Abstract
Purpose
To evaluate relations among resection volume, seizure outcome, and cognitive morbidity after temporal lobectomy for intractable epilepsy.
Methods
Thirty patients with mesial temporal sclerosis were evaluated with pre- and postoperative WAIS III, WMS III, and 3D coronal SPGR magnetic resonance imaging (MRI). Preoperative whole brain volumes were calculated with Statistical Parametric Mapping (SPM). Resection volume was calculated by manual tracing. Systat was used for statistical analysis.
Results
All resections included the temporal tip, at least 1 cm of the superior temporal gyrus and 3 cm to 5 cm of the middle and inferior temporal gyri. Left were significantly smaller than right temporal resections. Seizure free patients had significantly larger resections. Immediate verbal memory was significantly worse in patients after left temporal lobectomy. Surgical outcome and resection volume did not affect postoperative neuropsychological results.
Conclusions
Dominant temporal lobe resections are associated with immediate verbal memory deficits. Larger resection volume was associated with improved seizure control but not worse cognitive outcome.
Keywords: Epilepsy, Cognition, Neuropsychology, Temporal Lobe, Resection, Mesiotemporal Sclerosis
Introduction
The goal of surgery for mesial temporal lobe epilepsy (MTLE) is to perform a resection large enough to achieve seizure freedom, but small enough to avoid functional deficits. Some studies suggest that more extensive resections in patients with MTLE are more likely to achieve seizure control, although limited data are available (1). The relation between surgery extent and cognitive outcome is even less clear (2). We used MRI to measure resection volume and assess its relation to neuropsychological outcome in patients with MTS.
Methods
Patients
Thirty consecutive temporal epilepsy patients (14 male; mean age 38.7, range 18 to 57 years) had clinical evaluation with interictal EEG, ictal video-EEG, and MRI. With the exception of mesial temporal sclerosis (MTS), only patients who were nonlesional were included in the study. All patients had mesiotemporal sclerosis preoperatively by MRI criteria of T2 hyperintensity and volume loss as evaluated by an experienced radiologist or by post resection pathology. The study was approved by the NINDS Institutional Review Board. All patients were English speaking.
Neuropsychological Evaluations
All patients underwent neuropsychological evaluations with Weschler Adult Intelligence Score III (WAIS III) and Weschler Memory Score III (WMS III) preoperatively and a mean of 19 months post operatively. All patients had the same tests of memory and IQ before and after the surgery and all calculations were done using normative data and standard scores with a mean of 100 +/− 15. The WMS III was used to assess memory and learning of both verbal and visually presented material, immediate and delayed conditions. All have the same basic format: the information was presented in oral form for the verbal tests and as pictures for the visual tests with recall requested immediately after presentation. The patients were told that recall would be requested later and after a delay of approximately 25 minutes, recall was again requested. Verbal memory was assessed with two paragraph length stories read; verbal learning with paired associate learning. Visual memory was assessed with a ‘study’ period during which the patient looked at a standard set of faces each for 2 seconds followed by a recognition trial in which the study pictures were embedded in foils. Visual memory was also assessed with Family Pictures, a set of 4 pictures presented in sequence for 10 seconds each. The subject is then asked to recall the characters in each picture, their placement in a matrix and their activity in the picture.
The WAIS III is a multidimensional assessment of both verbal and nonverbal tasks that generate a Verbal IQ, Performance IQ and a Full Scale IQ. Word knowledge, fund of factual information, abstract reasoning, digit repetition and mental arithmetic contribute to the Verbal IQ. The Performance IQ is assessed with block design reproduction, matrix reasoning, picture completion, digit-symbol substitution and symbol matching. The Full Scale IQ is a weighted average.
Imaging
All patients had preoperative and postoperative images (3D SPGR, matrix: 256×256, 1.5 mm slices, TR: 27, TE:3, TA:20) on a GE Signa 1.5 Tesla MRI scanner (GE Medical Systems, Milwaukee, WI). The images were loaded into a unix based Sun system. MRI's were viewed in the coronal plane and manual tracing was performed in MEDx (Medical Numerics, Germantown, MD) to draw regions of interest (ROI's). Free borders including the medial, inferior, and lateral temporal convexities were traced according to symmetry and pre-resection images. These two dimensional ROI's were then reconstructed into three dimensional volumes that represented the absent brain tissue. The measurer was blinded to the clinical outcome of each patient at the time of analysis.
Hippocampal remnant shrinkage has been shown to begin within a week of surgery and stabilize by 3 months. All our patients were imaged more than 3 months after surgery, to avoid acute surgical changes that could affect the volume analysis.
Statistical Parametric Mapping (SPM) version 96 (Wellcome Department of Cognitive Neurology, London, England) was used on the MEDx platform to calculate the whole brain volume on the preoperative scans after manually defining the anterior commisure as the point of origin. After SPM segmentation, random sampling of each patient's scan was done to determine 4 segmentation ranges for background (0), white matter (177-183), gray matter (239-246), and CSF (113-119). A common low pass threshold of 177 was chosen for all patients to include gray and white matter and exclude CSF. Using a BET brain extraction program the extracerebral components were removed prior to calculating the volumes to ensure extraneous pixel intensities were not included. These volumes were then used to calculate the percentage of brain removed and normalize the raw resection volume values. SPM was not used to calculate post resection volumes as the segmentation program appears to be unreliable when large brain lesions are present (6) and our resections acted as lesions in the SPM algorithm.
Surgery
Temporal lobectomies were tailored to individual preresection evaluations. One patient had surgery at Children's National Medical Center; the remainder were performed by one surgeon (JH) at NIH. All resections included the temporal tip, a minimum of 1 cm of the anterior part of the superior temporal gyrus and between 3 cm to 5 cm of the middle and inferior temporal gyri. Surgical removal of the entire head of the hippocampus and the anterior part of the tail of the hippocampus was achieved in most patients. All patients had intraoperative electrocorticograms for the purpose of interictal spike detection, and 6 (all with a left hemisphere focus) had subdural electrode implantation for preoperative functional mapping. Language mapping performed either intraoperatively or via subdural electrodes included visual stimulus naming and counting during electrical stimulation. We used a bipolar Ojemann cortical stimulator (5 mm apart). Intraoperative stimulus pulse width was 1 msec, intensity 2-16 mA and stimulus frequency 50 Hz. With subdural electrodes, cortical stimulation was done mainly in referential mode and occasionally in bipolar mode, with stimulus pulse width 0.5-1 msec, frequency 50 Hz, and intensity 2-16 mA. Stimulation was at least 4 seconds, with monitoring of afterdischarges.
Outcome classification and Statistical Analysis
The primary outcome measure for the study was the relation of resection volume to seizure outcome. We also assessed the effect of side of surgery, resection volume, and seizure outcome on postoperative neuropsychological tests. The change in neuropsychological scores, absolute resection volumes, percent resections, side of surgery, and outcomes were compared using student's two sample t tests and analysis of variance with Systat (Systat inc., Point Richmond CA). Significance was set at p<0.05 after correction for multiple comparisons.
Results
There were 14 right and 16 left temporal lobectomies. Most patients continued to take the same medications after the surgery, at least until after their postoperative neuropsychological evaluation. Although several patients took fewer antiepileptic drugs AEDs after surgery, and none more, the mean number did not change. All patients were left language dominant based on Wada evaluation. All had MTS on pathological examination.
Although patients who had right and left resections were equally likely to become seizure-free, resection volume and percent resection were significantly higher in the 21 patients who were seizure free, compared to the nine who were not (table 1). Right nondominant side resections had significantly higher absolute volumes as well as percent resections (table 2).
Table 1.
Effect of resection size on outcome
| Seizure-free (21) | Persistent seizures (9) | ||
|---|---|---|---|
| Resection volume | 27441.2 ±10313.5 | 21037.0 ± 5050.8 | P<0.05 |
| Percent resection | 2.430±0.866 | 1.854±0.391 | P<0.05 |
Table 2.
Volume of right and left temporal resections
| Right temporal | Left temporal | ||
|---|---|---|---|
| Resection volume | 29958.9 ± 10400.6 | 21635.9 ± 6665.1 | P<0.02 |
| Percent resection | 2.65 ± 0.91 | 1.93 ± 0.51 | P<0.02 |
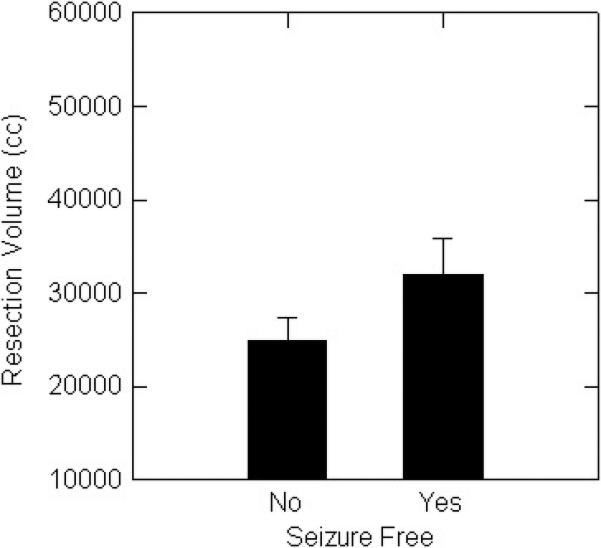
When left and right temporal resections were considered separately, among patients with left temporal lobectomies seizure-free patients had significantly greater resection volume (Figure 1A) and a trend toward greater percent resection (Figure 1B), than non seizure-free patients. For right temporal lobectomies, seizure-free patients had significantly greater percent resection (Figure 1C), and a trend toward larger resection volume (Figure 1D).
Figure 1.
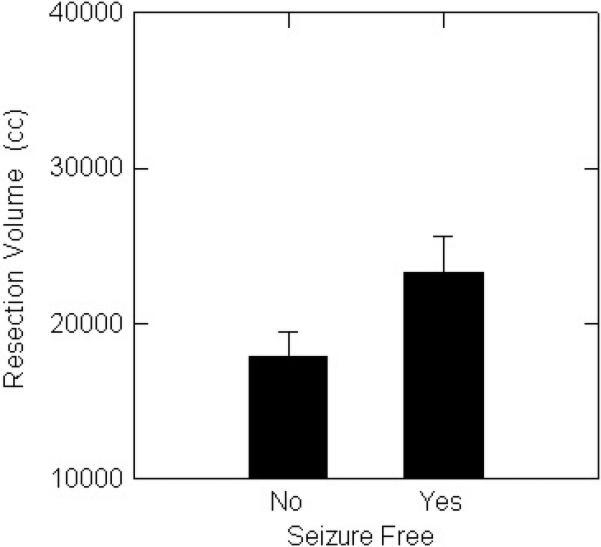
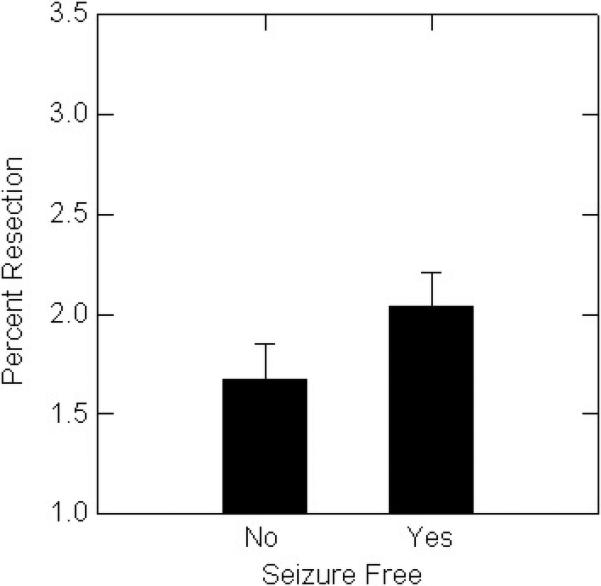

A. Seizure-free patients with left resections had significantly (p<0.05) larger percent resection than patients with persistent seizures.
B. There was a non-significant trend for seizure-free patients with left resections to have larger percent resection than patients with persistent seizures.
C. Seizure-free patients with right resections had significantly (p<0.05) larger volumes than patients with persistent seizures.
D. There was a non-significant trend for seizure-free patients with right resections to have larger volumes than patients with persistent seizures.
After surgery, there was a significant increase in Wechsler Performance and Full Scale scores, in the patients as a whole and those with a right temporal focus. (table 3). Left temporal patients had a non-significant tendency to have lower initial Verbal Intelligence Quotient scores.
Table 3.
Preoperative and Postoperative Wechsler Adult Intelligence Scale Scores
| Verbal | Performance | Full Scale | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | post | |
| All Patients | 96.6±16.5 | 97.0±17.4 | 93.9±12.4* | 98.6±14.9* | 95.4±14.4* | 98.6±16.9* |
| Left Focus | 91.9±12.1 | 91.5±10.5 | 91.7±12.3 | 95.1±12.5 | 91.5±11.6 | 92.8±11.0 |
| Right Focus | 101.9±19.6 | 103.4±21.6 | 96.4±12.5* | 102.5±16.9* | 99.7±16.5* | 105.4±20.2* |
P <0.01
There was a small but significant decline in immediate verbal memory in patients with left sided (−7.9 ± 18.3) when compared to those with right sided (3.3 ± 9.5) surgery (F-ratio 3.23; p < 0.05). Mean immediate verbal memory was non-significantly lower after surgery in patients with left-sided resection (85.0 ±19.3 versus 92.9 ±18.9; paired Student's t = 1.7: p= 0.10).
There was no relation between resection volume or percent resection and change in any of the neuropsychological parameters. For left temporal lobectomy patients, there was no relation between resection volume and auditory memory change (Figure 2A). For right temporal lobectomies, there was no relation between resection volume and visual memory change (Figure 2B). Outcome for seizure control did not affect neuropsychological results, for right or left resections, or the group as a whole.
Figure 2.
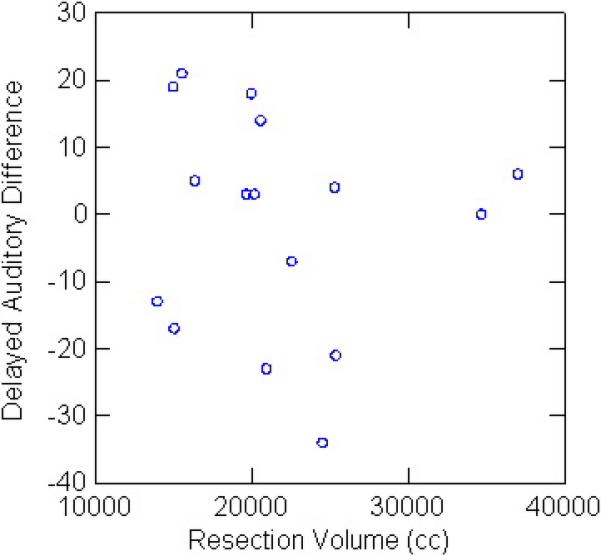
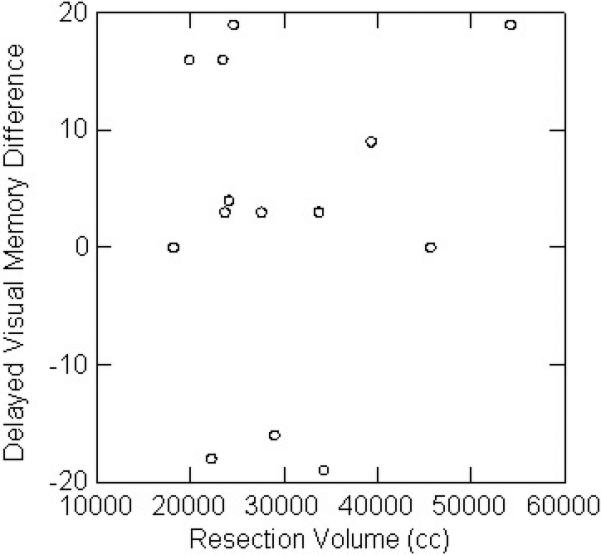
A. There was no relation between left temporal lobectomy volume and auditory memory change.
B. There was no relation between right temporal lobectomy volume and visual memory change.
Discussion
We found little evidence for worsening of neuropsychological performance in patients with temporal lobe epilepsy and MTS after resection. In contrast, larger resection volume was associated with better surgical outcome.
Several previous studies have found a relation between resection size and outcome. Using MRI volume studies, more extensive total temporal lobe resection was associated with a higher chance of good outcome (3). Longer hippocampal but not lateral temporal gyrus resection was associated with improved seizure outcome (4). Longer left inferior or basal temporal gyrus resection was associated with greater verbal memory decline. Some studies found no association between post-operative outcome and MRI measurement of resection extent (5)
Previous studies have shown a decline in neuropsychological parameters after temporal lobectomy that may be independent of resection volume (6, 7). Even selective amygdalohippocampectomy may lead to worse material-specific postoperative memory performance, although there was a greater effect on figural memory of resections including temporal pole and some lateral structures, regardless of laterality (8). One study found non-significant relations between decreased postoperative verbal retention and left mesial temporal resection extent, as well as visual material retention with right resection extent (9).
We found milder neuropsychological effects than reported in some previous studies. High preoperative scores, associated with worsening of memory and language parameters after left temporal lobe resection in one study, are unlikely to have affected our results, since preoperative performance was within the normal range (10). In our study, the number and type of AEDs were unchanged overall after surgery, although reductions in several patients may have ameliorated neuropsychological changes.
We tested patients at a mean of 19 months after surgery, which may not be long enough to show the full effects of resection. After amygdalo-hippocampectomy and neocortical temporal resection between 2.5 and 8 cm, verbal memory performance showed ongoing decline for up to 2 years after surgery; seizure outcome did not affect results (11).
All our patients had neuropsychological testing either during intraoperative electrocorticography or subdural recording. It is uncertain whether this could have affected our results. Comparisons of standard and tailored resections show conflicting outcome. In one study, left TLE patients who had standard resection showed gains, and patients with tailored resections losses, on several tasks. For the standard resection group, good cognitive outcome was associated with restricted superior temporal gyrus (STG) resection; larger standard STG resections led to decreased verbal intelligence (12). In contrast, tailored resection in patients with neocortical spikes on ECOG led to improved postoperative intelligence quotient and visual naming, while patients without preoperative spikes had postoperative declines; resection distance from the temporal tip did not affect outcome (13). Our data suggest that larger, tailored resections may be associated with better seizure outcome, but do not necessarily have worse cognitive effects.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, NINDS.
References
- 1.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 2.Hermann BP, Perrine K, Chelune GJ, Barr W, Loring DW, Strauss E, Trenerry MR, Westerveld M. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999;13:3–9. doi: 10.1037//0894-4105.13.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Awad IA, Katz A, Hahn JF, Kong AK, Ahl J, Luders H. Extent of Resection in Temporal Lobectomy for Epilepsy. I. Interobserver Analysis and Correlation with Seizure Outcome. Epilepsia. 1989;30:756–62. doi: 10.1111/j.1528-1157.1989.tb05335.x. [DOI] [PubMed] [Google Scholar]
- 4.Joo EY, Han HJ, Lee EK, Choi S, Jin JH, Kim JH, Tae WS, Seo DW, Hong S-C, Lee M, Hong SB. Resection extent versus postoperative outcomes of seizure and memory in mesial temporal lobe epilepsy. Seizure. 2005;14:541–551. doi: 10.1016/j.seizure.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Alsaadi TM, Ulmer JL, Mitchell MJ, Morris GL, Swanson SJ, Mueller WM. Magnetic resonance analysis of postsurgical temporal lobectomy. J Neuroimaging. 2001 Jul;11(3):243–7. doi: 10.1111/j.1552-6569.2001.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 6.Naugle RI, Chelune GJ, Cheek R, Luders H, Awad IA. Detection of Changes in Material-Specific Memory Following Temporal Lobectomy Using the Wechsler Memory Scale-Revised. Archives of Clinical Neuropsychology. 1993;8:381–395. [PubMed] [Google Scholar]
- 7.Lee TM, Yip JT, Jones-Gotman M. Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia. 2002;43:283–91. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- 8.Helmstaedter C, Oltmanns F, Schramm J, Lehmann TN. Differential effects of temporal pole resection with amygdalohippocampectomy versus selective amygdalohippocampectomy on material-specific memory in patients with mesial temporal lobe epilepsy. Epilepsia. 2008;49:88–97. doi: 10.1111/j.1528-1167.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- 9.Katz A, Awad IA, Kong AK, Chelune GJ, Naugle RI, Wyllie E, Beauchamp G, Lüders H. Extent of resection in temporal lobectomy for epilepsy. II. Memory changes and neurologic complications. Epilepsia. 1989 Nov-Dec;30(6):763–71. doi: 10.1111/j.1528-1157.1989.tb05336.x. [DOI] [PubMed] [Google Scholar]
- 10.Chelune GJ, Naugle RI, Luders H, Awad IA. Prediction of cognitive change as a function of preoperative ability status among temporal lobectomy patients seen at six months follow up. Neurology. 1991;41:399–404. doi: 10.1212/wnl.41.3.399. [DOI] [PubMed] [Google Scholar]
- 11.Alpherts WCJ, Vermeulen J, van Rijen PC, da Silva F H Lopes, van Veelen CWM. Verbal memory decline after temporal epilepsy surgery?: A 6-year multiple assessments follow-up study. Neurology. 2006 Aug 22;67(4):626–31. doi: 10.1212/01.wnl.0000230139.45304.eb. [DOI] [PubMed] [Google Scholar]
- 12.Alpherts WC, Vermeulen J, van Rijen PC, Lopes da Silva FH, van Veelen CW, Mvan Veelen CW. Standard versus tailored left temporal lobe resections: differences in cognitive outcome? Neuropsychologia. 2008 Jan 31;46(2):455–60. doi: 10.1016/j.neuropsychologia.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Leijten F, Alpherts W, Huffelen A, Vermeulen J, Rijen P. The Effects on Cognitive Performance of Tailored Resection in Surgery for Nonlesional Mesiotemporal Lobe Epilepsy. Epilepsia. 2005;46:431–439. doi: 10.1111/j.0013-9580.2005.33604.x. [DOI] [PubMed] [Google Scholar]


