SUMMARY
Increased transcriptional activity of β-catenin resulting from Wnt/Wingless-dependent or - independent signaling has been detected in many types of human cancer, but the underlying mechanism of Wnt-independent regulation remains unclear. We demonstrate here that EGFR activation results in disruption of the complex of β-catenin and α-catenin, thereby abrogating the inhibitory effect of α-catenin on β-catenin transactivation via CK2α-dependent phosphorylation of α-catenin at Ser641. ERK2, which is activated by EGFR signaling, directly binds to CK2α via the ERK2 docking groove and phosphorylates CK2α primarily at Thr360/Ser362, subsequently enhancing CK2α activity toward α-catenin phosphorylation. In addition, levels of α-catenin S641 phosphorylation correlate with levels of ERK1/2 activity in human glioblastoma specimens and with grades of glioma malignancy. This EGFR-ERK-CK2–mediated phosphorylation of α-catenin promotes β-catenin transactivation and tumor cell invasion. These findings highlight the importance of the crosstalk between EGFR and Wnt pathways in tumor development.
INTRODUCTION
Overexpression of epidermal growth factor (EGF) receptor (EGFR) has been reported in many human tumors, including lung, colon, breast, prostate, brain, head and neck, thyroid, ovarian, kidney, and bladder cancers as well as gliomas, and correlates with a poor clinical prognosis in the tumors (Moscatello et al., 1995; Nicholson et al., 2001). Activation of the receptor via EGF promotes migration of tumor cells (Lu et al., 2001). Cell migration itself is a highly coordinated process involving precise regulation of cell-cell adhesion and cell-to-extracellular matrix (ECM) adhesion (Lauffenburger and Horwitz, 1996; Ridley et al., 2003). Stimulation of epithelial cells with growth factors, including EGF (Lu et al., 2003; Muller et al., 2002), hepatocyte growth factor/scatter factor (HGF/SF) (Savagner et al., 1997; Weidner et al., 1990), fibroblast growth factor (FGF) (Valles et al., 1990), and transforming growth factor (TGF)-β (Miettinen et al., 1994), induces break-up of cell-cell junctions. This disruption of cell-cell junctions facilitates epithelial-mesenchymal transition (EMT) and tumor cell migration (Thiery and Sleeman, 2006).
β-catenin, a component of cell-cell adhesion structures, interacts with the cytoplasmic domain of E-cadherin and links E-cadherin to α-catenin, which in turn mediates anchorage of the E-cadherin complex to the cortical actin cytoskeleton (Nagafuchi, 2001; Perez-Moreno and Fuchs, 2006; Rimm et al., 1995). In addition to its role in cell-cell adherens junctions, β-catenin is also a key component of the Wnt/Wingless signaling pathway (Huang and He, 2008). Wnt signaling plays a central role in development, cell proliferation and differentiation (Wodarz and Nusse, 1998). In the absence of a Wnt signal, cytoplasmic β-catenin interacts with axin/conductin, glycogen synthase kinase-3β (GSK-3β), and the adenomatous polyposis coli protein (APC) (Hulsken et al., 1994). GSK-3β phosphorylates the N-terminal domain of β-catenin, which leads to β-catenin degradation via the SCF/ubiquitin/proteasome pathway (Clevers, 2006; Moon et al., 2004). Activation of the Wnt pathway inhibits GSK-3β-dependent phosphorylation of β-catenin. Stabilized, hypophosphorylated β-catenin translocates to the nucleus and interacts with transcription factors of the TCF/LEF-1 family, leading to the increased expression of genes such as c-myc and cyclin D1 (Clevers, 2006; Moon et al., 2004).
Mutations in APC, AXIN1, or CTNNB1 (which encodes β-catenin) enhance β-catenin stability and subsequent transactivation of TCF/LEF-1, and such transactivation is found in a wide variety of human cancers (Peifer and Polakis, 2000). However, mutations of Wnt pathway proteins that alter the stability of β-catenin are not the only factors that contribute to β-catenin activation (Lu and Hunter, 2004). For instance, in 12 of 20 (60.0%) endometrial cancers, β-catenin was found to accumulate in the nucleus, which is a hallmark of β-catenin activation—whereas there were only two instances of mutations in the CTNNB1 gene (Ashihara et al., 2002). Similarly, only 1 of 65 primary melanomas had detectable CTNNB1 mutations, with a third of the cases displaying nuclear accumulation of β-catenin (Rimm et al., 1999). Moreover, nearly 50% of hepatocellular carcinomas, in which the APC gene is rarely mutated, reveal nuclear accumulation of β-catenin protein, and genetic alterations in CTNNB1 are detected only in 16%–26% of the tumors (Polakis, 2000). In response to EGF stimulation, β-catenin translocates into the nucleus and increases its transactivation without altering its stability and phosphorylation level by GSK-3β (Lu et al., 2003). Leukemic stem cells in chronic myelogenous leukemia (CML) have high nuclear β-catenin levels, presumably driven by Bcr-Abl (Jamieson et al., 2004). EGF and HGF induce β-catenin signaling under conditions where they stimulate cell motility (Fang et al., 2007; Lu et al., 2003; Muller et al., 2002). Clearly, more than one mechanism regulates the activity of β-catenin (Lu and Hunter, 2004).
Although the adherens junction components, E-cadherin and β-catenin, have been intensively studied in terms of their roles in tumor development, α-catenin, which functions as a molecular switch that binds E-cadherin and β-catenin and regulates actin-filament assembly (Drees et al., 2005; Yamada et al., 2005), has received less attention (Benjamin and Nelson, 2008). Accumulated evidence points to α-catenin as a prognostic factor in cancer progression. Conditional deletion of α-catenin compromises intercellular adhesion and results in hyperproliferation of epidermal cells (Vasioukhin et al., 2001). Loss or downregulation of α-catenin has been detected in cell lines derived from leukemia, colon, prostate, and other cancers as well as primary human cancers (Benjamin and Nelson, 2008). The effect of α-catenin regulation on tumor development occurs at least partially through regulation of β-catenin, as evidenced by the fact that α-catenin overexpression leads to repression of β-catenin transcriptional activity, while α-catenin depletion has the opposite effect (Giannini et al., 2000; Hwang et al., 2005; Merdek et al., 2004). Nevertheless, the mechanism underlying this α-catenin-dependent regulation of β-catenin remains elusive.
EGFR activation initiates an array of protein phosphorylation-related signaling events mediated by protein kinases. Acting as serine (Ser, S) and threonine (Thr, T) protein kinases downstream from EGFR, mitogen-activated protein (MAP) kinases regulate a wide range of processes: cell growth and differentiation, gene expression, mitosis, cell motility, metabolism, cell survival and apoptosis, and embryogenesis (Chen et al., 2001). The classic MAP kinase family consists of three subfamilies: extracellular signal-regulated kinase (ERK; ERK1 and ERK2), c-Jun N-terminal kinase (JNK; JNK1, JNK2, and JNK3), and p38-MAP kinase (α, β, δ, and γ). In addition, ERKs 3–5 and 7–8 have been identified, although their function and regulation are less well characterized (Bogoyevitch and Court, 2004; Lu and Xu, 2006). Protein kinase CK2 is another important and highly pleiotropic protein kinase, and elevated CK2 kinase activity is frequently observed in many human tumors (Meggio and Pinna, 2003; Unger et al., 2004). CK2 can function as a tetramer, composed of two catalytic subunits—CK2α and/or CK2α′ (i.e., αα, αα′, α′α′)—and two regulatory subunits—CK2β, as well as a monomer (Filhol et al., 2004). The catalytic subunits can autophosphorylate themselves and CK2β (Litchfield, 2003; Meggio and Pinna, 2003). Although CK2 is traditionally regarded as a constitutively active kinase, studies have shown that CK2 is activated in response to a diverse array of growth factor stimuli including EGF treatment (Ackerman et al., 1990; Litchfield, 2003; Litchfield et al., 1994). Nevertheless, how CK2 is regulated in response to growth factors remains unknown.
In this report, we show that EGFR activation in cancer cells promotes β-catenin transcriptional activation by disrupting the association between β-catenin and α-catenin, which is mediated by CK2-phosphorylated α-catenin at S641. The EGF-induced CK2 activation, in turn, is mediated by ERK2-dependent C-terminal phosphorylation of CK2. EGF-induced disruption of the β-catenin/α-catenin interaction by this sequential phosphorylation relay promotes β-catenin transactivation and subsequent tumor cell invasion.
RESULTS
EGFR Activation Results in Disruption of β-Catenin and α-Catenin Complex and Promotes β-Catenin Transactivation
Stimulation of EGFR-overexpressing cancer cells with EGF disrupts cell-cell contacts and EMT (Lu et al., 2003; Muller et al., 2002). We previously showed that EGF stimulation results in translocation of β-catenin into the nucleus and increased transcriptional activity without altering its stability and phosphorylation level by GSK-3β (Lu et al., 2003). To examine the effect of EGFR activation on the β-catenin and α-catenin complex, we treated EGFR-overexpressing U87E, U373, and LN229 (data not shown) human glioblastoma (GBM) cells and A431 human epidermoid carcinoma cells with EGF. Immunoblotting of immunoprecipitated α-catenin with a β-catenin antibody showed that EGF induced disruption of the interaction between β-catenin and α-catenin in all tested cell lines (Fig. 1A) without significant alteration of expression levels of β-catenin and α-catenin in the tested time frame (Fig. S1A). In addition, β-catenin disassociates from α-catenin in both membrane and nucleus, as demonstrated in a subcellular fractionation analysis (Fig. 1B), whereas the amount of the membrane protein connexin-43 and the nuclear protein PCNA remained unchanged (Fig. S1B). The levels of immunoprecipitated α-catenin did not show a change in membrane or cytosolic fractions after EGF treatment because of saturation of the limited amount of anti-α-catenin antibody by α-catenin in either fraction. Consistent with a previous publication (Lu et al., 2003), EGF induced accumulation of nuclear β-catenin and reduced membrane-associated β-catenin. We did not detect any connexin-43 in the cytosolic or nuclear fractions or PCNA in the membrane or cytosolic fractions (Fig. S1B), suggesting that the subcellular fractionation was free of cross-contamination. Because the disruption of α-catenin association with β-catenin was detected in the cells expressing mutant (U87E and U373 cells) and wild type (WT) of PTEN (A431 and LN229 cells) (Maier et al., 1999; Parsa et al., 2007; She et al., 2005), this EGF-induced regulation is PTEN-independent.
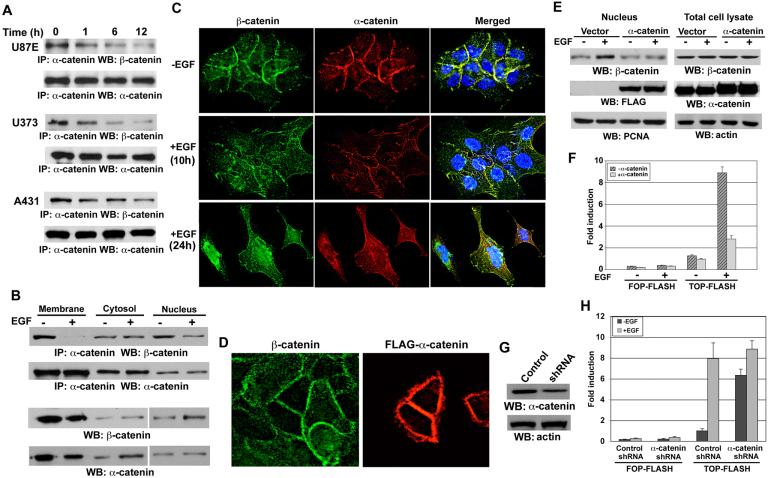
Figure 1. EGFR Activation Results in Disruption of β-catenin and α-catenin Complex and Promotes β-catenin Transactivation.
(A, B) Immunoprecipitation of α-catenin was followed by immunoblotting with a β-catenin antibody. (A) The indicated cell lines were treated with EGF (100 ng/ml) for the indicated time. (B) The subcellular fractionation was prepared from A431 cells treated with EGF (100 ng/ml) for 6 hr.
(C, D) A431 cells without (C) or with (D) transfection of FLAG-α-catenin were treated with EGF (100 ng/ml) for 10 or 24 hr (C) or 6 hr (D) and immunostained with the indicated antibodies.
(E) The nuclear fractions (left panel) or total cell lysate (right panel) were prepared from 293T cells expressing EGFR with or without FLAG-α-catenin. The cells were treated with EGF (100 ng/ml) for 6 hr and immunoblotting was performed with the indicated antibodies.
(F, H) The luciferase activity was determined after cells were treated with EGF (100 ng/ml) for 8 hr. The relative levels of luciferase activity were normalized to the levels of untreated cells and to the levels of luciferase activity of the Renilla control plasmid. Data represent the mean ± standard deviation of three independent experiments.
(F) pCep4-EGFR with either TOP-FLASH or FOP-FLASH were co-transfected with or without FLAG-α-catenin into 293T cells.
(G) A431 cells were stably expressed with control shRNA or α-catenin shRNA. Immunoblotting analyses were performed with the indicated antibodies.
(H) TOP-FLASH or FOP-FLASH was transfected into A431 cells that were stably expressed with control shRNA or α-catenin shRNA.
Immunofluorescence studies revealed that 10 hours of EGF treatment resulted in internalization of β-catenin and disruption of colocalization of β-catenin and α-catenin in cell-cell contacts; more pronounced effects were observed after 24 hr of treatment, showing EMT of A431 cells and nuclear accumulation of β-catenin (Fig. 1C). To test whether β-catenin transactivation contributes to EGF-induced EMT of A431 cells, we stably expressed a dominant negative mutant of β-catenin, β-Engrailed (β-Eng), in which the C-terminal transactivation domain of β-catenin is replaced with the transcriptional repression domain of Drosophila Engrailed (Montross et al., 2000). Expression of β-Eng, which blocked EGF-induced β-catenin transactivation (Lu et al., 2003), largely inhibited EGF-induced EMT and upregulation of vimentin (Fig. S1C), a marker of mesenchymal cells.
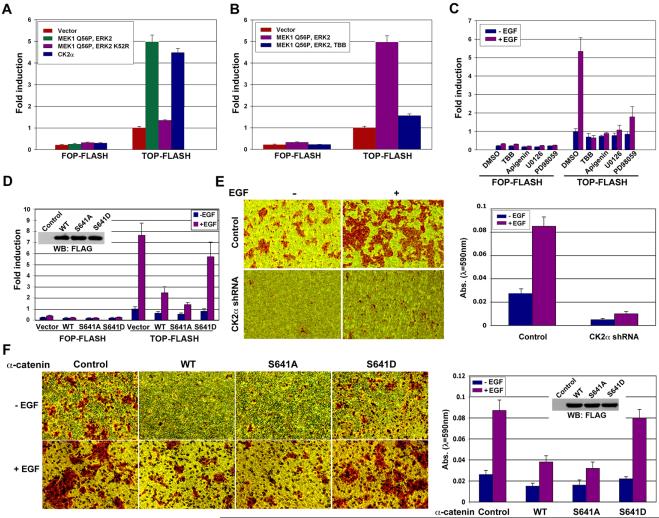
Intriguingly, overexpression of α-catenin resulted in reduced β-catenin internalization in A431 cells (Fig. 1D). These results are further supported by fractionation analyses showing that α-catenin overexpression blocked β-catenin nuclear accumulation induced by EGF stimulation (Fig. 1E). Furthermore, α-catenin overexpression largely inhibited EGF-induced β-catenin transactivation, which was evaluated by TCF/LEF-1 transcriptional activity measured by the TCF/LEF-1 luciferase reporter TOP-FLASH using FOP-FLASH as a control vector (Fig. 1F). Conversely, depletion of α-catenin through shRNA expression significantly enhanced TCF/LEF-1 transcriptional activity in both A431 (Fig. 1G, 1H) and U87E cells (data not shown), and this activity was further enhanced by EGF treatment, which could be due to a disruption of the interaction between the residual α-catenin and β-catenin and a combinational effect with other EGF-induced upregulated β-catenin transactivation. These results strongly suggest that EGFR activation results in disruption of the β-catenin and α-catenin complex, which in turn promotes β-catenin transactivation.
CK2α Interacts with and Phosphorylates α-Catenin at S641
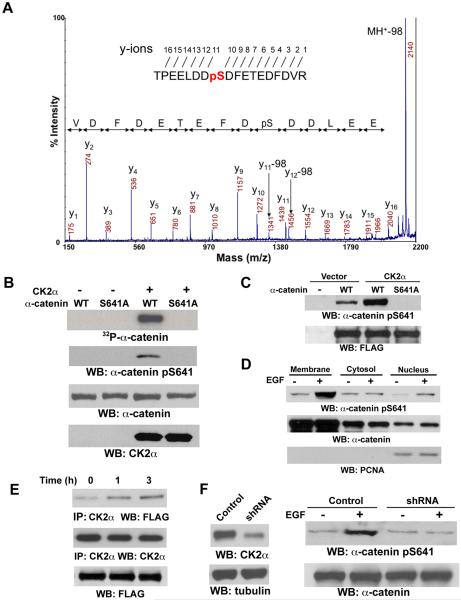
To examine whether the dissociation of α-catenin from β-catenin is due to a possible unknown post-translational modification on α-catenin, we analyzed a tryptic digest of α-catenin immunoprecipitated from EGF-stimulated A431 cells by mass spectrometry. The peptide coverage of α-catenin by mass spectrometry analysis was up to about 50% in some digests (Fig S2). One candidate phosphopeptide that spanned amino acids 634–651 was detected by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. S641 was then identified as a phosphorylation site in this α-catenin peptide by MALDI-TOF-TOF and confirmed by liquid chromatography-coupled ion trap mass spectrometry (LC-MS/MS) (Fig. 2A). The 641SDFE644 sequence of α-catenin is closely related to the CK2 phosphorylation motif S/TXXD/E (Litchfield, 2003). To determine whether CK2 phosphorylates α-catenin at S641, we performed in vitro kinase assays with purified CK2α mixed with purified WT His-α-catenin or His-α-catenin S641A mutant, in which Ser641 was mutated into alanine (Ala). As shown in Fig. 2B, CK2α was able to phosphorylate WT α-catenin but not α-catenin S641A mutant. In addition, phosphorylated WT α-catenin, but not α-catenin S641A mutant, was detected by an antibody that specifically recognized phosphorylated α-catenin S641 (Fig. 2B). To test whether CK2 is able to phosphorylate α-catenin in vivo, we co-transfected CK2α with FLAG-tagged WT α-catenin or α-catenin S641A mutant. Immunoblotting of immunoprecipitated FLAG-tagged α-catenins with the anti-phospho-α-catenin S641 antibody showed that CK2α phosphorylated WT α-catenin but not α-catenin S641A in vivo (Fig. 2C). A subcellular fractionation analysis revealed that enhanced α-catenin phosphorylation at S641 was detected in both membrane and nuclear fractions in response to EGF treatment (Fig. 2D). Furthermore, EGF treatment significantly enhanced the association between FLAG-α-catenin and endogenous CK2α (Fig. 2E). Depletion of CK2α through shRNA expression, which reduced both the expression (Fig. 2F) and activity of CK2α (Fig. S3), inhibited EGF-enhanced α-catenin phosphorylation at S641 (Fig. 2F). These results indicate that CK2α, which is downstream from EGFR activation, phosphorylates α-catenin at S641 both in vitro and in vivo.
Figure 2. CK2α Interacts with and Phosphorylates α-catenin at S641.
(B-E) Immunoblotting analyses were performed with the indicated antibodies.
(A) α-catenin immunoprecipitated from EGF-stimulated A431 cells was analyzed with mass spectrometry. Mass spectrometric analysis of a tryptic fragment 634-TPEELDDSDFETEDFDVR-651 indicates S641 was phosphorylated. The m/z difference between y-11 and y-10 matched with phospho-Ser.
(B) In vitro kinase assays were performed with purified bacterially expressed WT His-α-catenin or His-α-catenin S641A with or without CK2α.
(C) pRc/CMV2-HA-CK2α was co-transfected with pEGFP N1-FLAG-tagged WT α-catenin or α-catenin S641A mutant into 293T cells. Immunoblotting of immunoprecipitated FLAG-tagged α-catenins with the anti–α-catenin Ser641 antibody was performed.
(D) The subcellular fractionation was prepared from A431 cells treated with EGF (100 ng/ml) for 30 min.
(E) A431 cells expressing FLAG-tagged WT α-catenin were treated with or without EGF (100 ng/ml) for 1 or 3 hr. CK2α was immunoprecipitated.
(F) A431 cells with or without expression of CK2α shRNA or a control shRNA were treated with or without EGF (100 ng/ml) for 30 min.
CK2α-mediated Phosphorylation of α-Catenin at S641 Releases α-Catenin from Binding to β-Catenin
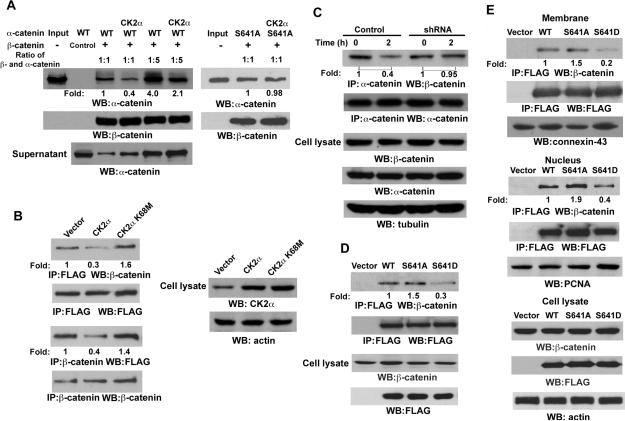
To determine whether CK2α-regulated phosphorylation of α-catenin affects the complex of α-catenin and β-catenin, we performed GST pull-down analyses by mixing purified WT His-α-catenin or His-α-catenin S641A mutant with purified GST-β-catenin (Fig. S4A) in the presence or absence of purified CK2α. Consistent with a previous report (Pokutta and Weis, 2000), α-catenin bound to β-catenin stoichiometrically (Fig. S4B). CK2α, which phosphorylates α-catenin, reduced the binding of WT α-catenin, but not α-catenin S641A mutant, to β-catenin (Fig. 3A, S4C). In addition, the amount of α-catenin binding to β-catenin inversely correlated with the amount of α-catenin in the supernatant. Immunoblotting of immunoprecipitated FLAG-tagged α-catenin with a β-catenin antibody showed that expression of CK2α, but not a CK2α K68M kinase-dead mutant, reduced the total amount of α-catenin binding to α-catenin (Fig. 3B, upper panel). These results were further confirmed by a reciprocal β-catenin immunoprecipitation, followed by immunoblotting with an anti-FLAG antibody (Fig. 3B, middle panel). In line with EGF-induced and CK2-dependent α-catenin phosphorylation, EGF treatment reduced α-catenin binding to β-catenin at endogenous levels, which can be blocked by expression of CK2α shRNA (Fig. 3C) or pretreatment with CK2 inhibitor, 4,5,6,7-tetrabromobenzotriazole (TBB), or apigenin in both A431 and U87E cells (data not shown). Furthermore, a phosphorylation-mimic α-catenin S641D mutant, in which Ser was mutated into Asp, showed a much-reduced binding to β-catenin in total cell lysate (Fig. 3D) or in the membrane and nuclear fraction (Fig. 3E) in contrast to WT α-catenin or α-catenin S641A mutant. These results indicate that phosphorylation of α-catenin at S641 by CK2α in response to EGF stimulation reduces α-catenin binding to β-catenin.
Figure 3. CK2 α-mediated Phosphorylation of α-catenin at S641 Releases α-catenin from Binding to α-catenin.
Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies.
(A) In vitro kinase assays were performed with purified bacterially expressed WT His-α-catenin with or without CK2α, which is followed by GST pull-down analyses with GST (control) or GST-α-catenin glutathione-agarose beads.
(B) FLAG-tagged α-catenin was co-transfected with pRc/CMV2-HA-CK2α or pRc/CMV2-HA-CK2α K68M into 293T cells.
(C) A431 cells expressing with or without CK2α shRNA were treated with or without EGF (100 ng/ml) for 2 hr.
(D) FLAG-tagged WT α-catenin, α-catenin S641A, or α-catenin S641D mutant were transfected into 293T cells. Immunoprecipitation of the total cell lysate with an anti-FLAG antibody was followed by immunoblotting analyses with the indicated antibodies.
(E) FLAG-tagged WT α-catenin, α-catenin S641A, or α-catenin S641D mutant were transfected into 293T cells. Immunoprecipitation of the membrane or nucleus fraction was performed with the indicated antibodies.
ERK2 Phosphorylates CK2α and Enhances CK2α Activity Toward α-Catenin in Response to EGF Stimulation
To investigate how CK2 is regulated in response to EGF, we investigated whether CK2 is post-translationally modified by EGF-induced activation of protein kinases including EGFR, Src, and ERK (Scaltriti and Baselga, 2006). Incubation of purified CK2α, which has been boiled and lost its activity, with purified active ERK2, ERK5, or immunoprecipitated EGFR and Src from EGF-stimulated A431 cells showed that ERK2 but not ERK5, EGFR, or Src (data not shown) phosphorylates CK2α in vitro (Fig. 4A). Sequence analysis of CK2α revealed that CK2α contains S/T-Proline, an ERK-consensus phosphorylation motif at T108, S294, T344, T360, S362, and S370 residues. Mass spectrometry analysis of ERK2-phosphorylated CK2α protein revealed T360 and S362 as potential phosphorylation sites (data not shown). Mutation of T360 and S362 into Ala reduced ERK2-dependent phosphorylation of CK2 in vitro, and further reduction was observed in the CK2α T360/S362A mutant, which has a combined mutation (Fig. 4B). These results indicate that ERK phosphorylates CK2α primarily at T360/S362. Consistent with these in vitro results, 32P-phosphate-metabolic labeling revealed that WT CK2α, but not CK2α T360/S362A, was phosphorylated in vivo by activated ERK2 induced by expression of a constitutively active MEK1 Q56P mutant (Fig. 4C). These results indicate that ERK phosphorylates CK2α at T360/S362.
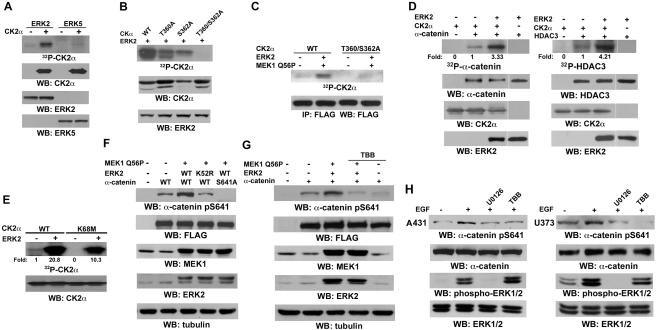
Figure 4. ERK1/2 Phosphorylates CK2α and Enhances CK2α Activity Toward α-catenin in Response to EGF Stimulation.
Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies.
(A) In vitro kinase assays were performed by mixing purified ERK2 or ERK5 with boiled CK2α.
(B) In vitro kinase assays were performed by mixing purified ERK2 with boiled CK2α, CK2α T360A, CK2α S362A, or T360/S362A mutants.
(C) 293T cells expressing MEK1 Q56P and ERK2 with FLAG-tagged WT CK2α or CK2α T360/S362A were metabolically labeled with 32P-phosphate for 12 hr. Immunoprecipitation with an anti-FLAG antibody was performed.
(D) Purified CK2α with or without purified His-α-catenin (left panel) or HDAC3 (right panel) was mixed with purified ERK2 in kinase assays. The imagines were quantified by scanning densitometry.
(E) In vitro kinase assays were performed by mixing non-boiled WT CK2α or CK2α K68M with or without purified ERK2.
(F, G) MEK1 Q56P was co-transfected with WT ERK2 or ERK2 K52R kinase-dead mutant together with WT α-catenin or α-catenin S641A into 293T cells. The cells were treated without (F) or with TBB (40 μM) (G) for 6 hr before harvesting.
(H) A431 or U373 cells were pretreated with U0126 (25 μM) or TBB (40 μM) for 30 min before EGF (100 ng/ml) stimulation for 30 min.
To determine the effect of CK2α phosphorylation by ERK2 on α-catenin phosphorylation, we incubated purified CK2α and α-catenin with or without active ERK2. As shown in Fig. 4D (left panel), active ERK2, which by itself does not phosphorylate α-catenin, significantly enhanced α-catenin phosphorylation by CK2α. Similarly, ERK2 also enhanced CK2α-induced phosphorylation of purified HDAC3 (Fig. 4D, right panel), a known substrate of CK2 (Zhang et al., 2005). These results strongly suggest that ERK2-mediated CK2α phosphorylation enhanced its activity towards its substrates in vitro. To examine the effect of ERK-mediated phosphorylation on CK2α autophosphorylation ability (Donella-Deana et al., 2001), we mixed purified WT CK2α and its K68M kinase-dead mutant with or without active ERK2. As shown in Fig. 4E, ERK2 significantly increased the phosphorylation level of WT CK2α (Lane 2), which was greater than the combined levels of autophosphorylated WT CK2α (Lane 1) and ERK2-phosphorylated CK2α K68M (Lane 4). These results indicate that CK2 phosphorylation by ERK increased the activity of CK2.
To examine whether activation of ERK2 affects phosphorylation of α-catenin S641 in vivo, we co-expressed active MEK1 Q56P mutant with WT ERK2 or ERK2 K52R kinase-dead mutant and with WT α-catenin or the α-catenin S641A mutant. Immunoblotting with the phospho-α-catenin S641 antibody revealed that activated ERK2, but not its kinase-dead mutant, significantly enhanced phosphorylation of WT α-catenin but not the α-catenin S641A mutant (Fig. 4F). In addition, this enhanced phosphorylation was blocked by treatment with the CK2 inhibitor TBB (Fig. 4G) or apigenin (data not shown). Consistently, EGF-enhanced phosphorylation of α-catenin S641 was blocked by pretreatment with MEK inhibitor U0126 or CK2 inhibitor TBB in A431, U373 (Fig. 4H), and U87E cells (data not shown), which is consistent with the effect of CK2α depletion on EGF-induced α-catenin phosphorylation (Fig. 2F). U0126 and TBB were used at a low dose that blocks phosphorylation and activation of ERK (Fig. 4H) and CK2α, respectively (Favata et al., 1998; Ruzzene et al., 2002). These results suggest that ERK2 phosphorylates CK2α, which in turn enhances CK2α-dependent phosphorylation of α-catenin at S641.
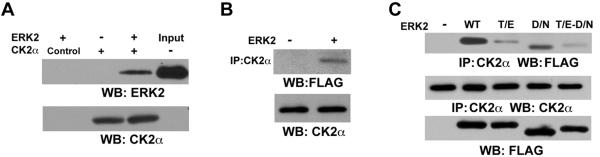
ERK2 Directly Binds to CK2α Through its Docking Groove
To examine whether ERK2 interacts with CK2α, we incubated immobilized purified His-CK2α with purified ERK2. Immunoblotting analysis with an anti-ERK2 antibody showed that both proteins bind to each other directly in vitro (Fig. 5A). The association of active ERK2 with endogenous CK2α in 293T cells was also detected by immunoblotting of immunoprecipitated CK2α with anti-FLAG-tagged ERK antibody (Fig. 5B). MAP kinases bind to their substrates through a docking groove comprised of an acidic common docking (CD) domain and Glu-Asp (ED) pockets (Lu and Xu, 2006). Immunoblotting of immunoprecipitated CK2α with anti-FLAG-tagged ERK2 antibody showed that mutation of either ERK2 CD domain (D316/319N) (Tanoue et al., 2000) or ED pocket (T157/158E) (Tanoue et al., 2001) reduced their binding to CK2α in contrast to WT ERK2, and a mutant (T/E-D/N) comprised mutations at both the CD domain and the ED pocket further reduced this binding (Fig. 5C). These results indicate that ERK2 binds to CK2α through its docking groove.
Fig. 5. ERK2 directly binds to CK2α through its docking groove.
Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies.
(A) Immobilized purified His-CK2α was mixed with purified ERK2 followed by washing three times with cell lysis buffer and immunoblotting analysis. Control, nickel-agarose beads.
(B) 293T cells were transfected with a control vector or vectors expressing MEK1 Q56P and FLAG-ERK2.
(C) A vector expressing MEK1 Q56P were co-transfected with FLAG-WT ERK2, FLAG-ERK2 T/E (T157/158E), FLAG-ERK2 D/N (D316/319N), or FLAG-ERK2 T/E-D/N mutant into 293T cells.
EGF-induced ERK2 Activation Promotes β-Catenin Transactivation and Tumor Cell Invasion by Enhancing CK2-phosphorylated α-Catenin at S641
To examine the effect of ERK2- and CK2-regulated α-catenin phosphorylation on β-catenin transactivation in response to EGFR activation, we first examined the activation of ERK2 and CK2 on TCF/LEF-1 transcriptional activity. The TCF/LEF-1 luciferase reporter analysis showed that expression of activated ERK2 and CK2α, but not an ERK2 kinase-dead mutant, significantly induced β-catenin transactivation (Fig. 6A). Furthermore, ERK-enhanced TCF/LEF-1 transcriptional activity was blocked by treatment with CK2 inhibitor TBB, indicating that CK2 regulated by ERK2 plays a role in β-catenin transactivation (Fig. 6B). In line with ERK and CK2 downstream from EGFR activation in regulation of α-catenin, pretreatment with CK2 inhibitor, TBB, apigenin, or MEK inhibitor U0126 or PD98059 largely blocked EGF-induced TCF/LEF-1 transcriptional activity (Fig. 6C). To test whether the effect of ERK1/2 and CK2 activation on β-catenin transactivation is, at least, partially via regulation of α-catenin, we co-transfected WT α-catenin, α-catenin S641A, or α-catenin S641D with TOP-FLASH or FOP-FLASH into A431 cells. Consistent with the results that phosphorylation of α-catenin regulates β-catenin/α-catenin complex stability, expression of α-catenin S641D, which has a much reduced binding ability to β-catenin, significantly lost its inhibitory effect on EGF-induced β-catenin transactivation in contrast to WT α-catenin or α-catenin S641A mutant (Fig. 6D). These results strongly suggest that EGF-induced phosphorylation of α-catenin S641 greatly abolished its negative regulation on β-catenin and promoted β-catenin transactivation.
Figure 6. EGF-induced ERK2 Activation Promotes β-catenin Transactivation and Tumor Cell Invasion by Enhancing CK2-phosphorylated α-catenin at S641.
(A-D) The relative levels of luciferase activity were normalized to the levels of untreated cells and to the levels of luciferase activity of the Renilla control plasmid. Data represent the mean ± standard deviation of three independent experiments.
(A) TOP-FLASH or FOP-FLASH was co-transfected with or without the indicated plasmids into 293T cells.
(B) TOP-FLASH or FOP-FLASH was co-transfected with or without vectors expressing MEK1 Q56P and ERK2 into 293T cells. The cells were pretreated with TBB (40 μM) for 6 hr before harvesting.
(C) TOP-FLASH or FOP-FLASH was co-transfected with a vector expressing EGFR into 293T cells. The cells were pretreated with TBB (40 μM), apigenin (40 μM), U0126 (25 μM), or PD98059 (30 μM) for 30 min before EGF (100 ng/ml) for 6 hr.
(D) TOP-FLASH or FOP-FLASH was co-transfected with vectors expressing FLAG-tagged WT α-catenin, α-catenin S641A, or α-catenin S641D into A431 cells. The cells were treated with EGF (100 ng/ml) for 8 hr. Immunoblotting with indicated antibodies was performed (left upper panel).
(E, F) A pool of A431 cells expressing with a control shRNA or CK2α shRNA (E) or stably transfected with a vector, FLAG-tagged WT α-catenin, α-catenin S641A, or α-catenin S641D (F) was plated at the top surface of the Matrigel in the absence or presence of EGF (100 ng/ml). One day after plating, cells that migrated to the opposite side of the insert were stained with crystal violet. Representative microphotographs are shown (left panel). The membranes with invaded cells were dissolved in 4% deoxycholic acid and read colorimetrically at 590 nm for quantification of invasion. Data represent the mean ± standard deviation of three independent experiments (right panel). Immunoblotting with indicated antibodies was performed (right upper panel).
β-catenin transcriptional activity has been closely related to tumor development. To examine the effect of phosphorylation of α-catenin by CK2 on tumor cell invasion, we performed Matrigel transwell invasion assays. As shown in Fig. 6E, CK2α depletion significantly inhibited EGF-induced cell invasion. This inhibition was unlikely to be caused by potential regulation of cell growth by CK2 depletion because we measured the tumor cell invasion in a 12-hr time-frame, during which we did not detect an effect of CK2α depletion on cell proliferation measured by cell counting (data not shown). In agreement with the results of α-catenin phosphorylation on β-catenin transactivation, stable expression of WT α-catenin or α-catenin S641A mutant in A431 cells or U87E cells (data not shown), which inhibit β-catenin transcriptional activity (Fig. 6D), greatly reduced EGF-induced cell invasion (Fig. 6F). In contrast, expression of α-catenin S641D, which has less of an effect on β-catenin transactivation, minimally affected EGF-induced cell invasion. These results indicate that α-catenin phosphorylation by CK2 promotes dissociation of α-catenin from β-catenin, thereby promoting β-catenin transactivation and tumor cell invasion.
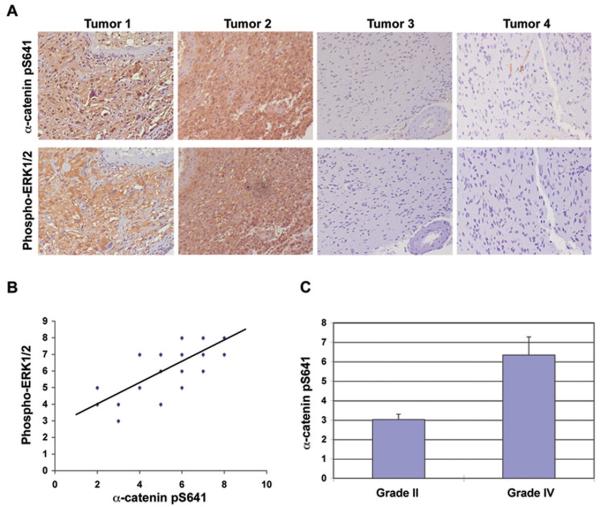
Levels of α-Catenin S641 Phosphorylation Correlate with Levels of ERK1/2 Activity in Human GBM and with Grades of Glioma Malignancy
Activated EGFR, together with its downstream ERK activation, has been detected in human GBM specimens (Feldkamp et al., 1999). We demonstrated that ERK1/2 activation is necessary and sufficient for phosphorylation of α-catenin at S641. To determine whether our findings have clinical relevance, we examined ERK1/2 activity and α-catenin phosphorylation in serial sections of 46 human primary GBM specimens (WHO grade IV) by immunohistochemical analyses. As shown in Fig. 7A, the levels of ERK1/2 activity were correlated with the levels of phosphorylation of α-catenin at S641. Quantification of the staining on the 0 to 8 scales showed that this correlation was significant in different specimens (Fig. 7B: r = 0.76, p < 0.0001). This clinical evidence strongly supported the role of ERK1/2 activation in phosphorylation of α-catenin at S641 in human GBM.
Figure 7. Levels of α-Catenin S641 Phosphorylation Correlate with Levels of ERK1/2 Activity in Human GBM and with Grades of Glioma Malignancy.
(A) Immunohistochemical staining with anti–phospho-ERK1/2 and anti–phospho-α-catenin S641A antibodies was performed on 46 GBM specimens. Representative photos of four tumors were shown.
(B) Immunohistochemical staining on the tissue sections was scored semi-quantitatively as described in Materials and Methods (Pearson product moment correlation test r = 0.76; p < 0.0001). Note that some of the dots on the graphs represented more than one specimen (some scores overlapped).
(C) Immunohistochemical staining of 23 diffuse astrocytoma specimens with an anti–phospho-α-catenin S641 antibody was performed and analyzed by comparing it with the staining of 46 GBM specimens (The student's t test, two tailed, p < 0.000001). Data represent the mean ± standard deviation of sample scores.
To examine whether the levels of α-catenin phosphorylation at S641 correlate with the grades of glioma malignancy, we compared α-catenin phosphorylation level in low-grade diffuse astrocytoma (WHO grade II with more than 5 years' median survival time) with high-grade GBM (with less than 1 year of median survival time) (Furnari et al., 2007). Immunohistochemical analyses of 23 human low-grade diffuse astrocytoma specimens showed significantly lower levels of phosphorylated α-catenin S641 than were present in GBM specimens (Fig. 7C).
DISCUSSION
β-catenin, functioning as a major component of both Wnt signaling and cell-cell adhesion, plays a central role in many aspects of cell functions and development (Huang and He, 2008; Wodarz and Nusse, 1998). β-catenin localized in different cellular compartments forms distinct complexes with unique function. In addition to activation of β-catenin by Wnt-dependent or activating mutations of Wnt components, Wnt-independent signaling is also involved in regulation of β-catenin transactivation and tumorigenesis (Giles et al., 2003; Lu and Hunter, 2004). For instance, β-catenin-TCF/LEF-1 signaling can be activated by growth factors, such as EGF, HGF, insulin-like growth factor (IGF)-I, IGF-II, and insulin (Desbois-Mouthon et al., 2001; Lu et al., 2003; Morali et al., 2001; Muller et al., 2002). EGF-induced β-catenin nuclear accumulation and transactivation does not accompany a detectable change in β-catenin's half-life or phosphorylation level by GSK-3β (Fang et al., 2007; Lu et al., 2003), implying that GSK-3β-independent regulation plays an instrumental role in EGF-induced β-catenin transactivation. Inhibition of β-catenin by β-Eng expression largely blocked EGF-induced EMT, implying a critical function of β-catenin in EGF-enhanced tumor cell motility.
The mechanistic regulation of interaction between β-catenin and α-catenin by α-catenin S641 phosphorylation requires further investigation. Previously, it was shown that the α-catenin 57–264 fragment directly binds to β-catenin (Pokutta and Weis, 2000). α-catenin S641 is in the intercellular adhesion modulation (M) domain (residues 509–643), which is required for mediating intercellular adhesion (Imamura et al., 1999; Kobielak and Fuchs, 2004). Phosphorylation of α-catenin at S641 by CK2α in response to EGF stimulation and ERK activation may lead to conformational change of α-catenin, which facilitates the disruption of α-catenin 57–264 binding to β-catenin and of intercellular adhesion. Abrogation of the inhibitory effect of α-catenin on β-catenin by releasing the binding of α-catenin from β-catenin promotes β-catenin transactivation.
We have previously shown that EGF treatment induces downregulation of caveolin-1, which promotes β-catenin transactivation (Lu et al., 2003). Notably, we have found that overexpression of WT caveolin-1 inhibited EGF-induced α-catenin phosphorylation at S641, whereas depletion of caveolin-1 by expression of antisense caveolin-1 RNA enhanced both basal and EGF-induced ERK activity and α-catenin phosphorylation (data not shown). These results imply that EGF-induced caveolin-1 downregulation and abrogation of the inhibitory effect of caveolin-1 on signaling molecules (Lu et al., 2003; Williams and Lisanti, 2005) activates ERK, leading to the CK2 phosphorylation and subsequent α-catenin phosphorylation. The elucidation of this EGF-induced phosphorylation cascades provides mechanistic insight into growth factor-induced non-canonical regulation of β-catenin.
The mechanism of CK2 activation has been equivocal. CK2 is activated in response to growth factor stimuli according to some research (Ackerman et al., 1990), whereas other reports support the concept that CK2 is a constitutively active kinase and its activity towards synthetic peptide substrates is not regulated by growth factors (Litchfield, 2003; Litchfield et al., 1994). Our results demonstrate that ERK phosphorylates CK2α, which enhances its autophosphorylation activity as well as its phosphorylation of α-catenin and HDAC3. These results suggest that the activity of a subcellular portion of CK2 can be enhanced by interaction with and phosphorylation by ERK and that the phosphorylation of CK2 substrates can be a combined effect of enhanced CK2 activity and increased binding of CK2 to its substrates. The increased activity of this subcellular-localized and ERK-interacted CK2 might not be able to significantly elevate the levels of total cellular activity of CK2.
CK2 regulates β-catenin via different mechanisms. For instance, integrated in the canonical regulation of β-catenin via ubiquitination and proteasomal degradation, CK2 can directly regulate cytoplasmic β-catenin stability by phosphorylating β-catenin or the involved E2 ubiquitin conjugating enzyme, UBC3B (Bek and Kemler, 2002; Seldin et al., 2005; Semplici et al., 2002; Song et al., 2003). In addition to regulation of β-catenin stability, it has been shown that CK2-mediated phosphorylation of LEF-1 enhanced its binding to nuclear β-catenin in Wnt-signaling cells (Wang and Jones, 2006). In our study, we demonstrate an important regulation of β-catenin by CK2-dependent abrogation of the inhibitory effect of α-catenin in response to EGF stimulation, providing further evidence that CK2 spatially regulates β-catenin function via distinct mechanisms in response to different extracellular stimuli.
CK2 exists as a monomeric subunit as well as a heterotetrameric complex, which differentiates CK2 cellular functions. α-catenin co-immunoprecipitates with CK2α, but not with CK2α′ or CK2β (data not shown), suggesting that monomeric CK2α rather than CK2 heterotetrameric complex phosphorylates α-catenin. In addition, the C-terminal domains of CK2α and CK2α′ are completely unrelated (Litchfield, 2003), and the C-terminal domains of CK2α′ lack the ERK-phosphorylation motif, further supporting the conclusion that CK2α, but not CK2α′, is pivotal for α-catenin phosphorylation. It has been shown that the C-terminal domains of CK2α are phosphorylated by Cdc2 and interact with the peptidyl-prolyl isomerase Pin1 in a cell cycle-dependent manner (Litchfield, 2003; Messenger et al., 2002). We find that EGF-stimulated CK2-dependent α-catenin phosphorylation was not affected by Cdc2 inhibition with a Cdc2-specific inhibitor roscovitine (data not shown), indicating that CK2α-regulated α-catenin phosphorylation is Cdc2-independent. In contrast, we found that ERK2 binds to CK2α directly, which is mediated by the ERK2 docking groove. In addition, active ERK2 sufficiently phosphorylates CK2α both in vitro and in vivo thereby enhancing α-catenin phosphorylation, and inhibition of ERK1/2 activation abrogated EGF-induced α-catenin phosphorylation. Importantly, analysis of human GBM specimens showed that activation of ERK1/2 correlates with phosphorylation of α-catenin at S641. Collectively, these results point to an essential role for ERK, which is downstream from EGFR, in positive regulation of CK2α-dependent α-catenin phosphorylation.
Adherens proteins at cell-cell adhesion, such as E-cadherin and β-catenin, can be phosphorylated by protein kinases, such as CK2, Src, Abl, Fer, and Fyn, which subsequently affect adherens protein association at the cell membrane (Lickert et al., 2000; Lilien and Balsamo, 2005; Piedra et al., 2003; Serres et al., 2000). Activated AKT stabilizes β-catenin through inhibition of GSK-3β (Cross et al., 1995) and directly phosphorylates β-catenin at S552, thereby promoting β-catenin transactivation (Fang et al., 2007). Further investigation is needed to determine whether β-catenin S552 phosphorylation contributes to disassembly of adherens protein. β-catenin still associates with α-catenin in some tumors, such as glioblastoma, which have no or very limited E-cadherin expression (Asano et al., 2000). Alpha-catenin associated with β-catenin primarily at the cell membrane in epithelial cells, but this interaction also existed in both cytosol and nucleus. EGF treatment interrupted this interaction in both membrane and nucleus, and α-catenin S641D phosphorylation-mimic mutant also lost its interaction with β-catenin in membrane and nucleus. In addition, ectopic expression of α-catenin increased the binding to β-catenin (data not shown) and blocked EGF-induced β-catenin accumulation in the nucleus, implying that α-catenin and β-catenin complex hinders β-catenin nuclear entry. Thus our results suggest that α-catenin phosphorylation by CK2 promotes β-catenin transactivation via at least two mechanisms: (1) Facilitating the release of membrane-localized β-catenin from α-catenin promotes β-catenin nuclear translocation; (2) Facilitating the release of nuclear-localized β-catenin from α-catenin binding promotes β-catenin transcriptional activity. The role of nuclear-localized α-catenin in β-catenin transactivation was also illustrated by the observation that levels of nuclear α-catenin are inversely correlated with TCF-dependent transcription in a colon cancer cell line and that nuclear α-catenin blocks the β-catenin-TCF complex with DNA (Giannini et al., 2000). Given that mislocalization or loss of α-catenin is associated with aggressive forms of cancer (Benjamin and Nelson, 2008), our results, which include identifying the relationship between levels of α-catenin S641 phosphorylation and grades of glioma malignancy, highlight a critical mechanism demonstrating the effect of enhanced β-catenin transactivation on cancer progression by disruption of α-catenin/β-catenin complex.
Activation of EGFR, elevated CK2 kinase activity, and increased β-catenin-TCF/LEF-1 transcriptional activity have all been separately reported to correlate with invasive stages of tumor development. We propose a mechanistic model for tumor cell invasion and metastasis that integrates these different components. EGFR activation results in the activation of ERK. ERK2, which interacts directly with CK2α regulated by ERK2 docking groove, phosphorylates CK2α, thereby enhancing CK2α activity toward phosphorylation of α-catenin at S641. The phosphorylated α-catenin loses its binding to β-catenin, which subsequently activates β-catenin-TCF/LEF-1 transcriptional activity. Our studies represent an important mechanism underlying the effects of EGF during tumor development. The demonstration of a mechanistic interplay between EGF and Wnt/Wingless signaling components provides an important insight for further understanding tumor cell invasion and metastasis.
EXPERIMENTAL PROCEDURES
Materials
Rabbit antibody recognizing phosphorylated α-catenin S641 was obtained from Signalway Antibody (Pearland, TX) and Cell Signaling Technology (Danvers, MA). Polyclonal antibodies for α-catenin and monoclonal antibodies for α-catenin and β-catenin (E-5) were acquired from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibodies for FLAG and tubulin, and EGF were purchased from Sigma (St. Louis, MO), and Hygromycin, G418, TBB, apigenin, and U0126 were purchased from EMD Biosciences (San Diego, CA). Active ERK2 was obtained from New England Biolabs (Ipswich, MA), Hoechst 33342, fluorescein isothiocyanate-conjugated anti-mouse antibody and Texas Red-conjugated anti-rabbit antibody were from Molecular Probes (Eugene, OR), and HyFect transfection reagents was from Denville Scientific (Metuchen, NJ). GelCode Blue Stain Reagent was obtained from Pierce (Rockford, IL).
Metabolic Labeling
Cells were labeled by incubating them with 1 mCi/ml of 32P-phosphate (MP Biochemicals, Solon, OH) in phosphate-free DMEM containing 10% dialyzed fetal bovine serum (Invitrogen, San Diego, CA) for 12 hours. Immunoprecipitated proteins were run on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membrane. Phosphorylated proteins were visualized by autoradiography.
Immunohistochemical Analysis
The tissue sections from paraffin-embedded GBM specimens were stained with an antibody against phospho-ERK1/2 (sc-7383; Santa Cruz Biotechnology, Santa Cruz, CA), α-catenin phosphorylation pSer641 antibody (Signalway Antibody, Pearland, TX) or nonspecific IgG as negative controls. We quantitatively scored the tissue sections according to the percentage of positive cells and staining intensity, as previously defined (Allred et al., 1998). We assigned the following proportion scores: 0 if 0% of the tumor cells showed positive staining, 1 if 0% to 1% of cells were stained, 2 if 1% to 10% stained, 3 if 11% to 30% stained, 4 if 31% to 70% stained, and 5 if 71% to 100% stained. We rated the intensity of staining on a scale of 0 to 3: 0, negative; 1, weak; 2, moderate; and 3, strong. We then combined the proportion and intensity scores to obtain a total score (range, 0–8), as described previously (Allred et al., 1998). The use of human glioma specimens and the database were approved by the institutional review board at The University of Texas M. D. Anderson Cancer Center.
The significance of differences of the data was determined by using the Student's t test (two-tailed), whereas Pearson's correlation test was used to determine the correlation of the expression level between phospho-ERK1/2 and phospho-α-catenin S641. P <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Hans Clevers (Netherlands Institute for Developmental Biology, Hubrecht Laboratory) for pTOP-FLASH and pFOP-FLASH and Pierre McCrea (The University of Texas M. D. Anderson Cancer Center) for the β-Eng vector. We thank Xiaomin Chen for his technical assistance and Sourav Ghosh and Kristi Speights for their critical reading of this manuscript.
This work was supported by an American Cancer Society Research Scholar Award, RSG-09-277-01-CSM (Z.L.); a Brain Tumor Society research grant (Z.L.); a Phi Beta Psi Sorority research grant (Z.L.), an institutional research grant from The University of Texas M. D. Anderson Cancer Center (Z.L.); and a National Cancer Institute grant, 5R01CA109035 (Z.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ackerman P, Glover CV, Osheroff N. Stimulation of casein kinase II by epidermal growth factor: relationship between the physiological activity of the kinase and the phosphorylation state of its beta subunit. Proc Natl Acad Sci U S A. 1990;87:821–825. doi: 10.1073/pnas.87.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- Asano K, Kubo O, Tajika Y, Takakura K, Suzuki S. Expression of cadherin and CSF dissemination in malignant astrocytic tumors. Neurosurg Rev. 2000;23:39–44. doi: 10.1007/s101430050030. [DOI] [PubMed] [Google Scholar]
- Ashihara K, Saito T, Mizumoto H, Nishimura M, Tanaka R, Kudo R. Mutation of beta-catenin gene in endometrial cancer but not in associated hyperplasia. Med Electron Microsc. 2002;35:9–15. doi: 10.1007/s007950200001. [DOI] [PubMed] [Google Scholar]
- Bek S, Kemler R. Protein kinase CKII regulates the interaction of beta-catenin with alpha-catenin and its protein stability. J Cell Sci. 2002;115:4743–4753. doi: 10.1242/jcs.00154. [DOI] [PubMed] [Google Scholar]
- Benjamin JM, Nelson WJ. Bench to bedside and back again: molecular mechanisms of alpha-catenin function and roles in tumorigenesis. Semin Cancer Biol. 2008;18:53–64. doi: 10.1016/j.semcancer.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch MA, Court NW. Counting on mitogen-activated protein kinases--ERKs 3, 4, 5, 6, 7 and 8. Cell Signal. 2004;16:1345–1354. doi: 10.1016/j.cellsig.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoel MJ, Bertrand F, Cherqui G, Perret C, Capeau J. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene. 2001;20:252–259. doi: 10.1038/sj.onc.1204064. [DOI] [PubMed] [Google Scholar]
- Donella-Deana A, Cesaro L, Sarno S, Brunati AM, Ruzzene M, Pinna LA. Autocatalytic tyrosine-phosphorylation of protein kinase CK2 alpha and alpha' subunits: implication of Tyr182. Biochem J. 2001;357:563–567. doi: 10.1042/0264-6021:3570563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Feldkamp MM, Lala P, Lau N, Roncari L, Guha A. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45:1442–1453. doi: 10.1097/00006123-199912000-00034. [DOI] [PubMed] [Google Scholar]
- Filhol O, Martiel JL, Cochet C. Protein kinase CK2: a new view of an old molecular complex. EMBO Rep. 2004;5:351–355. doi: 10.1038/sj.embor.7400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Giannini AL, Vivanco M, Kypta RM. alpha-catenin inhibits beta-catenin signaling by preventing formation of a beta-catenin*T-cell factor*DNA complex. J Biol Chem. 2000;275:21883–21888. doi: 10.1074/jbc.M001929200. [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J Cell Biol. 1994;127:2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SG, Yu SS, Ryu JH, Jeon HB, Yoo YJ, Eom SH, Chun JS. Regulation of beta-catenin signaling and maintenance of chondrocyte differentiation by ubiquitin-independent proteasomal degradation of alpha-catenin. J Biol Chem. 2005;280:12758–12765. doi: 10.1074/jbc.M413367200. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Kobielak A, Fuchs E. Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5:614–625. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lickert H, Bauer A, Kemler R, Stappert J. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. J Biol Chem. 2000;275:5090–5095. doi: 10.1074/jbc.275.7.5090. [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield DW, Dobrowolska G, Krebs EG. Regulation of casein kinase II by growth factors: a reevaluation. Cell Mol Biol Res. 1994;40:373–381. [PubMed] [Google Scholar]
- Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- Lu Z, Hunter T. Wnt-independent beta-catenin transactivation in tumor development. Cell Cycle. 2004;3:571–573. [PubMed] [Google Scholar]
- Lu Z, Jiang G, Blume-Jensen P, Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol. 2001;21:4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–631. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- Maier D, Jones G, Li X, Schonthal AH, Gratzl O, Van Meir EG, Merlo A. The PTEN lipid phosphatase domain is not required to inhibit invasion of glioma cells. Cancer Res. 1999;59:5479–5482. [PubMed] [Google Scholar]
- Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- Merdek KD, Nguyen NT, Toksoz D. Distinct activities of the alpha-catenin family, alpha-catulin and alpha-catenin, on beta-catenin-mediated signaling. Mol Cell Biol. 2004;24:2410–2422. doi: 10.1128/MCB.24.6.2410-2422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger MM, Saulnier RB, Gilchrist AD, Diamond P, Gorbsky GJ, Litchfield DW. Interactions between protein kinase CK2 and Pin1. Evidence for phosphorylation-dependent interactions. J Biol Chem. 2002;277:23054–23064. doi: 10.1074/jbc.M200111200. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montross WT, Ji H, McCrea PD. A beta-catenin/engrailed chimera selectively suppresses Wnt signaling. J Cell Sci. 2000;113(Pt 10):1759–1770. doi: 10.1242/jcs.113.10.1759. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Morali OG, Delmas V, Moore R, Jeanney C, Thiery JP, Larue L. IGF-II induces rapid beta-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene. 2001;20:4942–4950. doi: 10.1038/sj.onc.1204660. [DOI] [PubMed] [Google Scholar]
- Moscatello DK, Holgado-Madruga M, Godwin AK, Ramirez G, Gunn G, Zoltick PW, Biegel JA, Hayes RL, Wong AJ. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55:5536–5539. [PubMed] [Google Scholar]
- Muller T, Bain G, Wang X, Papkoff J. Regulation of epithelial cell migration and tumor formation by beta-catenin signaling. Exp Cell Res. 2002;280:119–133. doi: 10.1006/excr.2002.5630. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol. 2001;13:600–603. doi: 10.1016/s0955-0674(00)00257-x. [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Weis WI. Structure of the dimerization and beta-catenin-binding region of alpha-catenin. Mol Cell. 2000;5:533–543. doi: 10.1016/s1097-2765(00)80447-5. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science (New York, NY. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Caca K, Hu G, Harrison FB, Fearon ER. Frequent nuclear/cytoplasmic localization of beta-catenin without exon 3 mutations in malignant melanoma. Am J Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzene M, Penzo D, Pinna LA. Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem J. 2002;364:41–47. doi: 10.1042/bj3640041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- Seldin DC, Landesman-Bollag E, Farago M, Currier N, Lou D, Dominguez I. CK2 as a positive regulator of Wnt signalling and tumourigenesis. Mol Cell Biochem. 2005;274:63–67. doi: 10.1007/s11010-005-3078-0. [DOI] [PubMed] [Google Scholar]
- Semplici F, Meggio F, Pinna LA, Oliviero S. CK2-dependent phosphorylation of the E2 ubiquitin conjugating enzyme UBC3B induces its interaction with beta-TrCP and enhances beta-catenin degradation. Oncogene. 2002;21:3978–3987. doi: 10.1038/sj.onc.1205574. [DOI] [PubMed] [Google Scholar]
- Serres M, Filhol O, Lickert H, Grangeasse C, Chambaz EM, Stappert J, Vincent C, Schmitt D. The disruption of adherens junctions is associated with a decrease of E-cadherin phosphorylation by protein kinase CK2. Exp Cell Res. 2000;257:255–264. doi: 10.1006/excr.2000.4895. [DOI] [PubMed] [Google Scholar]
- She QB, Solit DB, Ye Q, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8:287–297. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DH, Dominguez I, Mizuno J, Kaut M, Mohr SC, Seldin DC. CK2 phosphorylation of the armadillo repeat region of beta-catenin potentiates Wnt signaling. J Biol Chem. 2003;278:24018–24025. doi: 10.1074/jbc.M212260200. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Maeda R, Adachi M, Nishida E. Identification of a docking groove on ERK and p38 MAP kinases that regulates the specificity of docking interactions. Embo J. 2001;20:466–479. doi: 10.1093/emboj/20.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Unger GM, Davis AT, Slaton JW, Ahmed K. Protein kinase CK2 as regulator of cell survival: implications for cancer therapy. Curr Cancer Drug Targets. 2004;4:77–84. doi: 10.2174/1568009043481687. [DOI] [PubMed] [Google Scholar]
- Valles AM, Boyer B, Badet J, Tucker GC, Barritault D, Thiery JP. Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line. Proc Natl Acad Sci U S A. 1990;87:1124–1128. doi: 10.1073/pnas.87.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Wang S, Jones KA. CK2 controls the recruitment of Wnt regulators to target genes in vivo. Curr Biol. 2006;16:2239–2244. doi: 10.1016/j.cub.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990;111:2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ozawa Y, Lee H, Wen YD, Tan TH, Wadzinski BE, Seto E. Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev. 2005;19:827–839. doi: 10.1101/gad.1286005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.