Abstract
Objective
We previously demonstrated that pharmacologic activation of AMP-activated protein kinase (AMPK) with 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR) 24 hours prior to (AICAR preconditioning; AICAR-PC) ischemia/reperfusion (I/R) prevents postischemic leukocyte-endothelial cell adhesive interactions (LEI) by a mechanism initiated by endothelial nitric oxide synthase (eNOS)-dependent NO production during the period of AICAR-PC. The major aim of this study was to examine the role of ATP-sensitive potassium (KATP) channels and heme oxygenase as mediators of the antiadhesive effects of AICAR-PC during I/R 24 hours later.
Methods
Intravital fluorescence microscopy was used to quantify LEI in the small intestine of AICAR-preconditioned C57BL/6J mice treated with KATP channel or heme oxygenase inhibitors during I/R 24 hours after AICAR-PC in separate experiments.
Results
I/R induced marked increases in LEI relative to sham control mice, proadhesive responses that were prevented by AICAR-PC 24 hours prior to I/R. The effects of AICAR-PC to prevent postischemic LEI were abolished by KATP channel or heme oxygenase inhibition during I/R.
Discussion
Our results indicate that the antiadhesive effects of AICAR-PC are mediated by KATP channel- and heme oxygenase-dependent mechanisms during I/R.
Keywords: ischemia, reperfusion, leukocyte rolling and adhesion, AMPK, preconditioning, KATP channels, heme oxygenase
A wide variety of diverse agents and stimuli (e.g., short bouts of ischemia, ethanol ingestion at low to moderate levels, treatment with adenosine receptor agonists, reactive oxygen species (ROS), opioids, calcitonin gene-related peptide (CGRP), bradykinin, or nitric oxide (NO) donors) have been shown to induce the development of a protective anti-inflammatory phenotype in postcapillary venules, such that these vessels fail to support adhesion molecule expression, leukocyte rolling and adhesion, and increased vascular permeability when the small bowel is subsequently exposed to prolonged I/R [8,9,24,25,36,37,50]. The agents and stimuli that induce development of such protected states are referred to as preconditioning stimuli. The cellular mechanisms involved in preconditioning are often operationally subdivided into three categories: triggers or initiators, downstream signaling cascades, and mediators or effectors of the preconditioned states. Triggers or initiators of preconditioning refer to chemical signals that are released and/or ion channels that are activated in response to exposure to preconditioning stimuli. These inaugurating events, in turn, activate downstream signaling cascades that include a variety of kinases that function to sequentially phosphorylate proteins involved in the activation of normally dormant stress-responsive transcription factors. Transcriptional upregulation of protective genes culminates in the synthesis of new proteins that serve as mediators or effectors of the beneficial actions of preconditioning during I/R 24 hours later. Most of the agents that induce the development of preconditioned states do so by a triggering mechanism that involves the formation of NO by endothelial nitric oxide synthase (eNOS).
We recently demonstrated that preconditioning with 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR), an activator of the ubiquitously expressed heterotrimeric serine/threonine kinase AMP-activated protein kinase (AMPK), triggers entrance into a anti-inflammatory phenotype (a phenomenon termed AICAR preconditioning; AICAR-PC) by an eNOS-dependent mechanism [12]. Interestingly, the glucose-lowering agent, metformin, has been shown to activate AMPK and prevent the signaling of inflammatory cytokines through nuclear factor kappa B and tumor necrosis factor a [18]. This study also demonstrated that adhesion molecule expression was reduced by metformin or AICAR administration, a protective effect that was abrogated by the addition of a siRNA directed toward AMPKα1. These activities have previously been attributed to eNOS by others [1,49]. A more recent study presented evidence of metformin's cardioprotective potential against I/R and its effect on AMPK activation and eNOS phosphorylation [3]. However, little is known about the effector mechanisms that are activated during I/R 24 hours after AMPK-dependent eNOS activation to mediate the reductions in postischemic leukocyte rolling and adhesion.
Many of the different preconditioning stimuli that have been studied to date appear to induce protection by KATP channel-dependent mechanisms. Most of this work is consistent with the concept that such preconditioning stimuli trigger entrance into a preconditioned state by activating surface (i.e., plasmalemmal) KATP (sKATP) channels while mitochondrial KATP (mitoKATP) channels serve as effectors that mediate protection during I/R 24 hours after the preconditioning stimulus is applied [31,32]. In light of these observations, we determined whether KATP channels serve as triggers versus effectors in AICAR-PC as the first goal of this study. Additionally, several lines of evidence suggested the possibility that heme oxygenase may serve as an effector of AICAR-PC. For example, AICAR-PC is triggered by the formation of eNOS-derived NO [12] and heme oxygenase activity is upregulated by NO donors [29]. In addition, the catalytic activity of heme oxygenase produces metabolites that exhibit powerful antiadhesive and antioxidant effects [14,20,30,42,43,46]. Thus, we hypothesized that this protein may serve as an important effector of AICAR-PC, mediating the antiadhesive actions of antecedent treatment with AICAR during I/R 24 hours later.
MATERIALS AND METHODS
Animals
Wild-type male C57BL/6J mice (six to seven weeks of age) were obtained from the Jackson Laboratories (Bar Harbor, Maine, USA). All mice were maintained on standard mouse chow and water ad libitum with 12-hour light-dark cycles, and used at 8–10 weeks of age. The experimental procedures have been described previously [25] and were performed according to the criteria outlined in the National Institutes of Health guidelines and were approved by the University of Missouri–Columbia Institutional Animal Care and Use Committee.
AICAR Preconditioning, Surgical Procedures, and Induction of I/R
Mice were preconditioned with the AMPK agonist, 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR; Sigma, St. Louis, MO, USA) (100 mg/kg, 0.5 mL) by intraperitoneal (i.p.) injection 24 hours prior to the induction of I/R in C57BL/6J mice. Twenty-four hours later, the mice were anesthetized initially by intramuscular (i.m.) injection of a mixture of ketamine (150 mg/kg body weight) and xylazine (7.5 mg/kg body weight). After attaining a surgical plane of anesthesia, a midline abdominal incision was performed and the superior mesenteric artery (SMA) was occluded with a microvascular clip for 0 (sham) or 45 minutes. After these procedures, the right carotid artery was cannulated and systemic arterial pressure was measured with a Statham P23A pressure transducer (Gould, Valley View, OH, USA) connected to the carotid artery catheter. Systemic blood pressure was recorded continuously with a personal computer (Power Macintosh 8600; Apple Cupertino, CA, USA) equipped with an analog-to-digital converter (MP 100; Biopac Systems, Goleta, CA, USA). The left jugular vein was cannulated for the administration of carboxyfluorescein diacetate, succinimidyl ester (CFDASE; Molecular Probes, Eugene, Oregon, USA), a fluorescent dye that labels leukocytes [40]. CFDASE was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 5 mg/mL, divided into 25-μL aliquots, and stored in light-tight containers at −20°C until use. During the preparation and storage of CFDASE, care was taken to minimize light exposure. After the 45-minute ischemic period, the clip was gently removed and leukocytes were labeled with CFDASE by intravenous (i.v.) administration of the fluorochrome solution (250 μg/mL saline) at a rate of 20 μL/min for five minutes. The sham group had an equivalent 45-minute period without occlusion of the SMA prior to CFDASE administration. Leukocyte/endothelial cell adhesive interactions were observed over minutes 30–40 and 60–70 of reperfusion, or at equivalent time points in sham groups, via intravital fluorescence microscopy.
Intravital Fluorescence Microscopy
The mice were positioned on a 20×30 cm acrylic plastic board in a manner that allowed a selected section of small intestine to be exteriorized and placed carefully and gently over a glass slide covering a 4×3 cm hole centered in the Plexiglas. The exposed small intestine was superfused with warmed (37°C) bicarbonate-buffered saline (BBS; pH 7.4) at 1.5 mL/min, using a peristaltic pump (Model M312; Gilson, Middleton, WI, USA). The exteriorized region of the small bowel was covered with BBS-soaked gauze to minimize tissue dehydration, temperature changes, and the influence of respiratory movements. The superfusate was maintained at 37±0.5°C by pumping the solution through a heat exchanger warmed by a constant temperature circulator (Model 1130; VWR, West Chester, PA, USA). Body temperature of the mouse was maintained between 36.5 and 37.5°C by use of a thermostatically controlled heat lamp. The Plexiglas board was mounted on the stage of an inverted microscope (Diaphot TMD-EF; Nikon, Melville, NY, USA), and the intestinal microcirculation was observed through a 20X objective lens. Fluorescence images of the microcirculation (excitation wavelength, 420–490 nm; emission wavelength, 520 nm) were detected with a charge-coupled device (CCD) camera (XC-77; Hamamatsu Photonics, Bridgewater, NJ, USA), a CCD camera control unit (C2400; Hamamatsu Photonics), and an intensifier head (M4314; Hamamatsu Photonics) attached to the camera. Micro-fluorographs were projected on a television monitor (PVM-1953MD; Sony, New York, NJ, USA) and recorded on DVD using a DVD video recorder (DMR-E50; Panasonic, Secaucus, NY, USA) for off-line quantification of measured variables during playback of the recorded images. A video time-date generator (WJ810; Panasonic) displayed the stop-watch function onto the monitor.
The intravital microscopic measurements described below were obtained over minutes 30–40 and 60–70 of reperfusion or at equivalent time points in the sham control groups, as described below. The intestinal segment was scanned from the oral to aboral section, and 10 single, unbranched venules (20–50 m in diameter, 100 μm in length) were observed for at least 30 seconds. Leukocyte-endothelial cell interactions (the numbers of rolling and firmly adherent leukocytes) were quantified in each of the 10 venules, followed by calculation of the mean value, which was used in the statistical analysis of the data. Circulating leukocytes were considered to be firmly adherent if they did not move along, or detach from, the venular wall for at least 30 seconds. Rolling cells are defined as cells crossing an imaginary line in the microvessel at a velocity that is significantly lower than centerline velocity; their numbers were expressed as rolling cells per minute. The numbers of rolling or adherent leukocytes were normalized by expressing each as the number of cells per square millimeter of vessel area.
Experimental Protocol
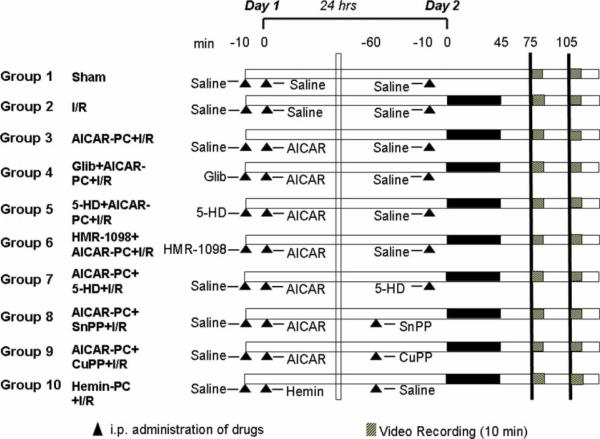
Figure 1 illustrates the general design of the experimental protocols for the study. Drug doses were selected based on previous experiments in our laboratory and reports in the literature [5,10,11,14,33,37].
Figure 1.
Schematic illustration of the experimental protocols assigned to each group. The numbers at the top of the diagram refer to minutes in the time line for the protocol on Days 1 and 2 (24 hours between both 0s). Hatched bars indicate when the 10-minute video recordings were obtained in the protocol. Solid bars depict the 45-minute period of ischemia. Triangles illustrate when administration of saline vehicle or drugs was accomplished in the protocol time line. I/R, ischemia and reperfusion. See the text for further details.
Group 1: Sham
As a time control for the effects of experimental duration, mice in this group (n=9) received an i.p. injection of 0.5 mL saline, which was used as a vehicle for AICAR and other pharmacologic agents in groups outlined below. Twenty-four hours later, the superior mesenteric artery was exposed but not subjected to occlusion, with leukocyte/endothelial cell adhesive interactions quantified at time points comparable to those described for mice subjected to 45 minutes of intestinal ischemia, followed by 70 minutes of reperfusion (Group 2, below).
Group 2: I/R Alone
Mice in this group (n=7) were treated as described for Group 1 above, except that I/R was induced 24 hours after the i.p. injection of saline vehicle on Day 1. Leukocyte rolling and adhesion were quantified during minutes 30–40 and 60–70 of reperfusion following 45 minutes of ischemia on Day 2.
Group 3: AICAR-PC+I/R
To confirm that AMPK activation with AICAR-PC reduces leukocyte rolling and adhesion, mice in this group (n=6) were treated with AICAR (Sigma) (100 mg/kg, 0.5 mL, i.p. injection) on Day 1. Twenty-four hours later (Day 2), the intestine was exposed to I/R and leukocyte rolling and adhesion were quantified, as described above.
Group 4: Glibenclamide+AICAR-PC+I/R
To determine if AMPK activation with AICAR-PC requires KATP channels as initiators, the nonspecific KATP channel inhibitor, glibenclamide, was given prior to AICAR-PC. Mice (n=6) were treated with glibenclamide (Sigma) (6 mg/kg, 0.3 mL, i.p. injection) on Day 1, 10 minutes prior to AICAR-PC (Sigma) (100 mg/kg, 0.5 mL, i.p. injection). Twenty-four hours later (Day 2), the intestine was exposed to I/R and leukocyte rolling and adhesion were quantified, as described above.
Group 5: 5-HD+AICAR-PC+I/R
To determine if AMPK activation with AICAR-PC requires mito-KATP channels as an initiator, on Day 1, the specific mitoKATP channel inhibitor, 5-hydroxydecanoic acid (5-HD), was given prior to AICAR-PC. Mice (n=8) were treated with 5-HD (Sigma) (10 mg/kg, 0.3 mL, i.p. injection) on Day 1, 10 minutes prior to AICAR-PC (Sigma) (100 mg/kg, 0.5 mL, i.p. injection). Twenty-four hours later (Day 2), the intestine was exposed to I/R and leukocyte rolling and adhesion were quantified, as described above.
Group 6: HMR-1098+AICAR-PC+I/R
To determine if AMPK activation with AICAR-PC requires sKATP channels as an initiator, on Day 1, the specific sKATP channel inhibitor, HMR-1098, was given prior to AICAR-PC. Mice (n=9) were treated with HMR-1098 (a generous gift from Garrett Gross, Medical College of Wiscosin Milwaukee, WI, USA) (6 mg/kg, 0.3 mL, i.p. injection) on Day 1, 10 minutes prior to AICAR-PC (Sigma) (100 mg/kg, 0.5 mL, i.p. injection). Twenty-four hours later (Day 2), the intestine was exposed to I/R and leukocyte rolling and adhesion were quantified, as described above.
Group 7: AICAR-PC+5-HD+I/R
To determine if AMPK activation with AICAR-PC requires mitoKATP channels as an effector, on Day 2, the specific mitoKATP channel inhibitor, 5-hydroxydecanoic acid (5-HD), was given just prior to I/R. Mice (n=6) were treated with AICAR (Sigma) (100 mg/kg, 0.5 mL, i.p. injection) on Day 1. Twenty-four hours later (Day 2), 5-HD (Sigma) (10 mg/kg, 0.3 mL, i.p. injection) was administered to mice 10 minutes prior to the induction of I/R and leukocyte rolling and adhesion were quantified, as described above.
Group 8: AICAR-PC+SnPP+I/R
To determine if AMPK activation with AICAR-PC requires heme oxygenase (HO) as an effector, on Day 2, the specific HO inhibitor, tin protoporphyrin-IX (SnPP), was given prior to I/R. On Day 1, mice (n=6) were treated with AICAR (Sigma) (100 mg/kg, 0.5 mL, i.p. injection). Twenty-four hours later (Day 2), SnPP (Porphyrin Products, Logan, Utah, USA) (50 mg/kg, 0.3 mL, i.p. injection, as described by Tulis et al. [42]) was administered one hour prior to I/R, followed by leukocyte rolling and adhesion quantification, as described above.
Group 9: AICAR-PC+CuPP+ I/R
To control for any nonspecific effects of protoporphyrins, the inactive copper protoporphyrin-IX (CuPP), was used on Day 2, 24 hours after AICAR-PC. On Day 1, mice (n=6) were treated with AICAR (Sigma) (100 mg/kg, 0.5 mL, i.p. injection). Twenty-four hours later (Day 2), CuPP (Porphyrin Products) (50 mg/kg, 0.3 mL, i.p. injection) was administered one hour prior to I/R, followed by leukocyte rolling and adhesion quantification, as described above.
Group 10: Hemin-PC+I/R
To determine if HO-1 induction with Hemin-PC is a sufficient stimulus to cause late phase protection against I/R, mice (n=6) were treated with hemin (Sigma) (50 mg/kg, 0.5 mL, i.p. injection) on Day 1. Twenty-four hours later (Day 2), intestinal I/R was induced and leukocyte rolling and adhesion were quantified, as described above.
Statistical Analysis
The data were analyzed with standard statistical analysis, such as analysis of variance (ANOVA) with Scheffe's (post-hoc) test for multiple comparisons. All values are expressed as the means±standard error of the mean (SEM). Statistical significances were defined at P<0.05.
RESULTS
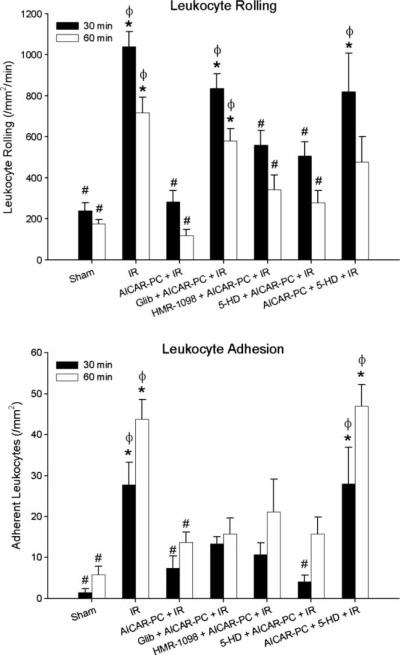
Figure 2 illustrates the effects of coincident administration of AICAR with the various KATP channel inhibitors 24 hours prior to I/R on postischemic leukocyte rolling and adhesion. I/R markedly increased leukocyte rolling (Figure 2, upper panel) and adhesion (Figure 2, lower panel), compared to sham (i.e., no ischemia) control mice. As we demonstrated previously [11], AICAR-PC completely prevented the postischemic increases in leukocyte rolling and adhesion in response to I/R. When the dual KATP channel antagonist (glibenclamide) was administered coincident with AICAR, it blocked the protective effects of AICAR-PC on leukocyte rolling and had no significant effect on leukocyte adhesion. Neither the selective surface KATP channel inhibitor (HMR-1098) nor the selective mitochondrial KATP channel inhibitor (5-HD) exerted significant effects on AICAR-PC to inhibit the reduction in postischemic leukocyte rolling or adhesion, although there was a tendency for increased leukocyte rolling. These results suggest that in contrast to most other preconditioning stimuli, the antiadhesive effects of AICAR-PC are not triggered on Day 1 by a surface KATP channel-dependent mechanism. On the other hand, treatment with the mitoKATP channel inhibitor, 5-HD, during I/R 24 hours after AICAR-PC abrogated the antiadhesive effects of this AMPK agonist to prevent leukocyte rolling and adhesion, indicating that mitoKATP channels may serve as a mediator of these anti-inflammatory effects on Day 2.
Figure 2.
Role of KATP channels in preconditioning with AICAR (AICAR-PC) 24 hours prior to ischemia/reperfusion (I/R) on postischemic leukocyte rolling (upper panel) and adhesion (lower panel) determined after 30 and 60 minutes of reperfusion in wild-type C57BL/6J mice. The nonspecific KATP channel inhibitor, glibenclamide (Glib), as well as the specific surface KATP channel inhibitor, HMR-1098, and the mitochondrial KATP channel-specific inhibitor, 5-HD, were administered either 10 minutes prior to preconditioning with AICAR on Day 1 or 10 minutes prior to I/R on Day 2. Open and solid bars represent data obtained at minutes 30–40 and 60–70 of reperfusion, respectively. * denotes values statistically different from control (P<0.05). # denotes values statistically different from I/R (P<0.05). ϕ denotes values statistically different from AICAR-PC+I/R.
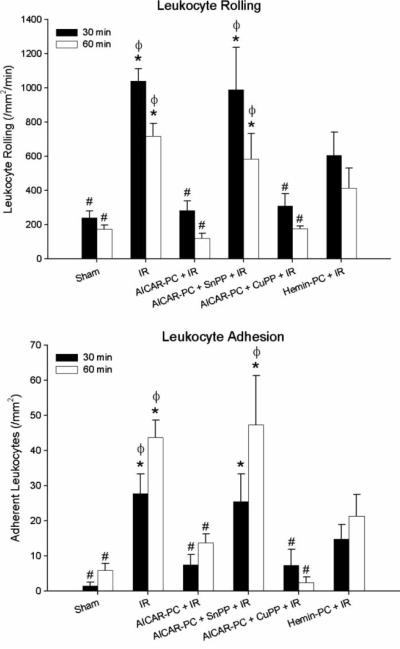
The data depicted in Figure 3 show the effects of HO inhibition during I/R on AICAR-PC and demonstrate that the antiadhesive actions of antecedent AICAR can be mimicked by preconditioning with hemin, a HO inducer. Treatment with SnPP, a potent inhibitor of HO, completely abolished the effects of antecedent AICAR to prevent postischemic leukocyte rolling and adhesion when administered during I/R 24 hours after AICAR-PC (Figure 3). Treatment with CuPP, a protoporphyrin that exhibits no inhibitory activity toward HO, was ineffective in preventing the antiadhesive effects of AICAR-PC when administered during I/R on Day 2. Interestingly, preconditioning with hemin, in lieu of AICAR, effectively reduced postischemic leukocyte rolling and adhesion. These observations suggest that HO is an important end effector of AICAR-PC.
Figure 3.
Role of heme oxygenase (HO) in preconditioning with AICAR (AICAR-PC) 24 hours prior to ischemia/reperfusion (I/R) on postischemic leukocyte rolling (upper panel) and adhesion (lower panel) determined after 30 and 60 minutes of reperfusion in wild-type C57BL/6J mice. The HO inhibitor, SnPP, or the inactive protoporphyrin, CuPP, was administered one hour prior to I/R on Day 2, 24 hours after preconditioning with AICAR on Day 1. The HO-1 inducer, hemin, was given on Day 1 in place of AICAR-PC to examine its ability to induce a protective phenotype during I/R 24 hours later on Day 2. Open and solid bars represent data obtained at minutes 30–40 and 60–70 of reperfusion, respectively. * denotes values statistically different from control (P<0.05). # denotes values statistically different from I/R (P<0.05). ϕ denotes values statistically different from AICAR-PC I/R.
DISCUSSION
AMPK is a ubiquitously expressed heterotrimeric serine/threonine kinase that is often referred to as a metabolic “master switch” due to its high sensitivity to changes in the AMP:ATP ratios and its centralized role in both short- and long-term metabolic signaling pathways [4,6,16,23]. The ability of AMPK activation to protect cellular energy levels and maintain the integrity of the mitochondrial membrane potential makes it an essential prosurvival signaling mediator in protecting cells and tissues from I/R injury [21]. In this regard, we recently demonstrated that an anti-inflammatory state could be induced by preconditioning with the AMPK activator, AICAR [11,12], such that postcapillary venules fail to support increased leukocyte rolling and adhesion during I/R 24 hours later. AICAR is first metabolized to ZMP, an AMP analog, which initially activates AMPK by binding to its gamma subunit [34]. This allosteric activation increases both AMPK activity and its affinity for upstream AMPK kinases, which in turn, phosphorylate AMPK at threonine-172 on the activation loop of the alpha subunit to further activate the enzyme. This latter process is responsible for the majority of AMPK activity [20,35,38,47,48].
Our earlier work indicated that AICAR-PC involves both the AMPK α1 and AMPK α2 isoforms and is triggered by eNOS activation [11,12]. However, little is known about the effector mechanisms that are activated during I/R 24 hours after exposure to this preconditioning stimulus. The fact that mitoKATP channels have been implicated as an important mediator for most forms of preconditioning, while treatment with KATP channel agonists prevent leukocyte infiltration when administered during reperfusion after prolonged ischemia [2,29,30,43,46], led us to first evaluate their role as an effector of AICAR-PC. Administration of the mitoKATP channel inhibitor, 5-HD, during I/R 24 hours after AICAR-PC effectively abolished the antiadhesive effects that are induced by this preconditioning stimulus (Figure 2).
Because strong evidence exists to support the notion that KATP channels play a dual role in many forms of preconditioning, with sKATP channels subserving a trigger function in the initiation of a protected state while mitoKATP channels serve as a mediator during I/R 24 hours later, we also evaluated the role of KATP channels as initiators for AICAR-PC. Treatment with either the sKATP channel (HMR-1098) or the mitoKATP channel (5-HD) blockers coincident with AICAR-PC failed to prevent the protective reduction in postischemic leukocyte adhesion, though a modest increase in I/R-induced leukocyte rolling was observed. The dual KATP channel blocker, glibenclamide, was able to prevent the anti-inflammatory effects of AICAR-PC on leukocyte rolling but was without effect on leukocyte adhesion when coadministered on Day 1. Our results are consistent with the concept that AICAR prevents leukocyte adhesion by a mechanism independent of KATP channel activation on Day 1 (Figure 2). Indeed, higher AICAR doses have been shown to inhibit, not promote, KATP channel activity in murine pancreatic islet cells [45]. Thus, a unique finding of the present study is the apparent lack of dependence of AICAR-PC on a sKATP channel-dependent triggering mechanism.
We hypothesized that HO might serve as an effector of AICAR-PC for the following reasons. First, HO activity is exquisitely sensitive to upregulation by NO donors [27] and NO appears to play an important role in initiating the effects of AICAR-PC [11]. Second, cAMP, a downstream signaling molecule that is produced in response to adenosine A2-receptor activation, increases HO-1 mRNA, protein, and activity [17], and produces a preconditioned phenotype in the small intestine [26]. Adenosine A2 receptor activation is another important trigger for AICAR-PC in the heart [7]. Third, induction of HO-1 suppresses P-selectin expression and leukocyte adhesion induced by hydrogen peroxide or I/R in the small intestine [19,44], inflammatory processes which are also prevented by AICAR-PC. Fourth, HO-1-derived CO also inhibits the expression of proinflammatory cytokines, as does AMPK activation [13,39]. Fifth, hemin-induced HO-1 expression exerts infarct-sparing effects in the setting of myocardial I/R [15], while the protective effects of ischemic-PC against I/R could be inhibited by pharmacologic inhibition of HO-1 with zinc protoporphyrin (ZnPP) or by prevention of its expression using siRNA approaches [22]. Moreover, HO-1 activity appears to be particularly rich in postcapillary venules of the small intestine [19]. Finally, and perhaps most importantly, the reaction products of HO-1-catalyzed heme degradation exert powerful antiadhesive and antioxidant effects [28,39,41]. Interestingly, hydrogen peroxide–induced leukocyte adhesion is inhibited by bilirubin, but not CO, whereas adhesive responses to lipopolysaccharide are attenuated by CO [20,33]. Although the mechanisms that account for these differential antiadhesive responses to the reaction products of HO activity are not known, they do indicate that the relative importance of each may depend on the nature of the initiating inflammatory stimulus.
In light of these considerations, we sought to determine whether treatment with SnPP, an HO inhibitor, would prevent the anti-inflammatory actions of AICAR-PC, which it did when administered during I/R 24 hours later (Figure 3). Importantly, administration of CuPP just prior to I/R, which demonstrates no inhibitory activity toward HO, failed to prevent the anti-inflammatory actions of antecedent AICAR (Figure 3). This latter result indicates that possible nonspecific effects due to the protoporphyrin moiety do not account for the ability of SnPP to prevent AICAR-PC. Finally, treatment with hemin, an agent known to induce the expression of HO-1, mimicked the antiadhesive actions of antecedent AICAR and prevented postischemic leukocyte rolling and adhesion when administered 24 hours prior to I/R (Figure 3). Similar results have been reported by Hayashi et al. [20], who demonstrated that increased leukocyte-endothelial cell adhesive interactions in the mesentery provoked by a hemorrhagic shock/resuscitation protocol were prevented by hemin-induced HO expression. While our studies do not shed light on the HO isoform involved in AICAR-PC, it is likely that induced expression of HO-1 plays a major role. Support for this notion is provided by our observation that SnPP treatment in the absence of I/R does not influence baseline leukocyte rolling or adhesion when measured 1 or 24 hours after administration of this HO inhibitor (unpublished observations), suggesting that constitutively expressed HO-2 does not play a role in baseline leukocyte adhesion.
CONCLUSIONS
Although the results of our study indicate that HO and mitoKATP channels both play important roles as effectors of the anti-inflammatory phenotype elicited by antecedent AICAR treatment (Figures 2 and 3), it is not clear whether these mediators work in concert or separately to limit postischemic leukocyte rolling and adhesion. It is tempting to speculate that because independent blockade of either effector during I/R completely prevented the beneficial antiadhesive actions of AICAR-PC, HO and mitoKATP channel activity may be coupled as sequential signaling elements to mediate these effects. However, it is also possible that both function independently to influence leukocyte rolling and adhesion, but the additive effects of both are required to limit postischemic adhesive responses. This latter concept implies that multiple effector activities may be required to reach a threshold for the development of the antiadhesive effects of AICAR-PC. Clearly, much additional work will be required to address these possibilities. Nevertheless, our results clearly indicate that both HO and mitoKATP channels serve as important effectors of AICAR-PC during I/R 24 hours later.
ACKNOWLEDGMENTS
Kazuhiro Kamada has returned to Japan and is currently with the Department of Inflammation and Immunology, Kyoto Prefectural University of Medicine, Graduate School of Medical Science (Kyoto, Japan). This work was supported by grants from the National Institutes of Health (AA-14945, HL-82816, HL-59976, and HL-73414).
Footnotes
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- 1.Blais V, Rivest S. Inhibitory action of nitric oxide on circulating tumor necrosis factor-induced NF-kappaB activity and COX-2 transcription in the endothelium of the brain capillaries. J Neuropathol Exp Neurol. 2001;60:893–905. doi: 10.1093/jnen/60.9.893. [DOI] [PubMed] [Google Scholar]
- 2.Broadhead MW, Kharbanda RK, Peters MJ, MacAllister RJ. KATP channel activation induces ischemic preconditioning of the endothelium in humans in vivo. Circulation. 2004;110:2077–2082. doi: 10.1161/01.CIR.0000144304.91010.F0. [DOI] [PubMed] [Google Scholar]
- 3.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 4.Carling D. AMP-activated protein kinase: balancing the scales. Biochimie. 2005;87:87–91. doi: 10.1016/j.biochi.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Carrasco-Chaumel E, Rosello-Catafau J, Bartrons R, Franco-Gou R, Xaus C, Casillas A, Gelpi E, Rodes J, Peralta C. Adenosine monophosphate-activated protein kinase and nitric oxide in rat steatotic liver transplantation. J Hepatol. 2005;43:997–1006. doi: 10.1016/j.jhep.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Chan AY, Dyck JR. Activation of AMP-activated protein kinase (AMPK) inhibits protein synthesis: a potential strategy to prevent the development of cardiac hypertrophy. Can J Physiol Pharmacol. 2005;83:24–28. doi: 10.1139/y04-107. [DOI] [PubMed] [Google Scholar]
- 7.Cronstein BN, Naime D, Ostad E. The anti-inflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayton C, Yamaguchi T, Kamada K, Carter P, Korthuis RJ. Antecedent ethanol ingestion prevents postischemic leukocyte adhesion and P-selectin expression by a protein kinase C-dependent mechanism. Dig Dis Sci. 2005;50:684–690. doi: 10.1007/s10620-005-2557-1. [DOI] [PubMed] [Google Scholar]
- 9.Dayton C, Yamaguchi T, Kamada K, Carter P, Korthuis RJ. Antecedent ethanol ingestion prevents postischemic P-selectin expression in murine small intestine. Microcirculation. 2004;11:709–718. doi: 10.1080/10739680490521014. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick CM, Shi Y, Hutchins WC, Su J, Gross GJ, Ostadal B, Tweddell JS, Baker JE. Cardio-protection in chronically hypoxic rabbits persists on exposure to normoxia: role of NOS and KATP channels. Am J Physiol Heart Circ Physiol. 2005;288:H62–H68. doi: 10.1152/ajpheart.00701.2004. [DOI] [PubMed] [Google Scholar]
- 11.Gaskin FS, Kamada K, Yusof M, Korthuis RJ. 5′-AMP-activated protein kinase activation prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol. 2007;292:H326–H332. doi: 10.1152/ajpheart.00744.2006. [DOI] [PubMed] [Google Scholar]
- 12.Gaskin FS, Kamada K, Yusof M, Rubin LJ, Korthuis RJ. Experimental Biology. The FASEB Journal; Washington, D.C.: 2007. Ethanol preconditioning is dependent on the activation of 5'-AMP-activated protein kinase. 2007. [Google Scholar]
- 13.Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004;24:479–487. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross ER, Nithipatikom K, Hsu AK, Peart JN, Falck JR, Campbell WB, Gross GJ. Cytochrome P450 omega-hydroxylase inhibition reduces infarct size during reperfusion via the sarcolemmal KATP channel. J Mol Cell Cardiol. 2004;37:1245–1249. doi: 10.1016/j.yjmcc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Hangaishi M, Ishizaka N, Aizawa T, Kurihara Y, Taguchi J, Nagai R, Kimura S, Ohno M. Induction of heme oxygenase-1 can act protectively against cardiac ischemia/reperfusion in vivo. Biochem Biophys Res Comm. 2000;279:582–588. doi: 10.1006/bbrc.2000.3973. [DOI] [PubMed] [Google Scholar]
- 16.Hardie DG. The AMP-activated protein kinase pathway—new players upstream and down-stream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 17.Haschemi A, Wagner O, Marculescu R, Wegiel B, Robson SC, Gagliani N, Gallo D, Chen JF, Bach FH, Otterbein LE. Cross-regulation of carbon monoxide and the adenosine A2a receptor in macrophages. J Immunol. 2007;178:5921–5929. doi: 10.4049/jimmunol.178.9.5921. [DOI] [PubMed] [Google Scholar]
- 18.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, Kitajima M, Makino N, Ishimura Y, Suematsu M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- 20.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 21.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 22.Jancso G, Cserepes B, Gasz B, Benko L, Borsiczky B, Ferenc A, Kurthy M, Racz B, Lantos J, Gal J, Arato E, Sinayc L, Weber G, Roth E. Expression and protective role of heme oxygenase-1 in delayed myocardial preconditioning. Ann N Y Acad Sci. 2007;1095:251–261. doi: 10.1196/annals.1397.029. [DOI] [PubMed] [Google Scholar]
- 23.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Kamada K, Dayton CB, Yamaguchi T, Korthuis RJ. Antecedent ethanol ingestion prevents post-ischemic microvascular dysfunction. Pathophysiology. 2004;10:131–137. doi: 10.1016/j.pathophys.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Kamada K, Gaskin FS, Yamaguchi T, Carter P, Yoshikawa T, Yusof M, Korthuis RJ. Role of calcitonin gene-related peptide in the postischemic anti-inflammatory effects of antecedent ethanol ingestion. Am J Physiol Heart Circ Physiol. 2006;290:H531–H537. doi: 10.1152/ajpheart.00839.2005. [DOI] [PubMed] [Google Scholar]
- 26.Korthuis RJ. cAMP reduces postischemic leukocyte rolling and adhesion via adenosine A2-receptor activation and HO-1. Circulation. 2004;110:2077–2082. [Google Scholar]
- 27.Leffler CW, Balabanova L, Fedinec AL, Parfenova H. Nitric oxide increases carbon monoxide production by piglet cerebral microvessels. Am J Physiol Heart Circ Physiol. 2005;289:H1442–H1447. doi: 10.1152/ajpheart.00464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maines MD, Panahian N. The heme oxygenase system and cellular defense mechanisms. Do HO-1 and HO-2 have different functions? Adv Exp Med Biol. 2001;502:249–272. doi: 10.1007/978-1-4757-3401-0_17. [DOI] [PubMed] [Google Scholar]
- 29.Mizumura T, Nithipatikom K, Gross GJ. Bimakalim, an ATP-sensitive potassium channel opener, mimics the effects of ischemic preconditioning to reduce infarct size, adenosine release, and neutrophil function in dogs. Circulation. 1995;92:1236–1245. doi: 10.1161/01.cir.92.5.1236. [DOI] [PubMed] [Google Scholar]
- 30.Mizumura T, Nithipatikom K, Gross GJ. Infarct size-reducing effect of nicorandil is mediated by the KATP channel but not by its nitrate-like properties in dogs. Cardiovascular Res. 1996;32:274–285. doi: 10.1016/0008-6363(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 31.Patel HH, Gross ER, Peart JN, Hsu AK, Gross GJ. Sarcolemmal KATP channel triggers delayed ischemic preconditioning in rats. Am J Physiol Heart Circ Physiol. 2005;288:H445–H447. doi: 10.1152/ajpheart.00031.2004. [DOI] [PubMed] [Google Scholar]
- 32.Patel HH, Hsu AK, Peart JN, Gross GJ. Sarcolemmal K(ATP) channel triggers opioid-induced delayed cardioprotection in the rat. Circ Res. 2002;91:186–188. doi: 10.1161/01.res.0000029085.69891.f2. [DOI] [PubMed] [Google Scholar]
- 33.Peralta C, Bartrons R, Serafin A, Blazquez C, Guzman M, Prats N, Xaus C, Cutillas B, Gelpi E, Rosello-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164–1173. doi: 10.1053/jhep.2001.29197. [DOI] [PubMed] [Google Scholar]
- 34.Sabina RL, Patterson D, Holmes EW. 5-Amino-4-imidazolecarboxamide riboside (Z-ribo-side) metabolism in eukaryotic cells. J Biol Chem. 1985;260:6107–6114. [PubMed] [Google Scholar]
- 35.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Bradykinin prevents postischemic leukocyte adhesion and emigration and attenuates microvascular barrier disruption. Am J Physiol. 1999;277:H161–H171. doi: 10.1152/ajpheart.1999.277.1.H161. [DOI] [PubMed] [Google Scholar]
- 37.Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Postischemic anti-inflammatory effects of bradykinin preconditioning. Am J Physiol Heart Circ Physiol. 2001;280:H441–H454. doi: 10.1152/ajpheart.2001.280.1.H441. [DOI] [PubMed] [Google Scholar]
- 38.Soltys CL, Kovacic S, Dyck JR. Activation of cardiac AMP-activated protein kinase by LKB1 expression or chemical hypoxia is blunted by increased Akt activity. Am J Physiol Heart Circ Physiol. 2006;290:H2472–H2479. doi: 10.1152/ajpheart.01206.2005. [DOI] [PubMed] [Google Scholar]
- 39.Song R, Kubo M, Morse D, Zhou Z, Zhang X, Dauber JH, Fabisiak J, Alber SM, Watkins SC, Zuckerbraun BS, Otterbein LE, Ning W, Oury TD, Lee PJ, McCurry KR, Choi AM. Carbon monoxide induces cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am J Pathol. 2003;163:231–242. doi: 10.1016/S0002-9440(10)63646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suematsu M, DeLano FA, Poole D, Engler RL, Miyasaka M, Zweifach BW, Schmid-Schönbein GW. Spatial and temporal correlation between leukocyte behavior and cell injury in postischemic rat skeletal muscle microcirculation. Lab Invest J Tech Meth Pathol. 1994;70:684–695. [PubMed] [Google Scholar]
- 41.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by micro-somal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tulis DA, Durante W, Peyton KJ, Evans AJ, Schafer AI. Heme oxygenase-1 attenuates vascular remodeling following balloon injury in rat carotid arteries. Atherosclerosis. 2001;155:113–122. doi: 10.1016/s0021-9150(00)00552-9. [DOI] [PubMed] [Google Scholar]
- 43.Uchiyama Y, Otani H, Wakeno M, Okada T, Uchiyama T, Sumida T, Kido M, Imamura H, Nakao S, Shingu K. Role of mitochondrial KATP channels and protein kinase C in ischaemic preconditioning. Clin Exp Pharmacol physiol. 2003;30:426–436. doi: 10.1046/j.1440-1681.2003.03853.x. [DOI] [PubMed] [Google Scholar]
- 44.Vachharajani TJ, Work J, Issekutz AC, Granger DN. Heme oxygenase modulates selectin expression in different regional vascular beds. Am J Physiol Heart Circ Physiol. 2000;278:H1613–H1617. doi: 10.1152/ajpheart.2000.278.5.H1613. [DOI] [PubMed] [Google Scholar]
- 45.Wang CZ, Wang Y, Di A, Magnuson MA, Ye H, Roe MW, Nelson DJ, Bell GI, Philipson LH. 5-amino-imidazole carboxamide riboside acutely potentiates glucose-stimulated insulin secretion from mouse pancreatic islets by KATP channel-dependent and pathways. Biochem Biophys Res Comm. 2005;330:1073–1079. doi: 10.1016/j.bbrc.2005.03.093. [DOI] [PubMed] [Google Scholar]
- 46.Wei W, Wei FC, Hung LM. Diazoxide ameliorates microcirculatory disturbances through PKC-dependent pathway in I/R-injured rat cremaster muscles. J Biomed Sci. 2005;12:521–529. doi: 10.1007/s11373-005-3730-4. [DOI] [PubMed] [Google Scholar]
- 47.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 49.Xenos ES, Stevens SL, Freeman MB, Cassada DC, Goldman MH. Nitric oxide mediates the effect of fluvastatin on intercellular adhesion molecule-1 and platelet endothelial cell adhesion molecule-1 expression on human endothelial cells. Ann Vasc Surg. 2005;19:386–392. doi: 10.1007/s10016-005-0011-7. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi T, Dayton C, Shigematsu T, Carter P, Yoshikawa T, Gute DC, Korthuis RJ. Preconditioning with ethanol prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol. 2002;283:H1019–H1030. doi: 10.1152/ajpheart.00173.2002. [DOI] [PubMed] [Google Scholar]