Abstract
Hibernation in Arctic ground squirrels (AGS), Spermophilus parryii, is characterized by a profound decrease in oxygen consumption and metabolic demand during torpor that is punctuated by periodic rewarming episodes, during which oxygen consumption increases dramatically. The extreme physiology of torpor or the surge in oxygen consumption during arousal may increase production of reactive oxygen species, making hibernation an injurious process for AGS. To determine if AGS tissues experience cellular stress during rewarming, we measured carbonyl proteins, lipid peroxide end products and percent oxidized glutathione in brown adipose tissue (BAT) and liver of torpid, hibernating (hAGS), late arousal (laAGS), and cold-adapted, euthermic AGS (eAGS). In BAT carbonyl proteins and lipid peroxide end products were higher in eAGS and laAGS than in hAGS. By contrast, in liver, no significant difference in carbonyl proteins was observed. In another group of animals, comparison of carbonyl proteins and percent oxidized glutathione in frontal cortex, liver, and BAT of eAGS and hAGS showed no evidence of oxidative stress associated with torpor. These results indicate that increased thermogenesis associated with arousal AGS results in tissue specific oxidative stress in BAT but not in liver. Moreover, torpor per se is largely devoid of oxidative stress, likely due to suppression of oxidative metabolism.
Keywords: Torpor, Spermophilus, Free radicals, Lipid peroxidation, Carbonyl protein
1. Introduction
It has been known since the 1990's that free radical metabolism is an integral part of the metabolic depression machinery associated with hibernation, estivation and tolerance to freezing, dehydration, hypoxia and anoxia (Hermes-Lima and Zenteno-Savin, 2002; Bickler and Buck, 2007). In the case of metabolic depression under anoxia/hypoxia or freezing, the transition towards aerobic metabolism may involve a process that is similar to ischemia with reperfusion of organs following stroke, cardiac arrest and other pathological conditions. Following ischemia/repefusion in humans and most mammals an overproduction of reactive oxygen species (ROS) devastates cellular integrity (Hermes-Lima and Zenteno-Savin, 2002). Direct observation of ROS formation in anoxic-tolerant turtles indicated very low levels of hydroxyl radical generation in brain during anoxia followed by a restoration in ROS formation upon reoxygenation (Milton et al., 2007). However, reperfusion in animals adapted to these challenges – anoxia/hypoxia or freezing – is a physiological process that is an integral part of their natural life cycle. In the case of transitions from aerobic hypometabolism (in estivation in land snails or hibernation in small mammals) to normal metabolic rate, there is an intermediate phase of very high oxygen consumption where increased mitochondrial ROS production may occur (Hermes-Lima and Zenteno-Savin, 2002; Ramos-Vasconcelos and Hermes-Lima, 2003). The return of normal aerobic metabolism from anoxia/hypoxia or from hypometabolism is accompanied in several cases by increased markers of oxidative stress: lipid peroxidation, protein carbonyl or oxidized glutathione (GSSG). Furthermore, an increase in antioxidant defenses during anoxia/hypoxia or estivation (Hermes-Lima and Storey, 1995; Lushchak et al., 2001; Hermes-Lima and Zenteno-Savin, 2002; Lushchak et al., 2005; Ramos-Vasconcelos et al., 2005; Bickler and Buck, 2007) is observed, indicating that animals emerging from a hypometabolic state may experience oxidative stress. In several cases, no sign of oxidative stress is observed upon return to normal aerobic metabolism, as would be seen if antioxidant defenses are effective (Hermes-Lima and Zenteno-Savin, 2002; Ramos-Vasconcelos et al., 2005).
ROS production and preparatory development of antioxidant defense prior to or during arousal from hibernation in Arctic ground squirrels (AGS), and 13-lined ground squirrels has also been investigated. Levels of plasma and cerebral spinal fluid (CSF) ascorbate are higher in hibernating AGS (with body temperatures near 2 °C) and 13-lined ground squirrels when compared to fully aroused animals (with body temperatures near 37 °C) (Drew et al., 1999, 2002). During arousal, plasma ascorbate levels in AGS decrease within 1 to 3 h and concentrations of uric acid, a potent antioxidant and product of ROS generating, xanthine oxidase catalyzed reactions, peak within 1 h and then quickly subside, possibly due to antioxidant quenching activity (Toien et al., 2001; Hermes-Lima, 2004).
Although no studies to date have uncovered evidence of oxidative stress in AGS during hibernation, failure to detect oxidative stress during hibernation or after arousal may be specific to the tissues studied and/or the methods used (Carey et al., 2000, 2003a; Ma et al., 2004, 2005). In the case of 13-lined ground squirrels, Carey and others (Carey et al., 2000, 2003a) showed increased oxidative stress in intestine of hibernating animals when compared with summer-euthermic animals. To further investigate if AGS experience oxidative stress during euthermy, arousal or prolonged torpor, levels of lipid peroxidation, carbonyl protein and the ratio between GSSG and GSH (called herein percent oxidized GSH) were quantified in BAT, liver, and frontal cortex from AGS as well as rat for comparison with a familiar species. BAT was studied as an important thermogenic tissue; liver was selected due to its important role in glycogen storage and metabolism; brain was studied because of its special vulnerability to ischemia/reperfusion injury. The rapid increase in temperature during arousal, powered by BAT, may be associated with overproduction of mitochondrial ROS in this thermogenic tissue (Nizielski et al., 1995; Cannon and Nedergaard, 2004). Thus, if oxidative metabolism is associated with oxidative stress upon arousal from hibernation, we predicted that BAT would show evidence of physiological oxidative stress.
In addition, previous results (Ma et al., 2004) showed that deliberate oxidation of plasma ascorbate by intravenous administration of ascorbate oxidase (AO) prior to arousal caused a decrease in plasma ascorbate to near-zero levels during torpor and after arousal (euthermic), but did not decrease post-arousal tissue levels of ascorbate, GSH, or urate in any tissue examined, except liver. In liver, where ascorbate is synthesized, ascorbate levels decreased during hibernation, and then exceeded euthermic levels after arousal. AO attenuated the rebound in liver ascorbate content seen after arousal but did not decrease urate or GSH arguing against an increase in AO-induced oxidative stress (Ma et al., 2004). Thus, an additional aim of the present study was to investigate the influence of AO treatment on more direct markers of oxidative stress in BAT and liver.
2. Material and methods
2.1. Chemicals
Baker's yeast glutathione reductase (GR), butylated hydroxytoluene (BHT), 2,4-dinitrophenyl-hydrazine, 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), NADPH, reduced glutathione (GSH), glutathione disulfide (GSSG), sulfosalicylic acid and thiobarbituric acid were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO, USA). Ascorbate oxidase (AO), isolated from zucchini (Cucurbita pepo medullosa) was obtained from Calzyme Laboratories (San Luis Obispo, CA, USA). All other reagents used were of analytical grade. All solutions were prepared with Milli-Q deionized water.
2.2. Animal handling and tissue collection
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Alaska Fairbanks and were in accordance with National Research Council “Guide for the Care and Use of Laboratory Animals”. Adult and juvenile (born the year they were trapped) Arctic ground squirrels (AGS, Spermophilus parryii) of both sexes were trapped during mid-July (1999, 2000 and 2002) in the northern foothills of the Brooks Range, AK, approximately 30 miles south of the Toolik Field Station of the University of Alaska Fairbanks (68° 38′ W; elevation 809 m) and transported to Fairbanks. The ground squirrels were housed individually at 18 °C, under natural light cycle for 64° latitude, and fed rodent chow and fresh carrots and apples ad libitum until early August. Sunflower seeds (10–15 seeds/day) were added to their diet 2 weeks prior to moving to environmental chambers. In mid-August, AGS were moved to environmental chambers set to an ambient temperature of 2 °C and 4:20 L:D. Animal experiments were conducted and tissue collected December through April with the following exceptions. Three animals trapped in 2002 who hibernated normally, were moved to Ta of 17 °C and 12:12 L:D on February 15, 2003 and returned to an ambient temperature of 2 °C and 4:20 L:D in mid-May of the same year. One of these animals began to hibernate June 30 and tissue was collected while torpid on August 11, 2003. Two of these animals remained euthermic and tissue was collected June 12, 2003.
Hibernation was assessed from core body temperature or by inactivity (indicated by the lack of disturbance of wood shavings placed on an animal's back 24 h previously). Deep hibernation was verified by rectal temperature at the time of euthanasia. All groups of AGS were matched as best as possible with regard to sex and age and consisted of approximately equal numbers of males, females, adults and juveniles. For reference to a familiar species, approximate age matched, male Wistar rats, 13 months old, were purchased from Simonson Labs (Gilroy, CA). Rats were housed in an ambient temperature of 20 °C and 12:12 L:D.
Telemetry transmitters, used to monitor core body temperature, and indwelling femoral arterial and venous cannulae were implanted in AGS treated with ascorbate oxidase (AO) or saline. Prior to surgery, described in detail in a previous study by Ma and coworkers (2004), AGS were placed at 21 °C and left overnight to arouse from hibernation. Animals were allowed one-day post-operative recovery at 18 °C before being returned to the environmental chamber to resume hibernation.
Ascorbate oxidase (96 IU/kg of AO in a volume of 0.6 mL/kg saline and sterilized via 0.2 μm filtration) or sterile saline (0.6 mL/kg of 0.9% NaCl) was administered by i.v. injection immediately before arousal from hibernation during four consecutive bouts of torpor with a mean (±SEM) torpor bout length of 6.1±0.9 days (AO group) and 5.5± 0.5 days (saline group). In each of the four episodes, arterial blood was sampled immediately prior to i.v. injection (T0) and 30 min after injection (T1) (Ma et al., 2004). An additional blood sample was collected after the fourth arousal bout when an animal had reached a body temperature of at least 35 °C [see Fig. 1 from Ma et al. (2004)]. Approximately 150 μL of blood was collected at each sampling point as described previously for ascorbate analysis. AO decreased plasma concentrations of ascorbate to below the limit of detection as described previously (Ma et al., 2004).
Fig. 1.
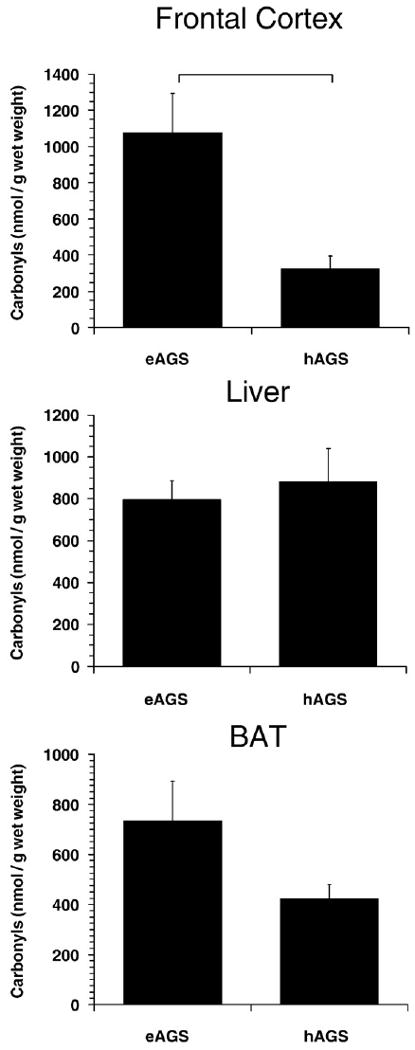
Carbonyl protein levels in frontal cortex, liver and BAT. In frontal cortex, carbonyl protein levels are significantly lower during hibernation. Horizontal bar indicates significant difference between groups (p<0.01, SNK, n=6 hAGS, n=5 eAGS). Rat data is described below, for comparison with a familiar species, but not shown to avoid over-interpretation of cross-species comparisons. Levels of CP in frontal cortex of rat were 797±132 nmol/g wet mass (p<0.05, SNK compared to hAGS, n=4 rats). Levels of CP in BAT from rat were 107±29 nmol/g wet mass (p<0.01, SNK compared to eAGS, n=4 rats).
Tissues were collected immediately after arterial blood was sampled at the end of the fourth arousal bout. Tissue was sampled from four groups of animals: euthermic, hibernating, and aroused-saline and aroused-AO. Before initial sampling in euthermic and aroused AGS, animals were lightly anesthetized with halothane (5% halothane mixed with medical grade O2 delivered at 1.5 L/min); hibernators were not initially anesthetized. Rectal temperatures were measured with a copper–constantan thermocouple. Following decapitation, samples of frontal cortex, brown adipose tissue (BAT) and liver were immediately dissected and frozen in liquid N2 as described by Drew et al. (1999) and Tøien et al. (2001). Frozen tissue samples were stored at −80 °C. Time from decapitation to freezing was less than 10 min. Characteristics of animal groups including days torpid are shown in Table 1.
Table 1.
Animal group characteristics.
| hAGSa | eAGSa | Rats | hAGSb | eAGSb | laAGSbc | |
|---|---|---|---|---|---|---|
| Sex | 4F, 2M | 3F, 3M | 4M | 2F, 3M | 3F, 2M | 2F, 3M |
| Age | 2A, 4J | 1A, 5J | 13 mo | 4A, 1J | 5A | 3A, 2J |
| Mass (g) | 591 | 658 | 601 | 677 | 888 | 639 |
| Initial body temperature (°C) | NA | NA | NA | NA | NA | 2.2 |
| Body temperature at time of tissue collection (°C) | 3.2 | 38 | 38 | 2.8 | 36 | 36 |
| Uninterrupted days in cold room | 234 | 239 | NA | 132 | 132 | 163 |
| Days torpid | 157 | 169d | NA | 93 | NAe | 131 |
| Days euthermic after arousal | NA | 78 | NA | NA | NA | <1 |
| Days torpid when tissue was collected | 6 | NA | NA | 9 | NA | 5 |
| Days torpid prior to last arousal | 9 | NA | NA | 13 | NA | 4 |
hAGS (hibernating AGS), eAGS (euthermic AGS), laAGS (late arousal AGS), Female (F), male (M), adult (A), juvenile (J).
Used to study effect of hibernation.
Used to study effect of arousal following hibernation.
laAGS were treated with saline prior to arousal.
3 of 5 AGS that hibernated. 2 of 5 AGS did not hibernate prior to tissue collection.
Euthanized before onset of hibernation.
The hibernating animals in this study were under torpor between 5 and 13 days. Euthermic AGS were those animals that did not hibernate in the laboratory; they were housed at 2 °C in the same room as the torpid animals until sacrifice and tissue collection.
2.3. Assays for lipid peroxidation and carbonyl protein
Thiobarbituric acid-reactive substances (TBARS) were quantified as an index of lipid peroxidation. Frozen samples were homogenized (1:20 w/v for all different tissues) in ice-cold 1.1% phosphoric acid containing 0.1 mM BHT. The following steps were conducted exactly as described by Ramos-Vasconcelos and Hermes-Lima (2003). Final TBARS values were expressed using the extinction coefficient of 156 mM−1. The spectrophotometric quantification of TBARS cannot be considered a technique to determine malondialdehyde in tissues because the assay overestimates the actual levels of malondialdehyde. However, it is considered effective for comparative studies since several other TBA-reactive aldehydes are also products of lipid peroxidation (Hermes-Lima and Storey, 1995; Hermes-Lima, 2004).
Oxidative damage to proteins was quantified as carbonyl protein using a method employing 2,4-dinitrophenyl-hydrazine. Frozen samples were homogenized (1:20 w/v for all tissues) in ice-cold 5% w/v sulfosalicylic acid and then centrifuged at 15,000 g in an Eppendorf microcentrifuge for 5 min. The following steps were conducted exactly as previously described (Ramos-Vasconcelos and Hermes-Lima, 2003). Final carbonyl protein values were expressed using the extinction coefficient of 22 mM−1.
2.4. Determination of glutathione levels
Glutathione equivalents (GSH-eq=GSH+2 GSSG) were quantified in tissue homogenized in 5% w/v sulfosalicylic acid by following the rate of GR-catalyzed reduction of DTNB by GSH at 412 nm and comparing this rate to a GSH standard curve. GR was included in the reaction mixture in a final concentration of 0.5 U per ml. Complete details of this procedure have been described previously (Ramos-Vasconcelos and Hermes-Lima, 2003).
To quantify only GSSG, Griffith's method (Griffith, 1980) was used with the modifications described by Ramos-Vasconcelos and Hermes-Lima (2003). 2-Vinylpyridine was employed to react with GSH, thus allowing only the measurement of GSSG. Levels of GSH were calculated as: GSH-eq−2×GSSG. Percent oxidized GSH was calculated as: 100×(2×GSSG/GSH-eq).
2.5. Data analysis
When assumptions for ANOVA were met, data were analyzed by one-way ANOVA across groups (hAGS, eAGS and rat) followed by Student–Newman Kuel's post-hoc comparisons. When tests of normality or equal variance failed, data were analyzed by Kruskal– Wallis one-way analysis of variance on ranks followed by Dunn's pairwise multiple comparisons. Level of significance was p<0.05. Data are reported as means±SEM. Results from rat were included for reference to a familiar species. These data were included in the statistical analyses and is reported in figure legends. Rat data were not presented graphically with AGS data to avoid over-interpretation of a species difference that may be related to a number of unrelated factors. AGS data were derived from determinations of animals trapped in 1999 and 2000 (one set of experiments) and those trapped in 2002 (other set of experiments).
3. Results
3.1. No evidence of oxidative stress in hibernation
To investigate if AGS experience oxidative stress during euthermy or prolonged torpor ROS-induced protein oxidation (determined as carbonyl proteins) as well as levels of oxidized GSH were quantified in BAT, liver and frontal cortex from eAGS and hAGS as well as from euthermic rat for reference to a familiar species. In frontal cortex carbonyl protein levels were significantly smaller in hAGS when compared with eAGS or rat (p<0.01; ANOVA, n=5–6) (Fig. 1). Neither liver nor BAT showed a significant difference in carbonyl protein levels between eAGS and hAGS (Fig. 1). There was a trend, however, for higher carbonyl proteins in BAT from eAGS compared to hAGS (p=0.056). Carbonyl protein levels in eAGS was about 7 times greater than in rat (p<0.01), likely due to thermogenic metabolic activity of BAT in eAGS housed at 2 °C.
Although the lower carbonyl proteins in frontal cortex from hAGS suggested a decrease in brain oxidative stress during hibernation, no significant difference between hAGS and eAGS in percent oxidized GSH was observed in frontal cortex (Fig. 2). Likewise, no significant differences were found in GSH equivalents (GSH-eq), GSH or GSSG in frontal cortex (Fig. 2).
Fig. 2.
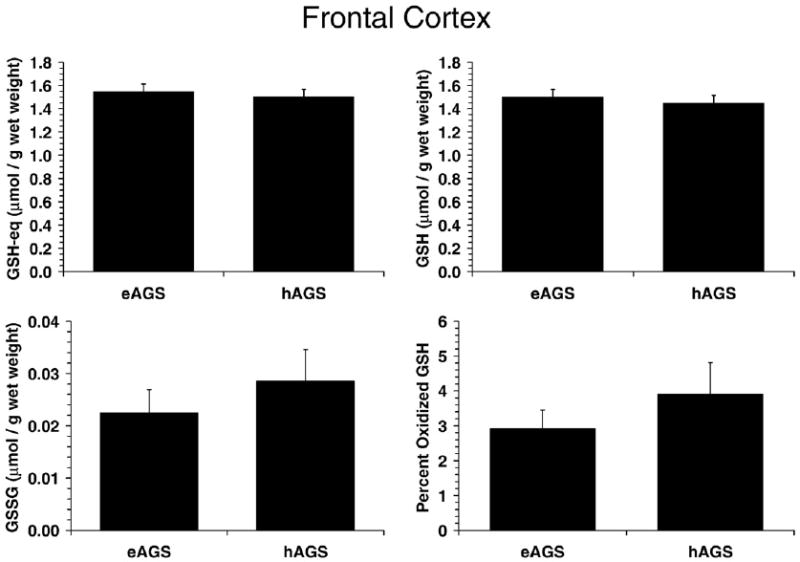
GSH parameters in brain. GSH parameters were not different in brain of hAGS and eAGS (n=6/group) and were comparable to values observed in rat (n=4 rats). Values (mean±SEM) for rat for GSH-eq, GSH, GSSG and percent oxidized GSH were: 1.7±0.14, 1.6±0.15, 0.034±0.011 (μmol/g wet mass) and 4.2±1.3% respectively.
In contrast, percent oxidized GSH was significantly higher in hAGS in liver (p<0.01, Kruskal–Wallis, n=6, Fig. 3) and BAT (p<0.001, ANOVA, n=5–6, Fig. 4) compared to eAGS. Because GSH metabolism is tied to flux of carbon through the pentose phosphate pathway as well as to detoxification of peroxides, GSH-eq, GSH and GSSG were analyzed to assess potential contributions of oxidative stress or changes in carbon flux. In liver, GSH-eq (p<0.005, ANOVA, n=6) and GSH levels (p<0.005, ANOVA, n=6) were lower in hAGS (Fig. 3). Because there was no difference in protein carbonyl content in liver of hAGS higher percent oxidized GSH in hAGS likely reflects a decrease in carbon flux through the pentose phosphate pathway during hibernation (see Discussion section) rather than an increase in ROS production. In BAT, the trends were similar as in liver however only the increased percent oxidized GSH in hAGS was significant (Fig. 4).
Fig. 3.
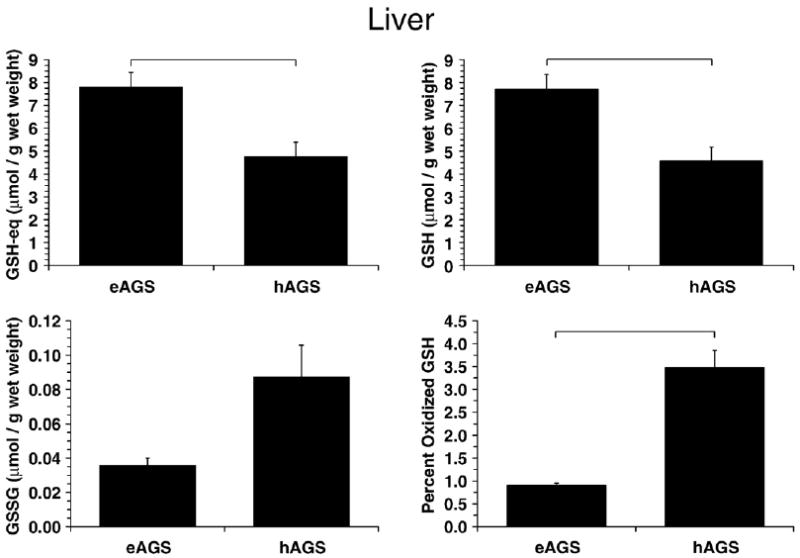
GSH parameters in liver. Percent oxidized GSH was higher in hAGS relative to eAGS (p<0.05, Dunns, n=6) while GSH-eq and GSH was lower in hAGS relative to eAGS (p<0.005, SNK, n=6) as indicated by horizontal bars. For comparison with a familiar species GSH-eq in rat (8.4±0.5 μmol/g wet mass, n=4) was higher than in hAGS (p<0.01, SNK, n=4–6). GSH in rat (8.1±0.5 μmol/g wet mass, n=4) was similar to eAGS but higher than in hAGS (p<0.01, SNK, n=4–6). GSSG was higher in eAGS than in rat (0.13±0.01 μmol/g wet mass, p<0.05, Dunns, n=4–6).
Fig. 4.
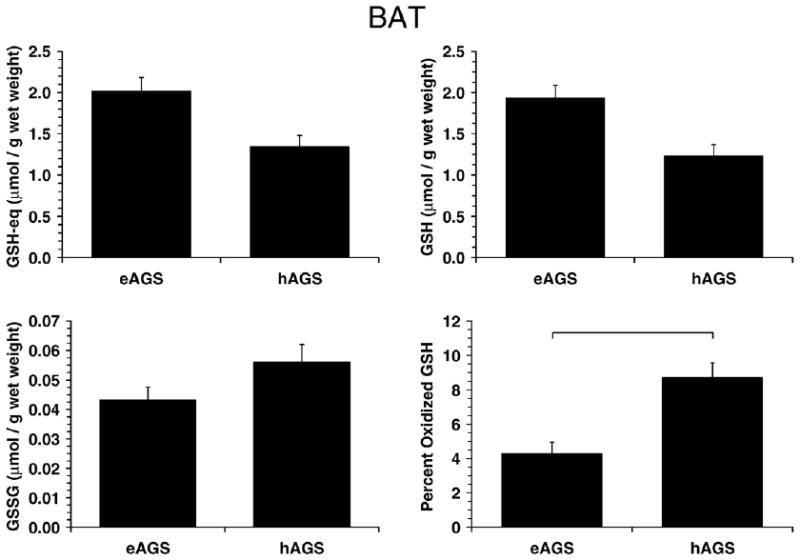
GSH parameters in BAT. Percent oxidized GSH in BAT was higher in hAGS relative to eAGS (p<0.001, SNK, n=6) as indicated by horizontal bar. For reference to a familiar species, GSH-eq was 3 fold higher in eAGS than in rat (0.71±0.2 μmol/g wet mass, n=4, p<0.01, Dunns); GSH was higher in eAGS than in rat (0.70±0.2 μmol/g wet mass, n=4, p<0.05, Dunns); and, GSSG was 10 fold higher in hAGS and eAGS than in rat (0.003±0.002 μmol/g wet mass, n=4) although the difference between rat and AGS was statistically significant only with comparison to eAGS (p<0.05, Dunns).
3.2. Oxidative stress in during late arousal
In a subsequent experiment, we investigated if arousal from hibernation produced oxidative stress in BAT, a tissue with high oxidative metabolism during arousal. Liver was selected as a less metabolically challenged tissue for comparison In addition, another marker of oxidative stress, TBARS (as an indicator of lipid peroxidation (Hermes-Lima, 2004)) was included to supplement interpretation of changes in carbonyl proteins. Moreover, we investigated the influence of tissue levels of ascorbate on the overall oxidative stress of BAT and liver by treating animals with ascorbate oxidase (AO) prior to arousal. Effect of AO on tissue levels of ascorbate in these animals has been reported previously. In liver, AO attenuates an increase in liver ascorbate content after arousal so that levels are similar to euthermic levels (Ma et al., 2004). It was predicted that if higher ascorbate levels in control animals (saline injected AGS) protect against oxidative stress, AO treatment would increase oxidative stress in liver after arousal.
Because tissue was collected from a separate set of eAGS and hAGS for this study it was first of interest to assess if differences between eAGS and hAGS observed earlier were reproducible in this independent set of tissues. Carbonyl protein levels in this second set of tissues (shown in Figs. 5 and 6) were qualitatively and quantitatively similar to levels in liver and BAT obtained in the earlier set of tissues (see Fig. 1). However, the previous trend for lower carbonyl protein levels in BAT during hibernation (Fig. 1) reached statistical significance in this second set of samples (Fig. 5). Moreover, treatment with AO did not have a significant effect on any parameter measured in liver or BAT when compared to saline treated AGS. Thus, the AO treated animals were excluded from subsequent comparisons between aroused AGS treated with saline, hAGS and eAGS. Levels of carbonyl proteins in BAT were significantly greater by late arousal when compared to hAGS (p<0.025, ANOVA, n=5) (Fig. 5). Similarly, levels of TBARS in BAT were larger in late arousal (p<0.025, Kruskal–Wallis, n=4–5), and the correlation between TBARS and carbonyl protein values in BAT was significant (r=0.62; p<0.025).
Fig. 5.
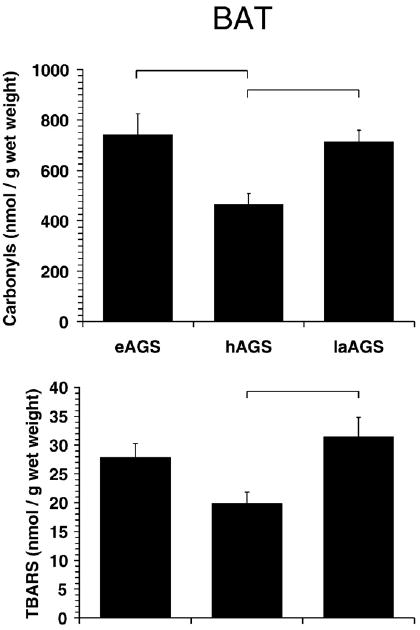
Oxidative stress is highest in BAT after arousal and during euthermy. Levels of carbonyl proteins were less in hAGS compared to laAGS (p<0.02, SNK, n=5) and compared to eAGS (p<0.02, SNK, n=5). Levels of TBARS were also less in hAGS compared to laAGS (p<0.05, Dunn's method, n=4–5) as indicated by horizontal bars. Data from animals treated with AO prior to arousal are described below, but not shown because AO did not affect any of the parameters measured. Level of protein carbonyls in BAT from animals treated with AO prior to arousal was 608±52 nmol/g wet mass and was not significantly different from late arousal (laAGS) animals treated with saline prior to arousal (p>0.30, t-test, n=5). Similarly, level of TBARS in animals treated with AO prior to arousal was 37±7.1 nmol/g wet mass and was not significantly different from laAGS treated with saline prior to arousal (p>0.5, t-test, n=5).
Fig. 6.
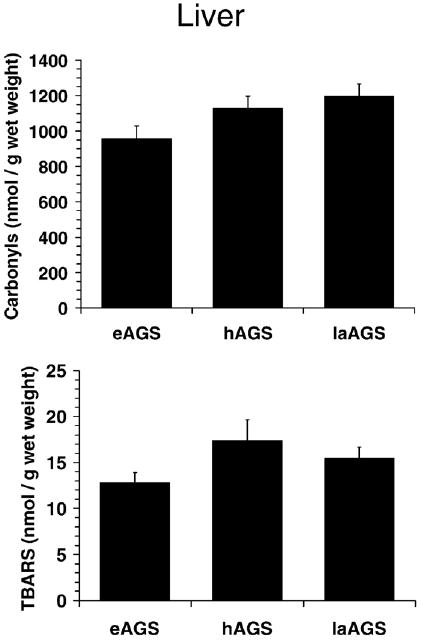
No evidence of oxidative stress was observed in liver after arousal. Level of protein carbonyls in liver from animals treated with AO prior to arousal was 1060± 73 nmol/g wet mass and was not significantly different from late arousal (laAGS) animals treated with saline prior to arousal (p>0.20, t-test, n=5). Similarly, levels of TBARS in animals treated with AO prior to arousal was 14.4±2.1 nmol/g wet mass and was not significantly different from laAGS (p>0.68, t-test, n=5).
Because tissue from these same animals was analyzed for uric acid and ascorbate previously and reported by Ma et al. (2004) association between these water soluble antioxidants and markers of oxidative stress was assessed using regression analysis. A positive correlation was found between levels of TBARS versus those of uric acid (r=0.75, p<0.005) or ascorbate (r=0.64, p<0.025), and between carbonyl protein versus ascorbate (r=0.72, p<0.005), but not versus uric acid (r=0.42, N.S.) in BAT of hAGS, eAGS and saAGS. Positive correlations show that higher levels of oxidative stress markers are associated with higher concentrations of ascorbate and uric acid in BAT. In addition, no correlation was found between levels of GSH (data from Ma et al. (2004) versus TBARS (r=0.05, N.S.) or carbonyl protein (r=0.29, N.S.) in BAT of hAGS, eAGS and saAGS.
In liver, a highly glycolytic tissue, no evidence of oxidative modification was found during late arousal (Fig. 6). It was also of interest to examine alterations in glutathione metabolism during late arousal. Again, because tissue was collected from a separate set of eAGS and hAGS for this study it was first of interest to assess if differences between eAGS and hAGS observed earlier were reproducible. Parameters of GSH in eAGS and hAGS were similar to results observed in the previous set of livers (Fig. 3) except; in this case, the increase in GSSG during hibernation reached statistical significance (Figs. 7 and 3). By late arousal, all parameters of GSH metabolism were comparable to euthermic levels. Percent oxidized GSH was lower in late arousal when compared to hAGS (p<0.001, ANOVA, n=5, Fig. 7), but was not different from eAGS. Moreover, hepatic GSSG levels in late arousal were significantly lower than during hibernation (p<0.02, ANOVA, n=5), but were not different from levels observed during euthermy (Fig. 7).
Fig. 7.
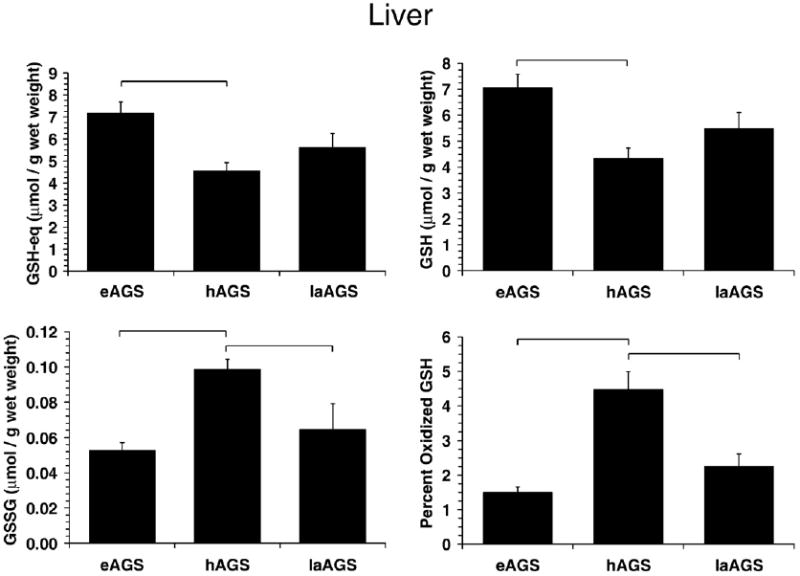
Alterations in GSH metabolism in liver during arousal results in an increase in percent oxidized GSH in hAGS relative to eAGS (p<0.001, SNK, n=5). A decrease in GSSG was also observed following arousal where GSSG was greater in hAGS than in laAGS (p<0.05, SNK, n=5). Horizontal bars indicates significant difference (p<0.05) between groups. There was no significant difference between animals treated with ascorbate oxidase or saline (laAGS) prior to arousal in any of the parameters measured (p>0.60, n=5). Values for AO treated animals are as follows: GSH-eq 5.50±0.49 μmol/g wet mass; GSH 5.37±0.49 μmol/g wet mass; GSSG 0.0674±0.011 μmol/g wet mass; percent oxidized GSH was 2.48±0.40.
4. Discussion
Several studies have indicated seasonal and state-dependent changes in antioxidant defenses in ground squirrels (Buzadzic et al., 1990; Buzadzic et al., 1992; Buzadzic et al., 1997; Blagojevic et al., 1998). However, only a few studies have monitored oxidative stress during hibernation or following arousal and those that have either focused on a different tissue (Carey et al., 2003b; Ma et al., 2005) or different animal (Osborne and Hashimoto, 2006), or used qualitative, immunohistochemical methods to assess relative differences in oxidatively modified biomolecules that may not show subtle differences evident from more quantitative analyses (Ma et al., 2005). Here we assessed oxidative stress in brown adipose tissue (BAT), liver and brain from winter-euthermic, winter-hibernating and 3-h aroused S. parryii, and observed statistically significant changes in lipid peroxidation (as TBARS) and carbonyl protein levels in BAT from torpid hibernators. No evidence of increased oxidative stress (as TBARS or carbonyl protein) was observed in any other tissue studied during hibernation or after arousal. These data suggest that brain, BAT and liver avoid oxidative stress during hibernation and that ROS generation in BAT during arousal causes oxidative stress of a physiological nature.
4.1. Oxidative stress in hibernation versus euthermy
Decreased carbonyl protein in frontal cortex of hAGS suggests that oxidative stress in brain is diminished in torpid squirrels than during euthermy, consistent with previous findings of decreased cellular stress in hibernating brain (Ma et al., 2005). These results extend previous observations using immunohistochemical analysis of oxidative stress markers in brain (Ma et al., 2005). As expected, quantitative analysis of carbonyl proteins detected a small but significant difference between eAGS and hAGS brain that was not evident from prior immunohistochemical analysis. In contrast to other tissues where urate decreases during hibernation, brain tissue concentrations of urate increase during hibernation in AGS (Toien et al., 2001; Ma et al., 2004). Such an increase could result from augmented oxidative stress or alterations in nucleotide metabolism (Ma et al., 2004). The present results argue for the latter since carbonyl protein, a more direct marker of oxidative stress (Hermes-Lima, 2004), decreases in frontal cortex during hibernation. Interestingly, a recent report in hamsters (Mesocricetus auratus) shows that brain tissue concentrations of urate decrease during torpor in this species (Osborne and Hashimoto, 2006), similar to the pattern seen in peripheral tissues in AGS.
Whether oxidative stress in AGS brain decreases during hibernation along with a fall in oxidative metabolism, or oxidative stress increases in euthermic AGS brain along with other aspects of cellular stress noted in brains of euthermic AGS (Ma et al., 2005; Zhu et al., 2005) is at yet unclear. Comparing the two groups, however, illustrates that oxidative stress is incresed in brain during euthermy. Moreover, Lee et al. (2002) observed increased levels of stress signaling proteins (TNF-alpha, JNK and Akt) in bat (Rhinolopus ferrumequinum) brain during early arousal from hibernation, which is accompanied by an increase in oxygen consumption from nearly zero to 11.9/kg/h within 30 min. JNK and ERK increase in brain of euthermic and aroused AGS when compared with hibernating animals (Zhu et al., 2005). Also, hypoxia-inducible factor 1α (HIF-1α) was found to be increased during arousal in the forebrain of AGS, indicative of insufficient tissue oxygenation (Ma et al., 2005). Thus, rewarming in AGS brain (but not hibernation per se) may be associated with oxidative stress and deserves further investigation.
While brain ascorbate and GSH remain stable throughout euthermy, hibernation and arousal, levels of ascorbate and GSH in liver and BAT decrease in torpid hibernators (Toien et al., 2001; Ma et al., 2004). The present results suggest that this decrease in low molecular weight, water-soluble antioxidants in liver and BAT, is not associated with oxidative stress. Neither liver nor BAT showed an increase in carbonyl proteins or TBARS in torpid squirrels. Indeed, these measures of oxidative stress tended to decrease in BAT of torpid animals. It is likely that the increase in percent oxidized GSH in liver and BAT during torpor (see Figs. 3 and 4) reflects decreased carbon flux through glycolysis and the pentose phosphate pathway. Decreased carbon flux through glucose utilization in torpid hibernators (Frerichs et al., 1995) would be expected to lead to a decrease in NADPH contents and subsequent decrease in glutathione reductase (GR) activity (such a decrease in activity would be due to a reduction in carbon flux through the pathway and not necessarily in GR concentration). Decreased GR activity would lead to an augment in GSSG levels and an increase in percent oxidized GSH that is unrelated to overall ROS production. Interestingly, Carey et al. (2003a) observed decreased GR activity in the gut of hibernating 13-lined ground squirrels. Decreased biosynthesis and/or increased export of liver GSH (for amino-acid transport) could also account for the decrease in hepatic GSH-eq in torpid hibernators. Biosynthesis of GSH is an energy consuming process (Hermes-Lima, 2004) and may be diminished during torpor in several organs.
The observed changes in nonenzymatic antioxidants would leave BAT and liver of hibernating AGS at risk for oxidative stress unless other antioxidant systems are enhanced to compensate for this decrease. While studies in other species suggest that activation of enzymatic antioxidants may be the case (Buzadzic et al., 1990) dynamic changes in antioxidants associated with hibernation vary with the animal species and tissue studied (Blagojevic et al., 1998; Drew et al., 1999; Carey et al., 2000; Osborne and Hashimoto, 2006). In line with these ideas, increased transcript levels of antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GPX) and glutathiose S-transferase were found in kidney of hibernating 13-lined ground squirrels (Eddy and Storey, 2002). Increased levels of peroxiredoxins – isoforms 1, 2 and 3 – where also found in BAT and heart of 13-lined ground squirrels under hibernation (Morin and Storey, 2007). Moreover, thioredoxin peroxidase is up-regulated in the heart of bat Myotis lucifugus during hibernation (Eddy et al., 2005), as well as GPX 1, glutathiose S-transferase A2 and peroxiredoxin 1 in skeletal muscle of hibernating bats (Tb=6 °C) in comparison with aroused animals (Tb=37 °C) (Storey, 2006). In addition, an increase in polyunsaturated fatty acid (PUFA) content in early torpor in several hibernating mammals presents a potentially pro-oxidant environment that likely requires enhanced antioxidant defenses to avoid oxidative stress (Munro and Thomas, 2004).
A very recent study by Page et al. (2009) indicated that hibernation in 13-lined squirrels prompted no changes in levels and activities of MnSOD and CuZnSOD in liver, brain and heart during hibernation. Even though an increase in hepatic GPX was observed in hibernating animals – suggesting some kind of response for oxidative stress in hibernation – no clear-cut trends were observed for catalase and GR activities in the 3 organs studied.
4.2. Oxidative stress after arousal
After the demanding, thermogenic process of arousal in which nonshivering thermogenesis and oxidative metabolism in BAT plays a critical role (Cannon and Nedergaard, 2004) oxidative stress in AGS BAT increases as evidenced by higher carbonyl proteins and lipid peroxidation. It is surprising that arousal does not produce greater oxidative stress in BAT than euthermy given that during arousal animals rewarm from near 0 °C to 37 °C while euthermic AGS maintain 37 °C; both at an ambient temperature of 2 °C. Similar levels of oxidative stress in BAT during euthermy and after arousal may be due to the accumulation of lipid peroxidation products (determined herein as TBARS) and carbonyl proteins over time in euthermic AGS in contrast to a more rapid, acute production of carbonyl proteins and TBARS during arousal. Alternatively, an increase in UPC-2 expression in white adipose tissue and UCP-3 in muscle of hibernating AGS (Boyer et al., 1998), two uncoupling proteins thought to attenuate mitochondrial ROS production (Brand and Esteves, 2005), may mitigate any rise in mitochondrial ROS generation during arousal. In addition, the observation of increased levels of peroxiredoxins in BAT of hibernating 13-lined ground squirrels (Morin and Storey, 2007) suggest that these enzymes could also play a role in the management of oxidative damage in AGS BAT during transition from hibernation to arousal states.
Current estimates indicate that 0.1% of oxygen consumed by mammalian mitochondria leads to ROS formation (Fridovich, 2004). A four-fold increase in oxygen consumption during rewarming in AGS (from ∼0.5 mL O2/g/h in hibernation to ∼2.0 mL O2/g/h (Ma et al., 2005) would therefore supply a reasonable rate of ROS formation to prompt oxidative stress – as observed in the case of free radical protein damage and lipid peroxidation in BAT (during AGS arousal).
Studies in anoxic tolerant turtles showed a dramatic decrease in cerebral in vivo ROS formation (quantified as salicylate hydroxylation – a marker for hydroxyl radical formation) from 4 h anoxia-exposed animals. Levels of ROS were back to control levels after 2 h of reoxygenation (Milton et al., 2007). Moreover ROS formation in isolated turtle brain sheets – determined by the DCF fluorescence technique – was also decreased during anoxia exposure, returning to control levels upon reoxygenation (Pamenter et al., 2007). These authors showed that brain ROS formation was of mitochondrial origin. The suppression of metabolic rate of AGS under hibernation – similar to what happens under anoxia exposure in turtles – might be a factor to promote a drastic reduction in mitochondrial ROS formation, even though there is plenty of oxygen in AGS tissues during hibernation. During rewarming, the reactivation of metabolic function and mitochondrial activity in AGS tissues could prompt a resurgence of ROS formation. This line of thought is against the idea that hibernation per se causes oxidative stress, as suggested by Carey and co-workers (2000, 2003b).
4.3. Changes in liver GSH metabolism during AGS arousal
A decrease in hepatic GSSG levels and in the percent oxidized GSH in late arousal (see Fig. 3) could be due to increased carbon flux through the pentose phosphate pathway and subsequent increase in production of NADPH and GR activity. Alternatively, these observations could result from redistribution of ascorbate to metabolically active tissues during arousal and subsequent increase in nonenzymatic GSH recycling. Ascorbate increases in liver after arousal and would therefore be available to maintain GSH in its reduced state (Toien et al., 2001; Ma et al., 2004). Pretreatment with AO attenuated the rebound in liver ascorbate levels after arousal (Ma et al., 2004), however did not increase the levels of GSSG or percent oxidized GSH arguing against a limiting role for ascorbate in nonenzymatic recycling of GSH during arousal.
As pointed out above (Section 4.1), levels of GSH, GSSG and GSH-eq in frontal cortex were unaffected in euthermic and torpid AGS, as well as during arousal (see Fig. 2), even though cerebral protein carbonyls were decreased in hibernation (see Fig. 1). This suggests that rates of GSH oxidation to GSSG in frontal cortex were counteracted by GR-catalyzed conversion of GSSG into GSH. Whether AGS frontal cortex GR activity is altered during hibernation or arousal, as compared to euthermia, needs further investigation.
4.4. Seasonal and inter-species comparisons
A caveat of this work is that euthermic ground squirrels used to compare with hibernators and arousing AGS were winter-euthermic animals. These were animals that did not enter hibernation at 2 °C in the laboratory during winter — the reason is yet to be determined. Therefore, several authors use these animals to compare physiological and biochemical parameters with torpid animals. It is possible that winter-euthermic AGS are under higher allostatic load (for the concept of allostasis see: McEwen and Wingfield, 2003; Landys et al., 2006) than summer-euthermics. We do not believe that this is the case since winter euthermic AGS show less activation of p38, a stress-activated protein kinase, than summer AGS (Zhu et al., 2005). Moreover it is problematic to compare summer and winter animals because of confounding effects of seasonality. It has been shown for several animals, ranging from invertebrates to mammals, that seasonality is a factor altering free radical metabolism (see: Blagojevic et al., 1998; Ramos-Vasconcelos et al., 2005; Furtado-Filho et al., 2007; Malanga et al., 2007). Thus, it is imperative to conduct experiments comparing hibernating with euthermic and arousing animals in the same season. In any case, comparisons between hibernators and arousing AGS have shown differences in the BAT levels of TBARS and carbonyl protein (see Fig. 5), which were not observed in liver (see Fig. 6).
In addition comparisons between carbonyl levels in rats and ground squirrels (considering eAGS) showed interesting results. Ground squirrels presented much higher BAT carbonyls (0.7 μmol/g wet wt. for eAGS) than rats (0.1 μmol/g wet wt.; see legend to Fig. 1). Moreover, GSH-eq concentration in rat BAT (0.7 μmol/g wet wt.) was considerably less than in BAT eAGS (2 μmol/g wet wt.). On the other hand, GSH-eq and carbonyls showed no relevant inter-species differences (rat versus eAGS) in liver and frontal cortex. The proposed high intensity of oxidative stress in AGS BAT – especially during thermogenesis associated with rewarming – would therefore require a compensatory level of antioxidant protection. GSH-eq seems to be an example for such protection in BAT.
In summary, we may conclude that there is enough evidence to indicate that a physiological oxidative stress is taking place in thermogenic BAT – but not in liver – of arousing AGS. In brain, oxidative stress seems to be decreased during AGS hibernation. Confirmatory information in brain will be possible after comparison of oxidative stress markers of hibernators versus arousing animals.
Acknowledgments
This project was supported by NIH-NS41069 (funded in part by NINDS, NIMH, NCRR and NCMHD) and US Army Medical Research and Materiel Command grant # 05178001 USAMRMC # 05178001 to KLD. M.H.-L. was supported by FINATEC, PRONEX, CNPq, Instituto do Milênio-CNPq (Redoxoma, Brazil) and by funds from Institute of Arctic Biology, University of Alaska Fairbanks. The authors acknowledge contributions by Dr. John Keller, Patricia Rivera, Kim Cozad, Christine Terzi and Cecília Carreiro. This paper is dedicated to former school teacher Eneida S. Hermes on the occasion of her 90th birthday, and to the memory of James Drew (1930-2008), airplane pilot and science lover.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit:
References
- Bickler PE, Buck LT. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu Rev Physiol. 2007;69:145–170. doi: 10.1146/annurev.physiol.69.031905.162529. [DOI] [PubMed] [Google Scholar]
- Blagojevic D, Buzadzic B, Korac B, Saicic ZS, Radojicic R, Spasic MB, Petrovic VM. Seasonal changes in the antioxidative defense in ground squirrels (Citellus citellus): possible role of GSH-Px. J Environ Pathol Toxicol Oncol. 1998;17:241–250. [PubMed] [Google Scholar]
- Boyer BB, Barnes BM, Lowell BB, Grujic D. Differential regulation of uncoupling protein gene homologues in multiple tissues of hibernating ground squirrels. Am J Physiol. 1998;275:R1232–1238. doi: 10.1152/ajpregu.1998.275.4.R1232. [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Buzadzic B, Spasic M, Saicic ZS, Radojicic R, Petrovic VM, Halliwell B. Antioxidant defenses in the ground squirrel Citellus citellus. 2. The effect of hibernation. Free Radic Biol Med. 1990;9:407–413. doi: 10.1016/0891-5849(90)90017-d. [DOI] [PubMed] [Google Scholar]
- Buzadzic B, Spasic MB, Saicic ZS, Radojicic R, Petrovic VM. Seasonal dependence of the activity of antioxidant defence enzymes in the ground squirrel (Citellus citellus): the effect of cold. Comp Biochem Physiol B. 1992;101:547–551. doi: 10.1016/0305-0491(92)90336-p. [DOI] [PubMed] [Google Scholar]
- Buzadzic B, Blagojevic D, Korac B, Saicic ZS, Spasic MB, Petrovic VM. Seasonal variation in the antioxidant defense system of the brain of the ground squirrel (Citellus citellus) and response to low temperature compared with rat. Comp Biochem Physiol C. 1997;117:141–149. doi: 10.1016/s0742-8413(97)00061-3. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Revs. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Carey HV, Frank CL, Seifert JP. Hibernation induces oxidative stress and activation of NK-kappaB in ground squirrel intestine. J Comp Physiol [B] 2000;170:551–559. doi: 10.1007/s003600000135. [DOI] [PubMed] [Google Scholar]
- Carey HV, Rhoads CA, Aw TY. Hibernation induces glutathione redox imbalance in ground squirrel intestine. J Comp Physiol [B] 2003a;173:269–276. doi: 10.1007/s00360-003-0330-3. [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003b;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Drew KL, Osborne PG, Frerichs KU, Hu Y, Koren RE, Hallenbeck JM, Rice ME. Ascorbate and glutathione regulation in hibernating ground squirrels. Brain Res. 1999;851:1–8. doi: 10.1016/s0006-8993(99)01969-1. [DOI] [PubMed] [Google Scholar]
- Drew KL, Toien O, Rivera PM, Smith MA, Perry G, Rice ME. Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp Biochem Physiol C. 2002;133:483–492. doi: 10.1016/s1532-0456(02)00118-7. [DOI] [PubMed] [Google Scholar]
- Eddy SF, Storey KB. Dynamic use of cDNA arrays: heterologous probing for gene discovery and exploration of animal adaptations in stressful environments. In: Storey KB, Storey JM, editors. Cell and Molecular Responses to Stress. Vol. 3. Elsevier Press; Amsterdam: 2002. pp. 315–325. [Google Scholar]
- Eddy SF, McNally JD, Storey KB. Up-regulation of a thioredoxin peroxidase-like protein, proliferation-associated gene, in hibernating bats. Arch Biochem Biophys. 2005;435:103–111. doi: 10.1016/j.abb.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Dienel GA, Cruz NF, Sokoloff L, Hallenbeck JM. Rates of glucose utilization in brain of active and hibernating ground squirrels. Am J Physiol. 1995;268:R445–453. doi: 10.1152/ajpregu.1995.268.2.R445. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell. 2004;3:13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- Furtado-Filho OV, Polcheira C, Machado DP, Mourão G, Hermes-Lima M. Selected oxidative stress markers in a South American crocodilian species. Comp Biochem Physiol C. 2007;146:241–254. doi: 10.1016/j.cbpc.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M. Oxygen in biology and biochemistry: role of free radicals. In: Storey KB, editor. Functional Metabolism: Regulation and Adaptation. John Wiley & Sons; New Jersey: 2004. pp. 319–368. [Google Scholar]
- Hermes-Lima M, Storey KB. Antioxidant defenses and metabolic depression in a pulmonate land snail. Am J Physiol. 1995;268:R1386–1393. doi: 10.1152/ajpregu.1995.268.6.R1386. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M, Zenteno-Savin T. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp Biochem Physiol C. 2002;133:537–556. doi: 10.1016/s1532-0456(02)00080-7. [DOI] [PubMed] [Google Scholar]
- Landys MM, Ramenofsky M, Wingfield JC. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol. 2006;148:132–149. doi: 10.1016/j.ygcen.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Lee M, Choi I, Park K. Activation of stress signaling molecules in bat brain during arousal from hibernation. J Neurochem. 2002;82:867–873. doi: 10.1046/j.1471-4159.2002.01022.x. [DOI] [PubMed] [Google Scholar]
- Lushchak VI, Lushchak LP, Mota AA, Hermes-Lima M. Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am J Physiol. 2001;280:R100–107. doi: 10.1152/ajpregu.2001.280.1.R100. [DOI] [PubMed] [Google Scholar]
- Lushchak VI, Bagnyukova TV, Lushchak OV, Storey JM, Storey KB. Hypoxia and recovery perturb free radical processes and antioxidant potential in common carp (Cyprinus carpio) tissues. Int J Biochem Cell Biol. 2005;37:1319–1330. doi: 10.1016/j.biocel.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Ma YL, Rice ME, Chao ML, Rivera PM, Zhao HW, Ross AP, Zhu X, Smith MA, Drew KL. Ascorbate distribution during hibernation is independent of ascorbate redox state. Free Radic Biol Med. 2004;37:511–520. doi: 10.1016/j.freeradbiomed.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Ma YL, Zhu X, Rivera PM, Toien O, Barnes BM, LaManna JC, Smith MA, Drew KL. Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul. 2005;289:R1297–1306. doi: 10.1152/ajpregu.00260.2005. [DOI] [PubMed] [Google Scholar]
- Malanga G, Estevez MS, Calvo J, Abele D, Puntarulo S. The effect of seasonality on oxidative metabolism in Nacella (Patinigera) magellanica. Comp Biochem Physiol A. 2007;146:551–558. doi: 10.1016/j.cbpa.2006.01.029. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Milton SL, Nayak G, Kesaraju S, Kara L, Prentice HM. Suppression of reactive oxygen species production enhances neuronal survival in vitro and in vivo in the anoxia-tolerant turtle Trachemys scripta. J Neurochem. 2007;101:993–1001. doi: 10.1111/j.1471-4159.2007.04466.x. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Storey KB. Antioxidant defense in hibernation: cloning and expression of peroxiredoxins from hibernating ground squirrels, Spermophilus tridecemlineatus. Arch Biochem Biophys. 2007;461:59–65. doi: 10.1016/j.abb.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Munro D, Thomas DW. The role of polyunsaturated fatty acids in the expression of torpor by mammals: a review. Zoology (Jena) 2004;107:29–48. doi: 10.1016/j.zool.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Nizielski SE, Billington CJ, Levine AS. Cold-induced alterations in uncoupling protein and its mRNA are seasonally dependent in ground squirrels. Am J Physiol. 1995;269:R357–364. doi: 10.1152/ajpregu.1995.269.2.R357. [DOI] [PubMed] [Google Scholar]
- Osborne PG, Hashimoto M. Brain antioxidant levels in hamsters during hibernation, arousal and cenothermia. Behav Brain Res. 2006;168:208–214. doi: 10.1016/j.bbr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Page MM, Peters CW, Staples JF, Stuart JA. Intracellular antioxidant enzymes are not globally upregulated during hibernation in the major oxidative tissues of the 13-lined ground squirrel Spermophilus tridecemlineatus. Comp Biochem Physiol A. 2009;152:115–122. doi: 10.1016/j.cbpa.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Pamenter ME, Richards MD, Buck LT. Anoxia-induced changes in reactive oxygen species and cyclic nucleotides in the painted turtle. J Comp Physiol B. 2007;177:473–481. doi: 10.1007/s00360-007-0145-8. [DOI] [PubMed] [Google Scholar]
- Ramos-Vasconcelos GR, Hermes-Lima M. Hypometabolism, antioxidant defenses and free radical metabolism in the pulmonate land snail Helix aspersa. J Exp Biol. 2003;206:675–685. doi: 10.1242/jeb.00124. [DOI] [PubMed] [Google Scholar]
- Ramos-Vasconcelos GR, Cardoso LA, Hermes-Lima M. Seasonal modulation of free radical metabolism in estivating land snails Helix aspersa. Comp Biochem Physiol C. 2005;140:165–174. doi: 10.1016/j.cca.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Storey KB. Gene hunting in hypoxia and exercise. In: Roach RC, Hackett PH, Wagner PD, editors. Hypoxia and Exercise Adv Exp Med Biol. Vol. 588. 2006. pp. 293–309. [DOI] [PubMed] [Google Scholar]
- Toien O, Drew KL, Chao ML, Rice ME. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am J Physiol. 2001;281:R572–583. doi: 10.1152/ajpregu.2001.281.2.R572. [DOI] [PubMed] [Google Scholar]
- Zhu X, Smith MA, Perry G, Wang Y, Ross AP, Zhao HW, Lamanna JC, Drew KL. MAPKs are differentially modulated in arctic ground squirrels during hibernation. J Neurosci Res. 2005;80:862–868. doi: 10.1002/jnr.20526. [DOI] [PubMed] [Google Scholar]


