Abstract
This study aims to start the development of a nanomedicine, containing indisulam solubilized in sterically stabilized micelles (SSM) composed of DSPE-PEG2000 or sterically stabilized mixed micelles (SSMM) composed of DSPE-PEG2000 plus egg phosphatidylcholine (EPC). Micelles were prepared by coprecipitation and reconstitution of drug and lipids. Particle size distributions of micellar formulations were determined by quasi-elastic light scattering (QELS). Amounts of solubilized drug were determined by Reverse Phase-High Performance Liquid Chromatography (RP-HPLC). In vitro cytotoxicity of indisulam in nanocarrier was determined on MCF-7 cell line by NCI developed Sulforhodamine B assay. Optimal solubilized indisulam concentrations in 5mM total lipid were 10 µg/ml for SSMM, and 400 µg/ml for SSM. HPLC results demonstrated encapsulation capacity of both micelles was over 95%. In vitro studies showed indisulam in micellar system was more effective than free indisulam. Optimized formulation was successfully freeze-dried without any addition of lyo- and cryo-protectants. We conclude SSM is a promising nanocarrier for indisulam, and indisulam-SSM should be developed further as a novel targeted nanomedicine.
Keywords: DSPE-PEG2000 and EPC; PEGylated Phospholipids; water-insoluble anticancer drug, indisulam; Sterically stabilized simple micelles; Sterically stabilized mixed micelles
INTRODUCTION
Recently, our laboratory have reported on the use of PEGylated phospholipids such as poly(ethylene glycol 2000) grafted 1,2-Distearoyl phosphoethanolamine, (DSPE-PEG2000), to form sterically stabilized micelles (SSM) or DSPE-PEG2000 plus egg phosphatidylcholine (EPC) to form sterically stabilized mixed micelles (SSMM) as targeted nanocarriers for the delivery of poorly water soluble drugs [1, 2]. Components of these micelles are FDA approved, safe, biocompatible, and relatively nontoxic [2, 3]. The PEG on the surface of the micelles renders them to be sterically stabilized, preventing opsonization and reticular endothelial system uptake [4].
These sterically stabilized micelles, because of their low critical micellar concentration (CMC) values and most likely because of strong interactions between the acyl chains in the core region, are also relatively stable on dilution [1, 5]. In addition, the small size (~15nm) of these carrier systems can provide targeted delivery to cancer tissue or other injured tissues by selective extravasation through leaky vasculature [6]. Furthermore, preparation of SSM is simple and efficient compared with bile salt/phospholipid mixed micelles or liposomes [7–9].
Indisulam or also called E7070, N-(3-chloro-7-indolyl)-1, 4-benzenedisulfonamide is a sulfonamide anticancer agent that disrupts the cell cycle progression at the G1/S phase [10–12]. Although the exact mechanism of action remains uncertain, some identified effects include induced expression of p53 and p21, inhibition of phosphorylation of the tumor supressor retinoblastoma protein (Rb), reduced expression of cyclins and cyclin-dependent kinases [13,14]; and inhibition of carbonic anhydrase IX [15]. Currently, indisulam is being tested in combination with capecitabine and carboplatin for clinical trials [16, 17]. Indisulam alone is shown to be effective on human breast cancer by in vitro and in vivo studies [12, 18, 19]. It was found to be considerably different from conventional anticancer drugs in clinical use with respect to its cell cycle effect and its tumor type selectivity [14, 20]. However, its efficacy in clinical trials for breast cancer was not up to expectations due to dose-limiting toxicities [19, 21]. Aqueous indisulam formulations tested in clinical trials are not made of nanoparticles and drug molecules can extravasate in any vasculature in the body resulting in high toxicity on healthy tissues. One of the reasons for hematological toxicity in these formulations tested in clinical trials is that indisulam binds to plasma proteins (albumin) and to erythrocytes (carbonic anhydrase) in a saturable manner [22]. However, if indisulam is solubilized in micelles, then the drug will not be free to interact with blood components and the nanosize of micelles will significantly reduce drug distribution and toxicity to normal tissues. In addition, the drug will be accumulated by passive targeting at the tumor sites by extravasations due to leaky vasculature [4]. Furthermore, nanomicelles can improve the solubility of hydrophobic compounds (aqueous solubility of indisulam is less than 5µg/ml at 37 °C, provided by Eisai, Inc.) in the aqueous medium to render them suitable for parenteral administration. Previously, we have shown improved solubility of other anticancer drugs such as camptothecin and paclitaxel using simple and mixed micelles [2, 7]. The purpose of this study was to investigate the solubility potential of SSM and SSMM for indisulam, and determine an optimized formulation to initiate the development of a novel nanomedicine for targeted treatment of breast cancer.
MATERIALS AND METHODS
Chemicals
1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy- poly(ethylene glycol 2000) (DSPE-PEG2000) and Egg-phosphatidylcholine (EPC) was obtained from LIPOID GmbH (Ludwigshafen, Germany). Indisulam was a gift from Eisai Inc. (Tokyo, Japan). All lipid samples were high performance liquid chromatography purified. HPLC-grade methanol, acetonitrile and phosphoric acid were purchased from Fisher Scientific (Ithasca, IL, USA). Eagle’s minimal essential medium (EMEM) was purchased from ATCC (Manassas, VA, USA) All other reagents were of analytical grade.
Preparation of Aqueous Dispersions of Indisulam
Simple and mixed micelles containing indisulam were prepared by coprecipitation method [1,2]. Briefly, for simple micelles, indisulam and poly-(ethylene glycol-2000)-grafted distearoyl phosphatidylethanolamine (DSPE-PEG2000) were dissolved in methanol. For mixed micelles, DSPE-PEG2000 and egg-phosphatidylcholine at optimal molar ratio (90:10) were coprecipitated along with indisulam [1]. The solvent was then removed by vacuum rotary evaporation under a stream of argon. Obtained dry film was further dried under vacuum overnight to remove any traces of remaining solvent. The dried film was rehydrated with isotonic 0.01 M Phosphate buffer, pH 6.0, at which indisulam is in the most stable form. The solution was then flushed with argon, sealed, and equilibrated for 2 hours for simple micelles and 12 hours for mixed micelles at room temperature which was approximately 25 °. The excess of unsolubilized indisulam was removed by centrifugation at 13,000 g for 5 min to obtain a clear dispersion. The maximum solubility of indisulam was determined in simple micelles of DSPE-PEG2000 by keeping the phospholipid concentration fixed and changing the drug concentration (drug:phospholipid molar ratios ranged from 0.005 to 1.04 for simple micelles, and 0.001 to 0.52 for mixed micelles) until a homogenous system was determined by a quasi-elastic light scattering as a single size peak population. Each formulation was prepared in triplicate. The prepared dispersions were then characterized for their particle size and assayed for the drug content. The optimal formulations of SSM or SSMM were chosen based on their formation of a homogenous system with maximum solubilization potential for indisulam.
Particle Size Determination
Particle size distribution and mean diameter of the prepared aqueous dispersions of indisulam were determined by quasi-elastic light scattering using a NICOMP 380 submicron Particle Sizer (Particle Sizing Systems, Santa Barbara, CA, USA) equipped with a 35mW helium-neon laser at 632.8 nm. Mean hydrodynamic particle diameters (đh) in the aqueous dispersions were obtained from the Stokes-Einstein relation using the measured diffusion of particles in solution (η = 0.933, T = 23°C, n = 1.33). Data were analyzed in terms of volume-and intensity-weighted distributions. Each reported experimental result is the average of at least three đh values obtained from analysis of the autocorrelation function accumulated for at least 20 minutes.
Assay of Solubilized Indisulam
The amounts of indisulam solubilized both in the SSM and the SSMM were determined by RP-HPLC. The clear aqueous dispersion was diluted with methanol and 20µl of each sample were injected into YMC-Pack-Pro-C18 column (5µm, 250 × 4.6 mm, 120 Å pore size). The column was eluted with acetonitrile/water/phosphoric acid (430:570:1, v/v/v) at flow rate of 1.2 ml/min. UV absorbance was measured at 223 nm. The drug concentration was calculated from standard curves. Each solution was analyzed in triplicates.
In Vitro Cytotoxicity
MCF-7 breast cancer cell line was used to evaluate the in vitro activity of the formulations. The cell line was maintained in Eagle’s minimal essential medium (EMEM) containing 2mM L-glutamine and Earle’s BSS adjusted to contain 1.5 g/l sodium bicarbonate, 0.1mM nonessential amino acids, 1mM sodium pyruvate and supplemented with 0.01 mg/ml fetal bovine insulin at 37 °C in a humidified, 5% CO2 atmosphere.
Optimum solutions of indisulam-SSM (composed of 5mM DSPE-PEG2000, 400 µg/ml indisulam) chosen from the solubilization studies were used as the test solutions. Drug-free SSM in 0.01M phosphate buffer (pH 6.0) were prepared at the same concentrations as the test solution and used as a negative control. Indisulam in 4% DMSO was used as a positive control. In vitro indisulam cytotoxicity was assessed using the sulforhodamine B assay adopted for a routine antitumor screening test in the National Cancer Institute [23, 24]. Briefly, serial dilutions with either phosphate buffer containing lipid or 4% DMSO were made to obtain indisulam concentrations from 0.1 – 50 mg/ml. MCF-7 cells (190µl ) were trypsinized and plated at a density of 1000 cells per well in a 96-well plate (final DMSO concentration was less then 0.5%). A total of 27 µl/well of the test solutions and controls groups of solvents were added to the microtiter plates. Each sample was evaluated in triplicate. The plates were then incubated for 72 hours in a humidified, 5% CO2 atmosphere.
After the incubation period, the cells were fixed to the plates by adding 100 µl/well of cold 20% trichloroacetic acid and incubated for 1 hour at 4°C. The plates were then washed, air-dried, and stained with 100 µl/well of 0.4% sulforhodamine B in 1% acetic acid for 30 minutes. Then the plates were washed with 1% acetic acid, and 200µl/well of 10mM Tris ([trishydroxymethyl]-aminomethane) buffer was added. The optical density was then read at 515 nm, and the readings obtained for the solvent controls were used to define 100% growth after normalizing for the value obtained for the zero-day control. These data were then expressed as survival percentage verses drug concentration, and ED50 values were calculated using nonlinear regression analysis.
Freeze-drying of Indisulam-SSM
The optimal solubilization ratio of indisulam in DSPE-PEG2000 micelles was determined as drug:lipid molar ratio 0.2:1. In our lyophilization studies, final lipid concentration of indisulam-SSM was 10 mM in order to form a good visually appealing cake [25]. The optimal indisulam-SSM formulation (composed of 10 mM DSPE-PEG2000, 800 µg/mL indisulam) based on indisulam solubilization experiments, without any modification or any addition of a cryoprotectant and lyoprotectant, was placed into 1.5ml glass vials at fill volumes of 1ml and subjected to a lyophilization cycle by FreeZone® 6 (Labconco, Kansas City, MO, USA) [26]. Briefly, prepared samples were stored at −20°C overnight, followed by freezing in liquid nitrogen for 3 minutes. Then frozen samples and controls were lyophilized. Micellar size and indisulam concentration were compared for stability before and after lyophilization.
Data and Statistical Analysis
All the data are expressed as means ± SD. Formulations and characterization data are averages of at least triplicate. In vitro cytotoxicity data are average of triplicates. ED50 values were calculated for each formulation and compared statistically using one-way analysis of variance. A p value < 0.05 was considered statistically significant.
RESULTS
Particle Size
A given phospholipid concentration can solubilize poorly water soluble drug only up to a certain concentration of the drug [26]. Once this maximal threshold is attained, populations of other species are observed by quasi-elastic light scattering. These particles that do not settle down after centrifugation are named as sterically stabilized particles (SSP), since most likely they are stabilized by DSPE-PEG2000 on their surface. Mean hydrodynamic diameters of SSM and SSMM without any presence of SSP were 12.6 ± 2.9 nm (n=20) and 13.0 ± 2.5 nm (n=20) respectively (Table 1).
Table 1.
Solubility of indisulam in 5mM SSM and SSMM; particle sizes of SSM and indisulam-SSM before and after lyophilization
| Formulation | Mean Particle Diameter | Optimum Drug Concentration | |
|---|---|---|---|
| Indisulam-SSMM (5mM lipids) |
12.6 ± 2.9 nm (n=20) | 10 µg/ml | |
| Indisulam-SSM (5mM lipids) |
13.0 ± 2.5 nm (n=20) | 400 µg/ml | |
| Before Lyophilization |
After Lyophilization |
||
| SSM (10mM lipids) | 10.4 ± 1.8 nm (n=3) |
10.7 ± 2.2 nm (n=3) |
0 |
| Indisulam-SSM (10mM lipids) |
10.8 ± 3.1 nm (n=3) |
10.3 ± 2.2 nm (n=3) |
800 µg/ml |
More (40×) drug solubilization is achieved in SSM than in SSMM. (n=20, mean ± SD, p>0.05, SSM mean particle diameter compared to SSMM). Particle sizes of SSM and Indisulam-SSM before and after lyophilization did not significantly change (p>0.05).
Solubilization of Indisulam
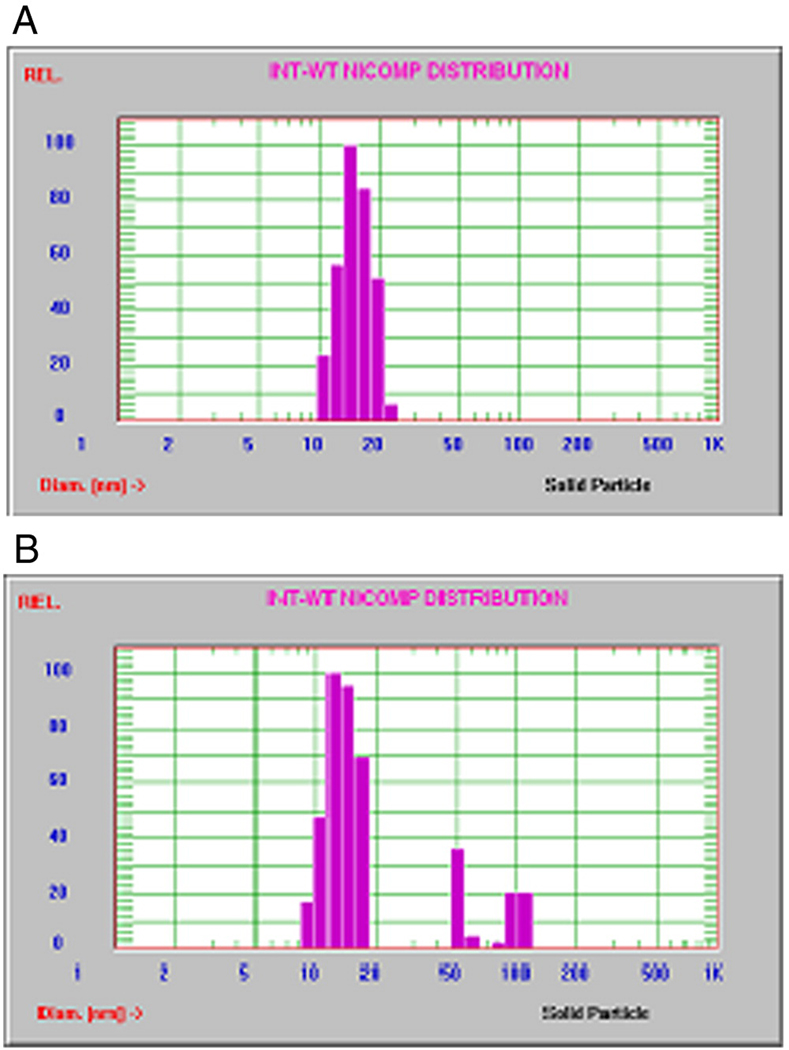
With 5mM fixed total phospholipid concentration of SSMM, we performed experiments at different indisulam concentrations ranging from 2µg/ml to 1000µg/ml. For concentrations at and below 10µg/ml, one uniform size distribution was observed by Nicomp analysis (Fig. 1A). Above this concentration, there were other populations of lipid coated particles coexisting with indisulam-SSMM as indicated by size distribution (Fig. 1B). Therefore, solubilization potential of 5mM SSMM for indisulam was low and only 10µg/ml (drug: lipid molar ratio 0.005: 1).
Figure 1.
Representative Nicomp size distribution of indisulam with SSMM; a) Indisulam-SSMM at optimum drug concentration of 10µg/ml, b) Indisulam-SSMM at excessive drug concentration of 25µg/ml.
Next, we tested SSM for its potential to solubilize indisulam. Sixteen different indisulam concentrations ranging from 2 to 2000 µg/ml were tested with 5mM SSM composed of just DSPE-PEG2000 alone. Surprisingly, all samples at and below 400µg/ml of indisulam had single uniform size distribution, and above this concentration SSP were observed (Fig. 2A and 2B).
Figure 2.
Representative Nicomp size distribution of indisulam with SSM; a) Indisulam-SSM at optimum drug concentration of 400µg/ml, b) Indisulam-SSM at excessive drug concentration of 500µg/ml.
The amount of solubilized drug in the system was calculated by standard curves of RP-HPLC. All standard curves were linear over the tested indisulam concentration range of 20–100 µg/ml. RP-HPLC results showed that at least 95% of the drug was incorporated in the system for all optimum drug concentrations.
In Vitro Cytotoxicity
We investigated whether the interaction between indisulam and the lipids in the formulation affected an anticancer activity of the drug. Since the solubility of indisulam in SSM was 40 fold higher than the solubility in SSMM, we performed in vitro cytotoxicity studies only with indisulam-SSM. The formulations of free indisulam in DMSO and indisulam-SSM were tested against MCF-7, a human breast cancer cell line.
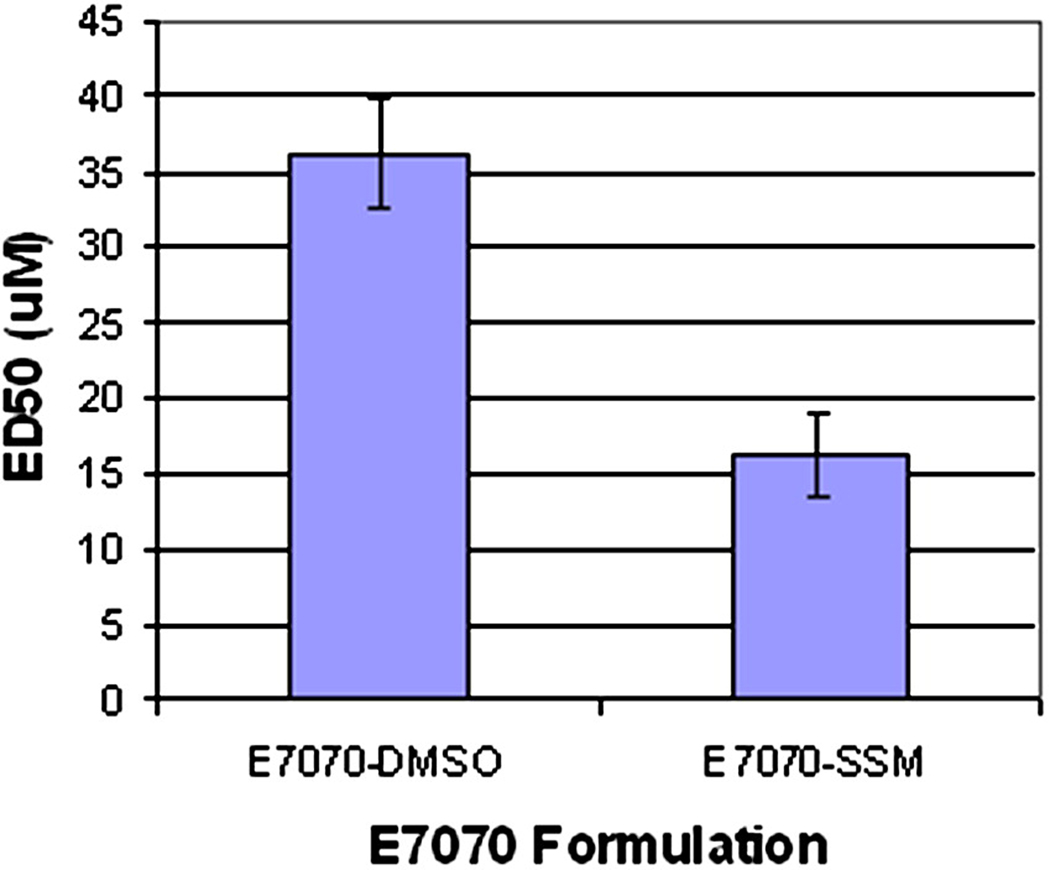
As shown on Figure 3, indisulam in simple micelles was readily available to interact with cancer cells and retained its anticancer activity. As shown on Figure 4, ED50 of indisulam in DMSO was 36.3 ± 3.6 µM, which is similar to the value reported by Nakatsu [18]. Indisulam in SSM proved to be more effective with ED50 value of 16.2 ± 2.7 µM.
Figure 3.
In vitro cytotoxicity of indisulam-SSM after 72 hours by Sulforhodamine B assay (n=3, mean ± SD, * p<0.05 compared to respective samples of indisulam in DMSO).
Figure 4.
ED50 values of indisulam in DMSO and indisulam-SSM (3.6.3 ± 3.6 µM and 16.2 ± 2.7 µM respectively).
Freeze Drying
PEGylated lipids are not stable in aqueous media and subject to degradation by hydrolysis and oxidation [27]. Therefore, we tested the option to keep the optimal indisulam-SSM formulation in dry form after lyophilization. Visual appearance of lyophilized cakes of indisulam-SSM was similar to SSM controls: white, cotton like cakes elegant in appearance. Particle sizes of both SSM alone and indisulam-SSM were not significantly different before and after lyophilization (Table 1). Based on HPLC results, all the drug molecules were incorporated in the micelles after lyophilization.
DISCUSSION
Drug incorporation did not change the particle size of simple and mixed micelles (Table 1), most probably because of the dominating effect of the PEG molecules to the overall size of micelles. Similarly addition of small sized drug molecules into the core did not make a significant change in size of SSM and SSMM.
Indisulam was solubilized 40 folds higher in SSM than in SSMM. The big difference in the solubilization potential of SSM and SSMM can be explained with the fact that indisulam is located at the interface between the hydrophobic core and relatively hydrophilic palisade region. For SSMM, most of this area may be occupied by EPC molecules, allowing only very few molecules of indisulam to reside, causing lower drug solubilization in SSMM.
Based on in vitro cytotoxicity experiments on MCF-7 cell line, indisulam in SSM proved to be more effective than in DMSO (Fig. 4). The reason for increased activity is yet to be investigated. However, it was not due to the additive effect of PEGylated lipids, since PEGylated lipids alone did not show any significant toxicity upto 150 µM.
Micellar formulations were successfully lyophilized. We believe that slightly smaller size for indisulam-SSM prepared for freeze drying (Table 1) is due to the higher concentration of lipids (5mM vs. 10mM), which may affect the extension of flexible PEG chains. However, particle size of samples in each case was not significantly different (p>0.05 5mM micellar samples compared to 10mM micellar samples).
In conclusion, molecular dispersion of indisulam in aqueous media was increased at least 80 folds when solubilized in SSM (drug concentration in aqueous media ≤ 5µg/ml verses in SSM 400 µg/ml). SSMM were not as efficient solubilizer as SSM, and only improved aqueous solubility by 2 folds. This was probably due to competition of indisulam molecules with EPC for the same location in SSMM micelles. Drug incorporation did not change the mean size of the micelles. Based on HPLC results more than 95% of the drug was incorporated in the optimized micellar system with no precipitation and SSP formation. In vitro studies using MCF-7 breast cancer cell line showed that indisulam-SSM is more effective than indisulam in DMSO. Optimized indisulam-SSM formulation was successfully lyophilized without any addition of lyo-and cryo-protectants indicating that acceptable shelf life can be achieved for this optimized indisulam formulation. We propose that indisulam-SSM should be further developed as a novel nanomedicine for targeted treatment of breast cancer.
Acknowledgments
This work was supported, in part, by Eisai Inc., NIH grant RO1 CA121797, NIH grant C06RR15482, VA Merit Review, and DOD contract W81XWH-07-1-0445.
Abbreviations
- E7070
N-(3-chloro-7-indolyl)-1, 4-benzenedisulfonamide
- BSS
balanced salt solution
- CMC
Critical Micellar Concentration
- DSPE-PEG2000
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy- poly(ethylene glycol 2000)
- EPC
egg phosphatidylcholine
- ED50
median effective dose
- EMEM
Eagle’s minimal essential medium
- FDA
Food and Drug Administration
- NCI
National Cancer Institute
- PEG
polyethylene glycol
- QELS
quasi-elastic light scattering
- RP-HPLC
Reverse Phase-High Performance Liquid Chromatography
- SRB
Sulforhodamine B
- SSM
Sterically Stabilized Micelles
- SSMM
Sterically Stabilized Mixed Micelles
- SSP
Sterically Stabilized Particles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ashok B, Arleth L, Hjelm RP, Rubinstein I, Onyuksel H. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: effects of PEG chain length and PC incorporation. J.Pharm.Sci. 2004 Oct;93(10):2476–2487. doi: 10.1002/jps.20150. [DOI] [PubMed] [Google Scholar]
- 2.Krishnadas A, Rubinstein I, Önyüksel H. Sterically Stabilized Phospholipid Mixed Micelles: In Vitro Evaluation as a Novel Carrier for Water-Insoluble Drugs. Pharm.Res. 2003;20(2):297–302. doi: 10.1023/a:1022243709003. [DOI] [PubMed] [Google Scholar]
- 3.Onyuksel H, Ikezaki H, Patel M, Gao XP, Rubinstein I. A novel formulation of VIP in sterically stabilized micelles amplifies vasodilation in vivo. Pharm.Res. 1999 Jan;16(1):155–160. doi: 10.1023/a:1018847501985. [DOI] [PubMed] [Google Scholar]
- 4.Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine. 2005 Sep;1(3):193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv.Drug Deliv.Rev. 2004 May 7;56(9):1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Lukyanov AN, Gao Z, Mazzola L, Torchilin VP. Polyethylene Glycol-Diacyllipid Micelles Demonstrate Increased Accumulation in Subcutaneous Tumors in Mice. Pharm.Res. 2002;19(10):1424–1429. doi: 10.1023/a:1020488012264. [DOI] [PubMed] [Google Scholar]
- 7.Koo OM, Rubinstein I, Onyuksel H. Camptothecin in sterically stabilized phospholipid micelles: a novel nanomedicine. Nanomedicine. 2005 Mar;1(1):77–84. doi: 10.1016/j.nano.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Hammad MA, Muller BW. Solubility and stability of lorazepam in bile salt/soya phosphatidylcholine-mixed micelles. Drug Dev.Ind.Pharm. 1999 Apr;25(4):409–417. doi: 10.1081/ddc-100102190. [DOI] [PubMed] [Google Scholar]
- 9.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv.Drug Deliv.Rev. 2004 Apr 29;56(8):1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Owa T, Yoshino H, Okauchi T, Yoshimatsu K, Ozawa Y, Sugi NH, et al. Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J.Med.Chem. 1999 Sep 23;42(19):3789–3799. doi: 10.1021/jm9902638. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Yamamoto N, Shimoyama T, Horiike A, Fujisaka Y, Takayama K, et al. Phase I pharmacokinetic and pharmacogenomic study of E7070 administered once every 21 days. Cancer Sci. 2005;96(10):721–728. doi: 10.1111/j.1349-7006.2005.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozawa Y, Sugi NH, Nagasu T, Owa T, Watanabe T, Koyanagi N, et al. E7070, a novel sulphonamide agent with potent antitumour activity in vitro and in vivo. Eur.J.Cancer. 2001 Nov;37(17):2275–2282. doi: 10.1016/s0959-8049(01)00275-1. [DOI] [PubMed] [Google Scholar]
- 13.Smyth J, Aamdal S, Awada A, Dittrich C, Caponigro F, Schoffski P, et al. Phase II study of E7070 in patients with metastatic melanoma. Annals of Oncology. 2005;16(1):158–161. doi: 10.1093/annonc/mdi016. [DOI] [PubMed] [Google Scholar]
- 14.Fukuoka K, Usuda J, Iwamoto Y, Fukumoto H, Nakamura T, Yoneda T, et al. Mechanisms of action of the novel sulfonamide anticancer agent E7070 on cell cycle progression in human non-small cell lung cancer cells. Invest.New Drugs. 2001;19(3):219–227. doi: 10.1023/a:1010608317361. [DOI] [PubMed] [Google Scholar]
- 15.Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg.Med.Chem.Lett. 2004;14(1):217–223. doi: 10.1016/j.bmcl.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 16.Tripathy D. Capecitabine in Combination with Novel Targeted Agents in the Management of Metastatic Breast Cancer: Underlying Rationale and Results of Clinical Trials. Oncologist. 2007;12(4):375. doi: 10.1634/theoncologist.12-4-375. [DOI] [PubMed] [Google Scholar]
- 17.Dittrich C, Zandvliet AS, Gneist M, Huitema AD, King AA, Wanders J. A phase I and pharmacokinetic study of indisulam in combination with carboplatin. Br.J.Cancer. 2007 Feb 26;96(4):559–566. doi: 10.1038/sj.bjc.6603606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakatsu N, Yoshida Y, Yamazaki K, Nakamura T, Dan S, Fukui Y, et al. Chemosensitivity profile of cancer cell lines and identification of genes determining chemosensitivity by an integrated bioinformatical approach using cDNA arrays. Mol.Cancer.Ther. 2005 Mar;4(3):399–412. doi: 10.1158/1535-7163.MCT-04-0234. [DOI] [PubMed] [Google Scholar]
- 19.Raymond E, ten Bokkel Huinink WW, Taieb J, Beijnen J, Faivre S, Wanders J, et al. Phase I and Pharmacokinetic Study of E7070, a Novel Chloroindolyl Sulfonamide Cell-Cycle Inhibitor, Administered as a One-Hour Infusion Every Three Weeks in Patients With Advanced Cancer. Journal of Clinical Oncology. 2002;20(16):3508. doi: 10.1200/JCO.2002.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Yokoi A, Kuromitsu J, Kawai T, Nagasu T, Sugi NH, Yoshimatsu K, et al. Profiling novel sulfonamide antitumor agents with cell-based phenotypic screens and array-based gene expression analysis. Mol.Cancer.Ther. 2002 Feb;1(4):275–286. [PubMed] [Google Scholar]
- 21.Punt C, Fumoleau P, van de Walle B, Faber M, Ravic M, Campone M, A study by the EORTC-Early Clinical Studies Group (ECSG) Phase I and pharmacokinetic study of E7070, a novel sulfonamide, given at a daily times five schedule in patients with solid tumors. Annals of Oncology. 2001;12(9):1289–1293. doi: 10.1023/a:1012287111922. [DOI] [PubMed] [Google Scholar]
- 22.Zandvliet AS, Copalu W, Schellens JH, Beijnen JH, Huitema AD. Saturable binding of indisulam to plasma proteins and distribution to human erythrocytes. Drug Metab.Dispos. 2006 Jun;34(6):1041–1046. doi: 10.1124/dmd.105.008326. [DOI] [PubMed] [Google Scholar]
- 23.Papazisis KT, Geromichalos GD, Dimitriadis KA, Kortsaris AH. Optimization of the sulforhodamine B colorimetric assay. J.Immunol.Methods. 1997 Oct 27;208(2):151–158. doi: 10.1016/s0022-1759(97)00137-3. [DOI] [PubMed] [Google Scholar]
- 24.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat.Protoc. 2006;1(3):1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 25.Lim SB, Rubinstein I, Onyüksel H. Freeze drying of peptide drugs self-associated with long-circulating, biocompatible and biodegradable sterically stabilized phospholipid nanomicelles. Int J Pharm. 2008;356(1–2):345–350. doi: 10.1016/j.ijpharm.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JX, Hansen CB, Allen TM, Boey A, Boch R. Lipid-derivatized poly (ethylene glycol) micellar formulations of benzoporphyrin derivatives. J.Controlled Release. 2003;86(2–3):323–338. doi: 10.1016/s0168-3659(02)00442-x. [DOI] [PubMed] [Google Scholar]
- 27.Hauss JD. Oral Lipid-Based Formulations: Enhancing the Bioavailability of Poorly Water-soluble Drugs (Drugs and the Pharmaceutical Sciences) Vol. 170. New York: Informa Healthcare; 2007. pp. 158–168. [Google Scholar]