Abstract
Background/Aims
Organophosphate poisoning has a high mortality rate. Recently, differences among organophosphorus insecticides in human self-poisoning were reported. This study investigated the prognostic risk factors and the mortality of different organophosphates following acute organophosphate poisoning.
Methods
This retrospective study included 68 patients with acute organophosphate poisoning. We investigated patient survival according to initial parameters, including the initial Acute Physiology and Chronic Health Evaluation (APACHE) II score, serum cholinesterase level, and hemoperfusion and evaluated the mortality according to organophosphate types.
Results
Thirteen of the 68 patients died. The agents responsible for mortality were different. The APACHE II score was a significant predictor of mortality (odds ratio [OR], 1.194; p<0.01; 95% confidence interval [CI], 1.089 to 1.309) and respiratory failure (OR, 1.273; p<0.01; 95% CI, 1.122 to 1.444). The mortality was 0% for dichlorvos, malathion, chlorpyrifos and profenofos. However, other organophosphates showed different mortality (16.7% for O-ethyl-O-4-nitrophenyl phenylphosphonothioate, 25% for phenthoate, 37.5% for phosphamidon, 50% for methidathion). The usefulness of hemoperfusion appears to be limited.
Conclusions
The initial APACHE II score is a useful prognostic indicator, and different organophosphates have different mortality.
Keywords: Organophosphate, Survival, APACHE
INTRODUCTION
Organophosphates (OP) inhibit acetylcholinesterase and cause excessive acetylcholine accumulation, which affects muscarinic and nicotinic receptors at synapses within the peripheral and central nervous systems [1]. OP poisoning causes neurotoxic sequela and has a high mortality rate [2-4].
The World Health Organization (WHO) estimates that the incidence of pesticide poisoning in developing countries has doubled during the past 10 years [5]. In Korea, the mortality due to poisoning has increased rapidly since 1998 [6]. Herbicides and pesticides constituted the largest proportion of deaths due to poisoning of all causes, although OP were not evaluated specifically [6].
Combined atropine and pralidoxime therapy is the cornerstone of OP poisoning treatment. Extracorporeal elimination may be a useful adjunctive strategy. However, the use of hemoperfusion in the management of severe OP poisoning remains controversial [7-10].
Recently, a difference among OP insecticides in human self-poisoning was reported. Moreover, the toxicity differed in humans and animals. These finding suggest that each OP has a different toxicity, although textbooks and articles have traditionally considered acute OP poisoning as a homogenous entity.
This study determined the predictors of a poor outcome in patients with acute OP poisoning and differences of toxicity according to OP type.
METHODS
Study population
This retrospective study examined the comprehensive medical, nursing, and intensive care records of 68 patients admitted to the Institute of Pesticide Poisoning at Soonchunhyang University Cheonan Hospital, Cheonan, Korea, for acute OP poisoning between January 2000 and December 2006. The Investigational Review Board at Soonchunhyang Cheonan Hospital approved this study.
Initial diagnoses were established in all cases based on cholinergic clinical features, the odor of OP in the gastric contents, history, and other circumstantial evidence, such as the poison or a label of an OP-containing product found by relatives. All patients were given the classic treatment for OP poisoning: gastric lavage, whole body surface washing, activated charcoal administration (1 g/kg by nasogastric tube), intravenous atropine and pralidoxime, and supportive measures such as mechanical ventilation (if necessary). Hemoperfusion (Absorba 300; Gambro, Hechingen, Germany) was performed for 3.5 hours when patients developed respiratory failure or mental changes. Complete blood cell counts, measures of liver and renal function, and arterial blood gases were obtained. The Acute Physiology and Chronic Health Evaluation (APACHE) II scores of all patients were determined. The 68 subjects were allocated to two groups: a respiratory failure group (the RF group) requiring mechanical ventilation, and a non-respiratory failure group (the non-RF group).
Statistical analysis
Data are presented as the mean±SD. Probability values of p<0.05 were considered significant, and all statistical analyses were performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were analyzed using the Student's t-test, and categorical variables were analyzed using the chi-square test. Binary logistic regression or multiple logistic regression analysis was used to identify factors associated with a poor outcome.
RESULTS
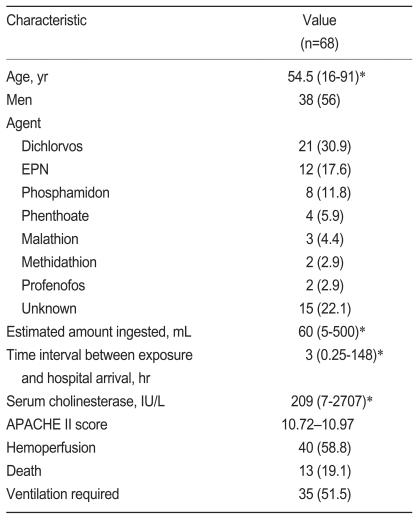
The general characteristics of the 68 study subjects, who all had acute OP poisoning at presentation, are summarized in Table 1. The mean amount of ingested OP was 60 mL (range, 5 to 500) and the agents responsible for poisoning were dichlorvos (n=21), O-ethyl-O-4-nitrophenyl phenylphosphonothioate (EPN, n=12), phosphamidon (n=8), phenthoate (n=4), malathion (n=3), methidathion (n=2), profenofos (n=2), chlorpyrifos (n=1), and an unidentified OP (n=15).
Table 1.
Characteristics of patients with acute organophosphate poisoning
Value are number (%) except where indicated otherwise.
EPN,O-ethyl-O-4-nitrophenyl phenylphosphonothioate; APACHE, Acute Physiology and Chronic Health Evaluation.
*Median (range).
†Mean±SD.
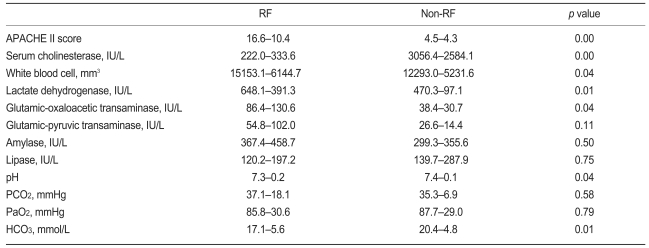
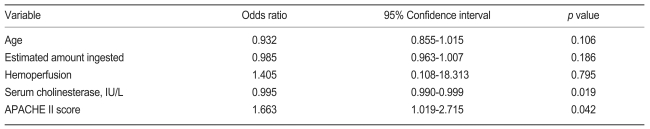
The RF group included 35 of the 68 patients. The mean duration of mechanical ventilation was 9.5±8.3 days. A tracheostomy was performed in 17 of these 35 patients. Table 2 shows the comparison of initial parameters in the RF and non-RF groups. The APACHE II score, white blood cell count, and lactate dehydrogenase and aspartate aminotransferase levels were higher in the RF group, and the serum cholinesterase and bicarbonate levels were lower than the non-RF group. No significant difference in the arterial oxygen partial pressures at presentation was observed between the two groups. The APACHE II score was a significant predictor of respiratory failure (odds ratio [OR], 1.273; p<0.01; 95% confidence interval [CI], 1.122 to 1.444). In addition, the APACHE II score was an important predictor of RF in the multiple logistic regression analysis (Table 3).
Table 2.
Comparison of the initial parameters in patients with or without respiratory failure (RF)
Values are mean±SD.
APACHE, Acute Physiology and Chronic Health Evaluation.
Table 3.
Multiple logistic regression analysis of the initial parameters associated with respiratory failure
APACHE, Acute Physiology and Chronic Health Evaluation.
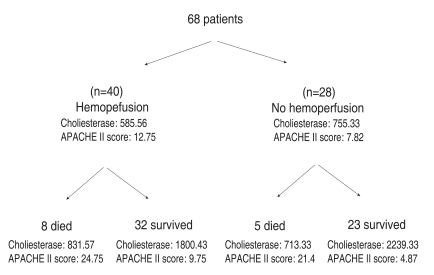
Information on the patients according to hemoperfusion treatment is presented in Fig. 1. The APACHE II scores were higher in patients with hemoperfusion treatment, but hemoperfusion treatment did not affect mortality. Regardless of hemoperfusion treatment, the APACHE II scores were higher and cholinesterase levels were lower in those who died.
Figure 1.
The comparison of clinical outcome in patients with or without hemoperfusion treatment. The Acute Physiology and Chronic Health Evaluation (APACHE) II scores were higher in patients with hemoperfusion treatment, but hemoperfusion treatment did not affect mortality.
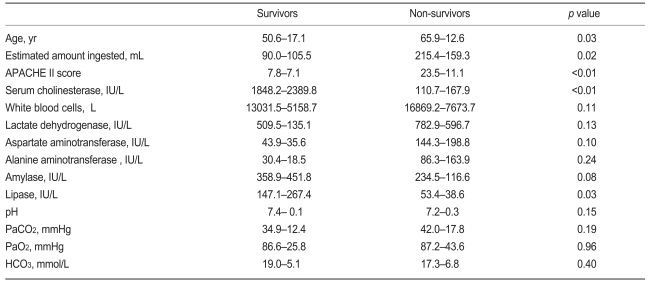
Thirteen of the 68 patients died. The clinical parameters are summarized in Table 4. The serum cholinesterase levels were lower in those who died. Twelve of the 35 RF group patients and only one of the non-RF patients died. Therefore, the need for mechanical ventilation was a significant predictor of a poor outcome (OR, 16.70; p<0.01; 95% CI, 2.03 to 137.60).
Table 4.
Comparison of the initial parameters between survivors and non-survivors
Values are mean±SD.
APACHE, Acute Physiology and Chronic Health Evaluation II score.
In addition, the APACHE II score was a significant predictor of mortality (OR, 1.194; p<0.01; 95% CI, 1.089 to 1.309). An APACHE II score <10 was a significant predictor of mortality (OR, 16.11; p<0.01; 95% CI, 3.17 to 81.73); a serum cholinesterase <1,000 IU/L was significantly associated with a poor outcome (OR, 0.676; p<0.05; 95% CI, 0.54 to 0.845).
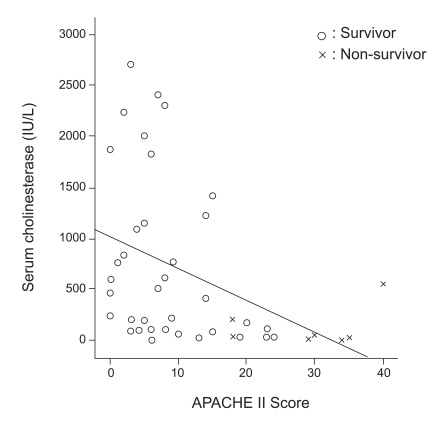
Pearson's correlation analysis revealed a significant negative correlation between the APACHE II score and serum cholinesterase level (r=0.415, p<0.01, Fig. 2).
Figure 2.
The association between serum cholinesterase and the Acute Physiology and Chronic Health Evaluation (APACHE) II. A negative correlation between the APACHE II score and serum cholinesterase level was significant.
An age over 60 years was also a significantly predictor of a poor outcome (OR, 6.852; p<0.01; 95% CI, 1.677 to 28.003). Hemoperfusion treatment was not related to a better outcome, although the patients who underwent hemoperfusion were in poorer condition (e.g., their APACHE II scores were higher).
Regarding the agents responsible, the death rate was 0% (0 of 21 of cases) for dichlorvos, 16.7% (2/12) for EPN, 37.5% (3/8) for phosphamidon, 25% (1/4) for phenthoate, 0% (0/3) for malathion, 50% (1/2) for methidathion, 0% (0/2) for profenofos, 0% (0/1) for chlorpyrifos, and 40% (6/15) for unidentified OP.
DISCUSSION
OP poisoning is a serious clinical entity and causes considerable mortality and polyneuropathy in survivors that is not always completely reversible. The estimated mortality following OP ingestion ranges from 20 to 50% [3,10-12].
In this study, the total mortality was 19% (13 deaths in 68 patients), and 12 of these deaths occurred in the 35 patients with respiratory failure. In our study, age, amount ingested, APACHE II score, initial cholinesterase level, and respiratory failure requiring mechanical ventilation were significantly associated with a poor outcome. The usefulness of the serum cholinesterase level in this context remains controversial [13,14]. In our patients, the cholinesterase level was correlated with the APACHE II score (Fig. 2), but this findings casts doubt on the usefulness of the cholinesterase level in patients with a low APACHE II score. The APACHE II score is a reliable general index that is useful for evaluating a wide spectrum of patients in intensive care units [15], and essentially shows that the degree of physiological derangement is closely correlated with outcome in critically ill patients. In a previous study, an APACHE II score >26 was a poor prognostic indicator in cases of OP poisoning [16], and the both APACHE II score and Glasgow coma scale predicted outcome [17,18]. In our study, the APACHE II score was correlated with respiratory failure and outcome. In particular, an APACHE II score >10 predicted a poor outcome.
Extracorporeal methods of blood purification have recently been recommended as a therapeutic approach to OP poisoning because of their ability to remove xenobiotics from blood, particularly hemoperfusion systems involving activated charcoal or resins [9,19,20]. However, the therapeutic benefits of this approach have not been substantiated by other investigators, and this has been explained by the large volume of distribution. The use of hemoperfusion for managing severe OP poisoning is also controversial [7,9]. In a study of 52 OP-poisoned patients, Altinop et al. [7] reported that hemoperfusion was useful in severe cases. By contrast, in our study, hemoperfusion was ineffective for improving survival, although it should be emphasized that the patients who were hemoperfused had high APACHE II scores, which makes it difficult to evaluate the effect of hemoperfusion. Randomized controlled studies are required to investigate the effect of hemoperfusion on survival in patients with severe OP poisoning.
Various types of OP were included in this study. No death occurred in 21 patients with dichlorvos poisoning, although the mean patient APACHE II score was 5.52±5.01. By contrast, 2 of 12 EPN cases, 3 of 8 phosphamidon cases, and 1 of 4 phenthoate cases died. This suggests that different OP have different toxicities. Eddlestone reported differences in the toxicity of OP [21]. In addition, the human toxicity differed from the animal toxicity suggested by WHO methods based on the toxicity in rats after oral dosing. That report suggested that each OP be considered as an individual poison. Our findings were consistent with that opinion.
We conclude that the initial APACHE II score is a useful prognostic indicator in cases of OP poisoning. Each OP might have a different toxicity. The management protocol for each OP in humans needs further study. Moreover, our results suggest that hemoperfusion is of limited benefit in cases of severe OP poisoning. Further randomized controlled studies of the beneficial effects of hemoperfusion on patient survival are required.
References
- 1.Goldfrank LR. Goldfrank's Toxicologic Emergencies. 7th ed. New York: McGraw Hill; 2002. pp. 1346–1360. [Google Scholar]
- 2.Bardin PG, van Eeden SF, Joubert JR. Intensive care management of acute organophosphate poisoning: a 7-year experience in the western Cape. S Afr Med J. 1987;72:593–597. [PubMed] [Google Scholar]
- 3.Munidasa UA, Gawarammana IB, Kularatne SA, Kumarasiri PV, Goonasekera CD. Survival pattern in patients with acute organophosphate poisoning receiving intensive care. J Toxicol Clin Toxicol. 2004;42:343–347. doi: 10.1081/clt-120039539. [DOI] [PubMed] [Google Scholar]
- 4.Rajapakse VP, Wijesekera S. Outcome of mechanical ventilation in Sri Lanka. Ann R Coll Surg Engl. 1989;71:344–346. [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2000-2001. Geneva: World Health Organization; 2001. WHO/PCS/01.4. [Google Scholar]
- 6.Shin SD, Suh GJ, Rhee JE, Sung JH, Kim JY. Epidemiologic characteristics of death by poisoning in 1991-2001 in Korea. J Korean Med Sci. 2004;19:186–194. doi: 10.3346/jkms.2004.19.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altintop L, Aygun D, Sahin H, et al. In acute organophosphate poisoning the efficacy of hemoperfusion on clinical status and mortality. J Intensive Care Med. 2005;20:346–350. doi: 10.1177/0885066605279834. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Chuecos J, del Carmen Jurado M, Paz Gimenez M, Martinez D, Menendez M. Experience with hemoperfusion for organophosphate poisoning. Crit Care Med. 1992;20:1538–1543. doi: 10.1097/00003246-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Okonek S. Probable progress in the therapy of organophosphate poisoning: extracoporeal hemodialysis and hemoperfusion. Arch Toxicol. 1976;35:221–227. doi: 10.1007/BF00293570. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka S, Yoshida M, Yamamura Y, Nishimura M, Takaesu Y. Study on acute organophosphorus poisoning-changes in the activity and isoenzyme patterns of serum cholinesterase in human poisoning. Nippon Eiseigaku Zasshi. 1993;48:955–965. doi: 10.1265/jjh.48.955. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita M, Tanaka J, Ando Y. Human mortality in organophosphate poisoning. Vet Hum Toxicol. 1997;39:84–85. [PubMed] [Google Scholar]
- 12.Wyckoff DW, Davies JE, Barquet A, Davis JH. Diagnostic and therapeutic problems of parathion poisonings. Ann Intern Med. 1968;68:875–882. doi: 10.7326/0003-4819-68-4-875. [DOI] [PubMed] [Google Scholar]
- 13.Aygun D, Doganay Z, Altintop L, et al. Serum acetylcholinesterase and prognosis of acute organophosphate poisoning. J Toxicol Clin Toxicol. 2002;40:903–910. doi: 10.1081/clt-120016962. [DOI] [PubMed] [Google Scholar]
- 14.Nouira S, Abroug F, Elatrous S, Boujdaria R, Bouchoucha S. Prognostic value of serum cholinesterase in organophosphate poisoning. Chest. 1994;106:1811–1814. doi: 10.1378/chest.106.6.1811. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 16.Lee P, Tai DY. Clinical features of patients with acute organophosphate poisoning requiring intensive care. Intensive Care Med. 2001;27:694–699. doi: 10.1007/s001340100895. [DOI] [PubMed] [Google Scholar]
- 17.Eizadi-Mood N, Saghaei M, Jabalameli M. Predicting outcomes in organophosphate poisoning based on APACHE II and modified APACHE II scores. Hum Exp Toxicol. 2007;26:573–578. doi: 10.1177/09603271060080076. [DOI] [PubMed] [Google Scholar]
- 18.Davies JO, Eddleston M, Buckley NA. Predicting outcome in acute organophosphorus poisoning with a poison severity score or the Glasgow coma scale. QJM. 2008;101:371–379. doi: 10.1093/qjmed/hcn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luzhnikov EA, Yaroslavsky AA, Molodenkov MN, Shurkalin BK, Evseev NG, Barsukov UF. Plasma perfusion through charcoal in methylparathion poisoning. Lancet. 1977;1:38–39. doi: 10.1016/s0140-6736(77)91670-1. [DOI] [PubMed] [Google Scholar]
- 20.Okonek S, Tonnis J, Baldamus CA, Hofmann A. Hemoperfusion versus hemodialysis in the management of patients severely poisoned by organophosphorus insecticides and bipyridyl herbicides. Artif Organs. 1979;3:341–345. doi: 10.1111/j.1525-1594.1979.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 21.Eddleston M, Eyer P, Worek F, et al. Differences between organophosphorus insecticides in human self-poisoning. Lancet. 2005;366:1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]