Abstract
The present study tested the long-term effects of dietary kudzu root extract supplementation on the regulation of arterial pressure, plasma glucose and circulating cholesterol in stroke-prone spontaneously hypertensive rats (SP-SHR). Female SP-SHR were maintained for 2 months on a polyphenol-free diet, with or without the addition of 0.2% kudzu root extract. One-half of the rats in each diet group were ovariectomized while the other half remained intact. Following 2 months on the diets, the 0.2% kudzu root extract supplementation (compared to control diet) significantly lowered arterial pressure (11–15 mm Hg), plasma cholesterol, fasting blood glucose (20%–30%) and fasting plasma insulin in both the ovariectomized and intact SP-SHR. These results indicate that long-term dietary kudzu root extract supplementation can improve glucose, lipid and blood pressure control in intact and ovariectomized SP-SHR.
Keywords: hypertension, glucose metabolism, lipids, polyphenols
Introduction
The metabolic syndrome affects nearly one-fourth of US adults (~47 million people), and a prominent feature of this syndrome is impaired glucose regulation (1). In the metabolic syndrome, insulin/glucose impairment, hypertension, obesity and hypercholesterolemia synergize to accelerate the development of cardiovascular disease, stroke and type 2 diabetes (2). Recently, there has been a growing interest in the ability of dietary polyphenols to reduce these three interacting factors. In rats, we and others have shown that several polyphenolic compounds decrease blood pressure, serum total cholesterol and insulin impairment (3–5).
Kudzu root (Radix pueraria) from Pueraria lobota, which is a rich source of isoflavone glucosides, has recently become commercially available in Western dietary supplements that have been marketed primarily for women’s health. The most abundant isoflavone of kudzu root is puerarin (daidzein 8-C-glucoside), but the extract also contains daidzein, daidzin (daidzein 7-O-glucoside) and other isoflavones (Fig. 1) (6)). The combined isofloavones in kudzu root extract are associated with antioxidant, antidipsotropic and related physiological effects (3,7)
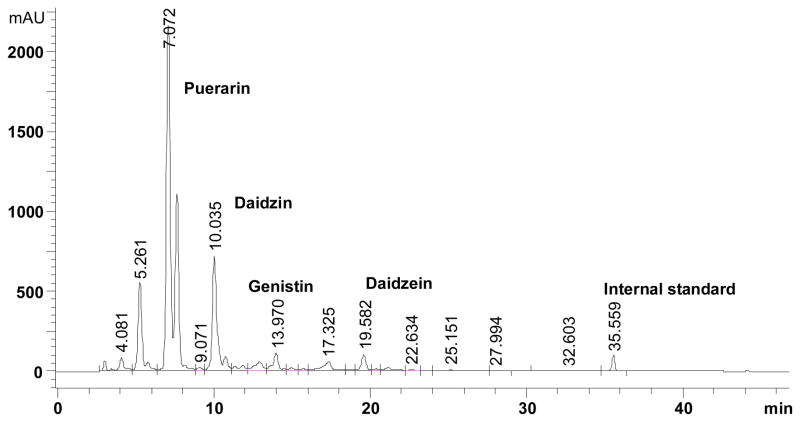
Figure 1.
HPLC chromatogram of the aqueous acetonitrile extract of kudzu root dietary preparation identifying the peaks for the major isoflavones in the supplement and retention times.
Our previous studies demonstrate that both dietary grape seed and soy isoflavone supplementation lower salt-sensitive hypertension in young spontaneously hypertensive rats (SHR) on a polyphenol free diet (8). We have also shown that several polyphenols have greater antihypertensive effects in ovariectomized compared to intact hypertensive rats (8), suggesting that these polyphenols act, at least in part, via activation of estrogenic pathways. Thus, this study tested the blood pressure lowering effects of kudzu root extract in ovariectomized and intact, hypertensive female rats.
Acute administration of puerarin, (the major isoflavone in kudzu root) significantly improves glucose tolerance in C57 BL/6J ob/ob mice (4), an animal model of Type 2 diabetes mellitus. Together with other information, this suggests that as puerarin is transited from the intestines to the blood, it inhibits glucose transport to the blood. In contrast, acute administration of the second most abundant isoflavone in kudzu root extract, daidzin (~7% w/w vs. puerarin 25% w/w), impairs glucose tolerance (4). Further studies have reported beneficial effects of acute puerarin administration on common pathogenic factors of metabolic syndrome (9, 10), not concerning the effects of long-term administration of these dietary polyphenols.
The present experiments hypothesized that dietary kudzu root extract supplementation improves blood pressure and metabolic indices in female stroke-prone spontaneously hypertensive rats (SP-SHR), a rat strain that is predisposed to hypertension and stroke and frequently used as a model for human metabolic syndrome (11).
Materials and Methods
Quantification of Kudzu Isoflavones
To determine the isoflavone content of the kudzu root extract, kudzu dietary supplement powder (20.96 mg; AMAX NutraSource, Eugene, OR) was extracted with 5 mL of 50% acetonitrile in water and the samples were tumbled for 2 h at room temperature. β-napthoflavone (Sigma Aldrich, MO, USA) was used as internal standard at 100 μM concentration. After extraction, all samples were centrifuged and an aliquot of the supernatant was analyzed by high-performance liquid chromatography (HPLC). It (10 μL) was injected onto a Brownlee 220 mm × 4.6 mm i.d. 300 Å pore size, Aquapore C8 reversed-phase column with a 150 mm × 3.2 mm i.d. RP-8 NewGuard guard column (Perkin Elmer, Norwalk, CT). The mobile phase consisted of solvent A (10% aqueous acetonitrile/0.1% trifluoroacetic acid) and solvent B (90% aqueous acetonitrile/0.1 % TFA). The samples were eluted from the HPLC column at a flow rate of 1.0 mL/min with a linear gradient increase of solvent B to 100% over 35 min. Over the next 8 min the column was flushed with 100% solvent B. Prior to the next injection, the column was equilibrated for 10 min with solvent A. The HPLC (1100 series Hewlett Packard/Agilent, Wilmington, DE) system was equipped with a quaternary pump, a refrigerated autosampler and a diode array detector. UV spectra were recorded over 225 to 400 nm with a step of 2 nm and a slit setting of 4 nm. Peak areas were measured at 262 nm, in agreement with studies of Yang, et al and our previously published work (6, 12). All data were processed using Chemstation Software for liquid chromatography 3D systems (Rev. A.08.03, Agilent, Wilmington, DE). Standard curves of puerarin, daidzin and daidzein (Indofine, Somerville, NJ, USA) were prepared and used for quantification of these isoflavones in the extract.
Liquid chromatography-mass spectrometry (LC-MS/MS) Quantification of Kudzu Isoflavones in Plasma
LC-MS/MS analyses of plasma samples were performed using a system consisting of a model SIL-HT refrigerated Shimadzu autosampler (Shimadzu Scientific Instruments, Inc. Columbia, MD), and an API 4000 Q TRAP (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada). Chromatography was carried out on a reversed-phase Phenomenex Synergi 4 micron Fusion-RP80 column (150 × 2.0 mm i.d.) pre-equilibrated with 10 mmol/L ammonium acetate (NH4OAc). The mobile phase consisted of a gradient of 10–70% acetonitrile in 10 mmol/L NH4OAc over 6 min with a flow rate of 0.2 mL/min Multiple reaction monitoring (MRM) was used to perform mass spectrometric quantification of puerarin (precursor ion to product ion transition from m/z 415/267). The column effluent was introduced into the mass spectrometer using electrospray ionization in the negative mode. The LC-MS/MS system was controlled by BioAnalyst 1.4 software. The analysis was carried out in the negative ion mode with declustering potential −110 V and ion spray voltage −4500 V. The temperature of turbo gas was 450°C. Nitrogen was used as the collision gas.
Diet Preparation
The Experimental diet was made by adding powdered kudzu root extract (0.2% w/w) to AIN-93M diet. The combination was blended overnight in a rotating mixer and pelleted (TestDiet, Richmond, IN). The control diet was a similarly pelleted AIN-93M diet with no additions (TestDiet, Richmond, IN). The diets were tested monthly to insure consistency of isoflavone content, using the HPLC method listed above. The assays of the percent dietary content of puerarin and daidzein from the random samples of the diets were in close agreement (< 5% variability from the original assay, see Results). The kudzu root extract concentration in the diet was chosen based on our preliminary and previous studies (5). Our pilot studies demonstrated that the extract could be given to rats for 3 mo at a concentration 20 times that used in this study with no toxic or observable pathological effects.
Animal experiments
SP-SHR were bred in our colony from parental lines from the National Heart, Lung and Blood Institute. They were housed three per cage at constant humidity (65 ± 5%), temperature (24°C ± 1°), and light-dark cycle (0600–1800 h, lights on), and allowed ad libitum access to tap water and diet throughout the experimental protocols. All experimental procedures were conducted under the oversight and approval of the Institutional Animal Care and Use Committee of the University of Alabama, Birmingham and in accordance with National Institutes of Health guidelines and the Guide to the Care and Use of Laboratory Animals.
All dams were maintained on a Teklad 2016 basic soy diet (Harlan, Indianapolis, IN) throughout their pregnancy and weaning. The pups continued on the soy-based diet after weaning. At one month of age, 28 female pups were anesthetized with 2–5% isoflorane and one-half of the animals in each group were ovariectomized under aseptic conditions (n=14), while the other 14 animals were not operated on (n=14). All animals recovered from the anesthesia for 1 week prior to division into two groups that were maintained on a polyphenol-free, AIN 93M diet with or without the addition of 0.2% kudzu root extract. Thus, seven animals in each of 4 groups (kudzu supplemented or non-supplemented diet with or without ovariectomy).
Following 2 months on diet, the rats were anesthetized (isoflurane), silastic femoral artery catheters were implanted, and after recovery the rats were individually housed. One day later, mean arterial pressure (MAP) and heart rate (HR) were measured in conscious, freely moving rats via a pressure transducer as previously described (BIOPAC System, Galeena, CA; (8)) After a 30 min stabilization period, MAP and heart rate were recorded for 30 min. After an overnight fast, blood was drawn via tail artery nick to measure plasma glucose (enzymatic glucose-oxidase reaction, GM7 analyzer, Analox USA, Lunenburg, MA). Blood for plasma insulin (Radioimmunoassay, Linco Research, St. Charles, MO) and plasma lipid concentrations (Vitros DT60 [Ektachem] analyzer, Ortho-Clinical Diagnostics, Rochester, NY) was collected via arterial catheters. Food intake and body weight were measured weekly throughout the experiment.
Statistical analysis
All experimental data were evaluated by two-way analysis of variance (ANOVA) followed by post hoc Tukey’s test to determine the source of main effects and interactions (SPSS, Chicago, IL). The significance criterion for all experiments was p < 0.05. All data are reported in the Results section as mean ± standard error.
Results
Quantification of isoflavones in kudzu root extract and its plasma concentration
HPLC analysis was used to quantify the amount of major isoflavones in the kudzu root extract, i.e., puerarin, daidzin and daidzein. Puerarin was the most abundant isoflavone (approximately 25.3%, w/w) followed by daidzin (7.1%, w/w) and daidzein (0.8%, w/w) in the kudzu root extract. In addition to these isoflavones, there are several other peaks including genistin (Fig. 1). We performed a preliminary investigation on bioavailability of puerarin in plasma samples collected from the ovariectomized groups that had been fed for 2 months with the same kudzu root extract supplemented (0.2%) AIN-93M diet that was used in the present study. The blood was sampled at 11:00 am, thus it is likely that this reflects a baseline concentration in the blood, since the rats would be expected to have few eating bouts after lights on at 6 am. The MRM analysis of plasma samples indicated the presence of unmetabolized puerarin. Using our previously reported LC-MS/MS method (13), puerarin was detected at the concentration of 49 ± 7 nmol/L in the treated ovariectomized animals, while in the control-fed group, it was below detection limits (< 1 nmol/L).
General effects on health
While kudzu treated (compared to control diet) animals had lower body weights (~8.5%; Table 1), these differences were not attributable to deviations in food consumption in the kudzu-fed rats. At the conclusion of the two-month feeding intervention, control-fed, intact animals had a higher body weight compared to kudzu-treated intact rats (Table 1). Similarly, the body weights of the ovariectomized rats on the control compared to kudzu diet were significantly higher. Daily food consumption did not differ between the control diet and kudzu treated groups in either intact rats or ovariectomized rats (Table 1). Similarly, no significant differences in water intake were observed between intact control and kudzu diet fed rats; however, both the kudzu-supplemented and the control diet ovariectomized rats had significantly greater water consumption than their counterparts in the intact groups (Table 1).
Table 1.
Physiological characteristics of intact and ovariectomized (Ovex) SP-SHR on the control and kudzu supplemented diets (Mean ± SE)
| Index | Intact control | Intact kudzu | Ovex Control | Ovex Kudzu |
|---|---|---|---|---|
| Arterial Pressure (mm Hg) | 182 ± 2 | 170 ± 3* | 199 ± 3 | 181 ± 4* |
| Heart Rate (bpm) | 389 ± 15 | 368 ± 13 | 397 ±15 | 378 ± 10 |
| Body Weight (g) | 213 ± 7 | 195 ± 4* | 235 ± 6† | 215 ± 5*† |
| Food Intake (g) | 13 ±1 | 12 ± 2 | 13 ±1 | 12 ± 2 |
| Water Intake (ml) | 20 ± 1 | 19 ± 1 | 25 ± 1† | 25 ± 1† |
p < 0.05 compared to control diet group with same treatment
p < 0.05 compared to intact group on same diet.
Cardiovascular Effects
Chronic dietary kudzu root extract supplementation resulted in a small but significant decrease in arterial pressure in both ovariectomized and intact animals (Table 1). The response in ovariectomized rats did not differ significantly from intact animals. Heart rates were not significantly different in control compared to kuzdu-supplemented SP-SHR in either the ovariectomized or the intact groups (Table 1).
Effects on metabolism and lipid profile
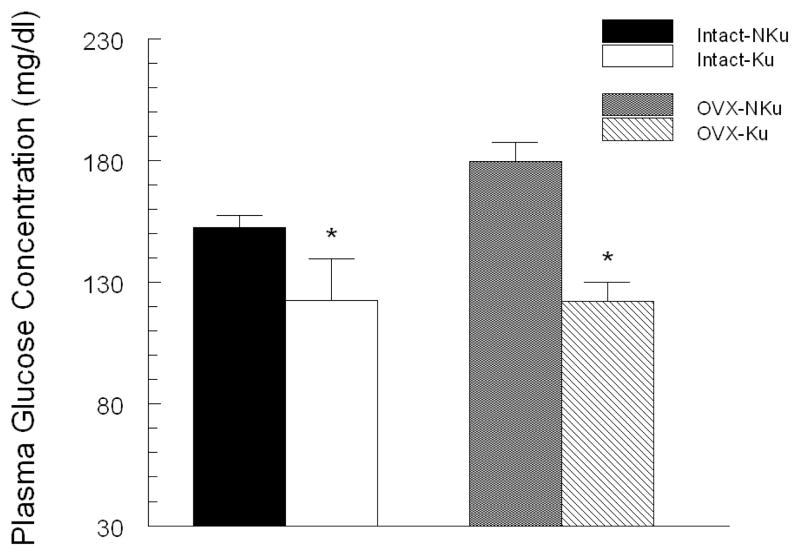
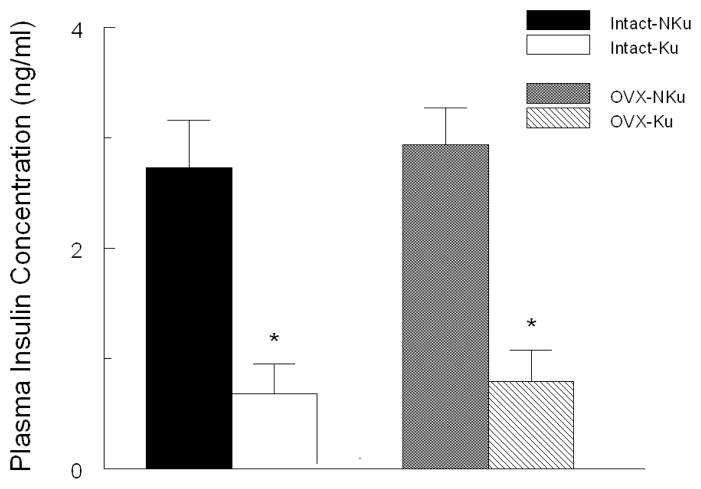
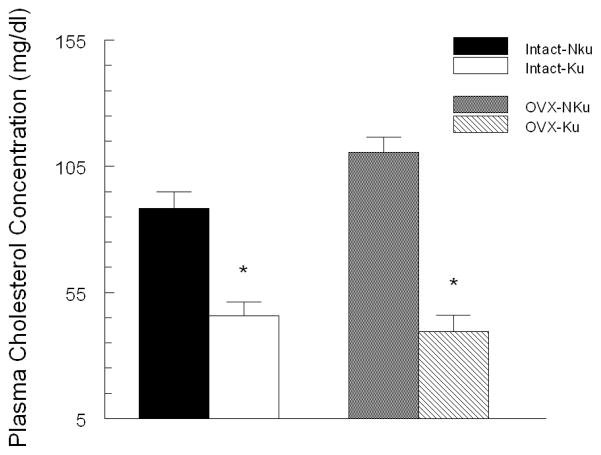
Following two months on diet, baseline fasting plasma glucose was significantly lower in the intact and ovariectomized rats fed the kudzu supplemented compared to control diet (Fig. 2). In both intact and ovariectomized groups, plasma insulin concentrations were significantly lower in the kudzu-fed, compared to control diet (Fig. 3). Chronic dietary kudzu also significantly lowered plasma total cholesterol concentrations, irrespective of ovariectomy status (Fig. 4). However, plasma triglycerides concentrations were not affected by the kudzu diet (data not shown).
Figure 2.
Chronic treatment with AIN-93 diet containing 0.2% kudzu root extract compared to non-supplemented AIN-93 diet, lowered plasma glucose concentration in both intact and ovariectomized (OVX) SP-SHR. * p < 0.05 compared to control (NKu) diet group with the same surgical treatment. n=7 per group.
Figure 3.
Chronic treatment with AIN-93 diet containing 0.2% kudzu root extract compared to non-supplemented AIN-93 diet, lowered plasma insulin in both intact and ovariectomized (OVX) SP-SHR. * p < 0.05 compared to control control (NKu) diet group with the same surgical treatment. n=7 per group.
Figure 4.
Chronic treatment with AIN-93 diet containing 0.2% kudzu root extract compared to non-supplemented AIN-93 diet, lowered plasma total cholesterol in both intact and ovariectomized (OVX) SP-SHR. * p < 0.05 compared to control (NKu) diet group with the same surgical treatment. n=7 per group.
Discussion
These results demonstrate that chronic dietary kudzu root extract improves plasma glucose, insulin and cholesterol concentrations in young female SP-SHR. Further, long-term intake of kudzu extract also decreases arterial pressure in this hypertensive model. The SHR model (including the SP-SHR) bears several characteristics that are similar to those associated with metabolic syndrome. While hypertension is the primary symptom in this model, the SHR also displays elevated resting plasma glucose and insulin concentrations, insulin resistance (14), endothelial dysfunction (15), oxidative stress (16) and hyperlipidemia (17). However, the SP-SHR does not become obese, nor does it develop full type 2 diabetes. Thus, the present results suggest that chronic administration of kudzu root isoflavones improves glucose and lipid metabolism and cardiovascular function, indicating that it may offer a useful tool to help reduce risk factors that contribute to the development of the metabolic syndrome.
This is the first report investigating potential for long-term kudzu supplementation to decrease these interacting factors of the metabolic syndrome in an animal model. Our initial focus was on the ability of kudzu supplementation to reduce hypertension in the SP-SHR. Hypertension is arguably the most important single risk factor for stroke, based on risk and prevalence. Reduction of hypertension by any of the current antihypertensive drugs lowers the risk of stroke (18). In addition to its effects on stroke, hypertension increases the risk of heart failure and coronary heart disease. For instance, the risk of developing left ventricular hypertrophy is greatly increased in chronic hypertensive patients. Further, cardiovascular disease is closely related to arterial blood pressure, with a 5 mm Hg decrease in arterial pressure equating to a 16% decrease in cardiovascular disease (19).
The decreases in mean arterial pressure that we observed in the SP-SHR that were treated with kudzu root extract compared to control diet are relatively small (< 15 mm Hg) compared to the increased hypertension observed in our other SHR studies. For instance, angiotensin converting enzyme inhibitors and other antihypertensive drugs typically decrease blood pressure by >20 mm Hg in SHR, e.g., (20). Further, a high (compared to basal) salt diet increases SHR blood pressure increases >20 mm Hg. Chronic treatment of ovariectomized SHR with soy or grape seed reduces blood pressure, but these two botanical supplements have little effect on blood pressure in intact female SHR. In contrast, kudzu root extract has relatively equal antihypertensive effects in intact and ovariectomized female SP-SHR, suggesting that the mechanism of action of these botanicals may differ.
Strategies currently recommended for decreasing hypertension and, thereby, reducing cardiovascular disease and stroke, depend primarily on pharmacological treatments but also include increasing daily activity and dietary interventions. Recent dietary strategies have included the intake of certain bioactive components in foods (21). The present findings suggest that polyphenols in kudzu root may provide a non-pharmacological complement to traditional approaches for treating hypertension. The ability of a well-tolerated, safe and low cost food additive to decrease hypertension is of considerable interest.
A second finding of the present study is that chronic kudzu root supplementation improves glycemic control, insulin sensitivity and total circulating cholesterol concentration in SP-SHR. Prominent contributors to the metabolic syndrome include insulin resistance, impaired glucose tolerance and elevated plasma lipids (22). The observation that chronic kudzu root extract decreases fasting blood glucose, total plasma cholesterol and insulin concentrations in an animal model of metabolic syndrome suggests that the polyphenols in kudzu root extract may provide a dietary supplement that significantly decreases the risk and severity of stroke and cardiovascular disease in at-risk individuals. These findings are consistent with others studies that have tested the acute effects of puerarin on these factors, e.g., (23, 24).
One potentially confounding factor in the interpretation of these findings is that the body weights of the kudzu-supplemented compared to control diet rats were ~8% lower. It should be noted that all rats gained considerable body weight over the course of the study (their body weight at the start of the dietary intervention was 75+/− 3 g) and were within the normal body weight range for this strain at the end of the study. We have previously maintained SP-SHR on these diets for 20 months and consistently noted that the appearance of the kudzu supplemented animals was equal or better than that of their control diet counterparts (unpublished data). Thus, the decreased body weight gain in the kudzu-supplemented SP-SHR does not appear to impair health in this strain. Further, on the kudzu-supplemented diet, the ovariectomized compared to the control SP-SHR had significantly greater body weights but no associated differences in plasma glucose, insulin or lipids. In studies in mice, kudzu root supplementation does not effect body weight, but it does improve plasma glucose concentration to about the extent as shown in the present studies (10). Further, in the present study we correlated body weights of rats with their baseline plasma glucose and lipid concentrations, and found no significant relationship in any of the four groups. One might conjecture that increased fat deposits or changes in lean body mass contribute to the lipid and glucose findings of the current study. In preliminary studies we have measured fat pads in kudzu-treated and control diet (for 6 months) SP-SHR and observed no diet-related differences in fat deposits (unpublished data). Together, these findings suggest that body weight per se is not a major contributor to the plasma glucose effects of kudzu root supplementation in SP-SHR. However, until a formal study of the effects of the diet on body composition are conducted, it remains possible that kudzu root acts on these parameters, in part, via alterations in body weight or body composition.
One mechanism that may contribute to the ability of kudzu root extract to regulate plasma glucose is alterations in the expression of intestinal glucose transporter(s) such as SGLT-1 and GLUT-2. These transporters normally move glucose into the blood, but acute exposure to puerarin appears to inhibit this transporter (4) potentially buffering glucose transport into the blood. Our studies in mice suggest that acute exposure to puerarin inhibits SGLT-1 transport (4).
A second mechanism by which kudzu polyphenols could improve glycemic control and reduce cholesterol is through activation of the peroxisome proliferator-activated receptor gamma (PPARγ) expression pathway. PPAR-γ is involved in the control of various aspects of lipid metabolism (25) and is highly expressed in adipose tissue. Adipogenesis is stimulated by PPAR-γ through the induction of genes mediating fatty acid metabolism, and increased adipogenesis is associated with decreased adiposity, and decreased release of detrimental cytokines from adipose tissue, thus reducing these major risk factors for insulin resistance and type 2 diabetes (26). Previous research has demonstrated that puerarin promotes PPARγ expression, partly through inhibition of abnormal TNF-alpha-induced intracellular-free Ca++ accumulation in endothelial cells. Studies from Chung and associates indicate that both in vitro and in vivo, puerarin and its glycosides induce LDL receptor promoter activity, increase mRNA and protein levels (27) and reduce HMG-CoA reductase mRNA and proteins (27). PPARγ stimulation also improves insulin-stimulated glucose disposal by muscle (28) and adiponectin translation (29).
A significant number of dietary plant compounds are phytoestrogenic, displaying estrogenic properties and possibly inducing their observed effects via interaction with estrogen receptors. Many of the compounds do not exert measurable effects in young, intact female animals, likely because of competition with circulating estrogen, but the effects of these polyphenols are significant in animals in which circulating estrogen is minimal, i.e., natural menopause or ovariectomy. We have shown previously that combined (but not individual) reduction of endogenous estrogen (via ovariectomy) and exogenous phytoestrogens/polphenols (dietary) leads to an exacerbation of hypertension in middle-aged SHR and SP-SHR. Addition of soy phytoestrogens (of which genistein conjugates are the most abundant) at dietary levels reduces hypertension in this model (30). In the present experiment, the effect of kudzu root extract on arterial pressure was not significantly greater in the ovariectomized compared to intact SP-SHR (Fig. 2–4 and Table 1). While potentially an estrogenic mechanism could underlie part of the observed antihypertensive and metabolic effects of the kudzu supplement, it is likely that a non-estrogenic component(s) play a prominent role in the effects of this botanical.
We suggest that individual phenolic compounds in kudzu extract are related to its beneficial effects. Kudzu root extract not only contains a high concentration of puerarin (25%) but also other phenolic compound. Together with our previous studies, the present results suggest that puerarin is the dominant polyphenol in the glucose effects of kudzu root extract supplementation (4).
In this study, resting plasma concentrations of unmetabolized puerarin were relatively low, indicating that puerarin does not accumulate in the blood following chronic administration of kudzu root extract. This is also in agreement with our recently report that a single oral administration of puerarin to rats results in a relatively rapid clearance of most puerarin from the blood (T½ = 1.7 ± 0.6 h), distribution of puerarin to several of body organs and a relatively rapid excretion of the compound (13).
In summary, the present results demonstrate that chronic administration of kudzu root extract decreases arterial pressure and reduces plasma glucose, insulin and cholesterol concentrations in a female rat model of the metabolic syndrome. While the exact mechanisms remain to be determined, the present results suggest that incorporation of kudzu root supplements into a diet modulates glucose, lipids and blood pressure. Since the supplement appears to have no adverse or toxic effects at these dietary levels in rats, it may be useful to consider the use of kudzu polyphenols as complements to strategies used to reduce metabolic disorders.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant AT 00477 from the National Center for Complementary and Alternative Medicine (to J.M.W.) and the Office of Dietary Supplements and Grants NS 041071 (to J.M.W.) and NS 047466 and NS 057098 (to J.M.W.) from the National Institute of Neurological Disorders and Stroke. The mass spectrometer was purchased from funds provided by NCRR grant S10 RR13795 and the UAB Health Services Foundation General Endowment Fund. Operation of the Mass Spectrometry Shared Facility comes from a NCI Core Support Grant (P30 CA13148) to the UAB Comprehensive Cancer Center. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements, or the NIH.
Abbreviations and Nomenclature
- HPLC
high-performance liquid chromatography
- Ku
rats on kudzu supplemented diets
- LC-MS/MS
Liquid chromatography-mass spectrometry
- MRM
multiple reaction monitoring
- NKu
rats on control diet
- OVX
ovariectomy
- SHR
Spontaneously Hypertensive rats
- SP-SHR
Stroke-prone spontaneously hypertensive rats
- TFA
trifluoroacetic acid
Footnotes
The authors have nothing to disclose
Literature Cited
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Kraja AT, Province MA, Huang P, Jarvis JP, Rice T, Cheverud JM, Rao DC. Trends in metabolic syndrome and gene networks in human and rodent models. Endocr Metab Immune Disord Drug Targets. 2008;8(3):198–207. doi: 10.2174/187153008785700145. [DOI] [PubMed] [Google Scholar]
- 3.Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC, Cheng JT. Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J Nat Prod. 2003;66(6):788–792. doi: 10.1021/np0203887. [DOI] [PubMed] [Google Scholar]
- 4.Meezan E, Meezan EM, Jones K, Moore R, Barnes S, Prasain JK. Contrasting effects of puerarin and daidzin on glucose homeostasis in mice. J Agric Food Chem. 2005;53(22):8760–8767. doi: 10.1021/jf058105e. [DOI] [PubMed] [Google Scholar]
- 5.Prasain JK, Jones K, Brissie N, Moore R, Wyss JM, Barnes S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2004;52(12):3708–3712. doi: 10.1021/jf040037t. [DOI] [PubMed] [Google Scholar]
- 6.Prasain JK, Jones K, Kirk M, Wilson L, Smith-Johnson M, Weaver C, Barnes S. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J Agric Food Chem. 2003;51(15):4213–4218. doi: 10.1021/jf030174a. [DOI] [PubMed] [Google Scholar]
- 7.Boue SM, Wiese TE, Nehls S, Burow ME, Elliott S, Carter-Wientjes CH, Shih BY, McLachlan JA, Cleveland TE. Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J Agric Food Chem. 2003;51(8):2193–2199. doi: 10.1021/jf021114s. [DOI] [PubMed] [Google Scholar]
- 8.Peng N, Clark JT, Prasain J, Kim H, White CR, Wyss JM. Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005;289(3):R771–R775. doi: 10.1152/ajpregu.00147.2005. [DOI] [PubMed] [Google Scholar]
- 9.Shi WG, Qu L, Wang JW. [Study on interventing effect of puerarin on insulin resistance in patients with coronary heart disease] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22(1):21–24. [PubMed] [Google Scholar]
- 10.Carlson S, Peng N, Prasain JK, Wyss JM. Effects of botanical dietary supplements on cardiovascular, cognitive, and metabolic function in males and females. Gend Med. 2008 ;5(Suppl A):S76–S90. doi: 10.1016/j.genm.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strahorn P, Graham D, Charchar FJ, Sattar N, McBride MW, Dominiczak AF. Genetic determinants of metabolic syndrome components in the stroke-prone spontaneously hypertensive rat. J Hypertens. 2005;23(12):2179–2186. doi: 10.1097/01.hjh.0000191904.26853.b8. [DOI] [PubMed] [Google Scholar]
- 12.Yang F, Ma Y, Ito Y. Preparative separation of isoflavone components in soybeans using high-speed counter-current chromatography. Vol. 293. 2001. pp. 271–274. [DOI] [PubMed] [Google Scholar]
- 13.Prasain JK, Peng N, Acosta E, Moore R, Arabshahi A, Meezan E, Barnes S, Wyss JM. Pharmacokinetic study of puerarin in rat serum by liquid chromatography tandem mass spectrometry. Biomed Chromatogr. 2007;21(4):410–414. doi: 10.1002/bmc.772. [DOI] [PubMed] [Google Scholar]
- 14.Collison M, Glazier AM, Graham D, Morton JJ, Dominiczak MH, Aitman TJ, Connell JM, Gould GW, Dominiczak AF. Cd36 and molecular mechanisms of insulin resistance in the stroke-prone spontaneously hypertensive rat. Diabetes. 2000;49(12):2222–2226. doi: 10.2337/diabetes.49.12.2222. [DOI] [PubMed] [Google Scholar]
- 15.Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43(1):19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Reckelhoff JF. Basic research into the mechanisms responsible for postmenopausal hypertension. Int J Clin Pract Suppl. 2004;(139):13–19. [PubMed] [Google Scholar]
- 17.Aitman TJ, Gotoda T, Evans AL, Imrie H, Heath KE, Trembling PM, Truman H, Wallace CA, Rahman A, Dore C, Flint J, Kren V, Zidek V, Kurtz TW, Pravenec M, Scott J. Quantitative trait loci for cellular defects in glucose and fatty acid metabolism in hypertensive rats. Nat Genet. 1997;16(2):197–201. doi: 10.1038/ng0697-197. [DOI] [PubMed] [Google Scholar]
- 18.Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370(9587):591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- 19.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 20.Fang Z, Berecek K, Wyss JM. Dietary NaCl-sensitive hypertension in one-year-old spontaneously hypertensive rats and lifetime captopril-treated Wistar Kyoto rats. Hypertension. 1998;32:36. [Google Scholar]
- 21.Samuels TA, Bolen S, Yeh HC, Abuid M, Marinopoulos SS, Weiner JP, McGuire M, Brancati FL. Missed opportunities in diabetes management: a longitudinal assessment of factors associated with sub-optimal quality. J Gen Intern Med. 2008;23(11):1770–1777. doi: 10.1007/s11606-008-0757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47(6):1093–1100. doi: 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133(5):1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 24.Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76(6):1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 25.Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiarelli F, Di MD. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: current evidence and future perspectives. Vasc Health Risk Manag. 2008;4(2):297–304. doi: 10.2147/vhrm.s993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung MJ, Sung NJ, Park CS, Kweon DK, Mantovani A, Moon TW, Lee SJ, Park KH. Antioxidative and hypocholesterolemic activities of water-soluble puerarin glycosides in HepG2 cells and in C57 BL/6J mice. Eur J Pharmacol. 2008;578(2–3):159–170. doi: 10.1016/j.ejphar.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 28.Sugden MC, Holness MJ. Role of nuclear receptors in the modulation of insulin secretion in lipid-induced insulin resistance. Biochem Soc Trans. 2008;36(Pt 5):891–900. doi: 10.1042/BST0360891. [DOI] [PubMed] [Google Scholar]
- 29.Banga A, Unal R, Tripathi P, Pokrovskaya I, Owens RJ, Kern PA, Ranganathan G. Adiponectin translation is increased by the PPAR{gamma} agonists pioglitazone and {omega}-3 fatty acids. Am J Physiol Endocrinol Metab. 2009;296(3):E480–E489. doi: 10.1152/ajpendo.90892.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang Z, Carlson SH, Chen YF, Oparil S, Wyss JM. Estrogen depletion induces NaCl-sensitive hypertension in female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2001;281(6):R1934–R1939. doi: 10.1152/ajpregu.2001.281.6.R1934. [DOI] [PubMed] [Google Scholar]