibodies were evident in experimentally infected dogs by IHA and ELISA at 4 weeks pi. Sylvio-X10–infected dogs died during the Week 5 pi, and no further serology could be performed. Dogs infected with the Zumpahuacan isolate exhibited a gradual increase in anti-T. cruzi antibodies during 4–10 weeks pi. Naturally infected dogs exhibited highest level of parasite-specific antibodies. Control dogs remained seronegative throughout the study (Table 1).
TABLE 1.
Serologic analysis of dogs naturally or experimentally infected with T. cruzi
| Weeks post-infection | ||||||
|---|---|---|---|---|---|---|
| T. cruzi infection | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 10 |
| IHA | ||||||
| Natural (A) | + | + | + | + | + | + |
| Zump (B) | − | − | + | + | + | + |
| Sylvio (C) | − | − | + | + | + | ND |
| None (D) | − | − | − | − | − | − |
| ELISA—OD450nm ± SD | ||||||
| Natural (A) | 1.85 ± 0.07 | 1.81 ± 0.03 | 1.88 ± 0.05 | 1.89 ± 0.02 | 1.88 ± 0.02 | 1.92 ± 0.05 |
| Zump (B) | 0.11 ± 0.03 | 0.14 ± 0.01 | 0.16 ± 0.05 | 0.25 ± 0.04 | 0.38 ± 0.07 | 1.05 ± 0.61 |
| Sylvio (C) | 0.09 ± 0.03 | 0.18 ± 0.03 | 0.16 ± 0.04 | 0.28 ± 0.04 | 0.53 ± 0.2 | ND |
| None (D) | 0.13 ± 0.01 | 0.13 ± 0.02 | 0.17 ± 0.03 | 0.13 ± 0.003 | 0.14 ± 0.02 | 0.15 ± 0.01 |
| Cut-off (CV) | 0.23 (0.21–0.26) | 0.23 (0.28–0.25) | 0.27 (0.25–0.30) | 0.23 (0.28–0.25) | 0.24 (0.22–0.27) | 0.25 (0.22–0.27) |
Sera samples were collected from dogs with natural T. cruzi infection (Group A) and dogs that were experimentally infected with Zumpahuacan (Zump, Group B) or Sylvio-X10 (Sylvio, Group C) isolates of T. cruzi at 1, 2, 3, 4, and 10 weeks post-infection. Normal, uninfected dogs (none, Group D) were used as controls. The sera level of anti-T. cruzi antibodies was determined by IHA and ELISA. IHA: +, positive at least at a 1/8 serum dilution; −, negative. The + and − represent mean of triplicate observations from four dogs in each group. ELISA: cut-off = average of negative controls +0.1 (±10%). Absorbance (OD450nm) ± SD values are representative of mean of triplicate observations per dog, four dogs per group, with variation coefficient subtracted at 10% level.
CV = coefficient of variation; ND = not determined because dogs died, and no samples were available.
Electrocardiography
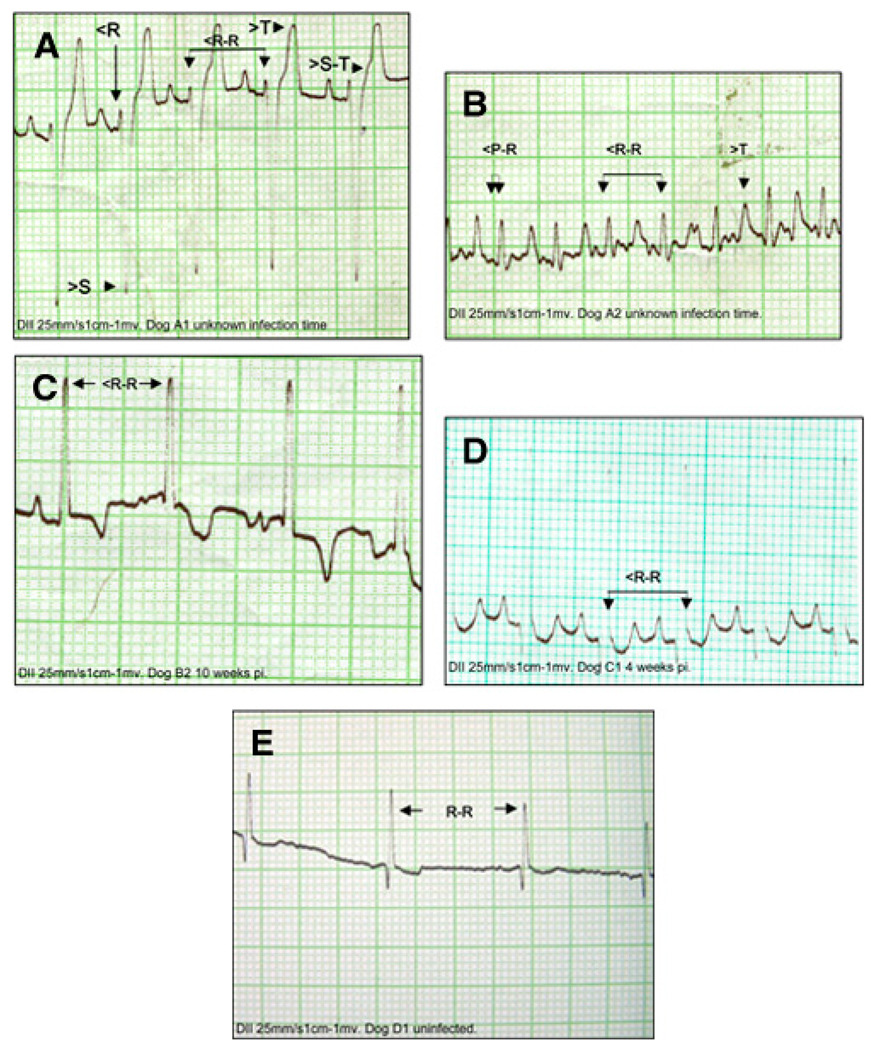
Electrocardiograms were obtained at weekly intervals. The Zumpahuacan-infected dogs exhibited discrete EKG abnormalities. These included, at 4 weeks pi, a reduction in S-T segment below 0.2 mV and a premature ventricular contraction (Figure 2C), indicative of ischemia and arrhythmia, respectively, that evolved toward EKG normalization by 8 weeks pi. Sylvio-X10–infected dogs exhibited sinus tachycardia at 4 weeks pi (Figure 2D) and died thereafter. Naturally infected dogs showed the most evident EKG alterations, including a reduced R-wave voltage, a deep S-wave, a ST-segment increase > 0.15 mV, and a T-wave increment that are indicative of pericardial effusion, right-atrium enlargement, and ischemia. Some naturally infected dogs also exhibited reduced P-R and R-R intervals and sinus tachycardia (Figure 2A and B). No EKG abnormalities were observed in healthy controls (Figure 2E).
FIGURE 2.
Electrocardiographic recordings taken at 25 mm/s. 1 mm = 0.04 seconds, 1 cm = 1 mV. Shown are lead II recordings from naturally infected dogs (A and B), Zumpahuacan-infected dog at 4 weeks pi (C), Sylvio-X10–infected dog at 4 weeks pi (D), and non-infected dog (E). EKGs show some of the conduction alterations found in infected dogs (interpretation can be seen in Table 2). R-R, normal R-R interval; >S-T, increased S-T segment; >T, increased T wave;<R, decreased R wave; >S, increased deep S wave; <R-R, decreased R-R interval; <P-R, reduced P-R interval. This figure appears in color at www.ajtmh.org.
Anatomo- and histo-pathologic findings
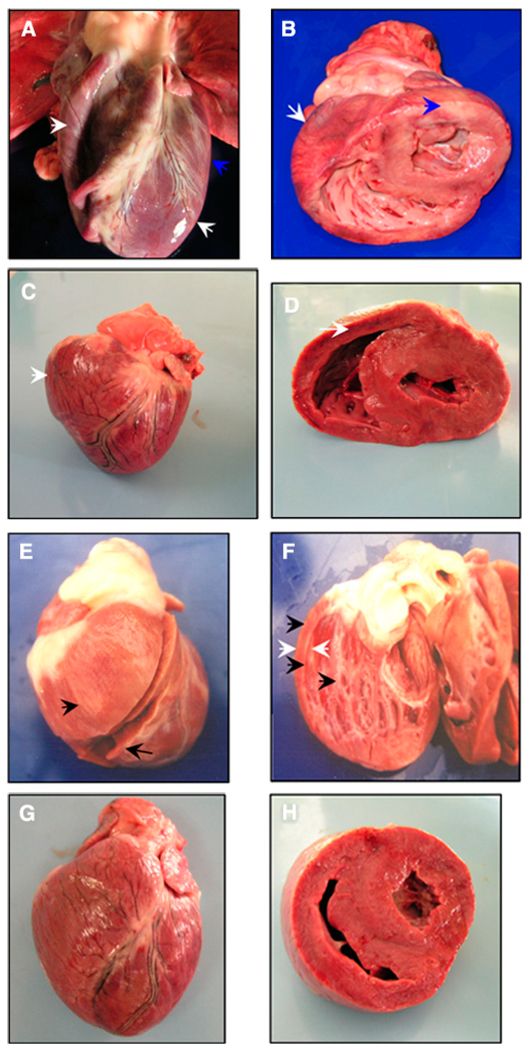
The anatomopathologic findings in infected dogs included dilated cardiomyopathy, cardiomegaly, hydropericardium, and focal and diffused myocarditis. Naturally infected dogs showed mild-to-severe bi-ventricle dilation that resulted in large cardiomegaly (Figure 3A and B). The Zumpahuacan- and Sylvio-X10–infected dogs displayed mild to moderate cardiopathic changes (e.g., hydropericardium and right ventricle dilation associated with moderate cardiomegaly; Figure 3C–F; Table 2). A healthy dog’s heart is shown for comparison (Figure 3G and H).
FIGURE 3.
Morphologic alterations of the Chagasic hearts from naturally and experimentally infected dogs. A and B, Heart morphology of a naturally infected dog with biventricular dilated cardiomyopathy (white arrows) and presence of pale striated epicardium (blue arrows). C and D, Heart from a Zumpahuacan-infected dog shows right-ventricle dilated cardiomyopathy and heart enlargement (white arrows). E and F, Heart from a Sylvio-X10–infected dog shows right-ventricle dilated cardiomyopathy with thin walls (white arrows) characterized by a rounded heart appearance and presence of whitish striates in epicardium, myocardium, and endocardium (black arrows). G and H, Heart from a healthy, non-infected dog. This figure appears in color at www.ajtmh.org.
TABLE 2.
Necropsy and histopathology alterations in dogs naturally or experimentally infected with T. cruzi
|
T. cruzi infection (group) |
||||
|---|---|---|---|---|
| Natural (A) | Zump (B) | Sylvio (C) | None (D) | |
| Anatomopathologic parameters |
||||
| Cardiomegaly | + to +++ | ++ | ++ | − |
| Hydropericardium | + to ++ | + | + to ++ | − |
| Left ventricular dilatation |
− to + | − | − | − |
| Right ventricular dilatation |
+ to +++ | ++ | ++ | − |
| Splenomegaly | + to +++ | + to ++ | − | − |
| Hepatomegaly | − to + | + to ++ | − | − |
| Hydroperitoneum | − to + | − to + | − to + | − |
| Histopathologic lesions | ||||
| Focal MLI | + to ++ | − | − | − |
| Diffused MLI | − | +++ | +++ | − |
| Necrosis of cardiomyocytes |
+ to +++ | + + to +++ | ++ | − |
| PEH | + | + + to +++ | ++ | − |
| Amastigote nests | − to + | + + to +++ | − to + | − |
Naturally infected dogs (A), and dogs experimentally infected with Zumpahuacan (B) or Sylvio-X10 (C) isolates were examined. Healthy, seronegative dogs (D) were used as controls. The representative findings from four dogs per group are documented here.
+ = mild; ++ = moderate; +++ = severe; − = no alteration detected; MLI = myocardial lymphoplasmacytic inflammation; PEH = perimysial and endomysial histolytic lymphoplasma-cytic cell infiltration.
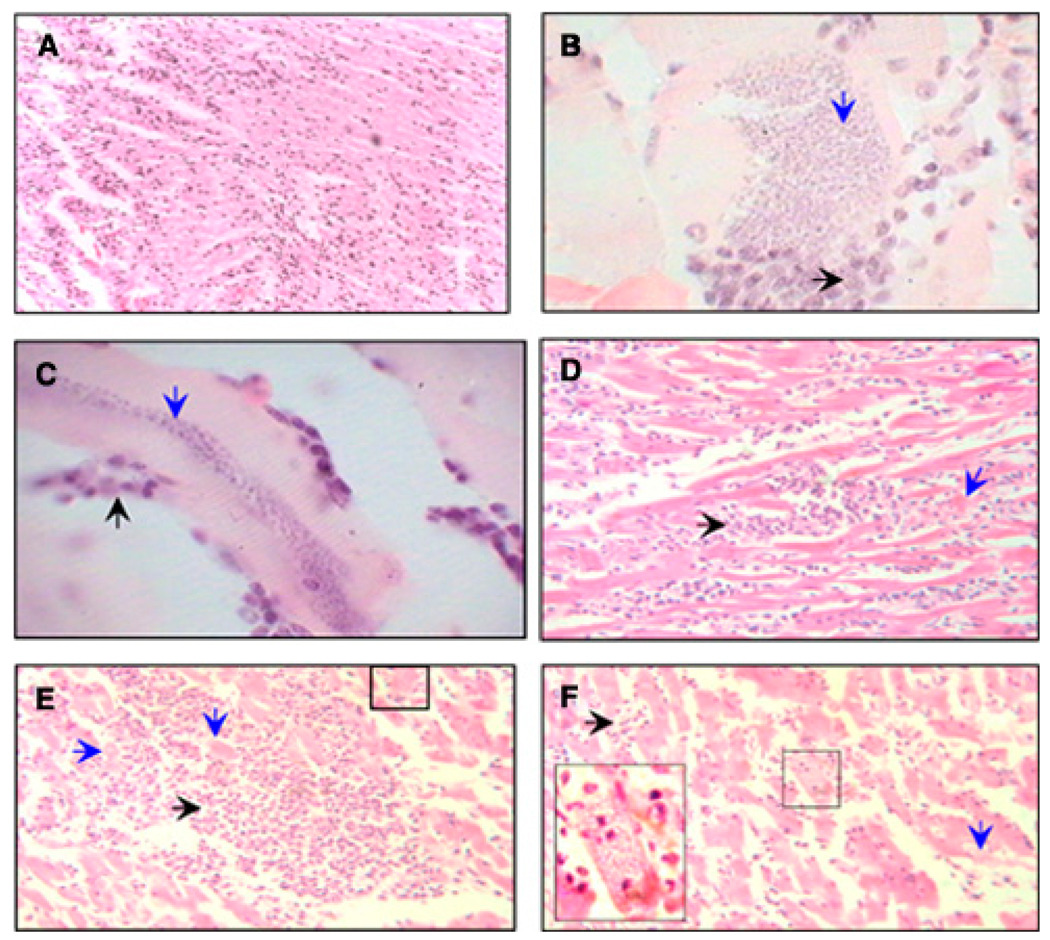
Histopathologic studies showed diffused and multifocal necrotic myocarditis, characterized by necrosis and fragmentation of myocardial fibers and perimysial and endomysial histiocytic and lymphoplasmacytic infiltration in the heart of naturally and experimentally infected dogs (Figure 4; Table 2). The extent of inflammatory infiltrate and tissue necrosis corresponded to the macroscopic pale zones (whitish lines) macroscopically noted in the heart of infected dogs (Figure 3). Severity of microscopic pathological findings, specifically diffused myocarditis, was more evident in experimentally infected than naturally infected dogs. Myocardial amastigote nests were detected in all infected dogs (Figure 4). Parasite foci were significantly higher in heart tissue of Zumpahuacan-infected dogs than naturally and Sylvio-X10–infected dogs (Table 2). Healthy control dogs exhibited no anatomo- and histo-pathologic abnormalities or parasite foci in the heart.
FIGURE 4.
Histopathological findings in naturally and experimentally infected dogs. Heart tissue sections (5 µm) were stained with hematoxylin and eosin. A, Note the infiltration of lymphoplasmacytic cells in myocardial section from a naturally infected dog (magnification: ×200). B and C, Transversal (B) and longitudinal sections (C) of muscular fiber from Zumpahuacan-infected dogs show the presence of large amastigote nests. In B, downward arrow shows the basophilic granular appearance of amastigotes inside of a muscular fiber and horizontal arrow shows the leukocyte inflammatory reaction against the infested fiber (×1,000). In C, the downward arrow shows the basophilic granular appearance of amastigotes in the middle of a muscular fiber, and the upward arrow shows the leukocyte inflammatory reaction against the infested fiber (magnification: ×1,000). D–F, Tissue sections from Sylvio-X10–infected dogs. D, Severe necrotic myocarditis characterized by leukocyte infiltration (horizontal arrow) with multifocal myofiber segmental necrosis (downward arrow) is evident. This infiltration corresponded to the macroscopic pale zones (whitish striates) observed in the heart. E, Dense focal interstitial leukocyte infiltration with severe multifocal myofiber segmental necrosis, loss of myocytes, and presence of a nest of amastigotes into a myofiber (small square). F, Moderate leukocyte infiltration (horizontal arrow) with multifocal myofiber segmental necrosis (downward arrow), and presence of a nest of amastigotes inside a myofiber (inset) (magnification: ×400). This figure appears in color at www.ajtmh.org.
DISCUSSION
We showed that experimental infection with the Zumpahuacan isolate produced an acute to indeterminate stage of infection that was also noted in naturally infected dogs from the rural areas of the State of Mexico. Infection of dogs with the Sylvio-X10 strain resulted in a lethal phenotype.
We performed an initial characterization of a canine model of acute chagasic myocarditis and indeterminate asymptomatic disease. We used Sylvio-X10 as a reference strain because it has been widely characterized in mouse models and proven to be pathogenically stable after many passages in in vitro culture.19,20 Infection of dogs with Sylvio-X10 was highly pathogenic; the symptoms of acute myocarditis and sudden death in Sylvio-X10–infected dogs resembled what has been noted in other hosts infected with virulent T. cruzi strains.21,22 Dogs experimentally infected with the Zumpahuacan isolate exhibited acute myocardial disturbances evidenced by electrocardio-graphic analyses. Naturally infected dogs showed clinical and pathologic signs of chronic Chagas disease. These data suggest that T. cruzi strains circulating in southern State of Mexico are able to produce various stages of Chagas disease.14
Dogs experimentally infected with Sylvio-X10 and Zumpahuacan strains developed anti-T. cruzi antibodies by 4 weeks pi. Our observations are in agreement with other reports where IgG response in infected dogs was noted at 3 weeks pi, and IgGs remained elevated with indeterminate to chronic progression of disease phase.13,15 Cardiac parasitic foci were maximum in Zumpahuacan-infected and moderate to none in Sylvio-X10–infected dogs. The elicitation of comparable IgGs and detection of fewer parasitic foci in myocardium of Sylvio-X10–infected dogs compared with Zumpahuacan-infected dogs suggests that sudden death of Sylvio-X10–infected dogs was not caused by the inability to mount anti-parasite antibodies or uncontrolled tissue parasite burden.
The interpretation of dog EKG readings according to Tilley23 and Sisson and others24 provides clues to clinical outcome of infected dogs. The Zumpahuacan-infected dogs exhibited conduction abnormalities (myocardial hypoxia, premature ventricular contraction, and sometimes sinus tachycardia) at 4 weeks pi that were normalized by 8 weeks pi, indicating the development of a clinically asymptomatic indeterminate stage. In comparison, Sylvio-X10–infected dogs exhibited sinus tachycardia and cardiac hypertrophy that likely contributed to diastolic insufficiency and myocardial hypoxia. We surmise that inability of the heart to compensate for conduction abnormalities resulted in acute myocardial hypoxia and sudden death in Sylvio-X10–infected dogs.
The notion of differential EKG abnormalities in dogs infected with various T. cruzi strains is supported by others. For example, no EKG alterations and pathologic changes were detected in dogs infected with 147 and SC-1 isolates,15,25 whereas 12SF-, Colombian-, or Be-78–infected dogs exhibited bradycardia, T-wave inversion, low-voltage QRS, S-T irregularities, or right bundle branch block (RBBB).26–28 Others showed decreased QRS voltage, repolarization disturbance, axis deviation, and indeterminate-rhythm in some, but not all, infected dogs.13 In our study, naturally infected dogs exhibited pericardiac effusion, right-ventricle enlargement, and myocardial hypoxia or right-ventricle and -atrium enlargement, accessory electric conduction defect, and sinus tachycardia. The extent of cardiomegaly and right-ventricle dilation was moderate in Sylvio-X10– and Zumpahuacan-infected dogs and mild-to-severe in naturally infected dogs. Overall, these data support the view that parasite strains, route of delivery, and number of injected parasites contribute to the differential clinical outcome of disease in T. cruzi–infected dogs.
Lesions related to cardiomyocyte fibrosis/necrosis are routinely detected in acutely infected dogs.13,29 Similarly, lesions consisting in damage to cell membrane, phlogistic aspect of cardiomyocytes, and fibrosis/necrosis have been reported in dogs28 and human patients in the indeterminate infection phase. We noted necrotic lesions representative of degenerative processes in cardiac tissue of infected dogs. Perimysial and endomysial lymphoplasmacytic infiltration in the heart of infected dogs was probably caused by evolution of adaptive immune responses and inflammatory processes that induce hyalinization and fibrosis. Others have reported the presence of myocardial necrosis14,15 and inflammatory infiltrate27,28 associated with fibrosis in dogs in indeterminate phases of T. cruzi infection and disease, thus providing support to our observations.
In summary, we showed that acute myocarditis and symptoms of indeterminate chronic Chagas disease develop in dogs experimentally or naturally infected with T. cruzi isolates from southern State of Mexico. Further studies evaluating the pathogenicity of Zumpahuacan and other isolates in dogs would advance the development of a canine model of Chagas disease and enhance our understanding of the risk of T. cruzi infection and its potential impact on inhabitants in this region of Mexico.
Acknowledgments
Financial support: This work was supported, in part, by grants from the Universidad Autónoma del Estado de México (UAEM) (2381/2006U) to J.C.V.C. and from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (AI072538) and American Heart Association (0855059F) to N.J.G. J.G.E.F. is supported by National Institutes of Health Grant A125489 and 84863 from CONACYT Mexico.
REFERENCES
- 1.World Bank W. Investing in Health. World Development Report. New York: Oxford University Press; 1993. [Google Scholar]
- 2.World Health Organization. Control of Chagas Disease: Second Report of the WHO Expert Committee. Geneva: UNDP/World Bank/WHO; 2002. [Google Scholar]
- 3.Rassi A, Jr, Rassi A, Little WC. Chagas’ heart disease. Clin Cardiol. 2000;23:883–889. doi: 10.1002/clc.4960231205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman-Bracho C. Epidemiology of Chagas disease in Mexico: an update. Trends Parasitol. 2001;17:372–376. doi: 10.1016/s1471-4922(01)01952-3. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Reyes A, Pickering-Lopez JM. Chagas disease in Mexico: an analysis of geographical distribution during the past 76 years—a review. Mem Inst Oswaldo Cruz. 2006;101:345–354. doi: 10.1590/s0074-02762006000400001. [DOI] [PubMed] [Google Scholar]
- 6.Estrada-Franco JG, Bhatia V, Diaz-Albiter H, Ochoa-Garcia L, Barbabosa A, Vazquez-Chagoyan J, Martinez-Perez MA, Guzman-Bracho C, Garg N. Human Trypanosoma cruzi infection and seropositivity in dogs, Mexico. Emerg Infect Dis. 2006;12:624–630. doi: 10.3201/eid1204.050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mott KE, Mota EA, Sherlock I, Hoff R, Muniz TM, Oliveira TS, Draper CC. Trypanosoma cruzi infection in dogs and cats and household seroreactivity to T. cruzi in a rural community in northeast Brazil. Am J Trop Med Hyg. 1978;27:1123–1127. doi: 10.4269/ajtmh.1978.27.1123. [DOI] [PubMed] [Google Scholar]
- 8.Gurtler RE, Cohen JE, Cecere MC, Lauricella MA, Chuit R, Segura EL. Influence of humans and domestic animals on the household prevalence of Trypanosoma cruzi in Triatoma infestans populations in northwest Argentina. Am J Trop Med Hyg. 1998;58:748–758. doi: 10.4269/ajtmh.1998.58.748. [DOI] [PubMed] [Google Scholar]
- 9.Laranja FS, Andrade ZA. Chronic cardiac form of chagas disease in dogs. Arq Bras Cardiol. 1980;35:377–380. [PubMed] [Google Scholar]
- 10.Andrade ZA, Andrade SG. Pathology of experimental Chagas disease in dogs. Mem Inst Oswaldo Cruz. 1980;75:77–95. doi: 10.1590/s0074-02761980000200008. [DOI] [PubMed] [Google Scholar]
- 11.Andrade ZA, Andrade SG, Sadigursky M, Maguire JH. Experimental Chagas’ disease in dogs. A pathologic and ECG study of the chronic indeterminate phase of the infection. Arch Pathol Lab Med. 1981;105:460–464. [PubMed] [Google Scholar]
- 12.Andrade ZA. Pathogenesis of Chagas disease. Res Immunol. 1991;142:126–129. doi: 10.1016/0923-2494(91)90021-a. [DOI] [PubMed] [Google Scholar]
- 13.de Lana M, Chiari E, Tafuri WL. Experimental Chagas’ disease in dogs. Mem Inst Oswaldo Cruz. 1992;87:59–71. doi: 10.1590/s0074-02761992000100011. [DOI] [PubMed] [Google Scholar]
- 14.Andrade ZA, Andrade SG, Sadigursky M, Wenthold RJ, Jr, Hilbert SL, Ferrans VJ. The indeterminate phase of Chagas’ disease: ultrastructural characterization of cardiac changes in the canine model. Am J Trop Med Hyg. 1997;57:328–336. doi: 10.4269/ajtmh.1997.57.328. [DOI] [PubMed] [Google Scholar]
- 15.Machado EM, Fernandes AJ, Murta SM, Vitor RW, Camilo DJ, Jr, Pinheiro SW, Lopes ER, Adad SJ, Romanha AJ, Pinto Dias JC. A study of experimental reinfection by Trypanosoma cruzi in dogs. Am J Trop Med Hyg. 2001;65:958–965. doi: 10.4269/ajtmh.2001.65.958. [DOI] [PubMed] [Google Scholar]
- 16.Lent H, Wygodzisnky P. Revision of the Triatominae (Hemiptera, Reduviidae) and their significance as vectors of Chagas’ disease. Bull Am Mus Nat Hist. 1979;163:284–286. [Google Scholar]
- 17.Ferreira AW, Camargo ME, Nakahara OS. Trypanosoma cruzi: immunoperoxidase antibody test for serologic diagnosis. Exp Parasitol. 1975;37:131–137. doi: 10.1016/0014-4894(75)90063-6. [DOI] [PubMed] [Google Scholar]
- 18.Bhatia V, Sinha M, Luxon B, Garg N. Utility of Trypanosoma cruzi sequence database for the identification of potential vaccine candidates: In silico and in vitro screening. Infect Immun. 2004;72:6245–6254. doi: 10.1128/IAI.72.11.6245-6254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postan M, Dvorak JA, McDaniel JP. Studies of Trypanosoma cruzi clones in inbred mice. I. A comparison of the course of infection of C3H/HEN- mice with two clones isolated from a common source. Am J Trop Med Hyg. 1983;32:497–506. doi: 10.4269/ajtmh.1983.32.497. [DOI] [PubMed] [Google Scholar]
- 20.Postan M, McDaniel JP, Dvorak JA. Comparative studies of the infection of Lewis rats with four Trypanosoma cruzi clones. Trans R Soc Trop Med Hyg. 1987;81:415–419. doi: 10.1016/0035-9203(87)90155-6. [DOI] [PubMed] [Google Scholar]
- 21.Postan M, McDaniel JP, Dvorak JA. Studies of Trypanosoma cruzi clones in inbred mice. II. Course of infection of C57BL/6 mice with single-cell-isolated stocks. Am J Trop Med Hyg. 1984;33:236–238. doi: 10.4269/ajtmh.1984.33.236. [DOI] [PubMed] [Google Scholar]
- 22.Rassi A, Jr, Rassi SG, Rassi A. Sudden death in Chagas’ disease. Arq Bras Cardiol. 2001;76:75–96. doi: 10.1590/s0066-782x2001000100008. [DOI] [PubMed] [Google Scholar]
- 23.Tilley LP. Essentials of Canine and Feline Electrocardiography: Interpretation and Treatment. Baltimore: Lippincott Williams & Wilkins; 1992. [Google Scholar]
- 24.Sisson D, O’Grady MR, Calvert CA. Myocardial diseases of dogs. In: Fox PR, Sisson D, Moïse NS, editors. Textbook of Canine and Feline Cardiology: Principles and Clinical Practice. Philadelphia: WB Saunders; 1999. p. 955. [Google Scholar]
- 25.Machado EM, Camilo Junior DJ, Pinheiro SW, Lopes ER, Fernandes AJ, Dias JC, Adad SJ. Morphometry of submucous and myenteric esophagic plexus of dogs experimentally reinfected with Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 2001;96:545–548. doi: 10.1590/s0074-02762001000400017. [DOI] [PubMed] [Google Scholar]
- 26.Andrade ZA, Andrade SG, Sadigursky M. Enhancement of chronic Trypanosoma cruzi myocarditis in dogs treated with low doses of cyclophosphamide. Am J Pathol. 1987;127:467–473. [PMC free article] [PubMed] [Google Scholar]
- 27.Caliari MV, de Lana M, Caja RA, Carneiro CM, Bahia MT, Santos CA, Magalhaes GA, Sampaio IB, Tafuri WL. Immuno-histochemical studies in acute and chronic canine chagasic cardiomyopathy. Virchows Arch. 2002;441:69–76. doi: 10.1007/s00428-001-0542-4. [DOI] [PubMed] [Google Scholar]
- 28.Caliari MV, do Pilar Machado R, de Lana M, Caja RA, Carneiro CM, Bahia MT, dos Santos CA, Magalhaes GA, Sampaio IB, Tafuri WL. Quantitative analysis of cardiac lesions in chronic canine chagasic cardiomyopathy. Rev Inst Med Trop Sao Paulo. 2002;44:273–278. doi: 10.1590/s0036-46652002000500008. [DOI] [PubMed] [Google Scholar]
- 29.Andrade ZA. Immunopathology of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94 Suppl 1:71–80. doi: 10.1590/s0074-02761999000700007. [DOI] [PubMed] [Google Scholar]