Abstract
Although many studies have examined the performance of animals in detecting a frequency change in a sequence of tones, few have measured animals' discrimination of the fundamental frequency (F0) of complex, naturalistic stimuli. Additionally, it is not yet clear if animals perceive the pitch of complex sounds along a continuous, low-to-high scale. Here, four ferrets (Mustela putorius) were trained on a two-alternative forced choice task to discriminate sounds that were higher or lower in F0 than a reference sound, using pure tones and artificial vowels as stimuli. Average Weber fractions for ferrets on this task varied from ~20 – 80% across references (200 - 1200 Hz), and these fractions were similar for pure tones and vowels. These thresholds are approximately 10 times higher than those typically reported for other mammals on frequency change detection tasks that use go/no-go designs. Naive human listeners outperformed ferrets on the present task, but they showed similar effects of stimulus type and reference F0. These results suggest that while non-human animals can be trained to label complex sounds as high or low in pitch, this task may be much more difficult for animals than simply detecting a frequency change.
I. INTRODUCTION
To interpret a vocal call appropriately, animals must perceive a number of attributes of complex sounds, including loudness, timbre, and pitch. In the context of human speech, pitch perception is particularly pertinent to identifying the speaker (Smith et al., 2005) and inferring their emotional state (Johnson, 1990), and is thought to play similarly important roles in vocal communication among non-human primates (Koda and Masataka, 2002; Kojima et al., 2003), songbirds (Nelson, 1989) and even frogs (Capranica, 1966). But although pitch is clearly a fundamental perceptual attribute of sound, few studies to date have examined how well animals can judge the physical correlate of pitch in naturalistic sounds – that is, the fundamental frequency (F0) of complex, periodic stimuli.
The physiological mechanisms underlying pitch perception for complex sounds are also incompletely understood. In the case of pure tones, perceived pitch correlates directly with frequency, and different pure tones maximally stimulate different parts of the cochlea. These widely appreciated and fundamental facts can lead to the tempting, but probably incorrect, conclusion that discriminations of the pitch of periodic sounds are the result of a simple frequency discrimination problem that can be easily solved through place coding in the tonotopically-organized ascending auditory pathway. However, most naturally occurring sounds are spectrally complex, (i.e. they carry acoustic energy at large numbers of frequencies). Consequently, the relationship between frequency content and perceived pitch is not straightforward, since there is no one-to-one relation between the fundamental frequency of complex sounds and the resulting pattern of activation within a tonotopic map. Instead, pitch perception may rely on a combination of spectral template matching and “time domain” information about the periodicity of sounds carried by temporally-phase-locked neural discharges early in the auditory pathway (Moore, 2003). These neural representations of sound periodicity need not necessarily result in an anatomically-ordered, topographic representation analogous to tonotopic maps. Some studies have provided evidence in favor of periodotopic arrangements which might serve as pitch maps at the level of the inferior colliculus in cats (Schreiner and Langner, 1988) and the primary auditory cortex in gerbils (Schulze and Langner, 1997; Schulze et al., 2002). Others have failed to find any clear topographic arrangement of periodicity preference in the auditory cortex of either ferrets (Nelken et al., 2008) or marmosets (Bendor and Wang, 2005). Therefore, it remains unclear whether or to what extent common physiological mechanisms are used to encode the fundamental frequency of pure tones and more naturalistic sounds.
For human listeners, the percept of pitch height, unlike that of timbre, can be described along a monotonic scale, from low to high, and one desirable feature of a topographic pitch map is that it might provide a simple neural mechanism by which listeners could judge the direction of a pitch change. Alternatively, specialized pitch “shift detection” operations have been proposed to underlie human listeners' ability to identify the direction of subtle F0 changes (Demany and Ramos, 2005). Before the relevance of such models to mammalian neurophysiology can be examined, it is first necessary to demonstrate that non-human animals do experience changes in the fundamental frequency of complex sounds as changes along an ordered, low-to-high pitch scale.
Numerous studies have shown that diverse species of animals are sensitive to changes in the F0 of tones or more complex stimuli. Most commonly, this is tested using a “go/no-go” task in which sounds are presented continuously and animals are conditioned to make a response if, and only if, the sound changes. This paradigm has been used to measure pure tone frequency discrimination thresholds in macaques (Sinnott et al., 1985; Pfingst, 1993), chinchillas (Nelson and Kiester, 1978; Shofner, 2000), cats (Elliott et al., 1960; Witte and Kipke, 2005), rats (Syka et al., 1996; Talwar and Gerstein, 1998; 1999), mice (Ehret, 1975), guinea pigs (Heffner et al., 1971), budgerigars (Dooling and Saunders, 1975), and electric fish (Marvit and Crawford, 2000). This experimental approach has also been used to demonstrate that electric fish, songbirds and chinchillas can detect changes in the F0 of complex sounds (Marvit and Crawford, 2000; Shofner, 2000; Dooling et al., 2002). What is not known, however, is whether the animals engaged in these tasks perceive the change in F0 as a change in pitch height, and whether they can discriminate upward from downward pitch changes. Recent studies of human listeners have emphasized that the ability to detect changes in the pitch of ongoing sounds does not necessarily imply that the listener can order these sounds along a pitch scale (Semal and Demany, 2006). While adults and children with cochlear implants are severely impaired on tasks that require discrimination of the direction of pitch changes (Fujita and Ito, 1999; Gfeller et al., 2002; Pressnitzer et al., 2005; Vongpaisal et al., 2006), children with cochlear implants have been shown to exhibit much finer pitch acuity in tasks that do not require them to report whether the pitch in a sequence of complex sounds has increased or decreased (Vongpaisal et al., 2006).
It is much more difficult to train animals on the types of psychophysical tasks required to address whether they order pitch height. Only a handful of studies of this kind have been undertaken so far, and none have used complex sounds. Go/no-go tasks, in which animals are trained to respond to a particular direction of frequency change (i.e., frequency increases or decreases) within a sequence of tones, have been used to demonstrate that primates (D'Amato, 1988; Brosch et al., 2004) and birds (Page et al., 1989; Cynx, 1995) do have the capacity to make relative pure tone frequency judgments. However, as noted above, the neural mechanisms of pure tone frequency discrimination may be quite different from those used to judge the pitch of a complex sound. Investigations of pitch discrimination in humans tend to favour two-alternative forced choice (2AFC) designs, in which the subject must not only detect pitch changes, but also identify the change on each trial as a relative increase or decrease in pitch from a standard reference value (Wier et al., 1977). This type of task is not commonly used in animal psychoacoustics, largely because of the difficulty in training animals on 2AFC auditory discrimination tasks (Burdick, 1979). However, these differences in task design make it problematic to compare the difference limens of humans and animals directly, since frequency discrimination performance has been shown to vary with task design (Nelson and Kiester, 1978; Burdick, 1980; Talwar and Gerstein, 1999).
Establishing a successful regime for training animals on 2AFC auditory discrimination tasks would also make novel investigations of the neurophysiological correlates of pitch perception possible. In the field of vision and somatosensory research, “neurometric” studies which combine electrophysiology and 2AFC discrimination tasks have identified the neural events that are likely to give rise to perceptual judgments (e.g. Liu and Newsome, 2005; de Lafuente and Romo, 2006). To use similar methodologies in hearing science, we require an animal model, like the ferret, that is suited to both psychophysical tasks and electrophysiological recordings.
In the present study we trained ferrets (Mustela putorius) to discriminate the direction of pitch changes on a positively conditioned, 2AFC discrimination task. On each trial, two artificial vowel sounds were presented in succession and the ferret had to indicate, by choosing a water spout either to the left or to the right, whether the second sound was higher or lower in pitch than the first. The anatomy and physiology of the ferret auditory system are well documented, and there are a number of observations that suggest that the ferret may be a suitable species in which to study the role of pitch cues in vocalization processing. Ferrets are highly sensitive to low-frequency pure tones (Kelly et al., 1986), there is evidence that they are sensitive to the harmonic fusion of tone complexes (Kalluri et al., 2008), and many of their vocalizations contain low-frequency energy and are strongly periodic, which gives them a clearly identifiable pitch. The responses of ferret auditory cortical neurons encode information about the F0 of artificial vowels (Bizley et al., 2009) and support the discrimination of human speech sounds (Mesgarani et al., 2008). However, the ability of ferrets to discriminate the pitch of complex sounds has not been previously measured.
We measured pitch discrimination in ferrets using both pure tones and artificial vowels in an identical paradigm, so that difference limens and Weber fractions could be directly compared. The artificial vowel stimuli were sufficiently complex to exhibit key features that are commonly found in vocalization sounds used in human and animal communication, yet simple enough to be described by a small number of numerical parameters. Finally, we also measured the discrimination performance of naive human listeners for comparison, using a similar paradigm.
II. METHODS
A. Subjects
Four adult pigmented ferrets (one male) were trained in this study. Ferrets were housed either singly (males) or in groups of two or three (females), with free access to high-protein food pellets and water bottles. On the day before training, water bottles were removed from the ferret's home cages and they were replaced on the last day of a training run. Training runs lasted for five days or less, with at least two days between each run. On training days, ferrets received drinking water as positive reinforcement while performing a sound discrimination task. Water consumption during training was measured, and was supplemented as wet food in home cages at the end of the day to ensure that each ferret received at least 70 ml of water per kilogram of body weight daily. Regular otoscopic and typanometry examinations were carried out to ensure that both ears of the animals were clean and healthy. All experimental procedures were approved by the local ethical review committee and were carried out under licence from the UK Home Office, in accordance with the Animals (Scientific Procedures) Act 1986.
B. Training apparatus
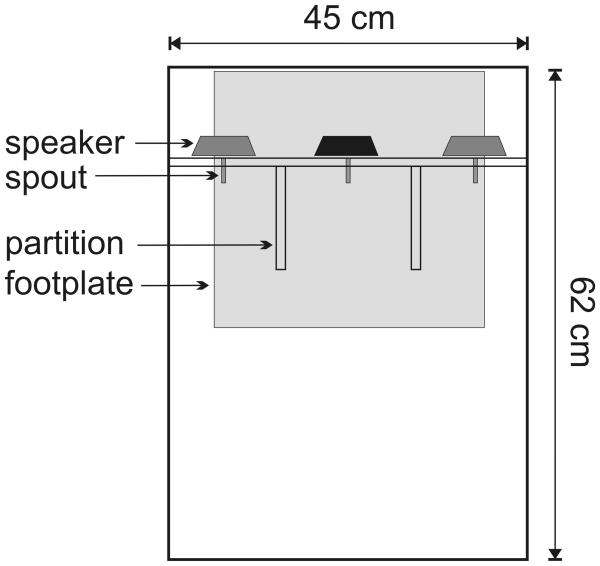
Ferrets were trained to discriminate sounds in custom-built testing chambers constructed from double glazing units that incorporated a sound-insulating vacuum. The ceiling of the chambers was covered in sound-absorbing foam. The testing chambers were approximately 45 cm wide, 62 cm long, and 54 cm high (Fig. 1). A plexiglass wall, 12 cm from the back of the box, separated the animal from the electronics and tubing of the apparatus. Three metallic water spouts were mounted on the plexiglass wall, one centrally positioned “start spout” and two “response spouts” positioned to the left and right. An aluminium 32×34 cm footplate covered the floor below the water spouts. When the ferret licked a water spout while standing on the footplate a small change in voltage between the steel spout and the aluminium plate resulted, allowing us to register the animal's licking responses using electronic circuitry, as described by Hayar et al. (2006). Sound stimuli as well as acoustic feedback signals were delivered via three loudspeakers (Visaton FRS 8) which were mounted above the spouts. These speakers produce a flat response (± 2 dB) from 200 Hz to 20 kHz, with an uncorrected 20 dB drop-off from 200-20 Hz. Plexiglass partitions, 13 cm long and 15 cm high, were positioned between the central spout and each of the peripheral spouts to increase the perceived cost involved in the ferrets' response, as initial testing had indicated that ferrets were less likely to pay careful attention to the acoustical cues if hopping between response spouts required essentially zero time or effort.
FIG. 1.
Schematic of the training apparatus, shown from above. Ferrets made behavioral responses and received water rewards from the three stainless steel water spouts while standing on the aluminium footplate. In the first training stage, sounds were presented from the central loudspeaker (black) as well as the two peripheral loudspeakers (gray). In later training stages and during testing, sounds were presented from the central loudspeaker only.
The behavioral task, data acquisition, and stimulus generation were all automated using custom software running on personal computers, which communicated with TDT RM1 real-time signal processors (Tucker-Davis Technologies, Alachua, FL).
C. Training
Ferrets were trained on a 2AFC discrimination task, using drinking water as a positive reinforcer. To assist learning, animals were trained in several stages of 2AFC tasks, and each ferret was advanced to the next stage when they reached a criterion of at least 85% correct on three consecutive sessions. Each of the five training stages and the final testing stage are described in detail below. Ferrets ran two training sessions daily within each 5-day training “run”, and typically completed between 60-150 trials per session.
During a pre-training stage, ferrets learned to lick the spouts in the testing chamber for a water reward. The animal was required to maintain contact with a spout for about one second before receiving a water reward from the spout. During pre-training, ferrets were required to alternate between the central and peripheral spouts in order to receive a water reward, but no sound stimuli were presented at this time. The amount of water used to reward a single response varied across animals, but for all animals the water reward presented from the peripheral response spouts (0.3 - 0.5 ml per trial) was larger than the water reward presented at the central start spout (0.1 - 0.2 ml per trial).
1. Training stage 1
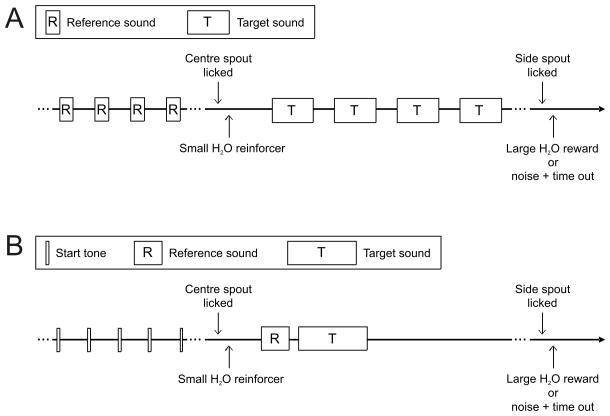
In the first training stage, the ferrets performed a pure tone frequency discrimination task, but with a localization cue to assist. At the start of each trial, a “reference signal” consisting of a continuously repeated pure tone (5 kHz, 100 ms duration, 150 ms inter-tone interval, 0.5 ms cosine ramped rise/fall) was presented from the central speaker until the ferret activated the central spout to start the trial (Fig. 2A). This activated a small water reinforcer at the central spout and was followed by a second “target” pulse of pure tones (300 ms duration, 150 ms inter-tone interval, 0.5 ms cosine ramped rise/fall) that differed in frequency from the initial reference tones. The target pulse continued to play until the ferret activated one of the peripheral spouts. If the target frequency was higher than the reference, the animal was required to move to the right response spout to receive a water reward. For frequencies lower than the reference the animal was required to move to the left. Responses at the central spout were ignored, and responses at the incorrect side spout resulted in a 500-ms broadband noise at the central speaker that indicated the onset of a 10-12 s timeout. In all training and testing conditions the central spout remained unresponsive for 2 s following a correct response to provide the animal with a quiet period between trials in which it could drink the water reward.
FIG. 2.
Discrimination trial schematics. (A) In the first three stages of training, a reference stimulus was repeated until the ferret initiated a trial by activating the central spout. Then a target stimulus of a different frequency was presented repeatedly until the animal responded. The ferrets' task was to indicate whether the fundamental frequency of the target sound was higher or lower than that of the reference by activating the right or left peripheral spouts, respectively. In training stages 1 and 2 the stimuli were pure tones, and in stage 3 they were click trains. (B) In training stages 4 and 5 and in testing, a “ready signal” consisting of a repeating 5000 Hz tone pip was presented to indicate to the animal that a trial could be started by licking the central “start” spout. The reference and target sounds were each then presented only once, in quick succession. As above, the ferrets' task was to indicate the direction of pitch change between the reference and target sounds by responding at the correct peripheral spout. The sounds to be discriminated were click trains in training stage 4 and an artificial vowel sound (formant-filtered click trains) in the final training and testing stages.
To make this task easy we provided an additional spatial cue during the initial training phases: the target tones were presented only from the loudspeaker above the correct water spout for a given trial. Thus, if the target was higher in frequency than the reference, then the target sounds were presented from the right peripheral speaker and right spout responses were rewarded with water.
If an animal responded incorrectly on a given trial, the same stimuli were presented on the next trial. Such “correction trials” continued until the animal responded correctly. For all training stages, the F0 of the reference sound was fixed during a session, and the target sound varied between two F0s across trials – one that was at least an octave higher than the reference and another that was at least an octave lower than the reference.
2. Training stage 2
Once animals learned to perform the above task, the localization cue was removed so that the ferret was left only with frequency as an acoustic cue to the correct response. For this and all further tasks, all stimuli were presented from the middle speaker above the start spout only.
3. Training stage 3
Once criterion had been reached on the pure tone frequency discrimination task, the tones were replaced with click trains, where the click rate (and hence the F0) of the reference (300-500 Hz) differed from the target sounds by at least an octave. Human listeners perceive this stimulus as a rich buzzing sound with a pitch corresponding to the click rate. Thus, the reference and target sounds were both broadband, but differed in F0.
4. Training stage 4
In stage 4 of training, the previous pitch discrimination task was modified so that the sounds to be discriminated were presented only once per trial, rather than being repeated until the animal made its response (Fig. 2B). Before each trial, a series of tone pips (5 kHz tone, 20 ms duration, 0.5 ms rise/fall time, 200 ms inter-tone interval) was presented as a “ready signal” to let the ferret know that the central spout could be triggered to start the trial. When the ferret triggered the central spout, a small water reward was administered from the spout on 10% of trials, chosen at random. On the remaining 90% of the trials, rewards were only given from the peripheral spouts for correct responses to the test sounds. The test sounds consisted of two consecutive click trains presented from the central speaker: a 200-ms-long reference click train (5 ms rise/fall time, with a click rate of approximately 400 Hz) followed by a 50-ms-long inter-stimulus interval of silence, and then a 500-ms-long target click train (5 ms rise/fall time, with click rates at least one octave away from that of the reference). As before, in order to receive a reward the ferret was required to activate the right peripheral spout if the target was higher in F0 than the reference sound, and to activate the left peripheral spout if the target pitch was lower in F0 than the reference. Incorrect responses were again negatively reinforced with a noise and timeout of 10-12 s, and were followed by correction trials. If an animal failed to make a response within 15 s of the onset of the target stimulus, the trial was reset without time-out or water reinforcement, forcing the animal to restart the trial.
5. Training stage 5
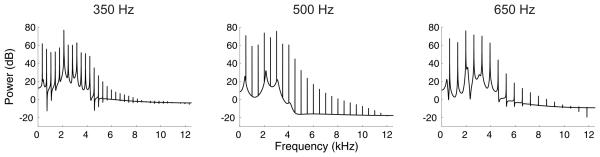
In this stage, click train stimuli were replaced with artificial vowel sounds that were created using custom software, based on an algorithm adapted from Malcom Slaney's Auditory Toolbox (http://cobweb.ecn.purdue.edu/~malcolm/interval/1998-010/). The vowel sounds were composed of click trains that were band-pass filtered to add “formants” centered at 430, 2132, 3070 and 4100 Hz (Fig. 3), and then given an envelope with 5 ms rise and fall times. These formants correspond to the first four formants of the English vowel /i/ (as in “pill”). The overall spectral distribution of the vowel was largely determined by the position of the formants and was thus similar across all F0s tested. The click rates of the target and reference sounds in this task were similar to those used in stage 4. The switch from unfiltered click trains to artificial vowels did not cause any transient drop in the animals' performance, indicating that the animals were able to generalize the pitch task very rapidly across the two types of complex sounds. Across trials, the sound levels of the reference and target vowels were varied independently. Each was set to one of 10 possible values, spanning a range of approximately 15 dB, and chosen at random with a uniform distribution. Sound levels were calibrated using a B&K sound level meter and free-field, ½ inch microphone type 4191 (Brüel & Kjær, Norcross, USA). The average sound level of the artificial vowel was approximately 80 dB SPL (± 3 dB across different F0s). The ± 7.5 dB random variation in sound level was introduced to ensure that ferrets were not inadvertently provided with relative level difference cues within particular spectral bands across the reference and target sounds. Once animals had reached criterion on a given session of this task, the two target F0s used were occasionally jittered (± 150 Hz) across additional sessions. By randomizing levels and target pitches we encouraged the animals to follow pitch cues, rather than mapping other acoustical features of the training targets onto the left and right spouts.
FIG. 3.
Power spectra of the artificial vowel stimulus used in this experiment (/i/), shown with a fundamental frequency of 350 (left panel), 500 (center panel) and 650 (right panel) Hz.
D. Pitch discrimination testing
Once ferrets performed at ≥ 85% correct in at least three consecutive sessions of training stage 5, we switched the ferrets from training to testing. The testing task was very similar to stage 5 of training, except that 30 different target F0s were now presented within a given session, rather than just two target F0s. Animals that had reached criterion on training stage 5 generalized to the 30-target task well, often performing at their best from the very first testing session. Within each weekly testing run, composed of 10 individual testing sessions, the F0 of the reference was held constant. Each weekly run was initiated with a session resembling training stage 5, wherein only two target sounds with fixed F0, one octave above and one octave below the reference, were used. Animals were required to perform at ≥ 85% on this task before progressing to the “variable target” condition, and most animals reached this criterion in 1 to 2 testing sessions. In the variable target condition, the F0 of the target sound was varied from trial to trial across 30 values. Fifteen of these targets had a higher F0 than the reference and 15 had a lower F0, with a sampling density of 12 steps per octave. To help ensure that the animals were still attending to the pitch of the sounds when the task was very difficult, if the ferret responded incorrectly when the target was within 5/12 octaves of the reference, then the correction trial was presented at the most extreme value in the range in the appropriate direction. Pitch discrimination testing was repeated across sessions using a constant reference F0 for typically 300-600 trials per reference. The animal was then restarted on stage 5 of training using a different reference F0 at the beginning of the next testing run (i.e. the following week). This procedure was repeated for a number of references between 200 - 1200 Hz.
E. Human psychophysics
Five adult humans (two male, ages 24 – 40 years) were tested on a similar pitch discrimination task, using both the artificial vowel and tones as stimuli. Human psychophysical procedures were carried out under the guidelines of the Central University Research Ethics Committee of the University of Oxford. We attempted to make the pitch discrimination task performed by human subjects as similar as possible to the task performed by the ferrets. The stimuli were presented from the same central speaker of the testing chamber in which ferrets were trained, and the sounds were presented with the same random level variation across trials as in the animals' task. The human subjects, being too large to fit in the ferret testing chamber, listened to the sounds through the opened lid, and initiated trials and responded by pressing keys on a keyboard positioned near the chamber. Upon initiation of each trial, a reference and a target sound, identical to those used with the ferrets, were presented. However, humans performed considerably better than ferrets at these discrimination tasks, so the range of periodicities sampled around each reference was adjusted based on pilot data (not shown), and was also occasionally readjusted for individual subjects, based on their performance on the first 50-100 trials. Instead of water rewards, subjects received feedback on a computer monitor after each trial, and incorrect choices resulted in a broadband noise and timeout of 2-4 seconds. Each subject completed 250-350 trials for each of four reference F0s, in both artificial vowel and tone versions of the discrimination task. The reference F0 was constant within any one testing run, tone and vowel trials were presented in blocks, and the order of reference periodicities presented was pseudo-randomized across subjects. Only one subject (H5) was musically trained, and none were given extensive training on the task prior to testing.
F. Data analysis
Correction trials were excluded from the data analysis, as were trials on any testing session in which the subject scored less than 60% correct overall. Each animal completed at least 300 trials for each reference F0, and in many cases the total number of trials was closer to 1000. Data were pooled across testing sessions that used a common reference F0.
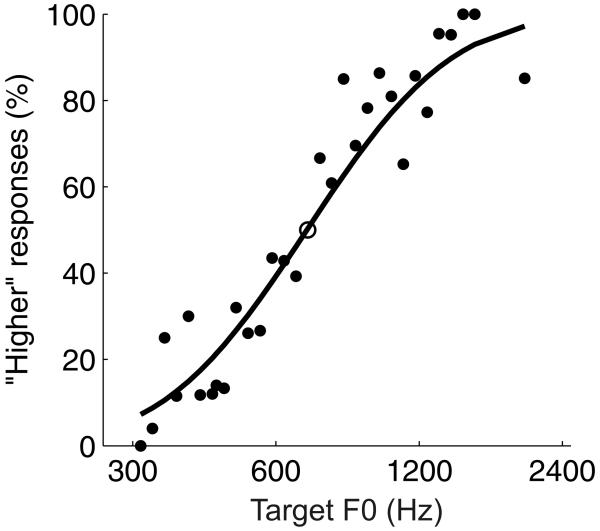
Figure 4 plots the performance of one ferret on trials in which the reference F0 (open circle) was 700 Hz. As expected, the ferret responded correctly more often when the F0 of the reference and target vowel were further apart. The functions relating the proportion of trials on which the animal responded at the right spout to the log of the target F0 were sigmoidal in shape and approximated a cumulative Gaussian distribution function (black dots, Fig. 4). Therefore, psychometric curves were estimated from ferrets' raw choice probabilities by fitting cumulative Gaussian distributions using probit generalized linear models (black line, Fig. 4). The difference limens for each reference F0 were calculated from the fitted psychometric curves as half of the distance between the F0 at which the right spout was chosen on 69.15% of trials, and the F0 at which the right spout was chosen on 30.85% of trials. A threshold of 69.15% was chosen because this level of performance on our 2-alternative discrimination task is equivalent to a d' of 1 (Wickens, 2002), making our results comparable to previous studies of frequency discrimination in non-human animals on go/no-go tasks. Weber fractions were calculated as the ratio of the difference limen for pitch divided by the F0 of the reference sound.
FIG. 4.
Performance of one ferret (subject F1) on the discrimination task when the reference vowel had a fundamental frequency of 700 Hz. The percentage of right spout choices is plotted as a function of the fundamental frequency of the target sound (black dots), and the reference F0 is indicated by an open circle plotted at 50% choice probability. The psychometric curve, a fitted cumulative Gaussian distribution function, is also shown (black line).
Pearson correlations and ANOVAs were used throughout to test whether differences in performance were significant and whether performance was related to parameters such as stimulus type (artificial vowel or pure tone) and reference pitch. A significance level (alpha) of 0.05 was used as a criterion for null hypothesis rejection, and where multiple comparisons were carried out across a number of subjects and/or reference values, significance levels were Bonferroni corrected.
III. RESULTS
A. Ferrets' discrimination of the pitch of artificial vowels
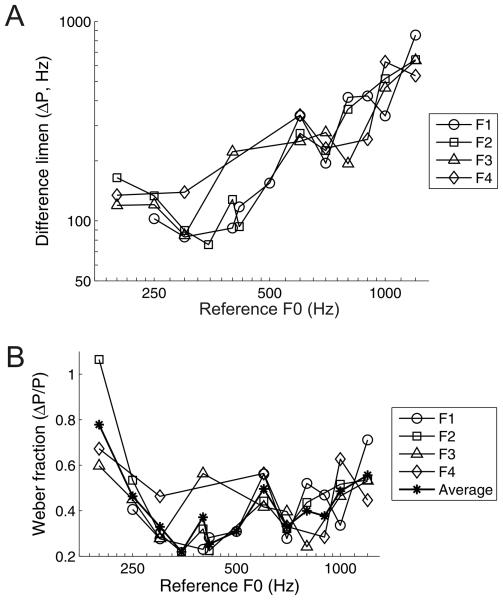
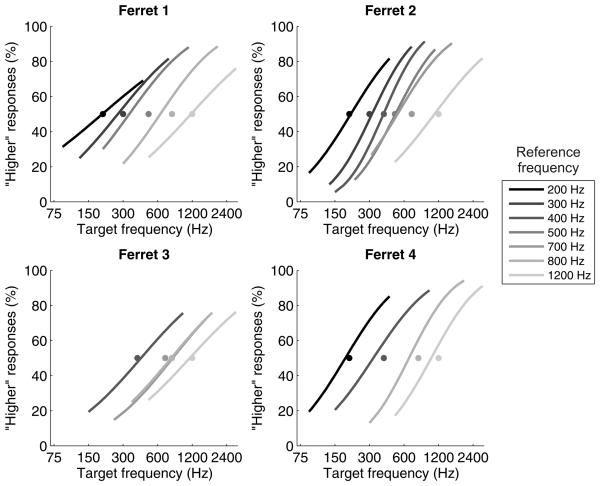
All four ferrets reached criterion on the five training stages after 1-2 weeks of training per stage. Figure 5 shows the psychometric functions obtained from all four ferrets for each reference F0 tested. The pitch acuity of ferrets is indicated by the steepness of the psychometric curves, and can also be expressed as the minimum F0 difference required for the animal to reach our criterion of 69.15% (i.e. the difference limen for pitch). Figure 6A illustrates how ferrets' difference limens for the pitch of the artificial vowel depend on the F0 of the reference. Pearson correlations showed that these difference limens increased significantly with the F0 of the reference vowel (r = 0.86, p < 0.001). Weber fractions were derived by normalizing each difference limen by the corresponding reference F0 (Fig. 6B). These normalized measures of pitch acuity showed no linear change across the range of reference F0s tested (r = −0.01, p = 0.951). Nevertheless, Weber fractions did differ significantly across reference F0s (1-way ANOVA; F(12,25) = 3.98, p = 0.002). Post hoc tests indicated that the Weber fraction measured using a reference of 200 Hz was significantly higher than the Weber fractions for references of 300 – 417 Hz and 700 Hz (Tukey's HSD test; p < 0.05).
FIG. 5.
Psychometric curves describe the performance of four ferrets trained to discriminate the pitch of an artificial vowel. Each panel shows the psychometric functions for a single animal, and the style and grayscale of each psychometric curve corresponds to the fundamental frequency of the reference vowel (see legend). The reference F0 for each curve is also indicated by a grayscale circle at 50% choice probability.
FIG. 6.
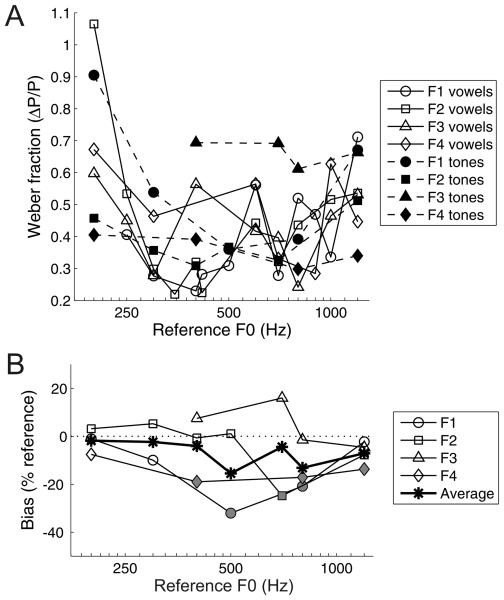
(A) Difference limens for pitch of four ferrets across a range of reference F0s. Data for each ferret are displayed with a unique symbol and connected with solid lines. (B) Weber fractions for the four ferrets (F1 - F4) across a range of reference F0s, calculated using the difference limens in (A). The average Weber fractions across all ferrets are shown by the asterisks.
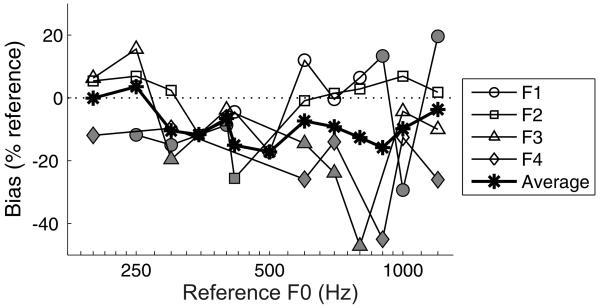
In Figure 5, some of the fitted psychometric curves do not pass through the reference F0 at the 50% choice probability point. These small shifts of the psychometric curve are partly attributable to statistical errors in the fit of the psychometric function, but might also indicate a response bias, i.e. when uncertain about the correct response, the ferret did not make “higher” (right) or “lower” (left) responses with exactly equal probability, but exhibited a small preference for either higher or lower responses. We quantified this bias in each psychometric curve as the distance, in Hz, between the 50% right choice probability point of the psychometric function and the reference F0. In this calculation, “higher” response biases are represented as negative values and “lower” biases as positive. In Figure 7, the biases are plotted as a percentage of the reference F0, and these values are compared across ferrets and across references. Some animals were more likely to show response biases than others, and most of the significant biases on this task (see Fig. 7 caption) were towards the right spout (i.e. higher pitch).
FIG. 7.
Bias of four ferrets on the pitch discrimination task. Biases were calculated as the distances, in Hertz, between the 50% right choice probability obtained from the fitted psychometric function and the corresponding reference F0, so that positive values indicate a bias towards the left (“lower”) spout and negative values indicate a bias towards the right (“higher”) spout. These biases are normalized by the reference F0. Data for different animals are plotted with different symbols, and bias values that are significantly different from zero (99.87% confidence intervals; 0.05 alpha with Bonferroni correction) are shown in gray, with non-significant values in white. The average percentage of bias across all four animals is plotted with asterisks. A dotted line is shown at a bias of zero, for reference.
To ensure that the ferrets were making pitch judgments, the intensities of the reference and target sounds were independently and randomly varied across trials. To confirm that the sound level of the target sounds did not affect discrimination performance, psychometric functions were fitted independently for target sounds at each intensity level for each reference F0. A 2-way ANOVA was then carried out on the slopes of the fitted psychometric functions (expressed as percent choice probability per octave), using ferret identity and target intensity as predictor variables. We found no significant effect of target sound intensity (F(19,436) = 1.09, p = 0.359), nor did the intensity significantly interact with the performance of individual ferrets (F(57,436) = 1.15, p = 0.221). Furthermore, responses on individual trials were not predicted by the intensity differences between the reference and target vowel (2-way ANOVA; F(28,569) = 1.25, p = 0.174) nor by the interaction between this intensity difference and ferret identity (F(84,569) = 1.23, p = 0.090). Therefore, across the 15 dB range tested, ferrets' performance on the pitch discrimination task did not depend on sound intensity.
Ferrets could adopt at least three strategies to solve our pitch discrimination task. They could compare the relative pitches of the target and reference presented on each trial, as human listeners report doing. They could also build up an internal “memory” of the reference during training at the beginning of the week and judge each target as high or low relative to this internalized reference. While this internal reference would be reinforced by the presentation of that same reference on each trial throughout the testing run, performance may persist without it. Finally, the ferrets may not compare the targets to a single reference at all, but instead compare a given target to internalized “low” and “high” target templates, for instance, the high and low pitches presented during training at the beginning of a weekly run. We attempted to assess whether animals were relying on the references presented at each trial by carrying out a week of “variable reference” testing sessions in which the reference pitch could take one of two values, approximately two octaves apart. Three targets were presented: one about an octave below the low reference, one about an octave above the high reference, and one centered between the low and high reference. If ferrets compared the pitch of the target to the reference pitch on each trial, they should perform this task well, responding “high” when the middle target was presented with the low reference and “low” when the middle target was presented with the higher target. If they matched targets to low and high templates, they might be expected to respond similarly to the middle target irrespective of the reference F0. If they rely on a stable memorized representation of the reference, they would be forced to adopt a new strategy in this paradigm because a stable reference is not presented, and so their performance on the variable reference task is less straightforward to predict.
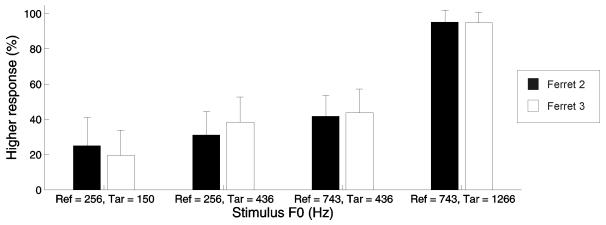
After four days of testing with two references, ferrets discriminated the highest and lowest targets well, but responded to the middle target at chance (Fig. 8). Responses to the middle target did not differ across the two references (Tukeys HSD test). This indicates that these animals were not accustomed to comparing the pitch of the target to that of the reference presented on each trial, but they instead used a strategy that utilized internal representations.
FIG. 8.
Performance of two ferrets when the reference roved between two F0 values across trials. Right spout choice probabilities (mean + standard deviation, across 8 consecutive testing sessions) are plotted across the four reference and target combinations presented in the “variable reference” testing sessions. This type of testing was carried out with Ferret 2 (black bars) and Ferret 3 (white bars).
B. Discrimination of the frequency of pure tones
Once trained to discriminate the pitch of artificial vowels, the same four ferrets were retested using pure tones instead of artificial vowels, but on an otherwise identical paradigm. On each trial, a reference tone was presented (200 ms duration, 20 ms rise/fall time), followed by a silent inter-stimulus interval (50 ms) and then a target tone (500 ms duration, 20 ms rise/fall time). Performance was again measured across a range of references between 200 and 1200 Hz. For each new reference tone, the animals were trained to criterion performance (85% correct) using two fixed targets before 30 new variable target frequencies were introduced in a single testing session, as described in Section IID. All ferrets generalized from the vowel to tone discrimination task well, and reached criterion on the latter within the first two weeks of training.
Ferrets' psychometric curves for the tone version of the pitch discrimination task are shown in Figure 9, while Figure 10A shows the Weber fractions calculated from these psychometric curves, together with the Weber fractions measured with artificial vowels for comparison. The Weber fractions obtained with the vowel and tone versions of this task were clearly similar overall, and these values did not differ significantly between the two stimulus types across the reference range (2-way ANOVA; F(1,43) = 1.36, p = 0.250). But note that one animal (F3) appeared to perform better on the vowel version of the task. Just as with the vowel data, the pure tone difference limens for pitch scaled with reference F0 (r = 0.88, p < 0.001), and when expressed as Weber fractions, discrimination performance did not show a systematic increase or decrease across the range of references tested (r = −0.04, p = 0.866). Small but significant response biases were again observed in some cases, as shown in Figure 10B, and they again tended to favour “higher” (right) responses.
FIG. 9.
Fitted psychometric curves showing the performance of four ferrets on a pure tone frequency discrimination task. The reference frequency for each curve is coded in grayscale, and the value of this frequency is also indicated by the position of the filled grayscale circles along the x-axis.
FIG. 10.
Performance of four ferrets on a tone discrimination task. (A) Weber fractions of four ferrets across a range of reference F0s, measured with either the artificial vowel (open symbols, solid lines) or with pure tones (filled symbols, dashed lines). Data for each ferret are displayed with a unique symbol, as shown in the legend. (B) Bias of four ferrets on the tone discrimination task. Biases were calculated and are presented as described for Fig. 7.
C. Discrimination of the pitch of artificial vowels by human listeners
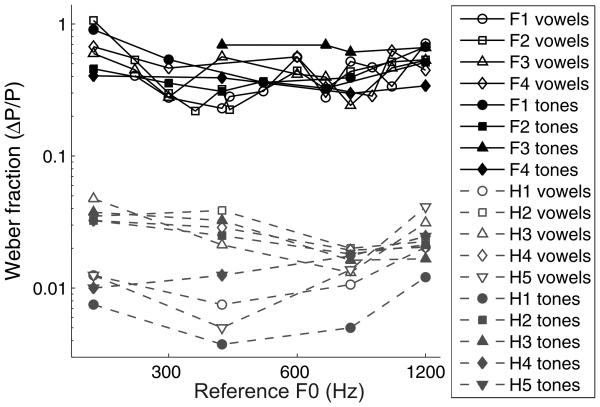
In Figure 11, the Weber fractions of human subjects are plotted together with the Weber fractions of ferrets on the same discrimination task. The pitch acuity of humans was clearly better than that of ferrets on these discrimination tasks (3-way ANOVA carried out on the difference limens across species, stimulus type, and reference periodicity; F(1,82) = 840.86, p < 0.001). As in the ferret data, the performance of the human listeners did not differ significantly when vowels or tones were used as stimuli (2-way ANOVA; F(1,35) = 1.81, p = 0.188), and difference limens for pitch scaled with the reference F0 in both the vowel (r = 0.74, p < 0.001) and tone (r = 0.78, p < 0.001) versions of the task, while Weber fractions did not change linearly across the range of references tested (r = −0.25, p = 0.297 for vowels; r = −0.04, p = 0.855 for tones). Analysis of the data collected for the subset of references that were tested in both human and ferret experiments showed that trends in difference limens across references did not differ between these two species (3-way ANOVA, interaction of species and reference F0; F(3,65) = 0.27, p = 0.846). A small but statistically significant bias was observed for 10 of the 40 tested conditions (p < 0.05/20, for 20 subject-by-reference conditions tested with vowels and with tones, data not shown). In the majority (8 of 10) of these cases, the bias was again towards “higher” responses.
FIG. 11.
Weber fractions of four ferrets and five human listeners on the pitch discrimination tasks. Data for each ferret are displayed with black symbols connected by black lines, and human data are displayed with gray symbols connected by gray lines. Weber fractions for each subject were measured using artificial vowels (open symbols) and tones (filled symbols).
IV. DISCUSSION
In natural environments, the fundamental frequency often correlates with the size of a vibrating object. Consequently, the ability to order complex sounds along a pitch scale can be useful, as it provides information about the physical properties of sound sources (Smith et al., 2005). Pitch ordering also plays an important role in vocal communication, as it often carries information not just about the gender and size, but also the emotional state of the vocalizing individual. The ability to perceive the pitch of periodic sounds along a continuous scale, from low to high, might facilitate estimating continuous valued properties of a sound source (heavy or light, large or small, relaxed or tense, empty or full). This would be a useful faculty for many species, but although there have been a number of previous investigations into frequency discrimination and, to a much lesser extent, the discrimination of periodic from non-periodic sounds in mammals, none so far have asked how well animals can judge the direction of a change in the F0 of complex sounds. Our results show that ferrets can distinguish artificial vowels with F0s that are higher than a reference from those that are lower, which suggests that they, like humans, perceive pitch along an ordered scale from low to high. We have also shown that ferrets' pitch discrimination performance for this class of stimuli closely matches their performance on an equivalent pure tone frequency discrimination task, even though the neural substrates for these two perceptual tasks could in principle be quite different.
A. Pitch direction judgments in animals
The few previous studies that have examined animals' ability to judge the direction of pitch changes have all used simple tones as stimuli. These studies have often been motivated by the question of whether animals can identify the direction of frequency changes by comparing the “relative” frequencies of several tones presented on a given trial, or if they instead compare the “absolute” frequency of each tone to an internal frequency representation (i.e., they possess and use “perfect pitch”). For instance, Cynx and colleagues carried out psychophysical tasks in birds that required the animals to respond to increases or decreases in frequency across a sequence of tones (Page et al., 1989; Cynx, 1995). While they showed that it is possible to train birds to make relative frequency judgments under carefully designed experimental conditions, these relative judgments do not appear to come easily. Their results suggest that both songbirds and non-songbirds prefer to label the absolute frequency of a sound to solve frequency discrimination tasks. Other groups have also experienced difficulty in training rats (D'Amato, 1988) and non-human primates (D'Amato, 1988; Izumi, 2001; Brosch et al., 2004) to respond to the direction of relative frequency changes within a tone sequence.
The design of our task encouraged subjects to respond to the relative change in F0 between the reference and target sound, and human listeners carrying out this task report basing their decisions on relative pitch judgments. However, since the reference sound remained constant throughout a given session, the ferrets could use other strategies to perform our task. For instance, they may have formed a internal, memorized representation of the reference during the first few trials in a session, and then compared each target against this memorized representation instead of, or in addition to, comparing it against the reference presented at the start of each trial. Alternatively, they could have formed representations of the “high” and “low” targets presented during early training session(s), and judged whether the target presented on each testing trial was more similar to this “high” or “low” template. The results of our testing studies using a reference that alternated randomly between two F0 values suggest that ferrets, like birds, rats and primates, may adopt such an “absolute” pitch-matching strategy, rather than comparing pitch values across sounds presented in sequence. Whichever of these approaches were employed by the animals, in order to perform the task, the ferrets had to be able to evaluate the targets along an ordered perceptual scale that correlates with the F0 of the sound. In other words, they had to rate the pitch height of the target.
A fourth strategy for solving our discrimination task may be proposed, which would not be consistent with the interpretation that ferrets order the F0 of sounds. That is, the ferrets may have simply memorized the two targets and labelled them as “left response” and “right response” sounds, without making any judgment on the target sounds' relative pitch. During the early training stages, where only two different target sounds were presented per session, this would be a viable strategy. However, if the animals were only capable of a simple recognition of memorized sound samples, then that would leave them poorly prepared for the first testing session, in which 30 new targets were introduced. We found that the animals adapted very quickly to the testing regime, and adopted a “go left for low and go right for high” strategy right from the first training session, which suggests that they quickly generalized their responses to the new target sounds as though they perceived the F0 of these harmonic sounds along an ordered pitch scale.
We have not been able to retrain our animals from this study on a task where references rove widely across more than 2 F0 values from trial to trial, which suggests that during their training they may have come to rely at least in part on a memorized pitch boundary. The perceptual demands of this task should not be very different, but from an animal training perspective it is much harder: randomized references make the stimulus set a great deal more variable, and it is correspondingly harder for the animal to discover the rules by which it is to respond to the stimuli. However, preliminary data from another laboratory suggests that ferrets can be trained to respond to the relative frequency of pure tones with roving references, provided the reference is varied from the beginning of training (Yin et al., 2007).
B. Frequency acuity of ferrets
Ferrets have been trained on a number of sound localization (Kelly and Kavanagh, 1994; Parsons et al., 1999; Kacelnik et al., 2006; Bizley et al., 2007; Nodal et al., 2008) and detection tasks (Kelly et al., 1986; Hine et al., 1994; Kelly et al., 1996; Fritz et al., 2003; Fritz et al., 2007) in the past, but rarely in discrimination tasks. In a series of studies by Shamma and colleagues, ferrets were trained on an aversively reinforced, go/no-go task to discriminate between two tones of fixed frequencies (Fritz et al., 2005) or to discriminate pure tones from inharmonic tone complexes (Kalluri et al., 2008). These experiments have demonstrated that ferrets can make discriminations based on simple spectral or harmonic cues, and that the response properties of primary auditory cortical neurons undergo systematic changes during this behavior. However, the present work is the first published measurement of frequency discrimination acuity in ferrets.
The pure tone Weber fractions of ferrets in our high/low discrimination task were higher than those previously reported for frequency change detection tasks carried out in other species (reviewed by Heffner et al., 1971; Fay 1988; Shofner, 2005). For example, on frequency discrimination tasks that use a 500-Hz reference tone, Weber fractions have been measured to be 3.4% in chinchillas (Nelson and Kiester, 1978), 1.6% in guinea pigs (Heffner et al., 1971), 1.4% in bushbabies (Heffner et al., 1969a), 2.5% in tree shrews (Heffner et al., 1969b), 3.6% in budgerigars (Dooling and Saunders, 1975) and 1.7% in cats (Elliott et al., 1960). In comparison, the average Weber fraction measured for ferrets in our direction judgment task at this reference frequency was about tenfold greater, at 36.4%. Even across studies that use the same species and very similar tasks, Weber fractions for pitch discrimination can vary widely. The average Weber fractions of chinchillas for a 250-Hz reference tone were measured on go/no-go tasks to be 4.6% by Nelson and Kiester (1978) and 21.2% by Shofner (2000). Large differences in pure tone frequency discrimination performance are also sometimes present across individual members of the same species within the same study. For instance, in one study, a group of nine male macaque monkeys tested with a 1000-Hz reference tone had individual Weber fractions that ranged from approximately 0.9 to 9.0% (Prosen et al., 1990). The pitch direction judgments of human listeners have also been observed to vary widely across individuals (Semal and Demany, 2006).
Nevertheless, the relatively high frequency difference limens measured here in ferrets warrant careful consideration. There are several factors which might explain the difference in frequency acuity measured on our current 2AFC task and previous go/no-go studies. They include differences in the sensory comparison required, cognitive demands, the number of stimulus exposures per trial, variation in sound levels, and the reverberant environment.
The design of the present task required animals to classify a stimulus pitch as “high” or “low” relative to a given reference (a two-alternative-forced-choice, or 2AFC, task), while in most other previous animal studies, the participants were required only to report any detectable change in a continuously repeated sound (a go/no-go task), and did not need to be able to distinguish pitch decreases from increases. Our design therefore requires a more demanding sensory judgment, and one might expect the animals' thresholds to be correspondingly higher.
Another, less intuitive, difficulty is associated with carrying out 2AFC frequency discrimination tasks in animals. Previous studies have suggested that when performing go/no-go tasks, animals tend to make response choices based on the “quality” of sounds (such as frequency or timbre), while in 2AFC tasks animals prefer to respond to the spatial location of the sound source (reviewed by Burdick, 1979). The reasons for this task specificity, which appears to be much more pronounced in the auditory than the visual modality, are still unclear, but they may include a difficulty in initially learning the rules required by a 2AFC task (which amount to simply “approach the source” in localization tasks), and the working memory challenge involved with arbitrarily mapping sound quality onto two response options. Sound quality discriminations have been shown to be more difficult to train on 2AFC tasks than in go/no-go alternatives in a number of species, including guinea pigs (Upton, 1929), chinchillas (Burdick, 1980), cats (Elliott et al., 1962), and monkeys (Elliott et al., 1971). Of particular interest to the present discussion is a frequency discrimination study by Dobrzecka and Konorski (1968), described in Burdick (1979). They report that dogs more accurately discriminated the frequencies of pairs of tones in a go/no-go task than in a two-choice procedure (but for an alternative interpretation see Neill and Harrison, 1987).
Our task design also differs from go/no-go studies of frequency discrimination in terms of the number of stimuli presented on each trial. In our task, as in most human studies, the ferrets heard only one instance of the reference and target before making their response choice on each trial. In the typical go/no-go frequency discrimination task, a sequence of reference tones is presented and the signal for detection is a frequency alteration in two or more of the tones near the end of this sequence. Therefore, in these studies, the animals are provided with “multiple looks” at the sounds to be discriminated in any one trial, whereas in our task a sensory decision is made after hearing each stimulus only once.
The randomization of stimulus level in the present study across 15 dB is larger than that used by most previous studies. We included this variation to prevent our animals from using simple spectral level cues, and to encourage them to rely solely on the F0 of sounds to solve the task. This manipulation might also encourage subjects to rely more heavily on temporal pitch cues and/or spectral matching of lower harmonics in our stimuli, since the higher harmonics may have been resolved at low sound levels but unresolved at higher ones.
Finally, the potential effect of reverberations on pitch discrimination is worth consideration. Here, stimuli were presented via a loudspeaker inside a chamber with vacuum glass walls, which provided good sound isolation but are also reverberant. In most human studies, sounds are presented over headphones to avoid reverberation from the environment. In a recent study, Sayles and Winter (2008) demonstrated that reverberation can significantly impair one set of pitch cues, namely those that may arise from regularities in the temporal envelope of the high frequency part of the sound signal. However, the signals we used in this experiment carry a substantial amount of pitch information that is far less affected by reverberation. This includes the temporal fine structure of the sound (i.e. frequency components at less than about 4 kHz to which auditory nerve fibres can phase lock), as well as resolvable low harmonics. In normal human listeners, spectral template matching is not thought to be the main strategy used to discriminate sounds that differ in pitch (Moore and Peters, 1992), but the presence of resolved harmonics can nevertheless contribute to better pitch discrimination thresholds (Bernstein and Oxenham, 2006). At present, little is known about the degree to which other mammals normally rely on spectral and temporal cues for pitch discrimination.
In summary, we attribute the relatively elevated Weber fractions reported here for pitch judgments in ferrets to the higher cognitive and sensory demands posed by a low/high pitch judgment compared to a mere change detection. This result is consistent with a study of pitch discrimination in children with cochlear implants, who achieved better pitch discrimination thresholds in a change detection task than in a task that required them to make pitch direction judgments (Vongpaisal et al., 2006). The authors of that study suggest that while subjects may have been able to solve the change detection task using spectral cues, these cues may have been insufficient to enable them to order the same sounds (synthesized piano notes) along a pitch scale. Thus the performance in a change detection task may not reflect subjects' ability to tell high from low pitch. Along similar lines, Semal and Demany (2006) have shown that listeners with frequency difference limens that are elevated but within the normal range find it easier to detect frequency changes than to identify the direction of those changes, while, counter-intuitively, listeners with the best frequency acuity have better thresholds on a pitch direction-identification task than on a change detection task. The authors hypothesize that the human auditory cortex may contain frequency “shift detectors”, which would enable the classification of small pitch changes (Demany and Ramos, 2005). The likely physiological basis for such shift detectors in the human brain, or indeed that of other mammals, remains unknown. From a comparative psychophysics point of view, it will be interesting for future studies to measure ferrets' difference limens for pitch on a go/no-go, F0 change detection task, in order to determine if and how thresholds on this task might differ from our current 2AFC measurements.
Our results indicate interesting parallels between humans and ferrets. For both species, Weber fractions changed across reference F0s in similar ways, and thresholds were indistinguishable in the pure tone and the artificial vowel versions of the task. However, humans discriminated changes in F0 consistently and substantially better than ferrets. This was not entirely surprising, given that previous studies have already established that the pure tone frequency discrimination performance of humans is superior to that of many other mammals across the reference range tested here (Fay, 1988). It has been proposed that the superior performance of humans in these tasks might be due to differences in basilar membrane mechanics or higher densities of ganglion cells in the human cochlea (Elliott et al., 1960). While humans' exceptional sensitivity to low-frequency pure tones may also facilitate pitch discrimination, ferrets are easily able to detect 200-1200 Hz tones presented at the levels used in the present study (Kelly et al., 1986). Furthermore, the fundamental frequencies of ferrets' vocalizations are within this frequency range (unpublished observations from the laboratory of Didier Depireux), so it is reasonable to expect that they might perceive the pitch of artificial vowels.
C. Pitch discrimination for complex sounds verses pure tones
We observed that, for both ferrets and humans, discrimination thresholds for the pitch of artificial vowels were not significantly different from those obtained with pure tone stimuli. However, previous studies of the pitch discrimination performance of humans and other species have shown that F0 acuity can depend on the type of periodic stimulus presented (reviewed by Shofner, 2005). For example, in chinchillas (Shofner, 2000) and humans (Henning and Grosberg, 1968; Moore et al., 1984), discrimination of the F0 of harmonic tone complexes can be more acute than that for pure tones at F0. In contrast, discrimination of the F0 of iterated rippled noise is poorer than pure tone frequency discrimination in humans (Yost, 1978) and chinchillas (Shofner et al., 2007), while these stimulus types yield similar discrimination thresholds in goldfish (Fay et al., 1983). Finally, discrimination of the modulation frequencies of sinusoidally amplitude-modulated noise bursts is poorer than the discrimination of pure tone frequencies in macaque monkeys (Moody, 1994), chinchillas (Long and Clark, 1984), parakeets (Dooling and Searcy, 1981) and humans (Formby, 1985).
Other authors have previously measured the pitch discrimination of human listeners using artificial vowel sounds (Flanagan and Saslow, 1958; Klatt, 1973) and subjects' thresholds on these tasks have been very modestly but consistently better than those for pure tone frequency discrimination. Weber fractions have been reported to be in the range of 0.23% - 0.40% on pitch discrimination tasks that use a 120 Hz reference vowel (Flanagan and Saslow, 1958; Klatt, 1973). These values are slightly better than the Weber fractions of 0.44% measured for 120 Hz pure tones by Flanagan and Saslow (1958). The variability in performance across subjects in our study may have been too large to observe more subtle effects of stimulus type on pitch discrimination, even though the expected effect of reference pitch on performance was clear in our data. The consensus between this study and previous ones seems to be that if there are differences between F0 difference limens for tones and vowels, these differences are small compared to the variation in difference limens across reference F0s.
D. Pitch discrimination thresholds of human listeners
The Weber fractions measured for human subjects in our study were larger than those previously reported (Flanagan and Saslow, 1958; Klatt, 1973; Wier et al., 1977), and there are a number of possible explanations for this discrepancy. First, this could be due to the smaller number of training and testing trials used in our study. Human subjects in our study carried out approximately 1200 pitch discrimination trials, while listeners in previous studies were much more highly practiced (Flanagan and Saslow, 1958; Klatt, 1973). Both human and animal frequency difference limens are known to continue to improve with training (Prosen et al., 1990; Demany and Semal, 2002; Banai and Ahissar, 2004; Delhommeau et al., 2005). The wide age range of our subjects may also have led to higher pitch thresholds, since the pitch discrimination thresholds of older adults for artificial vowels have been shown to be three times larger than those of young adults (Vongpaisal and Pichora-Fuller, 2007). Finally, while our sounds were presented via a loudspeaker, previous studies of pitch discrimination in humans have presented stimuli over headphones. As mentioned above, reverberation may have impaired the use of high frequency envelope pitch cues in our stimuli. While it is therefore likely that our results do not reflect the limit of pitch discrimination in humans under ideal listening conditions, we would argue that our results are representative of the pitch discrimination capability of average human listeners functioning in everyday acoustic environments.
E. Pitch discrimination as a function of the reference F0
For both ferret and human listeners, difference limens in Hz were larger for higher-pitched references - an effect that has also been demonstrated in previous studies of pure tone frequency discrimination in humans and non-human animals (Fay, 1988). Figure 11 shows that while Weber fractions generally decrease across the range of references between 200 - 500 Hz, they tend to show an opposite trend for the range of references above 500 Hz. This is manifest in both the human and ferret data, though it is more pronounced in the latter. A similar trend has been observed in past studies of pure-tone frequency discrimination in humans (Rosenblith and Stevens, 1953; Moore, 1973; Wier et al., 1977). While few non-human frequency discrimination studies have sampled reference frequencies below 500 Hz, those that have show compatible trends in the frequency difference limens of pigeons (Sinnott et al., 1980), bushbabies (Heffner et al., 1969a), chinchillas (Nelson and Kiester, 1978) and cats (Elliott et al., 1960). That is, difference limens are constant for references below ~500 Hz and rise thereafter. The physiological mechanisms underlying pitch discrimination remain poorly understood, but the prevalence of such common trends suggests that similar mechanisms may be at work across a wide variety of species.
F. Conclusions
This study shows that ferrets can be trained to judge the pitch of complex sounds along a low/high scale on a 2AFC task. Ferrets' acuity on this pitch discrimination task is dependent on the F0 of the reference sound. We observed very similar discrimination performance for pure tones, where the periodicity information is mapped onto the tonotopic axis of the ascending auditory pathway, and for complex sounds, where the existence of an anatomical pitch map remains uncertain. These effects were consistent across ferret and human data, although the pitch acuity of humans was much better overall than that of ferrets.
V. ACKNOWLEDGEMENTS
This research was supported by a Biotechnology and Biological Sciences Research Council Project Grant (BB/D009758/1) to J.W.H Schnupp, A.J. King, and J.K. Bizley, a Rothermere Fellowship and Hector Pilling Scholarship to K.M.M. Walker, and a Wellcome Trust Principal Research Fellowship to A.J. King. We wish to thank the undergraduate dissertation students who have assisted in carrying out this work. Finally, we are grateful to the Reviewers and Editor of this manuscript, whose insightful suggestions helped to improve the quality of our report.
Footnotes
PACS numbers: 43.66.Gf (Detection and discrimination of sound by animals), 43.66.Hg (Pitch), 43.66.Fe. (Discrimination: intensity and frequency), 43.80.Lb (Sound reception by animals: anatomy, physiology, auditory capacities, processing).
VI. REFERENCES
- Banai K, Ahissar M. Poor frequency discrimination probes dyslexics with particularly impaired working memory. Audiol. Neuro-otol. 2004;9:328–340. doi: 10.1159/000081282. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wang X. The neuronal representation of pitch in primate auditory cortex. Nature. 2005;436:1161–1165. doi: 10.1038/nature03867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein JGW, Oxenham AJ. The relationship between frequency selectivity and pitch discrimination: Effects of stimulus level. J. Acoust. Soc. Am. 2006;120:3916–3928. doi: 10.1121/1.2372451. [DOI] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Parsons CH, King AJ. Role of auditory cortex in sound localization in the midsagittal plane. J. Neurophysiol. 2007;98:1763–1774. doi: 10.1152/jn.00444.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Walker KMM, Silverman BW, King AJ, Schnupp JWH. Interdependent encoding of pitch, timbre and spatial location in auditory cortex. J. Neurosci. 2009;29:2064–2075. doi: 10.1523/JNEUROSCI.4755-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Bucks C, Scheich H. Macaque monkeys discriminate pitch relationships. Cognition. 2004;91:259–272. doi: 10.1016/j.cognition.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Burdick CK. The effect of behavioural paradigm on auditory discrimination learning: A literature review. J. Aud. Res. 1979;19:59–82. [PubMed] [Google Scholar]
- Burdick CK. Auditory discrimination learning by the chinchilla: comparison of go/no go and two-choice procedures. J. Aud. Res. 1980;20:1–29. [PubMed] [Google Scholar]
- Capranica RR. Vocal response of the bullfrog to natural and synthetic mating calls. J. Acoust, Soc. Am. 1966;40:1131–1139. [Google Scholar]
- Cynx J. Similarities in absolute and relative pitch perception in songbirds (starling and zebra finch) and a nonsongbird (pigeon) J. Comp. Psychol. 1995;109:261–267. [Google Scholar]
- D'Amato MR. A search for tonal pattern perception in cebus monkey: why monkeys can't hum a tune. Music Percept. 1988;5:452–480. [Google Scholar]
- de Lafuente V, Romo R. Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proc. Nat. Acad. Sci. U. S. A. 2006;103:14266–14271. doi: 10.1073/pnas.0605826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhommeau K, Micheyl C, Jouvent R. Generalization of frequency discrimination learning across frequencies and ears: implications for underlying neural mechanisms in humans. J. Assoc. Res. Otolaryngol. 2005;6:171–179. doi: 10.1007/s10162-005-5055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demany L, Ramos C. On the binding of successive sounds: perceiving shifts in non-perceived pitches. J. Acoust. Soc. Am. 2005;117:833–841. doi: 10.1121/1.1850209. [DOI] [PubMed] [Google Scholar]
- Demany L, Semal C. Learning to perceive pitch differences. J. Acoust. Soc. Am. 2002;111:1377–1388. doi: 10.1121/1.1445791. [DOI] [PubMed] [Google Scholar]
- Dobrzecka C, Konorski J. Acta. Biol. Exper. Vol. 28. Warsaw: 1968. Qualitative versus directional cues in differential conditioning. IV. Left leg - right leg differentiation to nondirectional cues; pp. 61–69. [PubMed] [Google Scholar]
- Dooling RJ, Leek MR, Gleich O, Dent ML. Auditory temporal resolution in birds: discrimination of harmonic complexes. J. Acoust. Soc. Am. 2002;112:748–759. doi: 10.1121/1.1494447. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Saunders JC. Hearing in the parakeet (Melopsittacus undulatus): absolute thresholds, critical ratios, frequency difference limens, and vocalizations. J. Comp. Physiol. Psychol. 1975;88:1–20. doi: 10.1037/h0076226. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Searcy MH. Amplitude modulation thresholds for the parakeet (Melopsittacus undulatus) J. Comp. Physiol. 1981;143:383–388. [Google Scholar]
- Ehret G. Frequency and intensity difference limens and nonlinearities in the ear of the housemouse (Mus. musculus) J. Comp. Physiol. 1975;102:321–336. [Google Scholar]
- Elliott DN, Frazier LA, Haydon RC. Relational and absolute cues in auditory discrimination by monkeys. Percept. Psychophys. 1971;10:278–282. [Google Scholar]
- Elliott DN, Frazier LA, Riach W. A tracking procedure for determining the cat's frequency discrimination. J. Exp. Anal. Behav. 1962;5:323–328. doi: 10.1901/jeab.1962.5-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DN, Stein L, Harrison MJ. Determination of absolute intensity thresholds and frequency difference thresholds in cats. J. Acoust. Soc. Am. 1960;32:380–384. [Google Scholar]
- Fay RR. Hearing in Vertebrates: A Psychophysics Databook. Hill-Fay Associates; Winnetka, Il: 1988. pp. 451–458. [Google Scholar]
- Fay RR, Yost WA, Coombs S. Psychophysics and neurophysiology of repetition noise processing in a vertebrate auditory system. Hear. Res. 1983;12:31–55. doi: 10.1016/0378-5955(83)90117-x. [DOI] [PubMed] [Google Scholar]
- Flanagan JL, Saslow MG. Pitch discrimination for synthetic vowels. J. Acoust. Soc. Am. 1958;30:435–442. [Google Scholar]
- Formby C. Differential sensitivity to tonal frequency and to the rate of amplitude modulation of broadband noise by normally hearing listeners. J. Acoust. Soc. Am. 1985;78:70–77. doi: 10.1121/1.392456. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, Shamma SA. Active listening: task-dependent plasticity of spectrotemporal receptive fields in primary auditory cortex. Hear. Res. 2005;206:159–176. doi: 10.1016/j.heares.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, Shamma SA. Adaptive changes in cortical receptive fields induced by attention to complex sounds. J. Neurophysiol. 2007;98:2337–2346. doi: 10.1152/jn.00552.2007. [DOI] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat. Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Fujita S, Ito J. Ability of nucleus cochlear implantees to recognize music. Ann. Otol. Rhinol. Laryngol. 1999;108:634–640. doi: 10.1177/000348949910800702. [DOI] [PubMed] [Google Scholar]
- Gfeller KE, Turner C, Woodworth G, Mehr M, Fearn R, Knutson J, Witt S, Stordahl J. Recognition of familiar melodies by adult cochlear implant recipients and normal-hearing adults. Coch. Implants Int. 2002;3:29–53. doi: 10.1179/cim.2002.3.1.29. [DOI] [PubMed] [Google Scholar]
- Hayar A, Bryant JL, Boughter JD, Heck DH. A low-cost solution to measure mouse licking in an electrophysiological setup with a standard analog-to-digital converter. J. Neurosci. Methods. 2006;153:203–207. doi: 10.1016/j.jneumeth.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Ravizza RJ, Masterton B. Hearing in primitive mammals IV: Bushbaby (Galago senegalensis) J. Aud. Res. 1969a;9:19–23. [Google Scholar]
- Heffner HE, Ravizza RJ, Masterton B. Hearing in primate mammals III: Tree shrew (Tupaia glis) J. Aud. Res. 1969b;9:12–18. [Google Scholar]
- Heffner R, Heffner H, Masterton B. Behavioral measurements of absolute and frequency-difference thresholds in guinea pig. J. Acoust. Soc. Am. 1971;49:1888–1895. doi: 10.1121/1.1912596. [DOI] [PubMed] [Google Scholar]
- Henning GB, Grosberg SL. Effect of harmonic components on frequency discrimination. J. Acoust. Soc. Am. 1968;44:1386–1389. doi: 10.1121/1.1911273. [DOI] [PubMed] [Google Scholar]
- Hine JE, Martin RL, Moore DR. Free-field binaural unmasking in ferrets. Behav. Neurosci. 1994;108:196–205. doi: 10.1037//0735-7044.108.1.196. [DOI] [PubMed] [Google Scholar]
- Izumi A. Relative pitch perception in Japanese monkeys (Macaca fuscata) J. Comp. Psychol. 2001;115:127–131. doi: 10.1037/0735-7036.115.2.127. [DOI] [PubMed] [Google Scholar]
- Johnson K. The role of perceived speaker identity in F0 normalization of vowels. J. Acoust. Soc. Am. 1990;88:642–654. doi: 10.1121/1.399767. [DOI] [PubMed] [Google Scholar]
- Kacelnik O, Nodal FR, Parsons CH, King AJ. Training-induced plasticity of auditory localization in adult mammals. PLoS Biol. 2006;4:e71. doi: 10.1371/journal.pbio.0040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri S, Depireux DA, Shamma SA. Perception and cortical neural coding of harmonic fusion in ferrets. J. Acoust. Soc. Am. 2008;123:2701–2716. doi: 10.1121/1.2902178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, Kavanagh GL. Sound localization after unilateral lesions of inferior colliculus in the ferret (Mustela putorius) J. Neurophysiol. 1994;71:1078–1087. doi: 10.1152/jn.1994.71.3.1078. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Kavanagh GL, Dalton JC. Hearing in the ferret (Mustela putorius): thresholds for pure tone detection. Hear. Res. 1986;24:269–275. doi: 10.1016/0378-5955(86)90025-0. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Rooney BJ, Phillips DP. Effects of bilateral auditory cortical lesions on gap-detection thresholds in the ferret (Mustela putorius) Behav. Neurosci. 1996;110:542–550. doi: 10.1037//0735-7044.110.3.542. [DOI] [PubMed] [Google Scholar]
- Klatt DH. Discrimination of fundamental frequency contours in synthetic speech: implications for models of pitch perception. J. Acoust. Soc. Am. 1973;53:8–16. doi: 10.1121/1.1913333. [DOI] [PubMed] [Google Scholar]
- Koda H, Masataka N. A pattern of common acoustic modification by human mothers to gain attention of a child and by macaques of others in their group. Psychol. Rep. 2002;91:421–422. doi: 10.2466/pr0.2002.91.2.421. [DOI] [PubMed] [Google Scholar]
- Kojima S, Izumi A, Ceugniet M. Identification of vocalizers by pant hoots, pant grunts and screams in a chimpanzee. Primates. 2003;44:225–230. doi: 10.1007/s10329-002-0014-8. [DOI] [PubMed] [Google Scholar]
- Liu J, Newsome WT. Correlation between speed perception and neural activity in the middle temporal visual area. J. Neurosci. 2005;25:711–722. doi: 10.1523/JNEUROSCI.4034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GR, Clark WW. Detection of frequency and rate modulation by the chinchilla. J. Acoust. Soc. Am. 1984;75:1184–1190. doi: 10.1121/1.390768. [DOI] [PubMed] [Google Scholar]
- Marvit P, Crawford JD. Auditory discrimination in a sound-producing electric fish (Pollimyrus): tone frequency and click-rate difference detection. J. Acoust. Soc. Am. 2000;108:1819–1825. doi: 10.1121/1.1287845. [DOI] [PubMed] [Google Scholar]
- Mesgarani N, David SV, Fritz JB, Shamma SA. Phoneme representation and classification in primary auditory cortex. J. Acoust. Soc. Am. 2008;123:899–909. doi: 10.1121/1.2816572. [DOI] [PubMed] [Google Scholar]
- Moody DB. Detection and discrimination of amplitude-modulated signals by macaque monkeys. J. Acoust. Soc. Am. 1994;95:3499–3510. doi: 10.1121/1.409967. [DOI] [PubMed] [Google Scholar]
- Moore BC. Frequency difference limens for short-duration tones. J. Acoust. Soc. Am. 1973;54:610–619. doi: 10.1121/1.1913640. [DOI] [PubMed] [Google Scholar]
- Moore BC. An Introduction to the Psychology of Hearing. Academic Press; London: 2003. pp. 195–231. Chap. 6. [Google Scholar]
- Moore BC, Glasberg BR, Shailer MJ. Frequency and intensity difference limens for harmonics within complex tones. J. Acoust. Soc. Am. 1984;75:550–561. doi: 10.1121/1.390527. [DOI] [PubMed] [Google Scholar]
- Moore BC, Peters RW. Pitch discrimination and phase sensitivity in young and elderly subjects and its relationship to frequency selectivity. J. Acoust. Soc. Am. 1992;91:2881–2893. doi: 10.1121/1.402925. [DOI] [PubMed] [Google Scholar]
- Neill JC, Harrison JM. Auditory discrimination: the Konorski quality-location effect. J. Exp. Anal. Behav. 1987;48:81–95. doi: 10.1901/jeab.1987.48-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken I, Bizley JK, Nodal FR, Ahmed B, King AJ, Schnupp JW. Responses of auditory cortex to complex stimuli: functional organization revealed using intrinsic optical signals. J. Neurophysiol. 2008;99:1928–1941. doi: 10.1152/jn.00469.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA. Song frequency as a cue for recognition of species and individuals in the field sparrow (Spizella pusilla) J. Comp. Psychol. 1989;103:171–176. doi: 10.1037/0735-7036.103.2.171. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Kiester TE. Frequency discrimination in the chinchilla. J. Acoust. Soc. Am. 1978;64:114–126. doi: 10.1121/1.381977. [DOI] [PubMed] [Google Scholar]
- Nodal FR, Bajo VM, Parsons CH, Schnupp JW, King AJ. Sound localization behavior in ferrets: comparison of acoustic orientation and approach-to-target responses. Neuroscience. 2008;154:397–408. doi: 10.1016/j.neuroscience.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SC, Hulse SH, Cynx J. Relative pitch perception in the European starling (Sturnus vulgaris): further evidence for an elusive phenomenon. J. Exp. Psychol. Anim. Behav. Process. 1989;15:137–146. [PubMed] [Google Scholar]
- Parsons CH, Lanyon RG, Schnupp JWH, King AJ. Effects of altering spectral cues in infancy on horizontal and vertical sound localization by adult ferrets. J. Neurophysiol. 1999;82:2294–2309. doi: 10.1152/jn.1999.82.5.2294. [DOI] [PubMed] [Google Scholar]
- Pfingst BE. Comparison of spectral and nonspectral frequency difference limens for human and nonhuman primates. J. Acoust. Soc. Am. 1993;93:2124–2129. doi: 10.1121/1.406673. [DOI] [PubMed] [Google Scholar]
- Pressnitzer D, Bestel J, Fraysee B. Music to electrical ears: Pitch and timbre perception by cochlear implant patients. Ann. N.Y. Acad. Sci. 2005;1060:343–345. doi: 10.1196/annals.1360.050. [DOI] [PubMed] [Google Scholar]
- Prosen CA, Moody DB, Sommers MS, Stebbins WC. Frequency discrimination in the monkey. J. Acoust. Soc. Am. 1990;88:2152–2158. doi: 10.1121/1.400112. [DOI] [PubMed] [Google Scholar]
- Rosenblith WA, Stevens KN. On the DL for frequency. J. Acoust. Soc. Am. 1953;25:980–985. [Google Scholar]
- Sayles M, Winter IM. Reverberation challenges the temporal representation of the pitch of complex sounds. Neuron. 2008;58:789–801. doi: 10.1016/j.neuron.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Langner G. Periodicity coding in the inferior colliculus of the cat. II. Topographical organization. J. Neurophysiol. 1988;60:1823–1840. doi: 10.1152/jn.1988.60.6.1823. [DOI] [PubMed] [Google Scholar]
- Schulze H, Hess A, Ohl FW, Scheich H. Superposition of horseshoe-like periodicity and linear tonotopic maps in auditory cortex of the Mongolian gerbil. Eur. J. Neurosci. 2002;15:1077–1084. doi: 10.1046/j.1460-9568.2002.01935.x. [DOI] [PubMed] [Google Scholar]
- Schulze H, Langner G. Periodicity coding in the primary auditory cortex of the Mongolian gerbil (Meriones unguiculatus): two different coding strategies for pitch and rhythm? J. Comp. Physiol. 1997;181:651–663. doi: 10.1007/s003590050147. [DOI] [PubMed] [Google Scholar]
- Semal C, Demany L. Individual differences in the sensitivity to pitch direction. J. Acoust. Soc. Am. 2006;120:3907–3915. doi: 10.1121/1.2357708. [DOI] [PubMed] [Google Scholar]
- Shofner WP. Comparison of frequency discrimination thresholds for complex and single tones in chinchillas. Hear. Res. 2000;149:106–114. doi: 10.1016/s0378-5955(00)00171-4. [DOI] [PubMed] [Google Scholar]
- Shofner WP. Comparative Aspects of Pitch Perception. In: Plack CJ, Oxenham AJ, Fay RR, Popper AN, editors. Pitch: Neual Coding and Perception. Springer Science + Business Media, Inc.; New York: 2005. pp. 56–98. [Google Scholar]
- Shofner WP, Yost WA, Whitmer WM. Pitch perception in chinchillas (Chinchilla laniger): stimulus generalization using rippled noise. J. Comp. Psychol. 2007;121:428–439. doi: 10.1037/0735-7036.121.4.428. [DOI] [PubMed] [Google Scholar]
- Sinnott JM, Petersen MR, Hopp SL. Frequency and intensity discrimination in humans and monkeys. J. Acoust. Soc. Am. 1985;78:1977–1985. doi: 10.1121/1.392654. [DOI] [PubMed] [Google Scholar]
- Sinnott JM, Sachs MB, Hienz RD. Aspects of frequency discrimination in passerine birds and pigeons. J. Comp. Physiol. Psychol. 1980;94:401–415. doi: 10.1037/h0077681. [DOI] [PubMed] [Google Scholar]
- Smith DR, Patterson RD, Turner R, Kawahara H, Irino T. The processing and perception of size information in speech sounds. J. Acoust. Soc. Am. 2005;117:305–318. doi: 10.1121/1.1828637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Brozek G, Jilek M. Auditory frequency and intensity discrimination in pigmented rats. Hear. Res. 1996;100:107–113. doi: 10.1016/0378-5955(96)00101-3. [DOI] [PubMed] [Google Scholar]
- Talwar SK, Gerstein GL. Auditory frequency discrimination in the white rat. Hear. Res. 1998;126:135–150. doi: 10.1016/s0378-5955(98)00162-2. [DOI] [PubMed] [Google Scholar]
- Talwar SK, Gerstein GL. A signal detection analysis of auditory-frequency discrimination in the rat. J. Acoust. Soc. Am. 1999;105:1784–1800. doi: 10.1121/1.426716. [DOI] [PubMed] [Google Scholar]
- Upton M. The auditory sensitivity of guinea pigs. Am. J. Psychol. 1929;41:412–421. [Google Scholar]
- Vongpaisal T, Pichora-Fuller MK. Effect of age on F0 difference limen and concurrent vowel identification. J. Speech. Lang. Hear. Res. 2007;50:1139–1156. doi: 10.1044/1092-4388(2007/079). [DOI] [PubMed] [Google Scholar]
- Vongpaisal T, Trehub SE, Schellenberg EG. Song recognition by children and adolescents with cochlear implants. J. Speech. Lang. Hear. Res. 2006;49:1091–1103. doi: 10.1044/1092-4388(2006/078). [DOI] [PubMed] [Google Scholar]
- Wickens TD. Elementary Signal Detection Theory. Oxford University Press; New York, NY: 2002. [Google Scholar]
- Wier CC, Jesteadt W, Green DM. Frequency discrimination as a function of frequency and sensation level. J. Acoust. Soc. Am. 1977;61:178–184. doi: 10.1121/1.381251. [DOI] [PubMed] [Google Scholar]
- Witte RS, Kipke DR. Enhanced contrast sensitivity in auditory cortex as cats learn to discriminate sound frequencies. Brain Res. Cogn. Brain Res. 2005;23:171–184. doi: 10.1016/j.cogbrainres.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Yin P, Fritz J, Shamma S. Can ferrets perceive relative pitch? In: Santi PA, editor. Assoc. Res. Otolaryngol. Abs. Denver; Colorado, USA: 2007. p. 141. [Google Scholar]
- Yost WA. Pitch and pitch discrimination of broadband signals with rippled power spectra. J. Acoust. Soc. Am. 1978;63:1166–1175. doi: 10.1121/1.381824. [DOI] [PubMed] [Google Scholar]